Abstract
 DNA interstrand crosslinks (ICLs) are the clinically most relevant adducts formed by many anti-tumor agents. To facilitate the study of biological responses triggered by ICLs, we developed a new approach toward the synthesis of mimics of nitrogen mustard ICLs. 7-Deazaguanine residues bearing acetaldehyde groups were incorporated into complementary strands of DNA and crosslink formation induced by double reductive amination. Our strategy enables the synthesis of major groove crosslinks in high yields and purity.
DNA interstrand crosslinks (ICLs) are the clinically most relevant adducts formed by many anti-tumor agents. To facilitate the study of biological responses triggered by ICLs, we developed a new approach toward the synthesis of mimics of nitrogen mustard ICLs. 7-Deazaguanine residues bearing acetaldehyde groups were incorporated into complementary strands of DNA and crosslink formation induced by double reductive amination. Our strategy enables the synthesis of major groove crosslinks in high yields and purity.
A variety of bifunctional electrophilic agents have the ability to covalently link two complementary strands of double stranded DNA, leading to the formation of DNA interstrand crosslinks (ICLs). Due to their ability to efficiently block replication and transcription, ICLs are among the most cytotoxic DNA lesions known. Their cytotoxic potential has found application in anticancer chemotherapy and crosslinking agents such as the nitrogen mustards, platinum complexes, chloro ethyl nitroso ureas and mitomycin C are among the most widely used antitumor agents today.1,2 Many factors contribute to the resistance of tumor cells to treatment with crosslinking agents and the removal of ICLs from DNA has emerged as one of the most important ones.3 Repair pathways for ICLs have their evolutionary origin in the necessity to counteract the threat posed by endogenous and exogenous metabolites, for example malonic dialdehyde and formaldehyde.4 ICL repair is an inherently complex process as the two strands need to be unhooked and repaired and no intact template is available for repair synthesis.2 This complexity provides one explanation why ICL repair pathways remain poorly understood.
A second problem in the study of ICL repair has been the limited availability of defined ICL adducts.5 ICLs were initially synthesized by the reaction of DNA with crosslinking agents followed by isolation and purification of the ICL.6-8 This approach yields mixtures of products (mono adducts, intra- and interstrand crosslinks) and the desired ICLs usually make up only a small fraction (typically 1-5%) of all the products formed. Two other more efficient approaches to the chemical synthesis of ICLs have subsequently been developed. The first one was based on the crosslinking of nucleosides outside of DNA followed by incorporation of the crosslinked dimer into DNA using solid-phase synthesis.9 This approach has been particularly successful in yielding ICLs that connect two bases through their Watson-Crick faces, although the solid-phase synthesis procedures are complicated by the connected nucleoside dimers. A second approach consists of the site-specific incorporation of post-synthetically modifiable crosslink precursors on one or two opposing strands of DNA, annealing of the two single strands and subsequent use of a specific coupling reaction to generate the ICL. This concept has been used in the synthesis of ICLs containing disulfide, psoralen, malondialdehyde and alkyl linkages.10,11 With the exception of disulfide ICLs, there has been a lack of efficient syntheses of ICLs formed in the major groove of DNA, where adducts by the clinically most important ICL-forming agents including the nitrogen mustards (NMs) are formed.
NMs preferentially form ICLs between the N7 positions of dG residues in a 5′-GNC sequence context and introduce a slight distortion into the DNA.6,7,12,13 Following the initial pioneering studies of NM ICLs by the Loechler and Hopkins groups no further efforts toward high yielding synthesis of these adducts have been reported.6
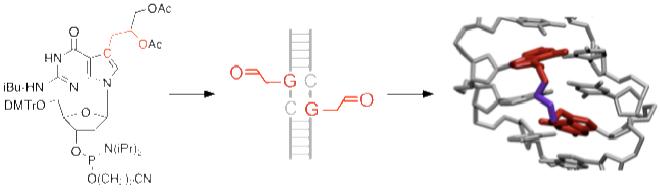
We designed a strategy for the synthesis of NM ICL mimics 2 based on the incorporation of ICL precursor nucleosides on opposing strands of DNA and the use of a subsequent specific coupling reaction to establish the ICL (Figure 1). We reasoned that alkylamine-containing crosslinks may be accessed by a double amination reaction from two aldehyde groups (3) on complementary DNA strands using an appropriate amine. Since guanine bases alkylated at the 7 position in the native NM ICL 1 are prone to depurination due to the positive charge on nitrogen, we decided to pursue the synthesis of the more stable, isosteric 7-deaza analogs 2. The increased hydrolytic stability of the 7-deaza compounds should make it possible to incorporate them into DNA using solid-phase synthesis and make them attractive substrates for biological studies. We envisioned that the aldehyde would be introduced into DNA masked as a protected diol 4,14 using standard phosphoramidite chemistry (Figure 1).
Figure 1.

Structures of NM ICLs 1 and strategy for the synthesis of NM ICL analog 2. NM ICLs (1) connect two complementary DNA strands by connecting two guanine bases through the N(7) positions. Our target NM ICL mimic 2 has the nitrogen at the 7 positions replaced with carbon to render the glycosidic bond of the ICL stable for synthesis and functional studies (X = amine-containing compound). We envisioned that ICL 2 could be obtained via double reductive amination of an aldehyde 3 with an appropriate amine, XH. 3 in turn could be derived from phosphoramidite 4, where the aldehyde is masked as a protected diol.
The synthesis of 4 (Scheme 1) started with 6-chloro-7-deaza-3′,5′-di-O-p-toluoyl-2′-deoxyguanosine 5,15 which was protected as an isobutyric amide at the N(2) position and selectively iodinated at C(7).16 A Stille coupling reaction was then used to introduce the allyl group in 6. Treatment with pyrimidine-2-carboxaldoxime17 restored the deazaguanine core and the toluoyl protecting groups in the carbohydrate moiety were replaced with TBDMS. The allyl group of 7 was then oxidized to corresponding diol (8) using osmium tetroxide and the newly generated hydroxyl groups were protected as acetate esters. Finally, the TBDMS protecting groups were removed and the sugar moiety was elaborated to the phosphoramidite 4 using standard procedures.
Scheme 1.

Synthesis of the ICL precursor phosphoramidite 4
Using solid phase synthesis, 4 was incorporated into two complementary DNA strands as a part of a 5′-d(GNC) sequence (10a-d, Figure 2A), which has been shown to be the preferred site for NM ICL formation.7 The two single-stranded oligonucleotides were purified by solid phase extraction using TOP-cartridges (Varian) and the incorporation and integrity of the diol was verified by ESI-MS (Suppl. Table 1). Exploratory experiments revealed that the diol-containing DNA could be oxidized to the aldehyde and derivatized with an aldehyde-reactive semicarbazide fluorescent dye (data not shown), validating our approach for the generation of the aldehyde precursor.
Figure 2.

Synthesis of DNA ICLs through reductive amination. A. Phosphoramidite 4 was incorporated into complementary strands of DNA and deprotected (10a-d). Following annealing and oxidization with NaIO4 to aldehydes 11a-d, reaction with ammonium choride, methylamine, hydrazine, ethylene diamine or dimethylethylene diamine followed by reduction with NaBH3CN was attempted to generate ICLs 12. B. Structures of the target ICLs 12a-h. C. Denaturing PAGE analysis of the reductive amination reaction of the two aldheyde containing 20-mers. The amine group X resulting from the reaction, as well as presence or absence of NaIO4 and NaBH3CN in the reaction mixture are indicated above the gel. The positions of marker 20-mer (for ssDNA) and 40-mer (for ICL containing DNA) oligomers are indicated. The gel was stained with methylene blue.
With the aldehyde containing single-stranded oligonucleotides in hand, we investigated the use of these building blocks in ICL formation. To circumvent the need for lengthy manipulations of the potentially unstable aldehyde functionalities, the two diol-containing strands were first annealed and subsequently oxidized with sodium periodate. Excess oxidizing reagent was quenched by addition of sodium sulfite and the aldehyde-containing oligonucleotides were treated with a variety of amines and NaBH3CN. Unexpectedly, incubation with NH4OAc or methylamine did not lead to any detectable formation of ICLs 12a or 12b, as only a band corresponding to the 20-mer single stranded oligonucleotide substrates was visible on a denaturing polyacrylamide gel (Figure 2C, lanes 2 and 3). By contrast, treatment with hydrazine, ethylenediamine or N,N′-dimethylethylenediamine resulted in the formation of a new major band with mobility corresponding to a size roughly double that of the starting 20-mers, indicating that the formation of ICLs 12c, 12d and 12e had occurred (Figure 2B, C, lanes 4, 7 and 10). Whlie the formation of ICLs by the ethylenediamines was dependent on the presence of NaBH3CN, hydrazine ICLs were also formed in the absence of any reducing agent, which could be explained by the formation of a stable hydrazone adduct (Figure 2C, compare lanes 4 and 5). Treatment with excess hydrazine and formaldehyde readily reversed of the non-reduced form of hydrazine ICL, but not the reduced form of 12c, indicating that the hydrazone linkage was indeed reduced by NaBH3CN. The formation of an ICL was dependent on the presence of aldehyde reactive groups on both strands of DNA, as no slower migrating band was formed if the aldehyde functionalized guanine residues were absent or present only on one of the two strands (data not shown). The ICL-containing oligonucleotides were purified by reverse-phase HPLC and subjected to MS and nucleoside composition analysis. ESI-MS analysis of the crosslinked 20-mer oligonucleotides revealed good agreement between the calculated and measured mass, but were not accurate enough to ascertain that the ICLs was present with two reduced amine bonds (Suppl. Table 1). To obtain more precise mass determinations, we repeated the synthesis to obtain the shorter 11-mer ICL-containing oligonucleotides 12f-h. In this case, the molecular weight could be determined for all the ICL-containing oligonucleotides with a precision of ± one mass unit (Suppl. Table 1) consistent wtih ICL formation and reduction of the imine and hydrazone linkages. We further attempted to analyze the ICL-containing oligonucleotides by nucleoside composition analysis and incubated them with phosphodiesterase I, exonuclease III and calf intestine phosphatase, conditions we had previously employed in the analysis of ICLs.11 HPLC analysis of the digestion of the dimethylethylenediamine ICL 12e yielded the expected peaks for the four native nucleosides dA, dC, dG and T and an additional slower eluting peak (Suppl. Figure 1). This peak was identified by MS as the expected crosslinked nucleoside dimer. Unexpectedly, digestion of the hydrazine or ethylenediamine ICLs did not result in the formation of a peak for the crosslinked dimers, as we observed either incomplete digestion or decomposition at the peak in the area of the dimethyl ethlylenediamine dimer (data not shown). Nonetheless, the data from MS analyses and digestion of the dimethyl ethlylenediamine ICL as well as the difference of stability of reduced and non-reduced ICLs and are only consistent with structures 12c-h.
The lack of ICL formation in the reductive amination reaction with ammonia and methylamine deserves some comment. It has previously been shown that since the distance between the N7-dG sites in 5′-d(GNC) sequences (∼8.9Å) is longer than the NM linkage (∼7.5Å) the NM ICLs must induce a bend into DNA (Figure 3).13 In our case, the intrinsically reversible initial imine formation step of the reductive amination reaction apparently does not provide enough strength to lead to the formation of ICLs 12a and 12b with these two amines. To exclude that the inability to form ICL 12a was due to reductive amination of both aldehyde groups with ammonia prior to ICL formation, we also attempted to generate ICL 12a by reaction between a 7-(2-oxoethyl)-7-deazaguanine and a 7-(2-aminoethyl)-7-deazaguanine residue on opposing strands in a d(GNC) sequence. This approach also did not lead to formation of ICL 12a (T.A. and O.D.S., data not shown), suggesting that indeed distance and reactivity constraints were responsible for the inability to complete the reductive amination with ammonia and methylamine. The formation of hydrazine ICLs (12c) and ethylenediamine ICLs (12d, 12e) on the other hand would not require the introduction of a bend in the DNA due to the longer linkage (8.9Å for hydrazine, 11.7Å for ethylenediamine), thereby facilitating ICL formation. An open question is how much the higher reactivity of hydrazine compared to an amine contributes to successful ICL formation in the case of 12c. Upon reaction with the aldehyde, hydrazine forms a hydrazone, which is much more stable than an imine and more reactive toward the aldehyde in the second reaction. Our observations that the reaction of the dialdehyde can lead to efficient ICL formation with hydrazine in the absence of a reducing agent supports this notion (Figure 2C, lane 5).
Figure 3.

Structure of B-from DNA highlighting the distance linked by a NM ICL. The distance between the two crosslinked N7 atoms of guanine is marked by a red dotted line. The nominal distance of an amine, hydrazine and ethylene diamine ICLs are indicated in the right panel.
In conclusion, we have developed synthetic methodology for the preparation of pure oligonucleotides containing site-specific ICLs in the major groove using a double reductive amination reaction of aldehyde functionalities in two complementary DNA strands at a scale of >10nmol. This method should be readily salable by a factor of 10 or 100 to generate ICLs in quantities that will enable detailed structural studies. These defined ICLs have already been proven to be valuable tools for the study of ICL repair mechanisms.18
Supplementary Material
Acknowledgment
We are grateful to Robert Rieger for MS spectral analyses supported by grant NIH/NCRR1 S10 RR023680-1 and to Arthur J. Campbell for discussions and help with the figures. This work was supported by the Swiss National Science Foundation (3130-054873.98), Swiss Cancer League (OCS-01413-08-2003) and the New York State Office of Science and Technology and Academic Research (NYSTAR) grant No. C040069.
Footnotes
Supporting Information Available: Supplementary Table 1, Supplementaty Figure 1, Experimental procedures, 1H, 13C and 31P-NMR spectra of all new compounds, ESI-MS spectra of oligonucleotides 10a-d and 12c-h are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Lawley PD, Phillips DH. Mutat. Res. 1996;355:13–40. doi: 10.1016/0027-5107(96)00020-6. [DOI] [PubMed] [Google Scholar]; Rajski SR, Williams RM. Chem. Rev. 1998;98:2723–2796. doi: 10.1021/cr9800199. [DOI] [PubMed] [Google Scholar]
- 2.Noll DM, Mason TM, Miller PS. Chem. Rev. 2006;106:277–301. doi: 10.1021/cr040478b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panasci L, Paiement JP, Christodoulopoulos G, Belenkov A, Malapetsa A, Aloyz R. Clin. Cancer Res. 2001;7:454–61. [PubMed] [Google Scholar]; McHugh PJ, Spanswick VJ, Hartley JA. Lancet Oncol. 2001;2:483–90. doi: 10.1016/S1470-2045(01)00454-5. [DOI] [PubMed] [Google Scholar]
- 4.Chaw YF, Crane LE, Lange P, Shapiro R. Biochemistry. 1980;19:5525–31. doi: 10.1021/bi00565a010. [DOI] [PubMed] [Google Scholar]; Stone MP, Cho YJ, Huang H, Kim HY, Kozekov ID, Kozekova A, Wang H, Minko IG, Lloyd RS, Harris TM, Rizzo CJ. Acc. Chem. Res. 2008;41:793–804. doi: 10.1021/ar700246x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noll DM, Noronha AM, Wilds CJ, Miller PS. Front. Biosci. 2004;9:421–37. doi: 10.2741/1246. [DOI] [PubMed] [Google Scholar]; Schärer OD. Chembiochem. 2005;6:27–32. doi: 10.1002/cbic.200400287. [DOI] [PubMed] [Google Scholar]
- 6.Ojwang JO, Grueneberg DA, Loechler EL. Cancer Res. 1989;49:6529–37. [PubMed] [Google Scholar]; Millard JT, Raucher S, Hopkins PB. J. Am. Chem. Soc. 1990;112:2459–2460. [Google Scholar]
- 7.Rink SM, Solomon MS, Taylor MJ, Rjur S, McLaughlin LW, Hopkins PB. J. Am. Chem. Soc. 1993;115:2551–2557. [Google Scholar]
- 8.Warren AJ, Hamilton JW. Chem Res Toxicol. 1996;9:1063–71. doi: 10.1021/tx960070c. [DOI] [PubMed] [Google Scholar]; Fischhaber PL, Gall AS, Duncan JA, Hopkins PB. Cancer Res. 1999;59:4363–8. [PubMed] [Google Scholar]
- 9.Harwood EA, Sigurdsson ST, Edfeldt NBF, Reid BR, Hopkins PB. J. Am. Chem. Soc. 1999;121:5081–5082. [Google Scholar]; Li HY, Qiu YL, Moyroud E, Kishi Y. Angew. Chem. Int. Ed. Engl. 2001;40:1471–1475. [PubMed] [Google Scholar]; Noll DM, Noronha AM, Miller PS. J. Am. Chem. Soc. 2001;123:3405–11. doi: 10.1021/ja003340t. [DOI] [PubMed] [Google Scholar]; Wilds CJ, Noronha AM, Robidoux S, Miller PS. J. Am. Chem. Soc. 2004;126:9257–9265. doi: 10.1021/ja0498540. [DOI] [PubMed] [Google Scholar]; Wilds CJ, Xu F, Noronha AM. Chem. Res. Toxicol. 2008;21:686–95. doi: 10.1021/tx700422h. [DOI] [PubMed] [Google Scholar]
- 10.Ferentz AE, Keating TE, Verdine GL. J. Am. Chem. Soc. 1993;115:9006–9014. [Google Scholar]; Erlanson DA, Chen L, Verdine GL. J. Am. Chem. Soc. 1993;115:12583–12584. [Google Scholar]; Kobertz WR, Essigmann JM. J. Am. Chem. Soc. 1997;119:5960–5961. [Google Scholar]; Dooley PA, Tsarouhtsis D, Korbel GA, Nechev LV, Shearer J, Zegar IS, Harris CM, Stone MP, Harris TM. J. Am. Chem. Soc. 2001;123:1730–9. doi: 10.1021/ja003163w. [DOI] [PubMed] [Google Scholar]; Hong IS, Greenberg MM. J. Am. Chem. Soc. 2005;127:10510–1. doi: 10.1021/ja053493m. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hong IS, Greenberg MM. J. Am. Chem. Soc. 2005;127:3692–3. doi: 10.1021/ja042434q. [DOI] [PubMed] [Google Scholar]
- 11.Alzeer J, Schärer OD. Nucleic Acids Res. 2006;34:4458–66. doi: 10.1093/nar/gkl587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rink SM, Hopkins PB. Biochemistry. 1995;34:1439–45. doi: 10.1021/bi00004a039. [DOI] [PubMed] [Google Scholar]; Dong Q, Barsky D, Colvin ME, Melius CF, Ludeman SM, Moravek JF, Colvin OM, Bigner DD, Modrich P, Friedman HS. Proc. Natl. Acad. Sci. U S A. 1995;92:12170–4. doi: 10.1073/pnas.92.26.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan Y-H, Gold B. J. Am. Chem. Soc. 1999;121:11942–11946. [Google Scholar]
- 14.Khullar S, Varaprasad CV, Johnson F. J. Med. Chem. 1999;42:947–50. doi: 10.1021/jm980605u. [DOI] [PubMed] [Google Scholar]
- 15.Seela F, Westermann B, Binding UJ. Chem. Soc. Perkin Trans. 1988;1:697–702. [Google Scholar]
- 16.Ramzaeva N, Seela F. Helv. Chim. Acta. 1995;78:1083–1090. [Google Scholar]
- 17.Buhr CA, Wagner RW, Grant D, Froehler BC. Nucleic Acids Res. 1996;24:2974–80. doi: 10.1093/nar/24.15.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raschle M, Knipsheer P, Enoiu M, Angelov T, Sun J, Griffith JD, Ellenberger TE, Schärer OD, Walter JC. Cell. 2008;134:969–80. doi: 10.1016/j.cell.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


