Abstract
Moderate to severe slipped capital femoral epiphysis leads to premature osteoarthritis resulting from femoroacetabular impingement. We believe surgical correction at the site of deformity through capital reorientation is the best procedure to fully correct the deformity but has traditionally been associated with high rates of osteonecrosis. We describe a modified capital reorientation procedure performed through a surgical dislocation approach. We followed 40 patients for a minimum of 1 year and 3 years from two institutions. No patient developed osteonecrosis or chondrolysis. Slip angle was corrected to 4° to 8° and the mean alpha angle after correction was 40.6°. Articular cartilage damage, full-thickness loss, and delamination were observed at the time of surgery, especially in the stable slips. This technique appears to have an acceptable complication rate and appears reproducible for full correction of moderate to severe slipped capital femoral epiphyses with open physes.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
In slipped capital femoral epiphyses (SCFE), severity of slippage correlates with poor long-term clinical outcome scores and radiographic evidence of osteoarthritis [11, 17, 51]. In situ fixation of higher-grade SCFE has a low surgical risk [11, 29] and has been advocated by authors who believe the deformed hip has the potential to remodel with some restoration of the disturbed anatomic axes [7, 8, 33, 50]. The remodeling potential remains controversial, however [7, 28, 55], and despite remodeling, the head-neck offset will remain abnormal likely leading to potential impingement of the femoral neck with the acetabular cartilage [53]. Impingement in SCFE has been associated with damage of the acetabular cartilage [37], which may explain early onset of osteoarthritis after SCFE [27]. If this is the case, there is a potential role for realignment procedures that can safely restore the mechanical alignment and the femoral head-neck contour.
Realignment procedures for moderate and severe slips have been described for the subcapital [16, 19, 35, 42, 45, 52], basicervical [5, 34, 57], intertrochanteric [46], and subtrochanteric levels [32, 56]. Osteonecrosis as a complication of the surgery is rare in stable SCFE pinned in situ [10, 13, 17, 61]; the risk of necrosis has been described as almost reciprocally proportional to the distance of correction from the physis [1, 13, 15, 22, 30], a phenomenon that can be explained by the vulnerability of the blood supply to the epiphysis [25]. On the other hand, realignment procedures at the level of the deformity (ie, subcapital level) can result in anatomic or near anatomic restoration of the proximal femur. As such, we believe they offer the best chance of correcting the anatomic deformities that can lead to early osteoarthritis.
To reduce the risk for osteonecrosis of the epiphysis during capital reorientation, tension of the posterosuperior retinaculum, containing the end branches of the medial femoral circumflex artery, is reportedly reduced by cuneiform wedge resection of varying size and location [12, 14, 16, 19, 45]. Removing the remainder of the growth plate provides an additional surgical step to accelerate consolidation and foster revascularization of the epiphysis [19]. Despite such caution, osteonecrosis reportedly occurs with an incidence of 10% to 100% [3, 4, 9, 10, 12, 14, 18, 20–22, 36, 43, 47, 54, 58, 59], and the combination of osteonecrosis and chondrolysis [60] up to 42% [3, 10, 12, 21, 22, 58].
Despite these complications, our understanding of the proximal femoral anatomy and blood supply suggests the challenges are mostly technical and can be solved with improved surgical technique. Based on this assumption, an attempt was made to improve the safety of Dunn’s subcapital realignment [16] by combining it with the surgical hip dislocation approach [23] that allows the development of an extended retinacular soft tissue flap. This combined approach provides extensive subperiosteal exposure of the circumference of the femoral neck [24, 38, 39], which facilitates trimming of the femoral neck and hence safe reduction of the femoral head.
The primary aim of our study was to demonstrate that the technique of capital realignment is feasible and reproducible and will restore hip anatomy and function. We specifically (1) ascertained the rate of major complications; (2) determined whether the procedure restored the slip angle, offset, and alpha angle; (3) determined whether postoperative hip range of motion would be restored; (4) ascertained short-term clinical scores (Merle d’Aubigné-Postel, Harris hip score, WOMAC); (5) determined the extent of intra-articular damage and related these to clinical stability and symptom duration; and finally (6) determined whether there was a relationship between clinical physeal stability and physeal stability seen at the time of surgery.
Materials and Methods
We retrospectively reviewed the medical records and radiographs of all 40 patients from two institutions (A and B) who had undergone a modified Dunn procedure. The modified Dunn procedure was devised at Institution A and had been performed at that institution since May 1998. The same technique has been used at Institution B since July 2001. We excluded patients with established necrosis before the index procedure and other medical conditions such as renal insufficiency. Included in this series was one patient with progression of the slip despite previous in situ pinning. The followup period for Cohort A was a minimum of 3 years (range, 3–8.4 years; average, 5.4 years). For Cohort B, the minimum followup period was 1 year (range, 1–4 years; average, 2.2 years). No patients were lost to followup. Institutional Review Board approval was obtained for this retrospective review.
The patient populations in the two institutions were dissimilar (Tables 1, 2). There was a predominance of women (A, 14 women and 16 men; B, nine women and one man) and unstable SCFE at Institution B (A, four of 30 unstable; B, eight of 10 unstable). However, the preoperative symptom duration was similar.
Table 1.
Demographics and preoperative slipped capital femoral epiphyses (SCFE) characteristics of Series A
| Patient number | Age | Gender | Side | Duration of symptoms | Slip angle | SCFE slip percentage | SCFE stability |
|---|---|---|---|---|---|---|---|
| 1 | 13 | M | L | 2 weeks | 70° | ++ | Stable |
| 2 | 12 | F | L | 6 months | 40° | ++ | Stable |
| 3 | 12 | M | L | 3 days | x | x | Unstable |
| 4 | 12 | F | R | 5 months | 50° | +++ | Stable |
| 5 | 16 | M | L | 1 week | x | x | Stable |
| 6 | 14 | M | L | 7 weeks | 30° | + | Stable |
| 7 | 11 | F | L | 2 months | 40° | +++ | Unstable |
| 8 | 13 | M | L | x | 30° | + | Stable |
| 9 | 13 | F | L | 4 months | 70° | +++ | Stable |
| 10 | 15 | M | L | 3 years | 45° | ++ | Stable |
| 11 | 18 | M | R | 2 months | 50° | + | Stable |
| 12 | 14 | F | L | 1 year | 70° | +++ | Stable |
| 13 | 13 | M | L | 2 weeks | 30° | ++ | Stable |
| 14 | 14 | M | L | 4 months | x | + | Stable |
| 15 | 11 | F | L | 5 months | 30° | +++ | Stable |
| 16 | 13 | M | L | 2 months | 40° | ++ | Stable |
| 17 | 14 | M | R | 9 months | 40° | +++ | Stable |
| 18 | 16 | M | L | x | 50° | +++ | Stable |
| 19 | 13 | F | L | 6 months | 50° | +++ | Stable |
| 20 | 14 | M | L | 5 months | 50° | ++ | Unstable |
| 21 | 11 | F | L | 4 months | 70° | +++ | Stable |
| 22 | 14 | M | R | 3 months | 50° | ++ | Stable |
| 23 | 11 | F | R | 3 weeks | 30° | ++ | Stable |
| 24 | 13 | F | L | 5 weeks | 40° | + | Stable |
| 25 | 12 | M | L | 7 weeks | x | x | Stable |
| 26 | 14 | F | R | x | 40° | +++ | Stable |
| 27 | 11 | F | L | 10 months | 45° | +++ | x |
| 28 | 10 | F | L | 2 weeks | x | x | Unstable |
| 29 | 14 | F | R | 1 year | 30° | + | Stable |
| 30 | 12 | M | L | 6 weeks | 50° | + | Stable |
M = male; F = female; L = left; R = right; x = no information; + = < 25%; ++ = 25% to 50%; +++ = > 50% of neck diameter.
Table 2.
Demographics and preoperative slipped capital femoral epiphyses (SCFE) characteristics of Series B
| Patient number | Age (years) | Gender | Side | Duration of symptoms | Slip angle | SCFE slip percentage | SCFE stability |
|---|---|---|---|---|---|---|---|
| 1 | 11 | F | R | 3 months | 34 | + | Unstable |
| 2 | 12 | F | L | 12 months | 59 | ++ | Stable |
| 3 | 11 | F | R | 7 days | 67 | +++ | Unstable |
| 4 | 14 | F | R | 12 months | 69 | ++ | Stable |
| 5 | 12 | F | L | 3 days | 64 | + | Unstable |
| 6 | 14 | M | R | 3 months | 55 | ++ | Unstable |
| 7 | 11 | F | L | 1 day | 69 | +++ | Unstable |
| 8 | 9 | F | L | 3 months | 44 | +++ | Unstable |
| 9 | 12 | F | L | 1 day | 53 | ++ | Unstable |
| 10 | 14 | F | R | 5 days | 52 | + | Unstable |
F = female; M = male; R = right; L = left; + = < 25% slip; ++ = 25% to 50% slip; +++ = > 50% of neck diameter.
Preoperative range of motion of the operated hip was inconsistently reported, especially in the unstable SCFE group. Additionally, as a result of the high percentage of hips with unstable SCFE, preoperative clinical outcome scores were believed not useful and hence not assessed.
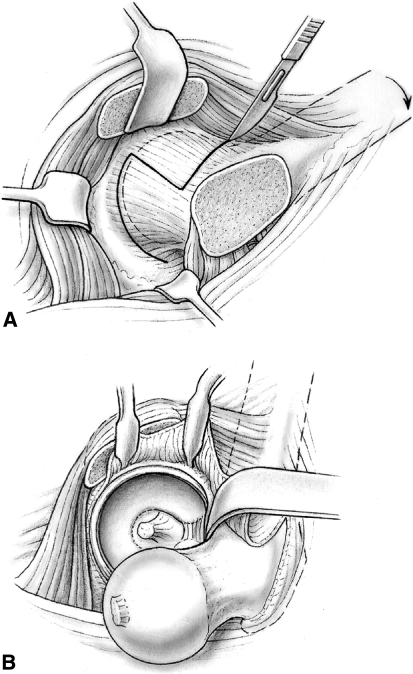
The patient was placed in a full lateral position and the transtrochanteric surgical dislocation of the hip performed [23]. In large patients, a longer incision was necessary and in the presence of a severe external rotation contracture, we judged it preferable to perform the osteotomy with curved chisels in the inferior-to-superior direction rather than with an oscillating saw in the posterior-to-anterior direction. The thickness of the trochanteric fragment should not exceed 1.5 cm. The mobile trochanter was flipped anterosuperiorly and the anterior hip capsule exposed. The arthrotomy was performed in a Z or reverse Z fashion to protect the soft tissues in the piriformis fossa. Care was taken not to damage the labrum when performing the arthrotomy close to the acetabular rim (Fig. 1A).
Fig. 1A–B.
(A) After the anterior hip capsule is cleared of soft tissue, the capsule is opened in a Z or reverse Z fashion to protect the femoral head blood supply (Reprinted with permission from Ganz R, Huff TW, Leunig M. Extended retinacular soft-tissue flap for intra-articular hip surgery: surgical technique, indications, and results of application. In: Azar FM, O’Connor MI, eds. Instructional Course Lectures 58. Rosemont, IL: American Academy of Orthopaedic Surgeons; in press). (B) The hip can then be anteriorly dislocated with hip adduction and external rotation and transection of the round ligament (Reprinted with permission from Ganz R, Huff TW, Leunig M. Extended retinacular soft-tissue flap for intra-articular hip surgery: surgical technique, indications, and results of application. In: Azar FM, O’Connor MI, eds. Instructional Course Lectures 58. Rosemont, IL: American Academy of Orthopaedic Surgeons; in press).
The hip was prepared for dislocation. When the stability of the physis was uncertain, we pinned the femoral epiphysis in situ using one or two threaded Kirschner wires before dislocation. We made no attempt to reduce the epiphysis to avoid any risk of stretching of the retinaculum. The epiphyseal perfusion was checked either by drilling a 2-mm hole in the anterior femoral head [26] or with a laser Doppler flowmetry probe (LDF) inserted into the epiphysis [48]. To dislocate the hip, we externally rotated and adducted the leg and placed it in the bag on the opposite side of the table. A hook was placed around the calcar, facilitating subluxation. We transected the round ligament with curved scissors (Fig. 1B). Dislocation of the hip was not necessary in SCFE with a slip angle of less than 30°, in which trimming of the anterior metaphysis may be sufficient to restore the anterior offset without weakening the femoral neck [41]. After dislocation, we documented the damage to the acetabular cartilage and labrum by the prominent anterior metaphysis [37] and the extent of impingement by the prominent metaphysis.
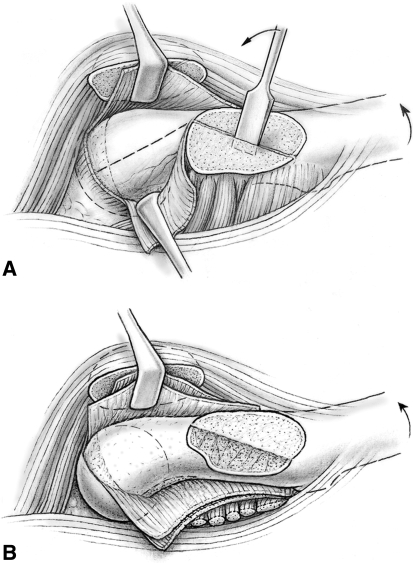
The first step of the development of an extended retinacular flap [24, 38, 39] was trimming of the posterosuperior portion of the stable greater trochanter down to the level of the femoral neck. This was performed with the head in the acetabulum (Fig. 2A). The portion of the trochanter proximal to the visible growth plate was mobilized using a chisel and the cancellous bone gently removed from the periosteum. The periosteum of the neck was incised along the anterior neck starting at the anterosuperior corner of the apophysis of the greater trochanter. Using a scalpel and periosteal elevator, the periosteum of the neck, including the retinaculum, was gradually released from the femoral neck. Care was taken not to cause tearing of the periosteum at the femoral head neck junction. Posterior subperiosteal dissection was continued down to the lesser trochanter. Any remaining ledge of the trochanteric bone was leveled with the femoral neck. The resulting soft tissue flap consisting of the retinaculum and external rotators held the vessels supplying the epiphysis. We freed the anteromedial periosteum from the femoral neck with the femoral head dislocated. Again, care was taken that the periosteum does not tear off the head; the medial flap contains a constant branch of the medial femoral circumflex artery supplying the inferomedial portion of the epiphysis (Fig. 2B). After complete periosteal dissection of the femoral neck, the femoral head was reduced in the acetabulum and perfusion checked.
Fig. 2A–B.
(A) The proximal portion of the stable trochanter is carefully removed. This allows full access to the posterior periosteum of the femoral neck for further subperiosteal dissection (Reprinted with permission from Leunig M, Slongo T, Ganz R. Subcapital realignment in slipped capital femoral epiphysis: surgical hip dislocation and trimming of the stable trochanter to protect the perfusion of the epiphysis. In: Duwelius PJ, Azar FM, eds. Instructional Course Lectures 57. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:499–507). (B) The anterior-medial periosteum of the femoral neck is incised and the entire femoral neck can be dissected subperiosteally while preserving the femoral head blood supply (Reprinted with permission from Ganz R, Huff TW, Leunig M. Extended retinacular soft-tissue flap for intra-articular hip surgery: surgical technique, indications, and results of application. In: Azar FM, O’Connor MI, eds. Instructional Course Lectures 58. Rosemont, IL: American Academy of Orthopaedic Surgeons; in press).
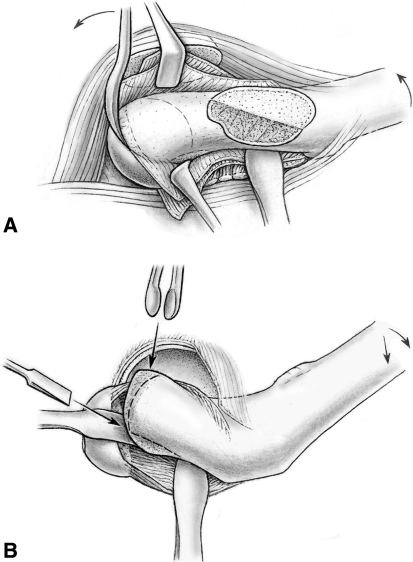
With the head dislocated again, we exposed the femoral neck with blunt retractors placed medially and laterally around the neck. Kirschner wires used to stabilize the femoral head during dislocation were removed per judgment of the surgeon. Using a curved 10-mm wide chisel, the epiphysis was gradually separated from the metaphysis by carefully levering the inserted instrument (Fig. 3A). Excision of a large bone wedge from the anterior metaphysis was usually not necessary for safe reduction of the femoral head unlike in a cuneiform osteotomy. Further careful levering with the chisel and external rotation of the leg allowed the epiphysis to be tilted posteriorly and the femoral neck to separate completely from the subperiosteal soft tissue envelope. In chronic SCFE, resection of a posteromedial callus bridge in flexion-external rotation can facilitate the separation of the head. By placing a small cotton swab into the acetabular cavity, inadvertent reduction of the mobile epiphysis into the acetabulum can be avoided. With the stump of the femoral neck exposed, the callus sitting on the surface of the posterior neck was removed with a straight chisel. The posterior surface of the femoral neck was leveled with the remaining neck and checked by palpation. The axial end surface of the femoral neck should be perpendicular to the axis of the neck and slightly rounded (Fig. 3B). The growth plate was exposed by manually stabilizing the femoral head while the neck was carefully turned inward (Fig. 4A). We avoided tension of the retinaculum by constant visual control. With the other hand, the surgeon removed residual tissue of the growth plate using a small curette. Special care was taken as the curette approached the retinacular attachment to avoid damage. Normally, the exposed epiphyseal bone would be visibly bleeding. After the callus was removed, the epiphysis was manually reduced onto the femoral neck while visually checking the tension of the retinaculum. If the retinaculum appeared under too much tension, then the reduction maneuver was stopped and the femoral neck inspected. Sometimes parts of the soft tissue flap were trapped and had to be unfolded. At times, additional shortening of the femoral neck was performed.
Fig. 3A–B.
(A) The femoral head can be separated from the femoral neck by gently levering through the physis (Reprinted with permission from Leunig M, Slongo T, Ganz R. Subcapital realignment in slipped capital femoral epiphysis: surgical hip dislocation and trimming of the stable trochanter to protect the perfusion of the epiphysis. In: Duwelius PJ, Azar FM, eds. Instructional Course Lectures 57. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:499–507). (B) This allows full access to the femoral neck callus, which can be safely removed without excessive shortening of the femoral neck (Reprinted with permission from Ganz R, Huff TW, Leunig M. Extended retinacular soft-tissue flap for intra-articular hip surgery: surgical technique, indications, and results of application. In: Azar FM, O’Connor MI, eds. Instructional Course Lectures 58. Rosemont, IL: American Academy of Orthopaedic Surgeons; in press).
Fig. 4A–B.
(A) While manually stabilizing the femoral head, the surgeon is able to safely curette out the remaining physis in the femoral head (Reprinted with permission from Leunig M, Slongo T, Ganz R. Subcapital realignment in slipped capital femoral epiphysis: surgical hip dislocation and trimming of the stable trochanter to protect the perfusion of the epiphysis. In: Duwelius PJ, Azar FM, eds. Instructional Course Lectures 57. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:499–507). (B) After gentle reduction of the femoral head back onto the femoral neck, the femoral head can be stabilized using a threaded wire placed through the fovea (Reprinted with permission from Ganz R, Huff TW, Leunig M. Extended retinacular soft-tissue flap for intra-articular hip surgery: surgical technique, indications, and results of application. In: Azar FM, O’Connor MI, eds. Instructional Course Lectures 58. Rosemont, IL: American Academy of Orthopaedic Surgeons; in press).
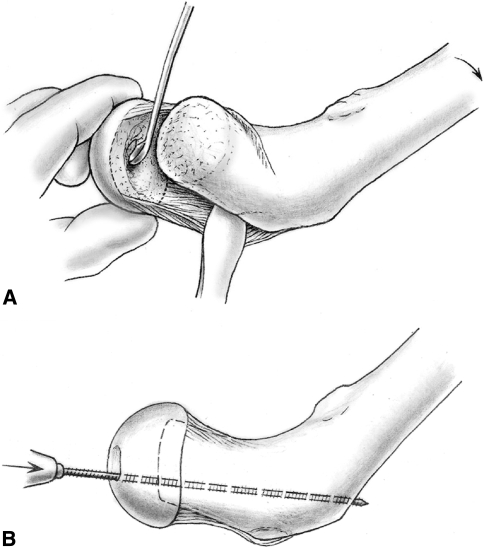
To minimize femoroacetabular impingement, we inspected the femoral head offset on the femoral neck and adjusted the offset by centering the femoral head on the neck as well as adjusting the thickness of the neck. Correct rotation was estimated with the position of the anterior retinacular border relative to the neck and with the position of the fovea capitis. To ensure correct varus-valgus alignment, we used fluoroscopy after preliminary wire fixation through the fovea capitis. The definitive fixation was achieved with a 3-mm fully threaded wire inserted through the fovea so it exited the lateral cortex below the greater trochanter (Fig. 4B). This wire must disappear into the fovea before the head is carefully repositioned into the acetabulum. One or two more wires were inserted from the lateral cortex for optimal fixation. After visual inspection of the retinacular flap, the final evaluation of the position of the head and of the wires with fluoroscopy was followed by a final check of the epiphyseal blood flow using a drill hole or LDF. We did not believe filling the gap between the head and neck with bone graft was necessary and presumed it might be detrimental to the blood supply and the head-neck offset. The capsule was closed loosely to avoid strangulation of the femoral head blood supply [48]. If tension of the piriformis muscle on the capsule was observed, we released the tendon. The greater trochanter was refixed with two to three 3.5-mm screws, if necessary, in a slightly advanced position (Fig. 5). The goal of the surgery was to obtain safe anatomic reduction of the femoral head with minimal shortening of the femoral neck and restoration of anterior head-neck offset as illustrated in a case of unstable SCFE treated with this modified Dunn procedure (Fig. 6A–F).
Fig. 5.
The femoral head is finally stabilized with additional threaded wires placed in the distal to proximal direction under fluoroscopy. The mobile trochanter is stabilized using cortical screws (Reprinted with permission from Leunig M, Slongo T, Ganz R. Subcapital realignment in slipped capital femoral epiphysis: surgical hip dislocation and trimming of the stable trochanter to protect the perfusion of the epiphysis. In: Duwelius PJ, Azar FM, eds. Instructional Course Lectures 57. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008:499–507).
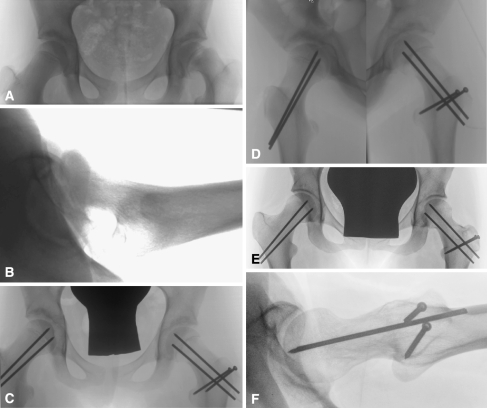
Fig. 6A–F.
This is an illustrative case of an 11-year-old female patient (Case 7) with an unstable left slipped capital femoral epiphyses (SCFE). (A) Anteroposterior and (B) lateral views before surgery. (C–D) Radiographs taken during the early postoperative period. The right hip was prophylactically pinned. (E–F) Five years after SCFE reduction. The physis and trochanteric osteotomy healed without complications. There is no evidence of osteonecrosis. The anterior head-neck offset if fully restored.
During the hospital stay and for approximately 3 weeks postoperatively, we used a continuous passive motion machine to minimize the risk of developing a flexion and external rotation contracture of the operated hip. The physical therapist only gave instructions on how to lift the operated leg by relying on the support of the contralateral leg, how to roll the foot on the floor without loading while walking on crutches, and how to walk up and down stairs. The patients were initially touchdown weightbearing for 6 to 8 weeks. Physical therapy for gait training and gentle range of motion were initiated immediately. Patients were allowed to use crutches immediately if they seemed able; otherwise, wheelchair use was encouraged. We did not institute a hip strengthening program until the trochanter was healed. We obtained radiographs at 4 weeks to determine whether touchdown weightbearing should be continued for another 4 to 5 weeks or whether weightbearing could be gradually increased. Full weightbearing was allowed when healing of the trochanteric osteotomy and femoral neck could be seen. Healing was judged by the blurring of the trochanteric osteotomy line and the proximal femoral physis. Continued healing of the femoral neck and progress of rehabilitation were re-evaluated 12 to 14 weeks after surgery. Thereafter, normal activities were permitted. Implant removal was scheduled for 6 months to 1 year after surgery.
We reviewed patient charts noting intraoperative findings and complications. During surgery, the stability of the physis was noted by the operating surgeon and graded as stable, easy surgical separation, and gross instability. Grossly unstable hips had torn anterior periosteum and without surgical stabilization, physeal separation would occur. In a case with a stable physis, the periosteum needed to be surgically dissected and physis surgically separated. In between these two scenarios, there were hips in which the physis was grossly stable and the periosteum intact, but with minimal effort, the physis separated once the femoral neck periosteum was freed. At the time of surgical dislocation, the extent and depth of acetabular cartilage damage was noted as well as the extent of labral damage. The area of acetabular cartilage damage (areas of delamination or full-thickness fibrillation or cartilage loss) was documented on a clock face diagram. The maximal depth of the damaged area from the edge of the bony acetabulum was documented. After femoral head reduction, a small drill hole was made in the femoral head and used to check femoral head vascularity. Laser Doppler flowmetry was used in nine cases at Institution A.
Postoperative clinical outcomes were assessed using the Merle d’Aubigné-Postel [44] and the Harris hip score [31] or the WOMAC [6]. At followup, the range of motion for both hips was recorded with special emphasis on internal and external rotation in flexion of 90°.
We used pre- and postoperative anteroposterior pelvic radiographs, and crosstable or frog lateral radiographs to determine the amount of slippage. Two of us (KC, CZ) measured the lateral radiographs by comparing the affected hip with the normal hip [56]. A slip angle less than 30° was estimated as mild, between 30° and 50° as moderate, and greater than 50° was classified as severe [8]. We quantified the anterior head-neck contour on the lateral radiographs at last followup using the Nötzli angle, a parameter associated with the development of femoroacetabular impingement [49]. We noted chondrolysis, joint space narrowing [2] over the observation period, and osteonecrosis. The minimum joint space is measured in the weightbearing portion of the femoral head in a radial direction going out from the center of the head.
We determined differences in pre- and postoperative slip angles using a paired t-test. Differences in the fraction of hips with articular cartilage damage in stable versus unstable hips were compared using chi square test. We compared differences in symptom duration using the Mann-Whitney test and slip angle using a two tailed t-test in hips with and without articular cartilage damage. The Kolmogorov-Smirnov test was used to determine whether the data met the assumption of normal distribution required for a parametric test. Analysis was performed using SPSS 15 software (SPSS Inc., Chicago, IL).
Results
No patients from either institution (Series A: Tables 1, 3, 5; Series B: Tables 2, 4, 6) developed osteonecrosis, infection, deep venous thrombosis, or nerve palsies. One patient developed symptomatic heterotopic ossification and one had residual impingement for which we performed revision surgery. Three patients developed delayed unions; none developed nonunions. In series A, three patients (Table 1; Patients 3, 18, and 29) were reoperated at 6 to 8 weeks postoperatively for breakage of a screw or wire fixation of the femoral epiphysis. The osteotomies in all three patients subsequently healed without further complications. There was no loss of fixation or breakage of screws in Series B. There were no patients with chondrolysis as evidenced by rapid-onset joint space loss and stiffness. In Case 3 of Series A, the patient had an unstable SCFE with femoral head-neck separation for 2 days before surgery (Table 1); the femoral head did not show LDF pulsations before and after realignment. At the time of revision surgery for loss of epiphysis fixation, the femoral head vascularity was checked again with the LDF. Pulsatile flow was noted and this patient went on to heal without osteonecrosis. There were no cases of femoral head avascularity after reduction in Series B. At followup, heterotopic ossifications were noted in three hips in Series A (Patients 6, 9, and 22 in Table 1). Patient 22 had reduced flexion resulting from the heterotopic ossification, which improved after resection at the time of hardware removal. No major heterotopic ossifications were noted in Series B. One patient in Series A (Patient 12 in Table 1) had residual impingement resulting from nonspheric deformity of the epiphysis in a chronic slip, which could not be treated during the index surgery. This patient underwent osteochondroplasty 2 years after capital realignment.
Table 3.
Radiographic and clinical results of Series A
| Patient number | Followup (months) | Head-neck offset | Alpha angle | ROM | Merle d’Aubigne score | Harris hip score | |||
|---|---|---|---|---|---|---|---|---|---|
| AP | Lateral | Flex | IR/flex | ER/flex | |||||
| 1 | 30 | − + | ++ | 44 | 110 | 45 | 45 | 18 | 100 |
| 2 | 56 | ++ | ++ | 60 | 110 | 30 | 50 | 18 | 100 |
| 3 | 66 | − + | − + | 42 | 100 | 20 | 30 | 18 | 100 |
| 4 | 64 | ++ | − + | 54 | 90 | 30 | 45 | 17 | 99.8 |
| 5 | 48 | ++ | + − | 39 | 100 | 45 | 30 | 18 | 100 |
| 6 | 75 | ++ | ++ | 49 | 120 | 30 | 40 | 18 | 100 |
| 7 | 51 | ++ | ++ | 32 | 120 | 45 | 50 | 18 | 100 |
| 8 | 53 | ++ | ++ | 40 | 100 | 30 | 45 | 18 | 100 |
| 9 | 53 | ++ | ++ | 40 | 80 | 10 | 40 | 18 | 99.4 |
| 10 | 74 | + − | − + | 39 | 90 | 5 | 30 | 17 | 98.5 |
| 11 | 61 | ++ | − + | 41 | 100 | 30 | 45 | 18 | 99.9 |
| 12 | 74 | ++ | ++ | 30 | 110 | 25 | 45 | 18 | 100 |
| 13 | 101 | ++ | − + | 40 | 120 | 30 | 30 | 18 | 100 |
| 14 | 78 | + − | ++ | 43 | 100 | 30 | 30 | 18 | 100 |
| 15 | 96 | ++ | ++ | 45 | 110 | 40 | 40 | 18 | 100 |
| 16 | 74 | + − | + − | 37 | 100 | 40 | 30 | 18 | 99.9 |
| 17 | 67 | ++ | ++ | 29 | 120 | 45 | 45 | 18 | 100 |
| 18 | 70 | + − | − + | 52 | 100 | 30 | 20 | 18 | 99.9 |
| 19 | 39 | + − | + − | 34 | 100 | 30 | 40 | 18 | 99.9 |
| 20 | 50 | ++ | + − | 27 | 100 | 10 | 40 | 18 | 99.8 |
| 21 | 60 | − + | + − | 28 | 110 | 20 | 50 | 18 | 96 |
| 22 | 76 | ++ | ++ | 36 | 100 | 20 | 40 | 17 | 99.9 |
| 23 | 48 | ++ | + − | 41 | 120 | 40 | 50 | 18 | 100 |
| 24 | 89 | + − | − + | 46 | 110 | 30 | 45 | 18 | 100 |
| 25 | 56 | − + | + − | 45 | 110 | 30 | 40 | 18 | 99.9 |
| 26 | 83 | + − | ++ | 44 | 110 | 15 | 30 | 18 | 100 |
| 27 | 57 | + − | ++ | 50 | 110 | 30 | 45 | 17 | 96 |
| 28 | 66 | + − | + − | 47 | 95 | 30 | 45 | 18 | 99.8 |
| 29 | 36 | ++ | ++ | 27 | 110 | 20 | 45 | 18 | 100 |
| 30 | 78 | ++ | ++ | − | 110 | 25 | 35 | 18 | 98.5 |
ROM = range of motion; AP = anteroposterior; Flex = flexion; IR = internal rotation; ER = external rotation; − = no crosstable lateral view available; ++ = identical anterior and posterior offset; − + = less anterior offset/less; medial offset; + − = less posterior offset/less lateral offset.
Table 5.
Intraoperative findings of Series A
| Patient number | Cartilage | Labrum | Epiphyseal stability | Bleeding of femoral epiphysis after reduction | ||
|---|---|---|---|---|---|---|
| Depth (mm) | Sector (hour) | Tear | Sector (hour) | |||
| 1 | 10 | 10-2 | Y | 10-2 | ++ | Yes |
| 2 | 10 | 10-1 | Y | 11-12 | + | Yes |
| 3 | 10 | 9-12 | Y | 9-1 | +++ | No |
| 4 | 10 | 12-3 | N | − | + | Yes |
| 5 | 10 | 10-11 | Y | 10-11 | + | Yes |
| 6 | 10 | 10-1 | Y | 11-1 | ++ | Yes |
| 7 | 8 | 10-1 | Y | 10-1 | ++ | Yes |
| 8 | 5 | 10-12 | Y | 10-12 | +++ | Yes |
| 9 | 15 | 9-12 | Y | 9-12 | ++ | Yes |
| 10 | 10 | 10-1 | Y | 10-1 | ++ | x |
| 11 | 2-20 | 11-2 | Y | 11-2 | + | Yes |
| 12 | 10 | 9-1 | Y | 9-1 | + | Yes |
| 13 | x | x | x | x | x | x |
| 14 | 10 | 12-2 | Y | 12-2 | + | Yes |
| 15 | 10 | 10-1 | Y | 10-1 | + | Yes |
| 16 | 10 | 10-12 | Y | 10-12 | ++ | Yes |
| 17 | 10 | 11-3 | Y | 11-3 | +++ | Yes |
| 18 | 10 | 10-1 | Y | 11-1 | + | Yes |
| 19 | 15 | 9-1 | N | − | + | Yes |
| 20 | 10 | 11-1 | Y | 11-1 | +++ | Yes |
| 21 | 10 | 10-12 | Y | 10-12 | ++ | Yes |
| 22 | 15 | 12-3 | Y | 11-3 | + | Yes |
| 23 | 10 | 11-3 | Y | 11-3 | ++ | Yes |
| 24 | 15 | 10-1 | Y | 10-2 | ++ | x |
| 25 | 10 | 11-1 | Y | 11-1 | + | x |
| 26 | 10 | 9-12 | Y | 9-12 | +++ | Yes |
| 27 | 15 | 9-1 | Y | 11-1 | ++ | Yes |
| 28 | x | x | x | x | x | Yes |
| 29 | 10 | 11-3 | Y | 11-3 | + | Yes |
| 30 | − | − | Y | 11-1 | ++ | Yes |
x = no information; Y = yes; N = no; + = stable epiphysis; ++ = easy surgical separation of physis; +++ = grossly unstable physis; − = no damage.
Table 4.
Radiographic and clinical results of Series B
| Patient number | Followup (months) | Slip angle correction | Postoperative slip angle | ROM | WOMAC | |||
|---|---|---|---|---|---|---|---|---|
| Flex | Flex IR | Flex ER | Pain | Function | ||||
| 1 | 48 | 29 | 6 | 100 | 30 | 50 | 5 | 5 |
| 2 | 42 | 57 | 2 | 90 | 30 | 50 | 0 | 8 |
| 3 | 48 | 63 | 4 | 100 | 30 | 50 | 0 | 0 |
| 4 | 24 | 64 | 5 | 100 | 30 | 50 | 0 | 0 |
| 5 | 12 | 52 | 12 | 120 | 30 | 60 | 0 | 0 |
| 6 | 12 | 54 | 1 | 90 | 20 | 50 | 2 | 12 |
| 7 | 12 | 53 | 16 | 100 | 30 | 50 | 2 | 0 |
| 8 | 24 | 24 | 20 | 100 | 20 | 50 | 0 | 0 |
| 9 | 20 | 43 | 10 | 90 | 30 | 50 | 2 | 5 |
| 10 | 21 | 42 | 10 | 100 | 30 | 50 | 1 | 0 |
ROM = range of motion; Flex = flexion; IR = internal rotation; ER = external rotation.
Table 6.
Intraoperative findings of Series B
| Patient number | Cartilage | Labrum | Epiphyseal stability | Bleeding of femoral epiphysis after reduction | ||
|---|---|---|---|---|---|---|
| Depth (mm) | Sector (hour) | Tear | Sector (hour) | |||
| 1 | x | x | Y | x | + | Yes |
| 2 | + | 12 | N | − | +++ | Yes |
| 3 | − | − | N | − | +++ | Yes |
| 4 | + | 10-2 | Y | 12-2 | + | Yes |
| 5 | − | − | Y | 11 | ++ | Yes |
| 6 | − | − | N | − | ++ | Yes |
| 7 | − | − | N | − | +++ | Yes |
| 8 | − | − | N | − | ++ | Yes |
| 9 | − | − | N | − | +++ | Yes |
| 10 | − | − | N | − | ++ | Yes |
x = no information; Y = yes; N = no; + = stable epiphysis; ++ = easy surgical separation of physis; +++ = grossly unstable physis; − = no damage.
Normal alpha angles were restored in Series A (Table 3) and slip angles were corrected in Series B (Table 4). The slip angle was corrected (p < 0.001) from an average of 56.6° (range, 34°–69°) to 8.6° in Series B. Furthermore, in Series A, the majority (27 of 30 hips) of cases had equal head-neck offset or sufficient anterior and lateral offset on postoperative radiographs (Table 3). The mean alpha angle after correction was 40.6° (range, 27°–60°) (Table 3).
Postoperative hip function was good with near normal range of motion (Tables 3, 4). The mean postoperative hip flexion, flexion internal rotation, and flexion external rotation were 104° (range, 80°–120°), 29° (range, 5°–45°), and 43° (range, 20°–60°), respectively.
Short-term postoperative clinical outcomes were near normal. In Series A, the Merle d’Aubigné-Postel score at the time of followup averaged 17.8 points. The outcome score of the operative side equaled the contralateral hip score, which had an average score of 17.7 points. The Harris hip score showed a similar good outcome with an average score of 99.6 for the operative side versus 99.5 for the contralateral hip. Similar to the results of Series A, patients in Series B had excellent clinical results with an average pain score of 1.2 and function score of 3.
Stability and duration of symptom of the SCFE (Tables 1, 2) was associated with acetabular cartilage damage at time of surgery (Tables 5, 6). More hips (p < 0.0001) with articular cartilage damage were seen in the stable SCFE (25 of 26 hips) versus the unstable SCFE (three of 10). The longer symptom duration was associated with (p < 0.01) cartilage damage: 6 months versus 1 month in hips with and without cartilage damage, respectively. The slip angles without and with cartilage damage were similar (p = 0.09).
Clinically assessed SCFE stability (Tables 1, 2) correlated with intraoperative physis stability (Tables 5, 6) only in the unstable SCFE. Most clinically unstable SCFE (11) had physes that were grossly unstable (five) or physes that were easily separated from the metaphyses (five). However, even in the clinically stable SCFE, 13 of 26 hips had physes that were either grossly unstable (four) or easily separated (nine).
Discussion
Moderate to severe slipped capital femoral epiphysis frequently leads to premature osteoarthrosis resulting from femoroacetabular impingement. While there are various approaches to minimize the risk of or delay the appearance of osteoarthrosis, we believe correction at the site of deformity is the best procedure to fully correct the deformity. However, reorientation of the femoral head with an osteotomy has traditionally been associated with high rates of osteonecrosis. Our primary aim of this article was therefore to determine whether this capital realignment technique was feasible and repeatable and would restore hip anatomy and function and provide good short-term outcome while avoiding osteonecrosis. Furthermore, we wanted to take this opportunity afforded us by this open approach to identify factors associated with articular cartilage damage and determine the relationship between clinically assessed SCFE stability with actual physeal stability seen intraoperatively.
Like with any single cohort study, there are major limitations, among which is lack of a comparison group. Without a truly randomized long-term study, it would be difficult to compare outcomes after various surgical treatment methods such as in situ pinning, capital realignment, and intertrochanteric osteotomy. We nonetheless found this complex technique can restore near normal anatomic alignment and can be performed with low complication rates at two centers. We therefore believe it is a reasonable option that has some theoretical advantages over other methods. Although we used nonvalidated grading schemes for articular cartilage damage and physeal stability, the observations that articular cartilage damage is related to symptom duration and clinically stable SCFE may also have an unstable physis are clinically important and should motivate future studies. Physeal instability facilitates surgical capital reduction; therefore, in stable SCFE, if acute surgical reconstruction is contemplated, then a surgical dislocation approach with inspection of the physeal stability may influence whether a capital reduction is performed versus an intertrochanteric level reconstruction. The fact that symptom duration is associated with articular cartilage damage brings into question whether osteoarthritis can truly be prevented in a hip that is reconstructed after years of impingement resulting from the SCFE deformity.
We observed low rates of major complications using this modified Dunn procedure for capital realignment. We had no cases of osteonecrosis in 40 cases. Previously reported results of capital realignment for SCFE demonstrated wide-ranging complication rates with the rate of osteonecrosis ranging from 10% to 100% [3, 4, 9, 10, 12, 14, 18, 20–22, 36, 43, 47, 54, 58, 59]. The surgical dislocation approach may facilitate capital realignment by allowing full exposure of the proximal femur and allows subperiosteal dissection of the entire femoral neck. We have demonstrated this technique can be applied at two independent centers with relatively low complication rates. In cases of open physis, capital realignment can be achieved without osteonecrosis.
Furthermore, this technique allows restoration of the proximal femoral anatomy with complete correction of the slip angle and head-neck offset. It does not result in the creation of secondary deformities. Full hip motion was restored and in the short term resulted in pain-free and fully functioning hips. Unlike the cuneiform osteotomies [19], which require substantial femoral neck shortening to ensure tension-free correction of the femoral epiphysis, this modified Dunn approach allows safe reduction by removal of the posterior callus and thinning of the femoral neck and hence should minimize leg length differences. A potential downside of the surgical dislocation approach is residual abductor weakness. In this young population, this did not appear to be a clinically noticeable problem; however, further study is necessary.
At the time of surgery, we noted substantial articular cartilage damage, especially in the stable SCFE and hips with a longer duration of symptoms. It is well established that full-thickness articular cartilage damage in adults will not spontaneously heal. If we assume the cartilage damage is the result of the SCFE deformity and if we were to simply in situ pin the SCFE, then the implication is that in the long term, these hips will develop osteoarthritis. However, long-term followup studies after SCFE treatment have demonstrated the outcome after in situ pinning is in general quite good [8]. These seemingly contradictory observations beg further study. Perhaps in the immature hips, there is some capacity for the articular cartilage to heal or perhaps careful study of hips after in situ pinning will reveal subclinical articular cartilage damage not obvious on radiographs. In either case, these sorts of clinical observations would be valuable in advancing our understanding of human osteoarthritis.
Even in the clinically stable SCFE, a surprising number of physes was mobile on direct inspection during surgery. In the case of moderate to severe clinically unstable SCFE, the rate of osteonecrosis with in situ pinning is high ranging from 10% to 40% [40]. It is the unstable SCFE in which direct visualization of the femoral head blood supply and careful dissection of the femoral neck periosteum through the surgical dislocation approach may theoretically decrease the rate of osteonecrosis. Therefore, we believe the unstable SCFE is the most important indication for this procedure. However, even in the clinically stable SCFE, often the physis is quite mobile suggesting the capital reduction procedure could be readily performed without major technical difficulties.
Our data suggest capital realignment of SCFE with open physes through the surgical dislocation approach can be performed with low complication rates. We believe the technique is most appropriate for moderate to severe SCFE and especially for unstable SCFE. The safe execution of this procedure requires full understanding of the vascular anatomy of the hip by the surgeon. This procedure restores the proximal femoral anatomy and although we do not have long-term results, we assume restoration of normal anatomy would lead to good long-term outcomes. This procedure is technically demanding; however, we believe it is worth the investment of effort and skill for a condition that could have lifelong consequences in an otherwise young and active population.
Acknowledgment
We thank Dr Martin Beck for assistance with this study.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Abraham E, Garst J, Barmada R. Treatment of moderate to severe slipped capital femoral epiphysis with extracapsular base-of-neck osteotomy. J Pediatr Orthop. 1993;13:294–302. [DOI] [PubMed]
- 2.Altman RD, Bloch DA, Dougados M, Hochberg M, Lohmander S, Pavelka K, Spector T, Vignon E. Measurement of structural progression in osteoarthritis of the hip: the Barcelona consensus group. Osteoarthritis Cartilage. 2004;12:515–524. [DOI] [PubMed]
- 3.Arnold P, Jani L, Soloniewicz A. Significance and results of subcapital osteotomy in severe slipped capital femoral epiphysis [in German]. Orthopade. 2002;31:908–913. [DOI] [PubMed]
- 4.Ballmer PM, Gilg M, Aebi B, Ganz R. Results following sub-capital and Imhauser-Weber osteotomy in femur head epiphyseolysis [in German]. Z Orthop Ihre Grenzgeb. 1990;128:63–66. [DOI] [PubMed]
- 5.Barmada R, Bruch RF, Gimbel JS, Ray RD. Base of the neck extracapsular osteotomy for correction of deformity in slipped capital femoral epiphysis. Clin Orthop Relat Res. 1978;132:98–101. [PubMed]
- 6.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed]
- 7.Bellemans J, Fabry G, Molenaers G, Lammens J, Moens P. Slipped capital femoral epiphysis: a long-term follow-up, with special emphasis on the capacities for remodeling. J Pediatr Orthop B. 1996;5:151–157. [PubMed]
- 8.Boyer DW, Mickelson MR, Ponseti IV. Slipped capital femoral epiphysis. Long-term follow-up study of one hundred and twenty-one patients. J Bone Joint Surg Am. 1981;63:85–95. [PubMed]
- 9.Broughton NS, Todd RC, Dunn DM, Angel JC. Open reduction of the severely slipped upper femoral epiphysis. J Bone Joint Surg Br. 1988;70:435–439. [DOI] [PubMed]
- 10.Carlioz H, Vogt JC, Barba L, Doursounian L. Treatment of slipped upper femoral epiphysis: 80 cases operated on over 10 years (1968–1978). J Pediatr Orthop. 1984;4:153–161. [DOI] [PubMed]
- 11.Carney BT, Weinstein SL, Noble J. Long-term follow-up of slipped capital femoral epiphysis. J Bone Joint Surg Am. 1991;73:667–674. [PubMed]
- 12.Clarke HJ, Wilkinson JA. Surgical treatment for severe slipping of the upper femoral epiphysis. J Bone Joint Surg Br. 1990;72:854–858. [DOI] [PubMed]
- 13.Crawford AH. Osteotomies in the treatment of slipped capital femoral epiphysis. Instr Course Lect. 1984;33:327–349. [PubMed]
- 14.DeRosa GP, Mullins RC, Kling TF Jr. Cuneiform osteotomy of the femoral neck in severe slipped capital femoral epiphysis. Clin Orthop Relat Res. 1996;322:48–60. [DOI] [PubMed]
- 15.Diab M, Hresko MT, Millis MB. Intertrochanteric versus subcapital osteotomy in slipped capital femoral epiphysis. Clin Orthop Relat Res. 2004;427:204–212. [DOI] [PubMed]
- 16.Dunn DM. The treatment of adolescent slipping of the upper femoral epiphysis. J Bone Joint Surg Br. 1964;46:621–629. [PubMed]
- 17.Engelhardt P. Juvenile Hüftkopflösung und Koxarthrose. Stuttgart: Enke Verlag; 1984.
- 18.Exner GU, Schai PA, Notzli HP. Treatment of acute slips and clinical results in slipped capital femoral epiphysis [in German]. Orthopade. 2002;31:857–865. [DOI] [PubMed]
- 19.Fish JB. Cuneiform osteotomy of the femoral neck in the treatment of slipped capital femoral epiphysis. J Bone Joint Surg Am. 1984;66:1153–1168. [PubMed]
- 20.Fish JB. Cuneiform osteotomy of the femoral neck in the treatment of slipped capital femoral epiphysis. A follow-up note. J Bone Joint Surg Am. 1994;76:46–59. [DOI] [PubMed]
- 21.Fron D, Forgues D, Mayrargue E, Halimi P, Herbaux B. Follow-up study of severe slipped capital femoral epiphysis treated with Dunn’s osteotomy. J Pediatr Orthop. 2000;20:320–325. [DOI] [PubMed]
- 22.Gage JR, Sundberg AB, Nolan DR, Sletten RG, Winter RB. Complications after cuneiform osteotomy for moderately or severely slipped capital femoral epiphysis. J Bone Joint Surg Am. 1978;60:157–165. [PubMed]
- 23.Ganz R, Gill TJ, Gautier E, Ganz K, Krugel N, Berlemann U. Surgical dislocation of the adult hip a technique with full access to the femoral head and acetabulum without the risk of avascular necrosis. J Bone Joint Surg Br. 2001;83:1119–1124. [DOI] [PubMed]
- 24.Ganz R, Huff T, Leunig M. Extended retinacular soft tissue flap for intraarticular surgery of the hip. Operative technique, indications and results of its application. Instr Course Lect. 2009; in press. [PubMed]
- 25.Gautier E, Ganz K, Krugel N, Gill T, Ganz R. Anatomy of the medial femoral circumflex artery and its surgical implications. J Bone Joint Surg Br. 2000;82:679–683. [DOI] [PubMed]
- 26.Gill TJ, Sledge JB, Ekkernkamp A, Ganz R. Intraoperative assessment of femoral head vascularity after femoral neck fracture. J Orthop Trauma. 1998;12:474–478. [DOI] [PubMed]
- 27.Goodman DA, Feighan JE, Smith AD, Latimer B, Buly RL, Cooperman DR. Subclinical slipped capital femoral epiphysis. Relationship to osteoarthrosis of the hip. J Bone Joint Surg Am. 1997;79:1489–1497. [DOI] [PubMed]
- 28.Guzzanti V, Falciglia F, Stanitski CL. Slipped capital femoral epiphysis in skeletally immature patients. J Bone Joint Surg Br. 2004;86:731–736. [DOI] [PubMed]
- 29.Hagglund G, Hannson LI, Sandstrom S. Slipped capital femoral epiphysis in southern Sweden. Long-term results after nailing/pinning. Clin Orthop Relat Res. 1987;217:190–200. [PubMed]
- 30.Hall JE. The results of treatment of slipped femoral epiphysis. J Bone Joint Surg Br. 1957;39:659–673. [DOI] [PubMed]
- 31.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed]
- 32.Imhäuser G. Die Imhäuser- Osteotomie bei floridem Gleitprozess. Bemerkungen zu der gleichlautenden Arbeit von Weber BG. Z Orthop Ihre Grenzgeb. 1966;102:327–329. [PubMed]
- 33.Jones JR, Paterson DC, Hillier TM, Foster BK. Remodelling after pinning for slipped capital femoral epiphysis. J Bone Joint Surg Br. 1990;72:568–573. [DOI] [PubMed]
- 34.Kramer WG, Craig WA, Noel S. Compensating osteotomy at the base of the femoral neck for slipped capital femoral epiphysis. J Bone Joint Surg Am. 1976;58:796–800. [PubMed]
- 35.Kraske P. Ueber die operative Behandlung der statischen Schenkelhalsverbiegung. Centralbl F Chir. 1896;6.
- 36.Lefort G, Cottalorda J, Bouche-Pillon MA, Lefebvre F, Daoud S. Open reduction by the Dunn technique in upper femoral epiphysiolysis. Report of 14 cases [in French]. Chir Pediatr. 1990;31:229–234. [PubMed]
- 37.Leunig M, Casillas MM, Hamlet M, Hersche O, Notzli H, Slongo T, Ganz R. Slipped capital femoral epiphysis: early mechanical damage to the acetabular cartilage by a prominent femoral metaphysis. Acta Orthop Scand. 2000;71:370–375. [DOI] [PubMed]
- 38.Leunig M, Slongo T, Ganz R. Subcapital realignment in slipped capital femoral epiphysis: surgical hip dislocation and trimming of the stable trochanter to protect the perfusion of the epiphysis. Instr Course Lect. 2008;57:499–507. [PubMed]
- 39.Leunig M, Slongo T, Kleinschmidt M, Ganz R. Subcapital correction osteotomy in slipped capital femoral epiphysis by means of surgical hip dislocation. Oper Orthop Traumatol. 2007;19:389–410. [DOI] [PubMed]
- 40.Loder RT. Unstable slipped capital femoral epiphysis. J Pediatr Orthop. 2001;21:694–699. [DOI] [PubMed]
- 41.Mardones RM, Gonzalez C, Chen Q, Zobitz M, Kaufman KR, Trousdale RT. Surgical treatment of femoroacetabular impingement: evaluation of the effect of the size of the resection. J Bone Joint Surg Am. 2005;87:273–279. [DOI] [PubMed]
- 42.Martin P. Slipped capital femoral epiphysis in the adolescent hip: a reconsideration of open reduction. J Bone Joint Surg Am. 1948;30:9–19. [PubMed]
- 43.Martin T, Fayad F. Severe upper femoral epiphysiolysis. Invasive reduction by Dunn’s technic: 11 cases [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1986;72:587–598. [PubMed]
- 44.Merle d’Aubigne R, Postel M. Functional results of hip arthroplasty with acrylic prosthesis. J Bone Joint Surg Am. 1954;36:451–475. [PubMed]
- 45.Müller M. Diagnosi e terapia delle disfuzione meccaniche dell’anca nel bambino quale profilassi dell’artrosi secondaria. Minerva Ortop. 1968;19:267–273. [PubMed]
- 46.Müller M. Die hüftnahen Osteotomien. Stuttgart, Germany: Thieme Verlag; 1971.
- 47.Nishiyama K, Sakamaki T, Ishii Y. Follow-up study of the subcapital wedge osteotomy for severe chronic slipped capital femoral epiphysis. J Pediatr Orthop. 1989;9:412–416. [PubMed]
- 48.Notzli HP, Siebenrock KA, Hempfing A, Ramseier LE, Ganz R. Perfusion of the femoral head during surgical dislocation of the hip. Monitoring by laser Doppler flowmetry. J Bone Joint Surg Br. 2002;84:300–304. [DOI] [PubMed]
- 49.Notzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84:556–560. [DOI] [PubMed]
- 50.O’Brien ET, Fahey JJ. Remodeling of the femoral neck after in situ pinning for slipped capital femoral epiphysis. J Bone Joint Surg Am. 1977;59:62–68. [PubMed]
- 51.Oram V. Epiphysiolysis of the head of the femur; a follow-up examination with special reference to end results and the social prognosis. Acta Orthop Scand. 1953;23:100–120. [DOI] [PubMed]
- 52.Pearl AJ, Woodward B, Kellyrp. Cuneiform osteotomy in the treatment of slipped capital femoral epiphysis. J Bone Joint Surg Am. 1961;43:947–954. [PubMed]
- 53.Rab GT. The geometry of slipped capital femoral epiphysis: implications for movement, impingement, and corrective osteotomy. J Pediatr Orthop. 1999;19:419–424. [DOI] [PubMed]
- 54.Rao JP, Francis AM, Siwek CW. The treatment of chronic slipped capital femoral epiphysis by biplane osteotomy. J Bone Joint Surg Am. 1984;66:1169–1175. [PubMed]
- 55.Siegel DB, Kasser JR, Sponseller P, Gelberman RH. Slipped capital femoral epiphysis. A quantitative analysis of motion, gait, and femoral remodeling after in situ fixation. J Bone Joint Surg Am. 1991;73:659–666. [PubMed]
- 56.Southwick WO. Osteotomy through the lesser trochanter for slipped capital femoral epiphysis. J Bone Joint Surg Am. 1967;49:807–835. [PubMed]
- 57.Sugioka Y, ed. Transtrochanteric Rotational Osteotomy of the Femoral Head. St Louis, MO: CV Mosby; 1980. [PubMed]
- 58.Szypryt EP, Clement DA, Colton CL. Open reduction or epiphysiodesis for slipped upper femoral epiphysis. A comparison of Dunn’s operation and the Heyman-Herndon procedure. J Bone Joint Surg Br. 1987;69:737–742. [DOI] [PubMed]
- 59.Velasco R, Schai PA, Exner GU. Slipped capital femoral epiphysis: a long-term follow-up study after open reduction of the femoral head combined with subcapital wedge resection. J Pediatr Orthop B. 1998;7:43–52. [PubMed]
- 60.Waldenstroem H. On necrosis of the joint cartilage by epiphysiolysis capitis femoris. Acta Orthop Scand. 1930;67:936. [PubMed]
- 61.Zilkens J, Lör F, Zilkens K, eds. Long-term Results After Conservatively Treated SCFE. Berlin, Germany: Springer; 1986.