Abstract
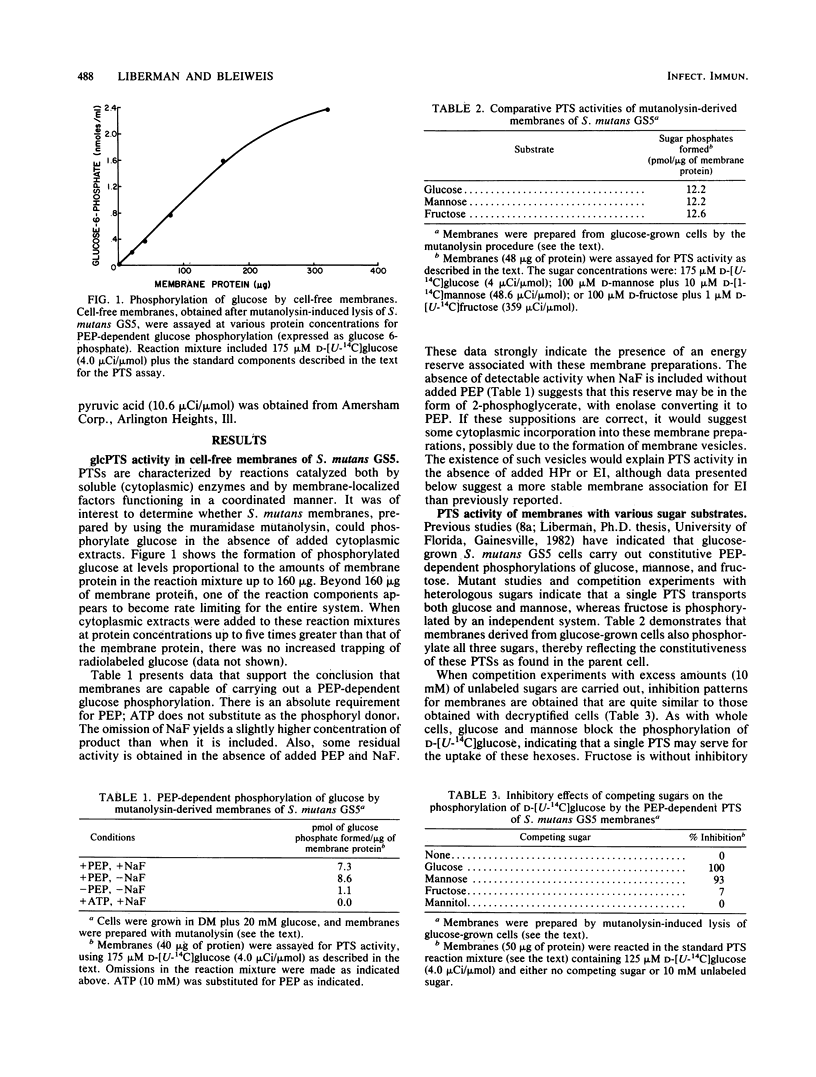
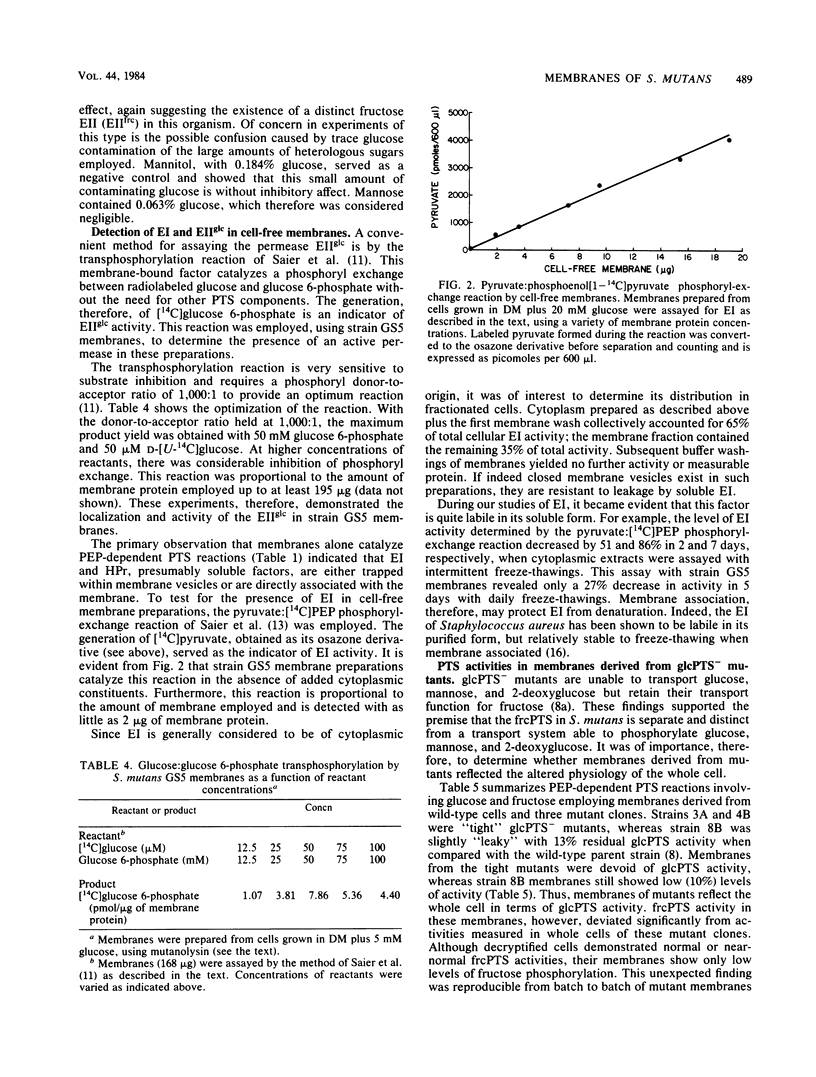
The glucose phosphotransferase system (PTS) of Streptococcus mutans GS5 has been partially characterized, using fractions derived from cells treated with the muramidase mutanolysin. Membranes retained functional PTS enzymes for the phosphoenolpyruvate-dependent phosphorylation of glucose, fructose, and mannose. This was confirmed by assaying membranes directly for enzyme I (EI) and enzyme IIglc (EIIglc) by employing specific phosphoryl-exchange reactions for each factor. Membranes prepared from glucose PTS- mutants, however, were either deficient in glucose phosphorylation or reflected the "leakiness" displayed by whole cells. Mutant membranes were unable to catalyze the glucose:glucose 6-phosphate transphosphorylation reaction, indicating a defective EIIglc in these fractions. Although total cellular EI activities in the mutant clones were about the same as that measured for the wild-type strain by employing the pyruvate:phosphoenolpyruvate phosphoryl-exchange reaction, mutant membranes were found to possess less than 10% of the specific EI activity of wild-type membranes. The cytoplasmic fractions of mutants, however, displayed markedly increased specific activities for this enzyme when compared with wild-type extracts. These results strongly suggest a molecular association of EI with a normal membrane protein, perhaps EIIglc, that is absent in mutants. This would explain the absence of fructose PTS activity in glucose PTS- mutant membranes despite the fact that whole cells of these clones are normal for this transport function.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dills S. S., Apperson A., Schmidt M. R., Saier M. H., Jr Carbohydrate transport in bacteria. Microbiol Rev. 1980 Sep;44(3):385–418. doi: 10.1128/mr.44.3.385-418.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dills S. S., Seno S. Regulation of hexitol catabolism in Streptococcus mutans. J Bacteriol. 1983 Feb;153(2):861–866. doi: 10.1128/jb.153.2.861-866.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham D. R., Phibbs P. V., Jr Fractionation and characterization of the phosphoenolpyruvate: fructose 1-phosphotransferase system from Pseudomonas aeruginosa. J Bacteriol. 1982 Feb;149(2):534–541. doi: 10.1128/jb.149.2.534-541.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs L. J., Post A., White E. R., Finkelstein J. A., Moeckel W. E., Holden K. G., Zarembo J. E., Weisbach J. A. Identification and quantitation of alditol acetates of neutral and amino sugars from mucins by automated gas-liquid chromatography. Anal Biochem. 1971 Oct;43(2):369–381. doi: 10.1016/0003-2697(71)90266-1. [DOI] [PubMed] [Google Scholar]
- Hamilton I. R., Lo G. C. Co-induction of beta-galactosidase and the lactose-P-enolpyruvate phosphotransferase system in Streptococcus salivarius and Streptococcus mutans. J Bacteriol. 1978 Dec;136(3):900–908. doi: 10.1128/jb.136.3.900-908.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. Transport studies in bacterial membrane vesicles. Science. 1974 Dec 6;186(4167):882–892. doi: 10.1126/science.186.4167.882. [DOI] [PubMed] [Google Scholar]
- Liberman E. S., Bleiweis A. S. Role of the phosphoenolpyruvate-dependent glucose phosphotransferase system of Streptococcus mutans GS5 in the regulation of lactose uptake. Infect Immun. 1984 Feb;43(2):536–542. doi: 10.1128/iai.43.2.536-542.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman E. S., Bleiweis A. S. Transport of glucose and mannose by a common phosphoenolpyruvate-dependent phosphotransferase system in Streptococcus mutans GS5. Infect Immun. 1984 Mar;43(3):1106–1109. doi: 10.1128/iai.43.3.1106-1109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr Bacterial phosphoenolpyruvate: sugar phosphotransferase systems: structural, functional, and evolutionary interrelationships. Bacteriol Rev. 1977 Dec;41(4):856–871. doi: 10.1128/br.41.4.856-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier M. H., Jr, Feucht B. U., Mora W. K. Sugar phosphate: sugar transphosphorylation and exchange group translocation catalyzed by the enzyme 11 complexes of the bacterial phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1977 Dec 25;252(24):8899–8907. [PubMed] [Google Scholar]
- Saier M. H., Jr, Newman M. J., Rephaeli A. W. Properties of a phosphoenolpyruvate: mannitol phosphotransferase system in Spirochaeta aurantia. J Biol Chem. 1977 Dec 25;252(24):8890–8898. [PubMed] [Google Scholar]
- Saier M. H., Jr, Schmidt M. R., Lin P. Phosphoryl exchange reaction catalyzed by enzyme I of the bacterial phosphoenolpyruvate: sugar phosphotransferase system. Kinetic characterization. J Biol Chem. 1980 Sep 25;255(18):8579–8584. [PubMed] [Google Scholar]
- Schachtele C. F., Mayo J. A. Phosphoenolpyruvate-dependent glucose transport in oral streptococci. J Dent Res. 1973 Nov-Dec;52(6):1209–1215. doi: 10.1177/00220345730520060801. [DOI] [PubMed] [Google Scholar]
- Siegel J. L., Hurst S. F., Liberman E. S., Coleman S. E., Bleiweis A. S. Mutanolysin-induced spheroplasts of Streptococcus mutants are true protoplasts. Infect Immun. 1981 Feb;31(2):808–815. doi: 10.1128/iai.31.2.808-815.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Nakazawa T., Hays J. B., Roseman S. Sugar transport. IV. Isolation and characterization of the lactose phosphotransferase system in Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):932–940. [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Slee A. M., Tanzer J. M. Effect of growth conditions on sucrose phosphotransferase activity of Streptococcus mutans. Infect Immun. 1980 Mar;27(3):922–927. doi: 10.1128/iai.27.3.922-927.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Turner K. W., Thomas T. D. Catabolite inhibition and sequential metabolism of sugars by Streptococcus lactis. J Bacteriol. 1978 Mar;133(3):1163–1174. doi: 10.1128/jb.133.3.1163-1174.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


