Abstract
Recurrence of Dupuytren’s contracture is common yet unpredictable, compromising surgical outcome. The α-smooth muscle actin-containing myofibroblast is the active contractile cellular component. Based on recent reports on β-catenin accumulation in Dupuytren’s disease, we investigated a possible relation with disease recurrence. We divided a collection of 143 nodules into those from patients with recurrent or nonrecurrent nodules and with a minimal 3-year followup. We randomly selected 12 and 11 samples of each group, respectively. We looked at Dupuytren’s diathesis, immunohistologic staining for β-catenin and α-smooth muscle actin, and Luck’s histologic stages (zones). The expression of selected Wnt genes was examined with TaqMan® PCR in separate histologic zones. All samples showed cytoplasmic and nuclear β-catenin accumulation in myofibroblasts in involutional zones. The risk score of Abe et al. and Dupuytren’s diathesis were greater in the recurrent group. Greater Wnt5a expression in the β-catenin-accumulating involutional zone was seen. We conclude intracellular β-catenin accumulation, possibly regulated by upstream Wnt signaling pathway activation and confined in myofibroblasts in the involutional zone of Dupuytren’s diathesis, is unrelated to disease recurrence. Clinical parameters for Dupuytren’s diathesis remain the best way to predict recurrence risk.
Introduction
Recurrence of Dupuytren’s contracture is relatively common after surgical correction, with reported rates varying from 2% to 63% [15]. A couple attempts to predict recurrence or extension of Dupuytren’s disease (DD) by developing scoring systems such as that of Abe et al. [1] have been made, with predictive risk values ranging from 23% to 71% [1, 10]. Such risk scores are based on clinical features. Currently, the clinical staging methods extracted from epidemiologic surveys are the only available tools to help predict recurrence rates. Parameters for these scoring systems are the personal history of the patient and severity of the contracture [7]. An early age of onset, positive family history, multiple ray involvement, and associated fibrotic disease (plantar or penile fibrosis) are predominant risk factors for DD [1, 9, 10, 15].
Surgical excision of the palmar fascia is the preferred treatment [3, 6]. Numerous surgical options are available using minimally invasive techniques (fasciotomy) or more aggressive methods, such as fascia resection, skin grafting, flap surgery, or even amputation. More invasive surgery with replacement of the involved skin by skin grafting or flap surgery can reduce recurrence risks [15]. A more accurate method for predicting disease recurrence may ease decision making between surgical options. Enhanced basic knowledge provides new insight into the underlying molecular activity control mechanisms responsible for recurrent DD [3, 6, 8, 24].
In 1959, Luck [14] classified the histology of nodules in DD into three stages based on fibroblast and intercellular matrix features: the proliferative stage, the involutional stage, and the residual stage. Rombouts et al. [20] added some prognostic value to this classification, suggesting recurrence was more frequent when the resected tissue samples of patients with DD had an abundance of proliferative tissue. Fifteen years after the introduction of Luck’s classification, the active contractile cell was identified as the α-smooth muscle actin (α-SMA)-containing myofibroblast [5, 16, 23]. These phenotypically modified fibroblasts have been described in inflammatory tissues, tumor stroma, and wound healing [8, 13]. Based on their contractile properties, these cells also are referred to as activated fibroblasts [13].
Some investigators have attributed an important role to the Wnt signaling pathway in fibrous tumors regulating fibroblast proliferation and cell fate or differentiation [4, 17, 23]. β-Catenin is a key protein in the canonical Wnt pathway [21]. Recent reports have shown increased cytoplasmic and nuclear levels in fibroproliferative diseases, including DD [3, 10, 17]. Wnts are the most recognized upstream regulators of β-catenin accumulation [4, 19]. Although Wnt expression per se has not been shown as the cause of β-catenin dysregulation in DD, Wnt 5a, 9a, 10b, and 11 are the major expressed Wnt family members in DD [19].
We investigated a possible use for β-catenin expression markers in predicting recurrence in DD. A possible role for Wnt pathway activation in β-catenin accumulation was explored by examining the coexpression of selected Wnt genes.
Materials and Methods
We obtained 143 fresh-frozen samples of removed nodules from patients with DD between February 2003 and September 2004 with a minimum 3-year followup (mean, 4 years 3 months; range, 3 years 2 months–5 years 2 months). These 143 tissue samples were evaluated for recurrence based on patients’ medical files and divided into recurrent or nonrecurrent subgroups with a minimum of 3-year followup. At random, we then selected patients for two groups: Group 1 included 12 patients with recurrent disease requiring revision surgery within 1 year after primary surgery and Group 2 included 11 patients with no recurrences within 3 years of clinical followup. From the patients’ medical files and a questionnaire, clinical data concerning DD, surgical treatment, and diathesis were gathered and compared between groups. Clinical data for gender, age of onset, and age at time of first surgical treatment were noted (Table 1). Attention was focused on hand involvement: bilateral disease, number of rays affected, radial side involvement, and metacarpophalangeal, proximal interphalangeal, or distal interphalangeal joint contracture. Dupuytren’s diathesis also was evaluated by obtaining a thorough family history and asking about ectopic fibrosis (Ledderhose’s plantar fibrosis or Peyronie’s penile fibrosis). The individual scores of Abe et al. [1] were calculated, with a score greater than 4 representing a high recurrence risk. Attention also was focused on the total number of operations and the most extensive surgical technique used in each patient (Table 1).
Table 1.
Demographic features of patients in Groups 1 and 2*
| Group-patient number | Recurrence (total number of operations) | Age at onset (years) | Age at surgery (years) | Gender | Bilateral | Fingers involved | Contracted joints | Total number of rays | Fibrosis | Score of Abe et al. [1] | Family | Associated diseases | Surgical technique revision |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-1 | 8x | 40 | 66 | Male | + | 1–5 | MCP, PIP, DIP | 10 | 5 | P, B | Melanoma | Z | |
| 1-1 | 6x | 28 | 33 | Male | + | 1–5 | MCP, PIP | 10 | Ledderhose | 7 | M | Hueston | |
| 1-3 | 2x | 24 | 62 | Male | + | 1, 4, 5 | MCP, PIP | 6 | Peyronie, Ledderhose | 7 | P | HemoA, Steinert, HCV | Flag flap |
| 1-4 | 3x | 65 | 78 | Male | + | 4, 5 | MCP, PIP | 3 | 2 | Baso, Vaquez | Z | ||
| 1-5 | 2x | 55 | 59 | Male | + | 1, 4, 5 | MCP, PIP, DIP | 4 | 4 | B | Trauma | Z | |
| 1-6 | 4x | 43 | 45 | Male | + | 1, 3–5 | MCP, PIP | 6 | 5 | Chase, Hueston | |||
| 1-7 | 4x | 50 | 58 | Male | + | 3–5 | MCP, PIP | 5 | 3 | M | HBV | Hueston | |
| 1-8 | 2x | 49 | 53 | Male | + | 4, 5 | MCP, PIP | 3 | 3 | B (I-10) | Z | ||
| 1-9 | 4x | 45 | 52 | Male | + | 1, 3, 5 | MCP | 4 | 5 | B (I-9) | Hueston | ||
| 1-10 | 4x | 48 | 53 | Female | + | 1, 5 | MCP PIP | 3 | 5 | Hueston, amp5 | |||
| 1-11 | 3x | 55 | 66 | Male | + | 4, 5 | MCP, PIP, DIP | 3 | 2 | Z | |||
| 1-12 | 3x | 48 | 57 | Male | + | 3–5 | MCP, PIP | 6 | Ledderhose | 5 | Gout | Chase, Hueston | |
| Mean | 3.6 | 46 | 53 | 100% | Radial side 58% | PIP 92% | 5.25 | 25% | 4.4 | 58% | |||
| 2-1 | 1x | 60 | 69 | Male | 3, 4 | MCP, PIP | 2 | 0 | Segm | ||||
| 2-2 | 1x | 55 | 60 | Male | + | 2–5 | MCP, PIP | 8 | 2 | Gout | Segm | ||
| 2-3 | 1x | 68 | 71 | Male | + | 4, 5 | MCP, PIP | 3 | 2 | Z | |||
| 2-4 | 1x | 58 | 62 | Male | 5 | MCP, PIP | 2 | Ledderhose | 3 | M | ACL | Z | |
| 2-5 | 1x | 58 | 69 | Male | 4, 5 | MCP, PIP | 2 | 1 | P, B, B | Z | |||
| 2-6 | 1x | 46 | 67 | Male | + | 4, 5 | MCP | 3 | 3 | Z | |||
| 2-7 | 1x | 51 | 56 | Male | + | 4 | MCP, PIP | 2 | 1 | Segm | |||
| 2-8 | 1x | 30 | 40 | Male | 3–5 | MCP | 3 | 2 | P | Segm | |||
| 2-9 | 1x | 54 | 55 | Male | 4, 5 | MCP, PIP | 2 | 1 | Z | ||||
| 2-10 | 1x | 58 | 62 | Male | + | 4, 5 | MCP | 3 | 2 | S, S, n, n, P | Segm | ||
| 2-11 | 1x | 57 | 60 | Male | 2–5 | MCP, PIP | 4 | 1 | Segm | ||||
| Mean | 1 | 54 | 60 | 45% | Radial side 0% | PIP 72% | 3.09 | 10% | 1.6 | 36% |
* Group 1 consists of the samples of 12 patients with multiple recurrences, requiring amputation in three cases; Group 2 consists of the samples of 11 patients with no recurrence within a minimum 3-years followup; MCP = metacarpophalangeal joint; PIP = proximal interphalangeal joint; DIP = distal interphalangeal joint; HemoA = hemophilia Type A; HCV = hepatitis C viral infection; HBV = hepatitis B viral infection; B = brother; P = father; M = mother; S = sister; n = niece/nephew; Chase = 4th ray amputation; Hueston = full-thickness skin graft; ACL = anterior cruciate ligament; Z = Brunner incision or Z plasty; amp5 = amputation of the fifth digit; Segm = segmental fasciectomy.
A senior pathologist (RS) first confirmed the diagnosis of Dupuytren’s nodules on a hematoxylin and eosin-stained sample. All samples were confirmed as Dupuytren’s nodules, with cross-sectional diameters ranging from 3 to 10 mm on microscopic evaluation with hematoxylin and eosin staining. We then prepared six neighboring sections (5 μm thick) per sample made directly adjacent to one another for interpretation of immunohistologic coexpression in similar cell groups. The 6% formalin-fixed, paraffin-embedded (FFPE) samples then were prepared for immunohistologic staining for β-catenin and α-SMA. After deparaffinating and rehydrating the samples and quenching endogenous peroxidase activity (in the samples for β-catenin staining, this was in citric acid, 10 mmol/L [pH 6], for 30 minutes in a 98°C water bath), the serial slides were blocked by serum bovine albumin and were incubated with the specific mouse monoclonal antibodies for β-catenin for 3 hours (Transduction Laboratories, San Diego, CA) and α-SMA for 3 hours (Dako, Glostrup, Denmark). For negative controls, we used a simultaneous identical procedure without antibody and a positive control for fibroblasts with vimentin for 30 minutes (Sigma-Aldrich Corp, St Louis, MO). After a wash step, we used EnVision™ antimouse immunoglobulin (Dako) for 30 minutes to allow observation. After washing in phosphate-buffered saline and bovine serum albumin, we stained the samples with diaminobenzidine under direct control using a microscope. Secondary staining was completed using hematoxylin and the samples were mounted.
Neighboring samples, stained with the primary antibodies for α-SMA and β-catenin, were studied in a blinded fashion by two observers (ID, ST). Histologic stages according to Luck [14] were identified based on microscopic evaluation of the cellular/matrix density and cellular orientation. The first histologic stage (the proliferative stage) was identified as cell-rich, little intercellular matrix, and no orientation of the cells. The second stage (involutional stage) was identified as cell-rich, little intercellular matrix, and obvious alignment of the cells. The third, residual stage was cell-poor with aligned cells and a collagen-rich intercellular matrix. The relative staining surface for β-catenin and the distribution of the three histologic stages were assessed by putting a millimetered geometric matrix on the samples with β-catenin staining and manually counting their relative surface ratio. Although Luck described his three stages as reflecting different times in the natural history of Dupuytren’s contracture, we found examples of all three stages in each specimen and therefore refer to these differing histologic pictures as zones.
On eight samples with sufficient tissue left for quantitative PCR (four in Group 1, four in Group 2), the three histologic zones were identified on the β-catenin-stained samples and separated on FFPE neighboring samples before RNA extraction. RNA was extracted from these FFPE sections using the RNeasy® FFPE kit (Qiagen, Inc, Valencia, CA) following the manufacturer’s instructions. The expression of Wnt 5a, 9a, 10b, and 11 was analyzed by TaqMan® PCR (Applied Biosystems, Foster City, CA) according to the protocols provided by the manufacturer. Expression of the housekeeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used for normalization of the different cellular density in the separate histologic zones. The mean calibrated normalized relative quantity values for the four investigated Wnts were calculated in the three histologic zones of all eight samples.
We compared the factors for DD and the score of Abe et al. [1] between the two groups using a two-sample t test. For immunohistologic evaluation, two statistical methods were used. First, correlation of the relative surface of the areas staining for β-catenin was compared between recurrent and nonrecurrent groups tested by using the Mann-Whitney U test. Second, we compared histologic areas between the recurrent and nonrecurrent groups. Because the patient-specific percentages of proliferation, involution, and residual zones are constrained to sum up to 100% per patient, classic statistical approaches are not appropriate to compare distribution between two groups. Therefore, analysis methods for compositional data have been used [2]. More specifically, using one of the zones as the arbitrary reference percentage, a log ratio transformation was used for the two other zones. Assuming a bivariate normal distribution, these log ratios then were compared between both groups using an F-test with two degrees of freedom. Percentages in each zone and their 95% confidence intervals were obtained by retransformation on the original scale (and using the delta rule). For the quantitative PCR, a two-sample t test was used to evaluate differences in Wnt expression in the histologic zones of all eight samples.
Results
Recurrence was present in 69 patients (48%). More risk factors for recurrence were seen in Group 1 (Table 1). These included an earlier mean age (p = 0.024) of onset (46 years, range, 28-65 years versus 54 years in Group 2), more patients (p = 0.029) with bilateral hand disease (100% versus 45%, respectively), greater percentage (p = 0.016) radial side involvement (58% versus 0%), greater numbers (p = 0.0089) of rays involved (mean of 5.25, range, 3–10 versus 3.09, range, 2–8), higher percentage of ectopic disease (25% versus 10%), and a greater percentage of positive family history (58% versus 36%). The score of Abe et al. [1] also was higher (p = 0.00012) in Group 1 (mean, 4.4; range, 2–7) compared with Group 2 (mean 1.6; range, 0–3). Both groups had a mean interval of 6 years between disease onset and first surgery. In Group 1, patients had reoperations for recurrence another 2.6 times (range, 1–7 times). Although first surgical procedures were segmental or total strand fasciectomy, surgical techniques were more aggressive in Group 1’s later revision surgery with flap surgery and even ray amputation.
Immunohistologic examination revealed diffuse nuclear and cytoplasmic staining for β-catenin in all samples. The proliferative and cell-poor residual zones showed no staining for β-catenin. In the involutional zones, β-catenin staining was obvious (Fig. 1).
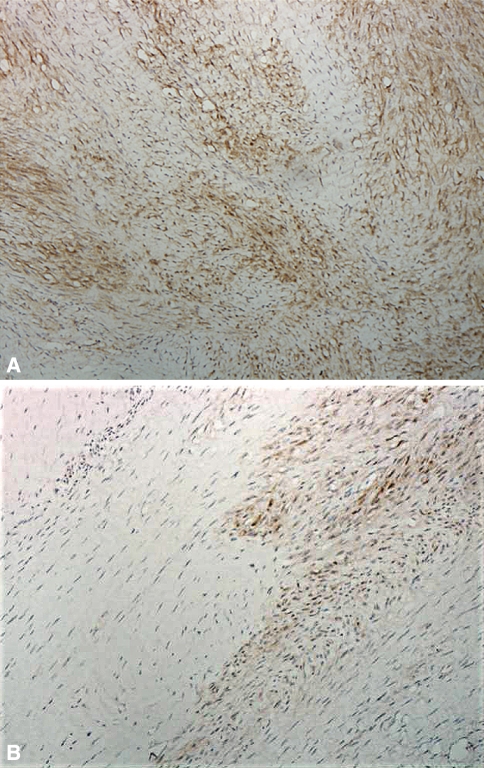
Fig. 1A–B.
Immunohistologic micrographs of a sample of a Dupuytren’s nodule show (A) an involutional zone with partially aligned staining myofibroblasts (larger spindle-shaped cells) with few fibers (Patient 1-8) and (B) an involutional β-catenin-staining zone (black arrow) adjacent to a nonstaining residual zone (white arrow) (Patient 1-12) (Stain, primary antibody for β-catenin; original magnification, ×400).
All samples showed immunohistologic staining of α-SMA to some extent. Involutional zones stained positive, whereas proliferative and residual zones expressed no staining for α-SMA. Thus, only the involutional zones stained positive for β-catenin and α-SMA (Fig. 2). Positive control showed diffuse cellular vimentin staining on all slides in the three histologic zones. In the negative control procedure, no staining was seen (Fig. 3). The surface ratio of the three histologic zones showed an overall percentage of 29% zones of proliferation, 11% zones of involution, and 60% residual zones (Fig. 4). In Group 1, there was a distribution of 13.6% (range, 4%–23%) zones of proliferation, 8.5% (range, 2.8%–14.1%) zones of involution, and 77.9% (range, 64%–91.8%) residual zones. In Group 2, there was a distribution of 40.4% (range, 21.7%–59%) zones of proliferation, 13.5% (range, 6.4%–20.5%) zones of involution, and 46.2% (range, 24.8%–67.6%) residual zones.
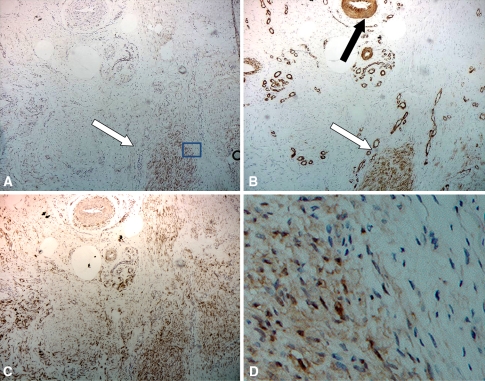
Fig. 2A–D.
Micrographs of neighboring samples stained with (A) β-catenin, (B) α-SMA, and (C) a positive control with vimentin in Patient 1-1 show a staining zone of involution (white arrow) surrounded by tissue in the proliferative zone with only vimentin staining fibroblasts and vascular infiltration (positive endothelial staining for α-SMA, black arrow) (original magnification, ×400). A magnification (×1000) of the selected staining area (D = square in micrograph A) with β-catenin staining shows nuclear and cytoplasmic accumulation in the myofibroblasts in contrast to the neighboring nonstaining fibroblasts.
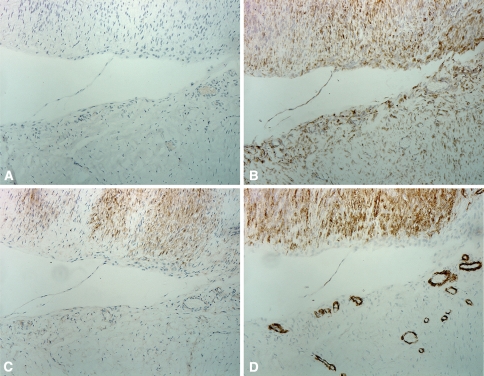
Fig. 3A–D.
Micrographs of neighboring tissue samples in Patient 1-2 show slides (A) without antibody (negative control), (B) vimentin staining fibroblasts (positive control), (C) β-catenin staining myofibroblasts, and (D) α-SMA marking myofibroblast. Histologically, the upper half of the image was identified as a contractile involutional zone with aligned myofibroblasts and the lower half is a proliferative zone with disorganized fibroblasts (Original magnification, ×400).
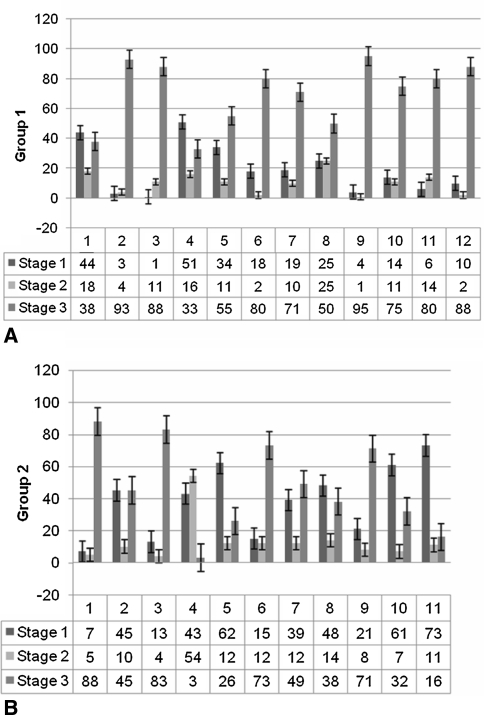
Fig. 4A–B.
The percentages of surface distribution ratio of the three histologic zones on every sample were measured with a geometric matrix in (A) Group 1 without recurrence and (B) Group 2 with recurrent DD. All zones were present in every sample in variable proportions. An overall distribution of 10% proliferative (Stage 1), 30% involutional (Stage 2), and 60% residual (Stage 3) zones was seen. In Group 1, residual zones were somewhat more pronounced, and in Group 2, the involutional zones were more prevalent. Proliferative zones had an equal distribution in both groups.
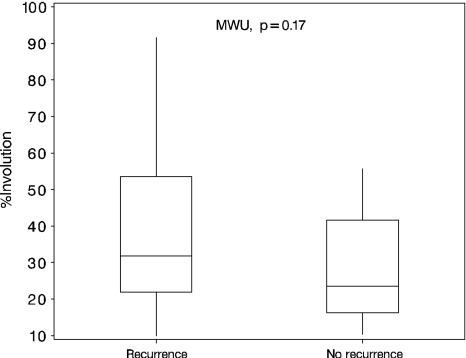
The distribution of β-catenin staining differed over the histologic zones between both groups (Table 2). We observed no difference in relative β-catenin staining surface in cell-rich areas (involutional compared with proliferative zones) between recurrent and nonrecurrent groups (Fig. 5).
Table 2.
Relative distribution of the proliferative, involutional, and residual zones*
| Zone | Group 1 | Group 2 | p Value |
|---|---|---|---|
| Proliferation | 13.6% (4%–23%) | 40.4% (21.7%–59% | 0.015 |
| Involution | 8.5% (2.8%–14.1%) | 13.5% (6.4%–20.5%) | 0.27 |
| Residual | 77.9% (64%–91.8%) | 46.2% (24.8%–67.6%) | 0.017 |
* Percentages (95% confidence interval) obtained after retransforming the results from the bivariate normal model on the original scale; p value refers to the comparison of both percentages.
Fig. 5.
Box plots of percent relative surface of the involutional zone as a percentage of the total amount of cell-rich areas (proliferative and involutional zones) show recurrent and nonrecurrent groups have obvious β-catenin accumulation with no difference between the groups (Mann-Whitney U test [MWU], p = 0.17).
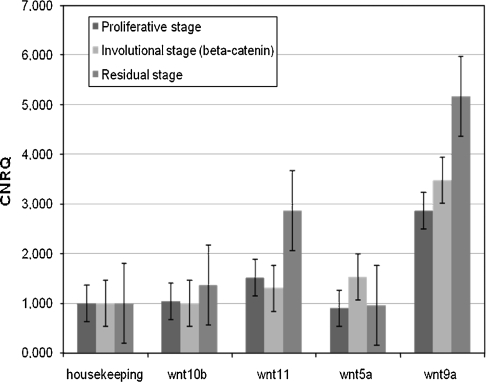
All four investigated Wnts (Wnt5a, 9a, 10b, 11) were readily detectible in all eight samples and in all three histologic zones of Groups 1 and 2 (Fig. 6). Wnt 9a showed the highest normalized relative quantity of expression followed by Wnt11. Although β-catenin accumulation was confined to the involutional zones, a higher Wnt expression was observed here, compared with the proliferative and residual zones. Wnt5a had a higher (p = 0.048) expression limited to the involutional zone.
Fig. 6.
Wnt expression in proliferative, involutional, and residual zones was compared using TaqMan® PCR in eight samples. We normalized using the housekeeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase). No differences in the β-catenin-accumulating involutional zone were seen, although Wnt5a showed slightly increased expression (p = 0.048). CNRQ = calibrated normalized relative quantity.
Discussion
Dupuytren’s disease can be therapy-resistant with unpredictable recurrence rates after surgery [15]. Although currently only clinical parameters are available to predict outcome and recurrence risks in DD, we explored β-catenin accumulation as a possible immunohistologic parameter in biopsies of patients with DD to predict recurrence. We also explored Wnt expression as a possible upstream regulator of β-catenin accumulation in DD.
The study has some major limitations. First, although sample numbers are limited, they were chosen randomly from a large population after separating recurrent (48%) and nonrecurrent (52%) groups with a minimum 3-year followup. Second, our sampling was limited to tissue obtained at the time of surgery; we cannot ensure those samples represent all tissue present. Next, immunohistologic staining in neighboring samples has inherent minor histologic differences. Also, antibody-dependent staining procedures demand different preparation methods of the tissue samples (a 98°C bath is necessary for quenching in the β-catenin staining procedure), meaning handling of the tissue is somewhat different between neighboring sections. Limitations of the quantitative PCR include a low sample size and the observer’s microdissection on nonstained FFPE samples based on neighboring β-catenin-stained samples. The relatively low expression consistency and limited number of investigated Wnt genes weaken conclusions on upstream Wnt regulation of β-catenin expression.
Reports on recurrence in DD are variable, ranging from 2% to greater than 63% owing to an inconsistent definition of recurrence and different followup periods [15]. Bulstrode et al. [5] stated recurrence is inevitable if one lives long enough. However, recurrence can be fast and aggressive in some cases, and here, more aggressive treatment with skin resection might be indicated [2, 15]. Reported complication rates in DD surgery vary from 17% to 50%, a good reason to keep the number of surgical procedures to a minimum [5]. We explored an immunohistologic difference of a high-risk group with recurrence within 1 year after surgery requiring revision surgery. Therefore, we defined recurrence within 1 year in the first group. Clinical parameters showed obvious DD in the recurrent group with a difference in the score of Abe et al. [1], with a score higher than 4 in the recurrent group, confirming good sample selection. Patients in the recurrent group had a younger age of onset, more severe hand involvement with bilateral disease, more severe contractures, and multiple ray and radial side involvement. Also, family aggregation and ectopic disease were more pronounced. These findings illustrate the prognostic value of clinical scores for recurrence risks in DD [1, 10].
We observed obvious cytoplasmic and nuclear β-catenin accumulation in all samples of DD, confirming previous findings by Montgomery et al. [17, 18], Varallo et al. [25], and Bowley et al. [4]. Histologic evaluation showed a gross distribution of 30% proliferative, 10% involutional, and 60% residual zones in all samples. The findings of Rombouts et al. [20], indicating a somewhat lower recurrence rate in cases in which residual zones were more pronounced, could not be confirmed in our study, in which a slight increase of residual zones was noted in the recurrent group. Based on α-SMA expression, we noted the myofibroblast is the predominant cell in the involutional zones of DD. The correlation between α-SMA expression and contractile properties of the myofibroblast contributing to the final finger contracture seen in DD was first observed in vitro by Tomasek and Rayan [23] in 1995. Luck [14] was the first to attribute the actual contraction to the cells seen in the involutional zones, even before the actual myofibroblast was discovered. Once the myofibroblast was identified, different studies have confirmed the presence of myofibroblast in the involutional zones [11, 12, 16]. We also found β-catenin limited to these myofibroblast cell groups, pointing toward a possible role for β-catenin in cellular activity in DD because the myofibroblast is the active component of the contracture [4, 8, 13, 23, 24]. However, no difference in β-catenin expression was observed between recurrent and nonrecurrent disease. This confirms the findings of the study by Wilbrand et al. [26] suggesting the presence of activation markers in connective tissue of DD is unrelated to disease recurrence.
β-Catenin is a central component of the canonical Wnt pathway, a common theme in embryonic development [21, 22]. The Wnt pathway has been implicated in the pathogenesis of many fibroproliferative diseases because abnormal levels of β-catenin are seen in cells in in vivo and in vitro studies and several family members have mutations in various human fibroproliferative malignancies but not in DD [3, 4, 17, 18, 21]. O’Gorman et al. [19] observed no clear underlying Wnt pathway activation as a mechanism for fibroblast activation or β-catenin accumulation in DD. We specifically highlighted the histologic zones in DD and draw attention to Wnt5a, which may have a slightly higher expression limited to the involutional β-catenin-accumulating zones in DD. Nevertheless, this finding may indicate an upstream regulating role for canonical Wnt pathway β-catenin accumulation in the involutional myofibroblast in DD.
Our observations suggest intracellular β-catenin accumulation is unrelated to disease recurrence. The risk score of Abe et al. [1] is well correlated with disease recurrence, and at this time, clinical parameters for DD remain the best tool to estimate individual recurrence risks. β-Catenin overexpression, possibly regulated by upstream Wnt signaling pathway activation, is confined in cellular subgroups identified as myofibroblasts in the involutional zone of DD.
Acknowledgments
We thank Steffen Fieuws, PhD, for his thorough work on the statistical analyses. We also thank Bart Biesmans and Jef De Schutter for help with Wnt pathway exploration in fibroproliferative disease.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Abe Y, Rokkaku T, Ofuchi S, Tokunaga S, Takahashi K, Moriya H. An objective method to evaluate the risk of recurrence and extension of Dupuytren’s disease. J Hand Surg Br. 2004;29:427–430. [DOI] [PubMed]
- 2.Aitchison J. The statistical analysis of compositional data. J R Stat Soc B. 1982;44:139–177.
- 3.Al-Qattan MM. Factors in the pathogenesis of Dupuytren’s contracture. J Hand SurgAm. 2006;31:1527–1534. [DOI] [PubMed]
- 4.Bowley E, O’Gorman DB, Gan BS. Beta-catenin signalling in fibroproliferative disease. J Surg Res. 2007;138:141–150. [DOI] [PubMed]
- 5.Bulstrode NW, Jemec B, Smith PJ. The complications of Dupuytren’s contracture surgery. J Hand Surg Am. 2005;30:1021–1025. [DOI] [PubMed]
- 6.Cordova A, Tripoli M, Corradino B, Napoli P, Moschella F. Dupuytren’s contracture: an update of biomolecular aspects and therapeutic perspectives. J Hand Surg Br. 2005;30:557–562. [DOI] [PubMed]
- 7.Dias JJ, Braybrooke J. Dupuytren’s contracture: an audit of the outcomes of surgery. J Hand Surg Br. 2006;31:514–521. [DOI] [PubMed]
- 8.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. [DOI] [PubMed]
- 9.Hindocha S, John S, Stanley JK, Watson SJ, Bayat A. The heritability of Dupuytren’s disease: familial aggregation and its clinical significance. J Hand Surg Am. 2006;31:204–210. [DOI] [PubMed]
- 10.Hindocha S, Stanley JK, Watson S, Bayat A. Dupuytren’s diathesis revisited: evaluation of prognostic indicators for risk of disease recurrence. J Hand Surg Am. 2006;31:1626–1634. [DOI] [PubMed]
- 11.Howard JC, Varallo VM, Ross DC, Roth JH, Faber KJ, Alman B, Gan BS. Elevated levels of beta-catenin and fibronectin in three-dimensional collagen cultures of Dupuytren’s disease cells are regulated by tension in vitro. BMC Musculoskelet Disord. 2003;4:16. [DOI] [PMC free article] [PubMed]
- 12.Iwasaki H, Müller H, Stutte HJ, Brennscheidt U. Palmar fibrosis (Dupuytren’s contracture). Virchows Arch A Pathol Anat Histopathol. 1984;405:41–53. [DOI] [PubMed]
- 13.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. [DOI] [PubMed]
- 14.Luck V. Dupuytren’s contracture. J Bone Joint Surg Am. 1959;41:635–664. [PubMed]
- 15.McFarlane RM, McGrouther DA, Flint MH. Dupuytren’s Disease. New York, NY: Churchill Livingstone; 1990.
- 16.Meister P, Gokel JM, Remberger K. Palmar fibrosis—“Dupuytren’s contracture”: a comparison of light electron and immunofluorescence microscopic findings. Pathol Res Pract. 1979;164:402–412. [DOI] [PubMed]
- 17.Montgomery E, Folpe AL. The diagnostic value of beta-catenin immunohistochemistry. Adv Anat Pathol. 2005;12:350–356. [DOI] [PubMed]
- 18.Montgomery E, Lee JH, Abraham SC, Wu TT. Superficial fibromatosis are genetically different from deep fibromatoses. Mod Pathol. 2001;14:695–701. [DOI] [PubMed]
- 19.O’Gorman DB, Wu Y, Seney S, Zhu RD, Gan BS. Wnt expression is not correlated with beta-catenin dysregulation in Dupuytren’s disease. J Negat Results Biomed. 2006;5:13. [DOI] [PMC free article] [PubMed]
- 20.Rombouts JJ, Noël H, Legrain Y, Munting E. Prediction of recurrence in the treatment of Dupuytren’s disease: evaluation of a histologic classification. J Hand Surg Am. 1989;14:644–652. [DOI] [PubMed]
- 21.Tejpar S, Nollet F, Li C, Wunder JS, Michils G, dal Cin P, Van Cutsem E, Bapat B, van Roy F, Cassiman JJ, Alman BA. Predominance of beta-catenin mutations and beta-catenin dysregulation in sporadic aggressive fibromatosis (desmoid tumor). Oncogene. 1999;18:6615–6620. [DOI] [PubMed]
- 22.Teo R, Mohrlen F, Plickert G, Muller WA, Frank U. An evolutionary conserved role of Wnt signaling in stem cell fate decision. Dev Biol. 2006;289:91–99. [DOI] [PubMed]
- 23.Tomasek J, Rayan GM. Correlation of alpha-smooth muscle actin expression and contraction in Dupuytren’s disease fibroblasts. J Hand Surg Am. 1995;20:450–455. [DOI] [PubMed]
- 24.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. [DOI] [PubMed]
- 25.Varallo VM, Gan BS, Seney S, Ross DC, Roth JH, Richerds RS, McFarlane RM, Alman B, Howard JC. Beta-catenin expression in Dupuytren’s disease: potential role for cell-matrix interactions in modulating beta-catenin levels in vivo and in vitro. Oncogene. 2003;22:3680–3684. [DOI] [PubMed]
- 26.Wilbrand S, Flodmark C, Ekborm A, Gerdin B. Activation markers of connective tissue in Dupuytren’s contracture: relation to postoperative outcome. Scand J Plast Reconstr Surg Hand Surg. 2003;37:283–292. [DOI] [PubMed]