Abstract
The surgical dislocation approach is useful in assessing and treating proximal femoral hip deformities commonly due to pediatric conditions. We sought to demonstrate the efficacy and problems associated with this technique. Diagnoses included slipped capital femoral epiphysis, Perthes disease, developmental dysplasia of the hip, osteonecrosis, and exostoses. Through this approach, femoral head-neck osteoplasty (22), intertrochanteric osteotomy (eight), femoral head-neck osteoplasty plus intertrochanteric osteotomy (15), femoral neck osteotomy (five), open reduction and internal fixation of an acute slipped capital femoral epiphysis with callus resection (five), open reduction and internal fixation of an acetabular fracture (one), trapdoor procedure (one), and acetabular rim osteoplasty (one) were performed. The average patient age was 16 years. The minimum followup was 12 months (average, 41.6 months; range, 12–73 months). Patients with Perthes disease and SCFE had preoperative and postoperative WOMAC scores of 9.6 and 5.1, and 7.9 and 3.5 respectively. In patients with unstable SCFEs, the average postoperative WOMAC score was 1.2. Seven patients underwent THAs and two patients underwent hip fusion. Complications in the 58 procedures included four cases of osteonecrosis: three after femoral neck osteotomy and one after intertrochanteric osteotomy. The surgical dislocation technique can be utilized to effectively treat these deformities and improve short-term symptoms. Although the technique is demanding, we believe surgical dislocation offers sufficient advantages in assessing and treating these complex deformities that it justifies judicious application.
Level of Evidence: Level IV, retrospective study, case series. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Developmental and acquired abnormalities of the hips are common in childhood and can lead to persistent hip deformity as the child grows into adulthood. Regardless of the underlying cause and initial treatment, certain common abnormalities in the shape and alignment of the proximal femur persist, which can result in femoroacetabular impingement. Femoroacetabular impingement can lead to pain and cartilage damage and predispose the hip to early osteoarthritis [1, 2, 5–8, 14–16].
Recent advances in surgical technique have permitted safe surgical dislocation of the adult hip, allowing not only full inspection of the joint but also dynamic assessment of femoroacetabular contact during hip motion. Reshaping the proximal femur through head-neck osteoplasty with or without a proximal femoral osteotomy can also be accomplished [4, 10, 15].
We questioned the effectiveness of the surgical dislocation approach in correction of pediatric and adolescent hip deformity. More specifically, we first asked whether patients achieved short-term pain relief by assessing the change in WOMAC scores [3] after surgery as well as looking at the conversion to arthroplasty or fusion, and if the underlying abnormality has any bearing on the clinical outcome. We then identified the complications (including nerve palsy, osteonecrosis, nonunion/delayed union of osteotomy) and determined whether complications were affected by the complexity of the reconstruction. Finally, we describe additional procedures performed after the index operation.
Materials and Methods
We retrospectively reviewed case records, radiographs, and pre- and posttreatment questionnaires of all 81 patients (84 hips) treated with the surgical dislocation technique for various causes (cam and pincer types of femoroacetabular impingement, Perthes disease, slipped capital femoral epiphysis or SCFE, osteonecrosis, synovial chondromatosis, exostosis, and acetabular fracture reduction) from August 2001 to January 2005. Through this surgical approach, osteoplasty, intertrochanteric osteotomy, or femoral neck osteotomy were performed alone or in combination. For this analysis we excluded the 27 patients with cam or pincer deformity unaccompanied by other recognized developmental hip conditions. This left 57 patients (58 hips) younger than 18 years of age at the time of a documented diagnosis of developmental or acquired hip pathology. The average age of the patients was 16 years (range, 8–38 years). The minimum followup period was 12 months (average, 41.6 months; range, 12–73 months). No patients were lost to followup prior to the 12-month minimum period. We saw no patients specifically for this study and rather obtained all information from medical records. We obtained prior IRB approval for this study.
WOMAC scores [3] were noted preoperatively, but WOMAC scores were not obtained in patients with acute trauma or those too young or mentally unable to comprehend the questionnaire. The underlying diagnoses included SCFE (29), Perthes disease (15), and osteonecrosis of the femoral head that was idiopathic or followed femoral neck fractures, SCFE, postfemoral nailing, and sickle cell anemia (eight) (Table 1). Symptoms of impingement were present in three patients in the study group who had previously undergone a Salter’s osteotomy for developmental dysplasia of the hip (DDH), growth arrest from treated DDH, and neonatal septic arthritis sequelae, respectively. One patient had a surgical dislocation to facilitate anatomic reduction of an acetabular fracture. An exostosis of the femoral neck was excised in one patient after a surgical dislocation. The procedure was also performed in one patient with protrusio acetabuli.
Table 1.
Patient demographics
| Diagnosis | Number of patients | Average age at surgery (years) | ||
|---|---|---|---|---|
| Male | Female | Total | ||
| Osteonecrosis | 5 | 3 | 8 | 16.4 |
| SCFE | 9 | 20 | 29 | 15.7 |
| Perthes disease | 6 | 9 | 15 | 19.3 |
| Impingement | 1 | 2 | 3 | 23.3 |
| Acetabular fracture | 1 | 0 | 1 | 11.7 |
| Exostoses | 0 | 1 | 1 | 8 |
| Protrusio acetabuli | 0 | 1 | 1 | 19.5 |
SCFE = slipped capital femoral epiphysis.
Many of our patients were referred with complex deformities and had undergone prior procedures. There were 35 prior procedures performed in 33 of the 58 hips (57%) before the surgical dislocation (Table 2). Seventeen hips underwent prior pinning for SCFE and two femoral neck fractures were closed reduced and pinned. Two patients with Perthes disease had prior arthroscopy; four had prior intertrochanteric osteotomy, and one had a shelf procedure. A patient with DDH had a prior innominate osteotomy and a patient with protrusio had a prior acetabuloplasty. One patient underwent intramedullary rodding of a femoral shaft fracture that went on to develop femoral head osteonecrosis.
Table 2.
Distribution of procedures performed before surgical dislocation
| Procedure | SCFE† | Protrusio acetabuli | Impingement secondary to DDH and neonatal sepsis | Perthes disease* | Osteonecrosis |
|---|---|---|---|---|---|
| Pinning in situ | 17 | – | – | – | 1 |
| Osteotomy | 1 | – | 1 | 4 | – |
| Pelvic osteotomy | – | 1 | 1 | 1 | – |
| Anterior drainage | – | – | 1 | – | – |
| Arthroscopy | – | – | – | 2 | – |
| Core decompression | – | – | – | 1 | – |
| Femoral neck fracture pinning | – | – | – | – | 2 |
| Femoral intramedullary nailing | – | – | – | – | 1 |
| Microfracture | – | – | – | 1 | – |
| Total | 18 | 1 | 3 | 9 | 4 |
*One patient with Perthes disease had a core decompression and an arthroscopy; †one patient with SCFE had a prior pinning followed by an osteotomy.
SCFE = slipped capital femoral epiphysis; DDH = developmental dysplasia of the hip.
The following procedures were performed during the index procedure: femoral head-neck junction osteoplasty alone (22), intertrochanteric osteotomy alone (eight), femoral head-neck osteoplasty plus intertrochanteric osteotomy (15), femoral neck osteotomy for stable SCFE and femoral neck fracture nonunion (five), open reduction and internal fixation of unstable SCFE with callus resection (five), open reduction and internal fixation of an acetabular fracture (one), trapdoor procedure (one), and acetabular rim osteoplasty (one) (Table 3). One patient with SCFE had bilateral procedures.
Table 3.
Procedures in addition to surgical dislocation of the hip
| Additional procedure | Perthes disease | SCFE | Osteonecrosis | Impingement secondary to DDH and neonatal sepsis | Exostoses | Protrusi acetabuli |
|---|---|---|---|---|---|---|
| Femoral head-neck osteoplasty (n = 22) | 9 | 7 | 2 | 3 | 1 | – |
| ITO (n = 8) | 2 | 5 | 1 | – | – | – |
| ITO + femoral head-neck osteoplasty (n = 15) | 4 | 8 | 3 | – | – | – |
| Femoral neck osteotomy (n = 5) | – | 4 | 1 | – | – | – |
| Trapdoor procedure (n = 1) | – | – | 1 | – | – | – |
| ORIF (n = 5) | – | 5 | – | – | – | – |
| ITO and rim osteoplasty (n = 1) | – | – | – | – | – | 1 |
One ORIF of the acetabular fracture is not included; SCFE = slipped capital femoral epiphysis; DDH = developmental dysplasia of the hip; ORIF = open reduction and internal fixation; ITO = intertrochanteric osteotomy.
All surgeries were performed by the senior authors (MBM, YJK) using the following technique. We draped the ipsilateral leg free with the patient in lateral decubitus position and used the surgical approach described by Ganz et al. [4]. The femoral head, proximal femur, acetabulum, and rim structures were inspected to assess for damage to the labrum and articular cartilage, and any labral tears or chondral flaps were débrided. Dynamic assessment of femoroacetabular contact was performed. In this series, labral repair or refixation was not performed. The hip was then reduced, and the osteoplasty of the metaphyseal prominence was performed. The hip was then carried through another range of motion to assess the effect of the osteoplasty on dynamic impingement. If no concomitant intertrochanteric osteotomy was performed, the greater trochanteric osteotomy was reduced to its bed and fixed in place.
We performed an intertrochanteric osteotomy in 23 patients to reorient the intact articular surface. Blade plate fixation was used, transfixing the trochanteric wafer with the blade of the implant and then inserting the blade into the head-neck fragment. In five patients with unstable SCFE, we performed an open reduction using the technique described by Leunig et al. [9]. In brief, after performing the trochanteric flip osteotomy and capsulotomy, the thinned periosteum along the anterior femoral neck was visualized. The slip was stabilized using two threaded Kirschner wires. The ligamentum teres was transected and the hip was dislocated anteriorly. We removed the proximal portion of the stable trochanter in a subperiosteal fashion using an osteotome and curettes. The femoral neck periosteum was gently mobilized posteriorly and anteriorly, protecting the vasculature by leaving the periosteal sleeve attached to the femoral head. We then removed the two threaded Kirschner wires and the femoral head was fully mobilized. Posterior callus was resected using a rongeur. We trimmed the femoral neck if we judged necessary until the head with its periosteal sleeve could be reduced anatomically without tension. The epiphysis was then fixed using threaded Kirschner wires and the trochanter reattached.
Postoperatively patients were permitted 1/6th of body weight bearing and placed on precautions that prevented adduction and external rotation for a total of 4 weeks. Passive abduction of 10° was permitted initially and active abduction was encouraged only after the trochanteric osteotomy healed. Flexion was allowed up to 80° and a CPM machine was used when an osteoplasty was done.
Patients were seen 4, 8, and 12 weeks postoperatively by the surgeon and physical therapist and radiographs were obtained to monitor trochanteric union. We obtained postoperative outcomes using the WOMAC questionnaire. The sums of the pain and function domains of the WOMAC questionnaire were recorded. For pain, the scale ranges from 0 to 20, with 0 to 5 considered mild, 6 to 10 moderate, and more than 10 severe. For function, the scale ranges from 0 (no disability) to 68 (extreme disability). Pre- and postoperative WOMAC scores were available in 34 (59.6%) patients and postoperative WOMAC scores were available in 55 patients (96.5%).
We obtained preoperative and postoperative standing anteroposterior pelvic radiographs and bilateral frog leg or true lateral radiographs. Radiographs were utilized to check for healing of the osteotomy and presence of osteonecrosis (GR and YJK). Osteonecrosis was diagnosed by radiographic signs such as increased density of the femoral head followed by eventual collapse. In case of SCFE undergoing capital reduciton, routine bone scans were obtained. Preoperative weight-bearing radiographs were deferred in unstable SCFEs.
Patients having a subsequent THA or hip arthrodesis were deemed failures. Major postoperative complications, such as nerve palsies, infections, nonunions, and hardware failures, were also noted.
Results
Patients with Perthes disease had average preoperative and postoperative WOMAC scores of 9.6 and 5.1, respectively. In patients with SCFE, the WOMAC scores reduced from a preoperative score of 7.9 to 3.5 postoperatively. Of this group, five patients had acute unstable SCFEs that underwent open reduction and callus resection with realignment of the capital epiphysis. The average postoperative WOMAC score was 1.2 in this group. Patients with osteonecrosis secondary to multiple etiologies had average preoperative and postoperative WOMAC scores of 7 and 6, respectively (Table 4). Ten of the total of 57 patients had postoperative pain scores greater than 10 (severe pain). Patients who had undergone prior surgery had an average postoperative WOMAC score of 5.7 whereas those who had not undergone prior surgery had an average postoperative WOMAC score of 4 (Table 5). Seven of the 57 patients had subsequent THAs and two underwent hip fusion and were considered failures (Table 6).
Table 4.
Average WOMAC scores in the major diagnosis groups
| Diagnosis group | Pain | Stiffness | Function | |||
|---|---|---|---|---|---|---|
| Preoperative | Postoperative | Preoperative | Postoperative | Preoperative | Postoperative | |
| Perthes disease | 9.6 | 5.1 | 3.6 | 2.7 | 22.6 | 14.3 |
| SCFE | 7.9 | 3.5 | 2.9 | 2.3 | 18.7 | 11.1 |
| Osteonecrosis | 7.0 | 6.0 | 3.7 | 4.0 | 23.5 | 24.0 |
| Impingement secondary to DDH and neonatal sepsis | 11.0 | 16.0 | 2.7 | 5.0 | 21.7 | 32.5 |
SCFE = slipped capital femoral epiphysis; DDH = developmental dysplasia of the hip.
Table 5.
WOMAC comparison of patients who did and did not undergo procedures before surgical dislocation
| Prior surgery | Pain | Stiffness | Function | |||
|---|---|---|---|---|---|---|
| Preoperative | Postoperative | Preoperative | Postoperative | Preoperative | Postoperative | |
| Yes | 9.0 (22/30) | 5.7 (29/30) | 3.5 (21/30) | 2.7 (22/30) | 22.1 (22/30) | 13.5 (22/30) |
| No | 8.0 (15/28) | 4.0 (26/28) | 2.5 (15/28) | 2.5 (18/28) | 19.6 (15/28) | 13.8 (18/28) |
Values are expressed as average WOMAC scores, with the numbers of patients having scores in parentheses.
Table 6.
Failures in each diagnosis group
| Diagnosis | THA | Hip arthrodesis |
|---|---|---|
| Perthes disease | 3 | – |
| SCFE | – | 2 |
| Osteonecrosis | 1 | – |
| Impingement secondary to DDH and neonatal sepsis | 2 | – |
| Protrusio acetabuli | 1 | – |
| Total | 7 | 2 |
SCFE = slipped capital femoral epiphysis; DDH = developmental dysplasia of the hip.
One patient developed a superficial peroneal nerve palsy, which was believed secondary to compression boots and completely resolved on followup. There was one common peroneal nerve palsy (foot drop), which partially recovered (active dorsiflexion to neutral), but the patient was still using a leaf spring ankle foot orthosis and had some ankle pain at last followup.
Four of the 57 patients (7%) developed osteonecrosis after a surgical dislocation, three after a femoral neck osteotomy and one after an intertrochanteric osteotomy. Two patients with severe SCFE with closed or partially closed physis treated with surgical dislocation/femoral neck osteotomy developed severe osteonecrosis and are scheduled for or underwent a hip fusion. In addition, a 14-year-old boy with a severe healed post-SCFE deformity who underwent a surgical dislocation and an intertrochanteric osteotomy developed mild anterior segmental osteonecrosis, which was managed nonoperatively. This patient has mild morning stiffness but no pain and is taking active part in sports at 3-year followup. A 16-year-old boy who sustained an unrecognized femoral neck fracture in a skiing accident was referred to our institution 3 months after injury with a varus nonunion and a severe limp developed osteonecrosis after femoral neck osteotomy.
Two patients with stable SCFE that had undergone prior surgical dislocation continued to have persistent deformity that needed a flexion valgus osteotomy to relieve symptoms of impingement and restore the functional arc of motion. One patient with Perthes disease underwent a subsequent flexion valgus deformity to reposition the viable part of her femoral head into the weightbearing zone. The patient with exostoses had two subsequent varus intertrochanteric osteotomies for recurrent valgus deformity. Three patients with SCFE that had undergone surgical dislocation developed a contralateral SCFE that needed pinning. Two patients went on to contralateral epiphysiodesis for leg-length discrepancy. Four patients underwent hip arthroscopy after the index procedure. Fourteen percent of the patients underwent additional surgery on the ipsilateral hip after the index surgical dislocation.
Discussion
We aimed to determine whether pediatric and adolescent patients with hip deformity in whom we used the surgical dislocation approach achieved short-term pain relief, by assessing the change in WOMAC scores after surgery as well as looking at the conversion to arthroplasty or fusion. Additionally, we looked at our complication rate and determined whether it was affected by the complexity of the reconstruction.
We were limited by the heterogeneity of the patient group and the retrospective nature of the research. As a rough measure we considered complexity related to the number of additional procedures performed on the hip at time of the surgical dislocation approach. We studied outcomes in the short term but would need a longer followup to substantiate our present findings. We can therefore only comment on short term complications and outcomes. There is potential to have a high complication rate with surgical dislocation combined with osteotomy and this treatment should be reserved for centers with a great deal of expertise in treating these problems. Finally, the WOMAC score has not been validated in a pediatric population, although we believe it useful. Despite these limitations we believe the technique useful in pediatric conditions and believe the information a basis for further study.
Many of the hips we treated have complex and severe deformities requiring complex reconstructions not amenable to arthroscopic or limited open approaches. This is in contrast to adult FAI where the anterior head-neck junction prominence is focal and can be readily determined through a limited open surgical or an arthroscopic approach. Clinical manifestation is usually in the form of loss of motion in certain planes or hip pain or a combination of both symptoms in otherwise young, healthy, active individuals. We believe that surgical dislocation of the hip permits precise assessment of impingement to better plan reshaping of the femoral head-neck junction and redirectional osteotomies. The deformation of the labrum during hip motion due to abnormal femoral head-neck morphology can be directly visualized and treated by excising the offending area. The case of a 10 year old girl with healed Perthes disease illustrates this point (Fig. 1). The aspherical contour of the femoral head is very gradual such that without direct visualization, it would be difficult to judge the areas of the head-neck junction that would need resection to make the femoral head more spherical. We performed osteoplasty of the femoral head and neck for impingement secondary to SCFE, Perthes disease, growth arrest secondary to DDH, and proximal femoral exostoses. A maximum of 30% of the anterolateral quadrant of the femoral head neck junction was resected, keeping in mind the change in the pattern of the femoral head-neck response and the propensity to fracture with axial loads [10]. Intertrochanteric osteotomy was combined with osteoplasty to treat cases with severe deformity and marked limitations in range of motion even after performing an osteoplasty. By direct visualization it is possible to explore the corrections that could be obtained by osteotomy to allow normal walking and sitting, which has been described previously using a computer model of SCFE [12]. By combining an osteoplasty at time of the intertrochanteric osteotomy, the amount of correction through the osteotomy may be lessened (Fig. 2), which would minimize the proximal femoral deformity that may hinder future arthroplasty. Moreover, long-term outcome studies after intertrochanteric osteotomy for SCFE without concomitant osteoplasty demonstrate hip osteoarthritis may progress [13]. This may be due to preexisting cartilage damage as well as the untreated impingement due to the prominence in the head-neck junction.
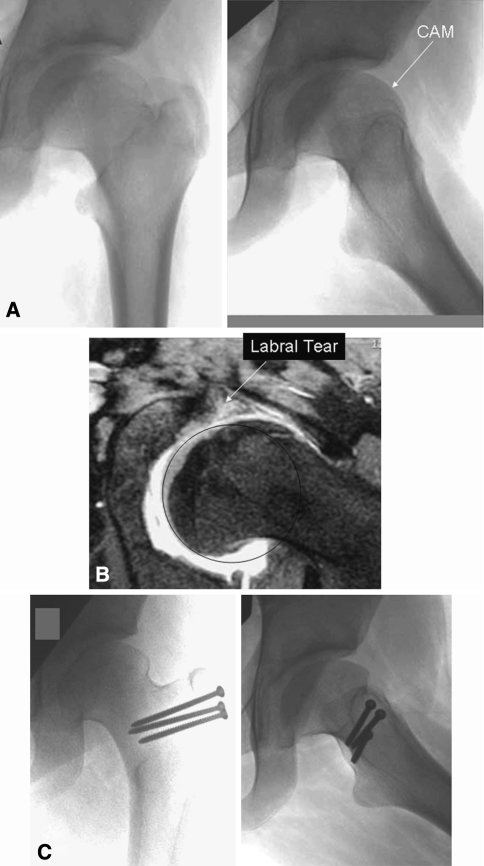
Fig. 1A–C.
(A) Preoperative AP and frog lateral radiographs of the left hip in a 10-year-old girl with healed Perthes disease shows the presence of an aspherical head with cam lesion. (B) An MRI image shows a labral tear caused by cam impingement in the same patient with Perthes disease. (C) Postoperative AP and frog lateral radiographs show improvement in sphericity of femoral head after surgical dislocation and head neck osteochondroplasty.
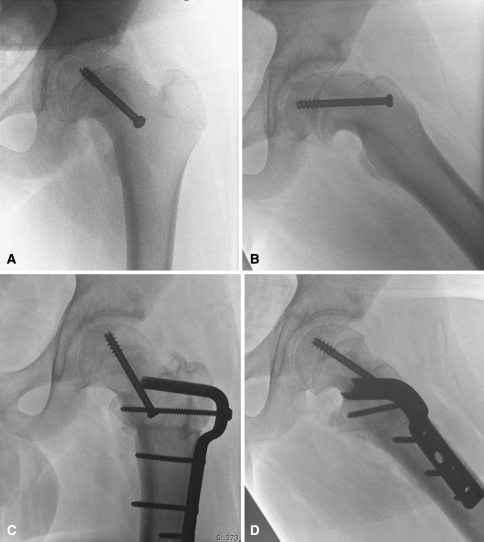
Fig. 2A–D.
(A) Preoperative AP and (B) frog lateral radiographs of the left hip in a 14-year-old boy show healed moderately slipped capital epiphysis treated with in situ transphyseal screw fixation. (C) Post-operative radiographs show improved alignment of the femoral head with the trochanter refixed in the anatomic position. (D) The frog lateral view shows diminution of size of the cam lesion and anatomic alignment of the proximal femur with the femoral shaft after surgical dislocation, osteochondroplasty, and intertrochanteric flexion osteotomy.
Patients with SCFE appeared to derive greater benefit from the procedure than patients with Perthes disease or osteonecrosis. We attribute this finding to the underlying articular cartilage damage and the inability to restore normal anatomy in the latter two conditions. Moreover, in our study, patients with SCFE were younger at the time of surgery than those with Perthes disease.
Surgical dislocation can be safely used to anatomically reduce unstable SCFEs. Unlike in chronic SCFE where the physis must be surgically resected, in the unstable SCFE the physis is already unstable and the amount of callus that needs to be resected is less; therefore, it is a much less technically difficult procedure than that for the stable SCFE. We used the approach in five cases with unstable SCFE. In addition to having good outcomes, none of the cases developed osteonecrosis.
Three of the four cases of osteonecrosis of the femoral head after surgical dislocation occurred in patients who had undergone either femoral neck or intertrochanteric osteotomy for correction of deformity after stable SCFE. The fourth case of osteonecrosis occurred in a patient with an undiagnosed femoral neck fracture in whom treatment was delayed. In this setting, it was difficult to ascribe the femoral head osteonecrosis to the femoral neck osteotomy. Active monitoring of intraoperative femoral blood flow using devices like laser Doppler flowmetry may make complex reconstruction through this approach safer [11].
The complication rate appears to be the related to the complexity of the reconstruction at time of dislocation. At present, although a true femoral neck osteotomy in a mature hip may be technically possible, it should be avoided due to the high rates of osteonecrosis. If it is attempted then some form of femoral head blood flow monitoring is recommended and the benefits of this procedure weighed against the risks. In contrast, simple osteoplasty through a dislocation approach appears to be safe and can be effective. In cases of mild deformities, a simple osteoplasty may be sufficient and more effective and less morbid than a traditional intertrochanteric osteotomy without an osteoplasty. Using SCFE as an example, our current approach is to first perform a femoral head-neck junction osteoplasty and inspect the hip motion and impingement; if the range of motion is still quite limited then an intertrochanteric osteotomy is additionally performed. Only in extreme deformities is a femoral neck procedure contemplated. This approach takes full advantage of the surgical dislocation technique, will address the CAM type impingement effectively, and will minimize the risk of osteonecrosis.
In conclusion, we believe the surgical dislocation approach can be used effectively to treat different causes of pediatric hip deformity and improve symptoms in the short term. The risk of complications increased with the complexity of the reconstruction, such as when surgical dislocation is combined with concomitant osteotomy for more severe deformity and hence should be applied judiciously.
Acknowledgment
We thank Cathy Matero for her assistance in performing the study.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87:1012–1018. [DOI] [PubMed]
- 2.Beck M, Leunig M, Parvizi J, Boutier V, Wyss D, Ganz R. Anterior femoroacetabular impingement: Part II. Midterm results of surgical treatment. Clin Orthop Relat Res. 2004;418:67–73. [DOI] [PubMed]
- 3.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed]
- 4.Ganz R, Gill TJ, Gautier E, Ganz K, Krügel N, Berlemann U. Surgical dislocation of the adult hip a technique with full access to the femoral head and acetabulum without the risk of osteonecrosis. J Bone Joint Surg Br. 2001;83:1119–1124. [DOI] [PubMed]
- 5.Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112–120. [DOI] [PubMed]
- 6.Ito K, Minka MA 2nd, Leunig M, Werlen S, Ganz R. Femoroacetabular impingement and the cam-effect: a MRI-based quantitative anatomical study of the femoral head-neck offset. J Bone Joint Surg Br. 2001;83:171–176. [DOI] [PubMed]
- 7.Lavigne M, Parvizi J, Beck M, Siebenrock KA, Ganz R, Leunig M. Anterior femoroacetabular impingement: Part I. Techniques of joint preserving surgery. Clin Orthop Relat Res. 2004;418:61–66. [DOI] [PubMed]
- 8.Leunig M, Casillas MM, Hamlet M, Hersche O, Nötzli H, Slongo T, Ganz R. Slipped capital femoral epiphysis: early mechanical damage to the acetabular cartilage by a prominent femoral metaphysic. Acta Orthop Scand. 2000;71:370–375. [DOI] [PubMed]
- 9.Leunig M, Slongo T, Kleinschmidt M, Ganz R. Subcapital correction osteotomy in slipped capital femoral epiphysis by means of surgical hip dislocation. Oper Orthop Traumatol. 2007;19:389–410. [DOI] [PubMed]
- 10.Mardones RM, Gonzalez C, Chen Q, Zobitz M, Kaufman KR, Trousdale RT. Surgical treatment of femoroacetabular impingement: evaluation of the effect of the size of the resection. J Bone Joint Surg Am. 2006;88 Suppl 1 Pt 1:84–91. [DOI] [PubMed]
- 11.Nötzli HP, Siebenrock KA, Hempfing A, Ramseier LE, Ganz R. Perfusion of the femoral head during surgical dislocation of the hip: monitoring by laser Doppler flowmetry. J Bone Joint Surg Br. 2002;84:300–304. [DOI] [PubMed]
- 12.Rab GT. The geometry of slipped capital femoral epiphysis: implications for movement, impingement and corrective osteotomy. J Pediatr Orthop. 1999;19:419–424. [DOI] [PubMed]
- 13.Schai PA, Exner GU, Hänsch O. Prevention of secondary coxarthrosis in slipped capital femoral epiphysis: a long-term follow-up study after corrective intertrochanteric osteotomy. J Pediatr Orthop B. 1996;5:135–143. [DOI] [PubMed]
- 14.Siebenrock KA, Wahab KH, Werlen S, Kalhor M, Leunig M, Ganz R. Abnormal extension of the femoral head epiphysis as a cause of cam impingement. Clin Orthop Relat Res. 2004;418:54–60. [DOI] [PubMed]
- 15.Spencer S, Millis MB, Kim YJ. Early results of treatment of hip impingement syndrome in slipped capital femoral epiphysis and pistol grip deformity of the femoral head-neck junction using the surgical dislocation technique. J Pediatr Orthop. 2006;26:281–285. [DOI] [PubMed]
- 16.Tannast M, Goricki D, Beck M, Murphy SB, Siebenrock KA. Hip damage occurs at the zone of femoroacetabular impingement. Clin Orthop Relat Res. 2008;466:273–280. [DOI] [PMC free article] [PubMed]