Abstract
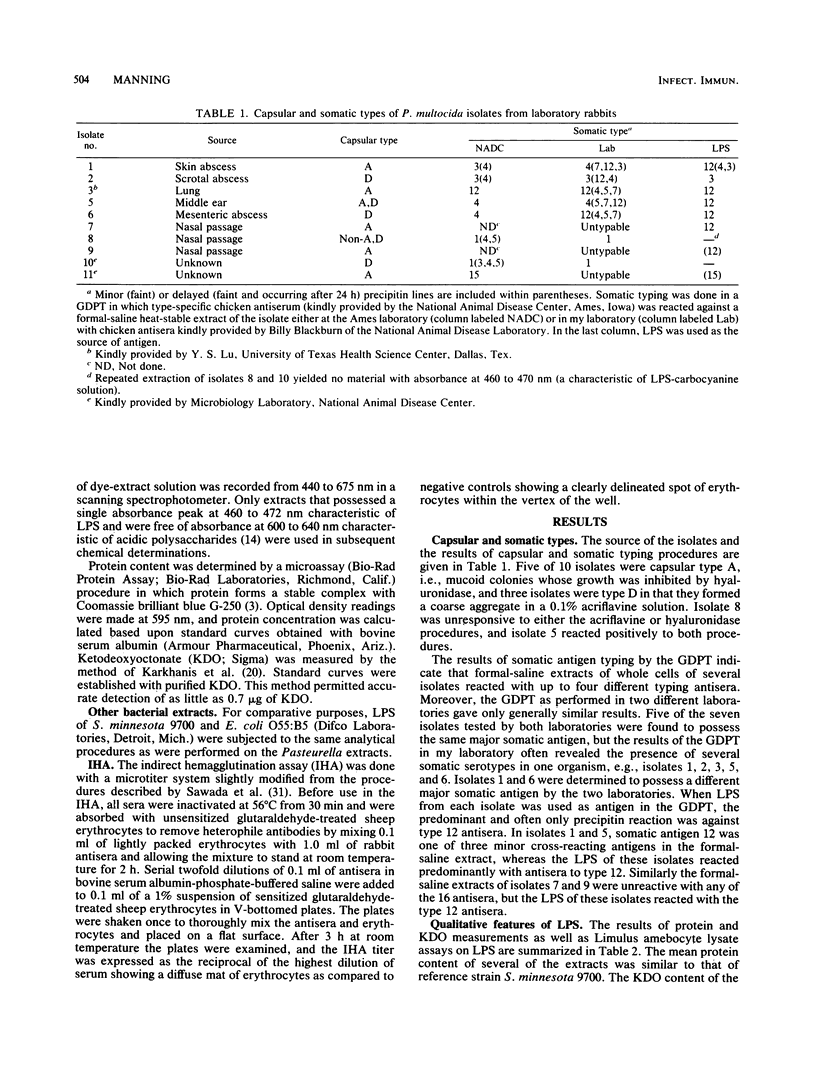
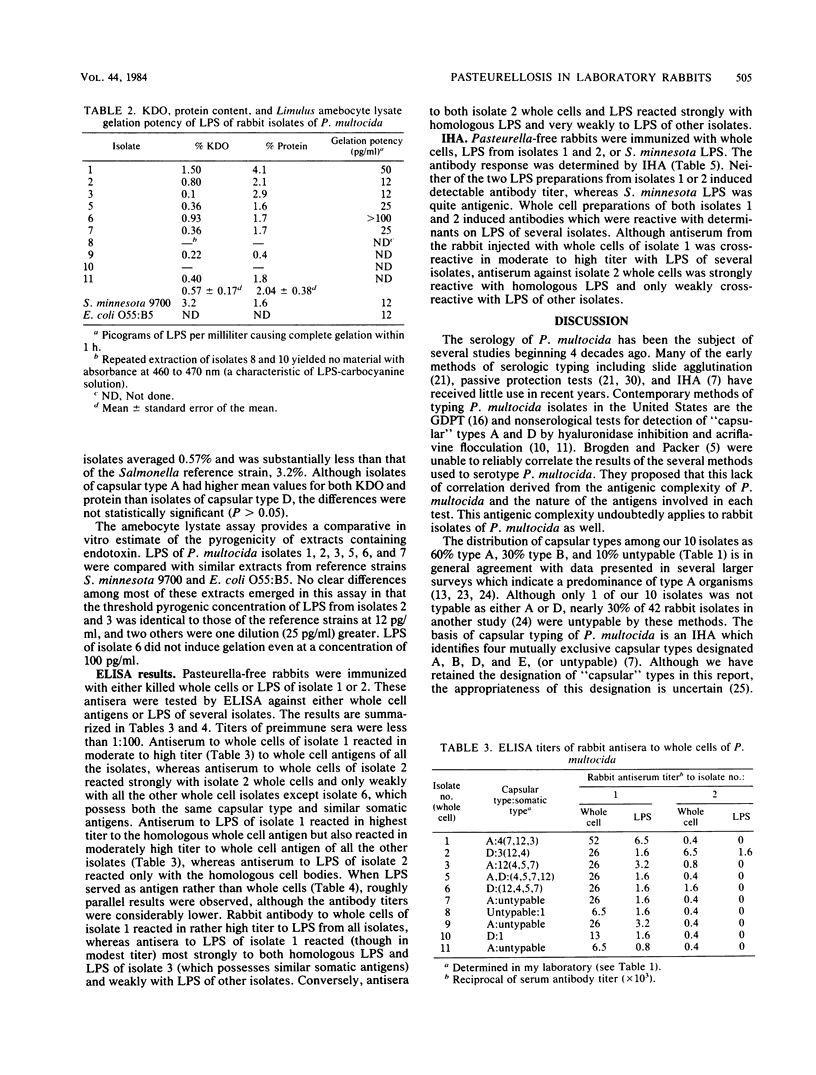
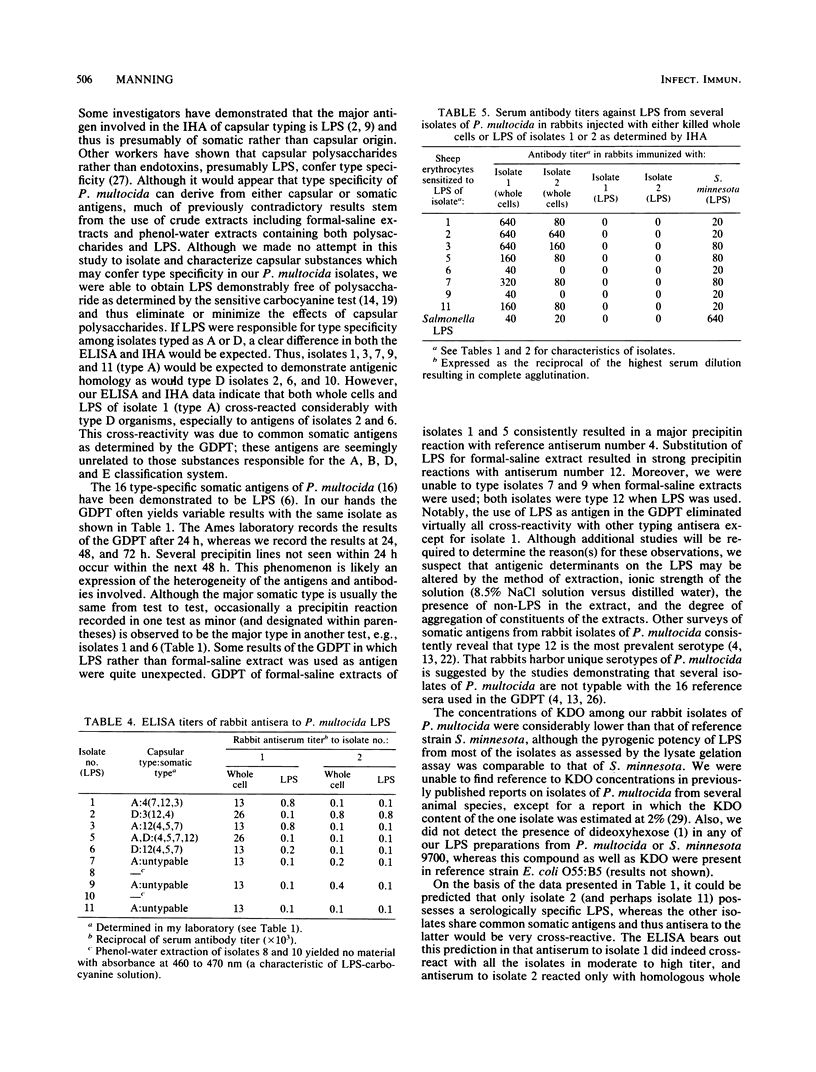
Whole cells and lipopolysaccharides (LPS) of 10 isolates of Pasteurella multocida from laboratory rabbits were subjected to chemical and serological analysis. LPS of most of these isolates possessed pyrogenic potency comparable to LPS from Salmonella minnesota 9700, although their average ketodeoxyoctonate content was only 18% of that of salmonella. A gel diffusion precipitin test for somatic antigens extracted in a formal-saline solution demonstrated several isolates with three to four somatic antigens, with some variation in the major somatic type from one test to another. Conversely, the use of LPS as antigen in the gel diffusion precipitin test (i) eliminated cross-reactivity with reference antisera and (ii) often resulted in the organism being typed as serotype 12 even when the type 12 antigen was a minor antigen in the formal-saline extracts. Antisera from specific pathogen-free rabbits immunized with either whole cells or LPS of two isolates were tested against whole cells of LPS of the 10 isolates by enzyme immunoassay and indirect hemagglutination. Both whole cells and LPS of one of the isolates (isolate 2) were serologically specific, whereas those of the other isolate (isolate 1) were moderately to strongly cross-reactive with other isolates. The data indicate that although LPS is the major antigen responsible for typing based on the gel diffusion precipitin test, substances other than LPS (probably capsular polysaccharide) are responsible for the type specificity that forms the basis for the A, B, D, or E classification of this organism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anacker R. L., Bickel W. D., Haskins W. T., Milner K. C., Ribi E., Rudbach J. A. Frequency of occurrence of native hapten among enterobacterial species. J Bacteriol. 1966 Apr;91(4):1427–1433. doi: 10.1128/jb.91.4.1427-1433.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAIN R. V., KNOX K. W. The antigens of Pasteurella multocida type I. II. Lipopolysaccharides. Immunology. 1961 Apr;4:122–129. [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brogden K. A., Packer R. A. Comparison of Pasteurella multocida serotyping systems. Am J Vet Res. 1979 Sep;40(9):1332–1335. [PubMed] [Google Scholar]
- Brogden K. A. Physiological and serological characteristics of 48 Pasteurella multocida cultures from rabbits. J Clin Microbiol. 1980 Jun;11(6):646–649. doi: 10.1128/jcm.11.6.646-649.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden K. A., Rebers P. A. Serologic examination of the Westphal-type lipopolysaccharides of Pasteurella multocida. Am J Vet Res. 1978 Oct;39(10):1680–1682. [PubMed] [Google Scholar]
- CARTER G. R. Studies on Pasteurella multocida. I. A hemagglutination test for the identification of serological types. Am J Vet Res. 1955 Jul;16(60):481–484. [PubMed] [Google Scholar]
- Carter G. R., Rundell S. W. Identification of type A strains of P multocida using staphylococcal hyaluronidase. Vet Rec. 1975 Apr 12;96(15):343–343. doi: 10.1136/vr.96.15.343. [DOI] [PubMed] [Google Scholar]
- Carter G. R., Subronto P. Identification of type D strains of Pasteurella multocida with acriflavine. Am J Vet Res. 1973 Feb;34(2):293–294. [PubMed] [Google Scholar]
- Chengappa M. M., Myers R. C., Carter G. R. A streptomycin dependent live Pasteurella multocida vaccine for the prevention of rabbit pasteurellosis. Lab Anim Sci. 1980 Jun;30(3):515–518. [PubMed] [Google Scholar]
- Chengappa M. M., Myers R. C., Carter G. R. Capsular and somatic types of Pasteurella multocida from rabbits. Can J Comp Med. 1982 Oct;46(4):437–439. [PMC free article] [PubMed] [Google Scholar]
- Edstrom R. D. A colorimetric method for the determination of mucopolysaccharides and other acidic polymers. Anal Biochem. 1969 Jun;29(3):421–432. doi: 10.1016/0003-2697(69)90327-3. [DOI] [PubMed] [Google Scholar]
- Heddleston K. L., Gallagher J. E., Rebers P. A. Fowl cholera: gel diffusion precipitin test for serotyping Pasteruella multocida from avian species. Avian Dis. 1972 Jul-Sep;16(4):925–936. [PubMed] [Google Scholar]
- Heddleston K. L., Rebers P. A. Properties of free endotoxin from Pasteurella multocida. Am J Vet Res. 1975 Apr;36(4 Pt 2):573–574. [PubMed] [Google Scholar]
- Hofing G. L., Rush H. G., Petkus A. R., Glorioso J. C. In vitro killing of Pasteurella multocida: the effect of rabbit granulocyte and specific antibody source. Am J Vet Res. 1979 May;40(5):679–683. [PubMed] [Google Scholar]
- Janda J., Work E. A colorimetric estimation of lipopolysaccharides. FEBS Lett. 1971 Sep 1;16(4):343–345. doi: 10.1016/0014-5793(71)80386-1. [DOI] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Lu Y. S., Pakes S. P. Protection of rabbits against experimental pasteurellosis by a streptomycin-dependent Pasteurella multocida serotype 3:A live mutant vaccine. Infect Immun. 1981 Dec;34(3):1018–1024. doi: 10.1128/iai.34.3.1018-1024.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y. S., Pakes S. P., Stefanu C. Capsular and somatic serotypes of Pasteurella multocida isolates recovered from healthy and diseased rabbits in Texas. J Clin Microbiol. 1983 Aug;18(2):292–295. doi: 10.1128/jcm.18.2.292-295.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y. S., Ringler D. H., Park J. S. Characterization of Pasteurella multocida isolates from the nares of healthy rabbits with pneumonia. Lab Anim Sci. 1978 Dec;28(6):691–697. [PubMed] [Google Scholar]
- Manning P. J. Serology of Pasteurella multocida in laboratory rabbits: a review. Lab Anim Sci. 1982 Dec;32(6):666–671. [PubMed] [Google Scholar]
- Mushin R., Schoenbaum M. A strain of Pasteurella multocida associated with infections in rabbit colonies. Lab Anim. 1980 Oct;14(4):353–356. doi: 10.1258/002367780781071030. [DOI] [PubMed] [Google Scholar]
- Penn C. W., Nagy L. K. Capsular and somatic antigens of Pasteurella multocida, types B and E. Res Vet Sci. 1974 Mar;16(2):251–259. [PubMed] [Google Scholar]
- Rebers P. A., Heddleston K. L., Rhoades K. R. Isolation from Pasteurella multocida of a lipopolysaccharide antigen with immunizing and toxic properties. J Bacteriol. 1967 Jan;93(1):7–14. doi: 10.1128/jb.93.1.7-14.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada T., Rimler R. B., Rhoades K. R. Indirect hemagglutination test that uses glutaraldehyde-fixed sheep erythrocytes sensitized with extract antigens for detection of Pasteurella antibody. J Clin Microbiol. 1982 May;15(5):752–756. doi: 10.1128/jcm.15.5.752-756.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang J. C., Wang C. S., Alaupovic P. Degradative effect of phenol on endotoxin and lipopolysaccharide preparations from Serratia marcescens. J Bacteriol. 1974 Feb;117(2):786–795. doi: 10.1128/jb.117.2.786-795.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


