Abstract
Objective
Our objective was to determine a mechanism for the thrombocytopenia of murine Wiskott-Aldrich syndrome (WAS).
Materials and Methods
Consumption rates of WAS protein (WASP)( −) and wild-type (WT) platelets were measured by injection of 5-chloromethylfluorescein diacetate (CMFDA)-labeled platelets into WT or WASP(−) recipients, and by in vivo biotinylation. Platelet and reticulated platelet counts were performed using quantitative flow cytometry. Bone marrow megakaryocyte number and ploidy was assessed by flow cytometry. Phagocytosis of CMFDA-labeled, opsonized platelets was assessed using bone marrow–derived macrophages. Serum antiplatelet antibodies were assayed via their binding to WT platelets.
Results
CMFDA-labeled WASP(−) platelets are consumed more rapidly than WT platelets in either WT or WASP(−) recipients. In vivo biotinylation studies corroborate these findings and show a normal consumption rate for WASP(−) reticulated platelets. The number of reticulated platelets is reduced in WASP(−) mice, but a significant number of the mice show an increased proportion of reticulated platelets and more severe thrombocytopenia. Sera from some of the latter group contain antiplatelet antibodies. Compared to WT platelets, WASP(−) platelets opsonized with anti-CD61 or 6A6 antibody are taken up more rapidly by bone marrow–derived macrophages. In vivo consumption rates of WASP(−) platelets are more accelerated by opsonization than are those of WT platelets.
Conclusion
Both rapid clearance and impaired production contribute to the thrombocytopenia of murine WAS. Increased susceptibility of opsonized WASP(−) platelets to phagocytosis leads to increased in vivo clearance. This correlates with a higher incidence of individuals with an elevated fraction of reticulated platelets, a more severe thrombocytopenia, and antiplatelet antibodies.
The Wiskott-Aldrich syndrome (WAS) is an X-linked recessive condition of variable penetrance, classically described as a triad of immunodeficiency, eczema, and thrombocytopenia [1,2]. It is caused by mutations that reduce the level of the WAS protein (WASP). WASP is a 54-kDa polypeptide that is expressed primarily, but not exclusively [3,4], in hematopoietic cells. WASP transmits and integrates signals arising at the cell membrane that result in actin polymerization. This can, in turn, have multiple effects, including but not limited to changes in cell shape and motility. While its function in T cells and macrophages has been studied in some detail [5,6], its biological role in platelets is not clear.
WAS patients can have severe thrombocytopenia, with platelet counts ranging from 10,000 to 50,000 per uL in one series [7]. In some cases, the thrombocytopenia is the predominant clinical abnormality. These cases, formerly termed X-linked thrombocytopenia, are frequently due to missense mutations in the first four exons of the gene [8], and can show a fluctuating course resembling immune thrombocytopenic purpura [9]. Platelet volume is in all cases low, a relatively specific finding. The thrombocytopenia of WAS responds well to splenectomy, although in the context of an immunodeficiency this approach is often avoided by clinicians. Even after splenectomy, however, WAS patients have a high incidence of immune thrombocytopenic purpura [10]. Other autoimmune conditions, the most common being autoimmune hemolytic anemia, are seen in as much as 40% of WAS patients [11].
Clinical platelet turnover studies have confirmed rapid platelet turnover in WAS patients [12–15]. There is evidence in favor of reduced or abnormal platelet production as well [15–17], but not all the relevant studies have supported this [18]. Reported abnormalities in the function of WASP(−) platelets include a slight increase in collagen-induced aggregation and a significant increase in the duration of calcium influx after thrombin stimulation [19,20]. Structural and biochemical abnormalities that have been demonstrated in WASP(−) platelets include reduced numbers of both α and dense granules, increased microparticle release, and increased surface phosphatidyl serine [12–14,19]. However, no consistent abnormalities in overall actin polymerization, platelet shape change, or Arp2/3 complex activation after platelet stimulation have been observed [20–23]. Murine knockout models of WAS have been independently derived by two laboratories [24,25]. They show a mild immunodeficiency and thrombocytopenia, the latter in spite of a marked increase in extramedullary hematopoiesis [26].
Here we address the roles of altered platelet production and consumption in the thrombocytopenia of murine WAS, and present evidence for increased phagocytosis of platelets, reduced thrombopoiesis, and development of antiplatelet antibodies as contributing factors.
Materials and methods
Reagents
Hamster anti-mouse CD61, rat fluorescein isothiocyanate (FITC)-labeled anti-mouse CD41, phycoerythrin (PE)-labeled rat anti-mouse CD41, streptavidin-PE, FITC rat anti-mouse CD47, FITC goat anti-rat IgG, PE-labeled anti-American and -Syrian hamster IgG antibody, anti-CD62P, Cy5.5 Annexin-V, Anti BCLXL (2H12), and FITC goat anti-mouse IgG/IgM were obtained from BD Biosciences (San Jose, CA, USA). Rabbit polyclonal anti-WASP antiserum was a gift of Dr. Hans Ochs. 6A6 antibody was originally derived by Dr. R. A. Good [27]. 6A6-IgG1 antibody was derived as described previously [28]. Rat anti-mouse CD42B (4A5) was a gift of Dr. Sam Burstein (University of Oklahoma). CMFDA was obtained from Molecular Probes Inc. (Invitrogen, Carlsbad, CA, USA).
Mouse strains
WASP(−) mice on the 129SvEv background [24] were bred onto the C57Bl/6J (Jackson Laboratory, Bar Harbor, ME, USA) background for four to eight generations (N4 to N8). CD47−/−(C57Bl/6J) mice were obtained from the Jackson Laboratory and bred in the Memphis VA Medical Center’s animal facility. WASP(−/−) females were bred with CD47(−/−) males and the progeny interbred to produce WASP(−), 47(−/−) males. Splenectomies were performed under isoflurane or ketamine anesthesia.
Consumption of CMFDA-labeled platelets
Male WASP(−) donors (N8) and age-matched male WT controls (Jackson Laboratory) were bled via tail vein directly into microfuge tubes containing 800 uL buffer A, which consisted of modified Tyrode’s buffer (20 mM HEPES, 137 mM NaCl, 13.8 mM NaHCO3, 2.5 mM KCl, 0.36 mM NaH2PO4-H2O, 5.5 mM glucose, 0.25% bovine serum albumin, 1 mM MgCl2) supplemented with 90 uL 3.8% sodium citrate, and 1 ug/mL PGE-1 (Sigma, St Louis, MO, USA). For phagocytosis studies, buffer A was supplemented with 2 uM β-mercaptoethanol at this step only. Platelets were separated from other blood components via Ficoll density gradient (Fico/Lite platelets; Atlanta Biologicals (Atlanta, GA, USA); 350 relative centrifugal force, 15 minutes, room temperature), diluted in modified Tyrode’s buffer supplemented with PGE-1, and centrifuged at 6000 RCF for 5 minutes. Pellets were resuspended in modified Tyrode’s buffer supplemented with PGE-1, counted using a Beckman-Coulter (Fullerton, CA, USA) Model Z2 Coulter particle count and size analyzer, and labeled in the same buffer at 2.9 × 105 per uL with 2.5 uM CMFDA (Molecular Probes, Eugene, OR, USA) for 20 minutes at 37°C. The labeled platelets were washed with 5 volumes of modified Tyrode’s buffer and resuspended in same. Opsonization, when used, was performed at this stage with 150 ng anti-CD61 antibody per million platelets (room temperature, 20 minutes) followed by addition of more modified Tyrode’s buffer, centrifugation at 6000 RCF for 5 minutes, and resuspension in modified Tyrode’s buffer. Labeled platelets were injected via tail vein at 0.4 to 1.0 × 108 platelets per mouse in a net volume of approximately 0.5 mL. Mice were bled retro-orbitally (5–10 uL) at 5 minutes after injection to measure baseline labeling using a Becton Dickinson FACS-Calibur flow cytometer. This was repeated at the subsequent time points indicated in the figures. Linear and nonlinear regression curve fitting was performed with Microsoft Excel.
In vivo Platelet Labeling. The procedure was essentially as described [29]. Briefly, sulfo-NHS-biotin (Pierce, Rockford IL, USA) was dissolved in 150 mM NaCl and immediately injected via tail vein at 35 ug per gram body weight. Recipients (all male) were bled retro-orbitally at the times indicated. Labeled platelets were detected by flow cytometry in whole blood diluted in phosphate-buffered saline (PBS)/2% inactivated fetal bovine serum/0.02% NaN3, using streptavidin-PE (BD Biosciences). After antibody binding, the cells/platelets were centrifuged at 1700 RCF for 5 minutes and resuspended in thiazole orange (“Retic-Count”; BD Biosciences). After 30-minute room temperature incubation, the cells/platelets were analyzed by flow cytometry and appropriately color compensated.
Megakaryocyte number and ploidy
These studies were done essentially as described previously [30]. Briefly, bone marrow (all from males) was flushed from femur and tibia of each mouse in 2 mL Citrate, adenosine, theophylline, in calcium- and magnesium- free Hank’s balanced salt solution (CATCH) medium supplemented with 8 uM PGE-1 (Sigma-Aldrich, St. Louis, MO, USA). CATCH Medium (pH 7.0) consists of Hank’s balanced salt solution (HBSS) (Gibco) supplemented with .268 g/L adenosine (Sigma-Aldrich), 0.36 g/L theophylline (Sigma-Aldrich), 3.6g/L sodium citrate (Fisher), 35 g/L bovine serum albumin (Sigma), and 6.0 g/L HEPES (Sigma). After filtration through 105-uM monofilament nylon mesh (Small Parts Inc, Miami, FL, USA), marrow suspensions were pelleted at 400g for 5 minutes and resuspended in CATCH medium. Nonspecific secondary antibody binding was inhibited with normal goat serum (Life Technologies, Grand Island, NY, USA), followed by binding of rat anti-CD42b (4A5), and FITC-labeled goat anti-rat IgG F(ab′)2 (Biosource, Camarillo, CA, USA). After binding of propidium iodide (50 ug/mL in 0.1% sodium citrate), RNase treatment (bovine pancreas RNase, 50 ug/mL; Calbiochem, San Diego, CA, USA), and filtration through 105-uM nylon mesh, the cells were analyzed on a FACSCalibur flow cytometer (Becton Dickinson). 4A5-positive cells, including cells with N2 and N4 ploidy, were interpreted as megakaryocytes, and the number at each ploidy level was quantified by propidium iodide fluorescence.
Platelet and reticulated platelet counts
Platelet counts shown in Figure 1C were measured with a HemaVet modified Coulter counter (CDC Technologies, Seymour, CT, USA). All other platelet counts were performed as described previously [31]. Briefly, 5 uL blood was obtained retro-orbitally using a heparinized capillary tube, and diluted into 45 uL 0.11M sodium citrate, FITC-anti–CD41 antibody was bound, followed by dilution to 1 mL in PBS/0.2% bovine serum albumin/0.1% dextrose/0.1% formalin containing 15 uL Spherotech Accucount fluorescent particles (Spherotech Inc, Libertyville, IL, USA). Platelet and fluorescent particle counts were measured by flow cytometry, and platelet counts inferred from the known concentration of the fluorescent particles. The accuracy of the method was verified by parallel counting of platelet preparations in a Coulter Model Z2 Particle Count and Size Analyzer (Beckman Coulter). For measurement of “reticulated platelets,” platelets were resuspended in a thiazole orange solution (“Retic-Count,” BD Biosciences) (30 minutes, room temperature), then analyzed by flow cytometry. Data was analyzed using Flowjo software (Tree Star, Inc., Ashland, OR, USA).
Figure 1.
Platelet and reticulated platelet counts. (A) Platelets were counted by quantitative flow cytometry. Population characteristics: wild-type (WT) (C57Bl/6J), n = 24, age 7 to 16 weeks; mean age 10.6 weeks, mean platelets 1236 K/uL; WASP(−)(N8), n = 70, age 6 to 19 weeks, mean age 9.1 weeks, mean platelets 578 K/uL; CD47(−/−), n = 11, age 8 to 19 weeks, mean age 9.4 weeks, mean platelets 1052; WASP(−), CD47(−/−), n = 23, age 7 to 14 weeks, mean age 8.8 weeks, mean platelets 617 K/uL. (B) The fraction of reticulated platelets were quantified simultaneously with the platelet counts shown in (A). Mean percent reticulated platelets are 5.98 [wasp(−)] vs 5.76(WT). (C) Reticulated platelet count was determined from the information in (B).
Bone marrow– derived macrophages
Bone marrow–derived macrophages were prepared as follows. Male WT (C57Bl/6J) or WASP(−)(N8) donors were sacrificed by cervical dislocation, and bone marrow cells were eluted from femurs and tibias with PBS. Nine volumes of red cell lysis buffer (154 mM NH4Cl, 10 mM KHCO3, 1 mM disodium ethylene diamine tetraacetic acid) were added, and cells were immediately pelleted at 420g for 6 minutes. Cells were resuspended in 10 mL Dulbecco’s modified Eagle’s medium (DMEM; CellGro, Mediatech Inc, Herndon, VA, USA) supplemented with penicillin/streptomycin, 10% heat inactivated fetal bovine serum (DMEM/IFBS; CellGro, Mediatech Inc), which was in turn supplemented with 10% to 20% L929-cell–conditioned medium (activity of the conditioned medium varied between preparations). Cells were cultured overnight in one 10-cm diameter tissue culture–treated dish. Nonadherent cells were removed and cultured at approximately 500,000 per mL in the same medium for 3 days, and in fresh medium for another 2 to 3 days. The subsequent adherent macrophages were rinsed twice with PBS and treated with 0.1 mL per square cm of Accutase (Chemicon International, Temecula, CA, USA) at 37°C for 15 minutes. Residual adherent cells were then scraped into PBS, pooled with the Accutase-detached cells, pelleted at 420g for 6 minutes, resuspended in DMEM/IFBS, and transferred to 24-well dish wells at 1 mL per well, 300,000 cells per mL. These cells were cultured overnight prior to phagocytosis assays.
Phagocytosis assays
This procedure was adapted from that described by Olsson et al. [32]. Platelets were prepared as described above (male donors were used) for ex vivo platelet labeling. After CMFDA labeling, comparable gMFI values for different labeled platelet preparations within each experiment were verified by flow cytometry. Platelets were then opsonized with hamster anti-mouse CD61 (BD Biosciences) at 0.15 μg per million platelets, at a platelet concentration of 3 × 105 per μL, in modified Tyrode’s buffer, at room temperature, for 20 minutes. Platelets were then diluted with 5 volumes modified Tyrode’s buffer, pelleted at 6000g for 5 minutes, and re-suspended in DMEM/IFBS at a concentration of 2 to 3 × 105 per μL. 3 × 107 platelets were then added to each well of macrophages, and the plates were centrifuged for 1 minute at 200g to move the platelets into the vicinity of the macrophages. After incubation at 37°C for 30 minutes, the wells were washed three times with HBSS (Life Technologies, Gaithersburg, MD). Then 250 μL Accutase was added per well, and after 20 minutes at 37°C the cells were removed via vigorous pipetting. After binding of “Fc block” (rat anti-mouse CD16/CD32; BD Biosciences) and subsequent binding of PE-labeled rat anti-mouse CD41 (BD Biosciences), the cells were analyzed by flow cytometry. Cells showing CMFDA and no surface CD41 were counted as having ingested platelets, while those showing both CD41 expression and CMFDA uptake were inferred to have adsorbed platelets.
Detection of antiplatelet antibodies
Mice were bled retro-orbitally and sera separated in serum separator tubes (Microtainer, Becton Dickinson) by centrifugation at 6700 RCF for 2 minutes. Sera were stored in aliquots at −70°C. Platelets from WT donors were prepared as for CMFDA labeling (above), mixed with one volume 2% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) for 5 minutes at room temperature, centrifuged for 5 minutes at 6000 RCF, and resuspended in modified Tyrode’s buffer. The 106 platelets (~ 2 uL) were mixed with 20 uL heat-inactivated serum to be tested, PBS, and (if indicated) 6A6 antibody in a final volume of 40 uL, and left at 37°C for 1 hour with intermittent agitation. Platelets were diluted in modified Tyrode’s buffer, centrifuged at 6000 RCF for 5 minutes, and resuspended for binding of FITC goat anti-mouse IgG/M. Reactions were left on ice for 30 minutes, then diluted in PBS/2% IFBS/0.02% sodium azide and immediately analyzed by flow cytometry.
Measurement of antigen and protein levels
For CD47 levels, WASP(−)(N8) and WT platelets were prepared via differential centrifugation [32], then bound to FITC-labeled rat anti-mouse CD47 (BD Biosciences) for 30 minutes at room temperature in buffered saline glucose citrate buffer [32], diluted in PBS, and analyzed by flow cytometry. Platelets for the studies below were prepared as for CMFDA labeling, including exposure during preparation to PGE-1. For CD61 levels, the same antibody used for the phagocytosis studies was bound to platelets and detected with PE-labeled anti-American and -Syrian hamster IgG antibody (BD Biosciences) in PBS. 6A6 antigen levels were assessed with 6A6 antibody, followed by exposure to PE goat anti-mouse antibody. Phosphatidyl serine exposure was measured by binding Cy5.5 Annexin V (BD Biosciences) before and after 24-hour incubation in HBSS (pH 6.6; Sigma Biochemical). CD62P (P-selectin) expression was measured by binding of FITC-anti-CD62P. As a positive control, 106 WT platelets were exposed to 0.1 U thrombin from bovine plasma (Sigma-Aldrich) in complete Tyrode’s buffer for 10 minutes at 37°C, followed by fixation in 2% paraformaldehyde (room temperature, 30 minutes), addition of PBS/2% inactivated fetal bovine serum, centrifugation at 6000 RCF for 5 minutes, and exposure to FITC-anti-CD62P. For BCLXL levels, Western blots were performed by standard methods.
Results
Platelet and reticulated platelet counts by quantitative flow cytometry
After backcrossing of WASP(−) mice onto the C57Bl/6J background for eight generations, we evaluated platelet counts using a three-parameter method in place of the two parameters (impedance and light scatter) used in commercial hematology analyzers. Platelet populations in whole blood were identified by forward scatter, side scatter, and CD41 expression, and quantified by comparison to an internal control [31]. Figure 1A shows a more significant thrombocytopenia in WASP(−) mice (relative to WT) than had been previously reported by us [33] and others [24]. The cluster of WASP(−) mice showing a still greater thrombocytopenia (100 to 300 K/uL) is not statistically significant (Student’s t-test for proportions).
Reticulated platelets were simultaneously counted via the method of Ault et al. [34], with gating as shown in Figure 5C. Figure 1B shows that while the mean percentage of reticulated platelets (RP) is not elevated in WASP(−) mice, our colony contained a subpopulation of mice for which this parameter was elevated (p = 0.016, Student’s one-tailed t-test for proportion of platelets with >8% reticulated forms). Absolute number of RP was significantly diminished in WASP(−) mice (32.3 thousand/uL, vs 69.6 thousand for WT, p <10−18) (Fig. 3C). While this suggests that platelet production is reduced, there are stoichiometric issues with this interpretation (see Discussion).
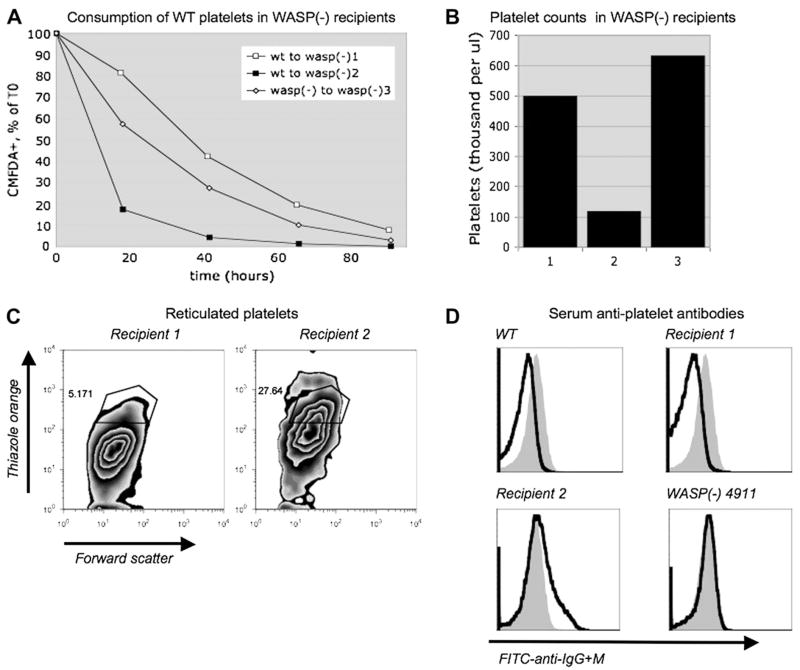
Figure 5.
Platelet-associated antibodies in thrombocytopenic WASP(−) mice. (A) Pooled CMFDA-labeled wild-type (WT) or WASP(−) platelets were injected into tail veins of three WASP(−) recipients as described in Figure 1, then quantified at the time points shown via retro-orbital sampling and flow cytometry. (B) At the 18-hour time point, platelet counts were performed as described in Figure 3. (C) 11 days after tail vein injection, reticulated platelets were quantified as described in Figure 3. (D) Black lines: 19 days after tail vein injection, serum was obtained from the recipients shown, from an unmanipulated WT mouse, and from a WASP(−) mouse with an increased fraction of reticulated platelets (4911; see text). Platelets from another unmanipulated WT mouse were exposed to the sera, then exposed to fluorescein isothiocyanate–labeled anti-IgG+M antibody. Solid gray histograms show platelets exposed to WT serum with 6A6 antibody added (9.6 ng per million platelets).
Figure 3.
In vivo biotinylation of WASP(−) platelets. (A) Simultaneous analysis of biotinylation and thiazole orange staining. Wild-type (WT) (n = 3) and WASP(−) (n = 3) (C57Bl/6J, N8, 12–15 weeks) mice were injected with 32 to 36 ug sulfo-NHS-biotin per gram body weight. Mice were bled at the indicated times, and the platelets were exposed to phycoerythrin (PE)-streptavidin and thiazole orange before analysis by flow cytometry. Profiles from representative WT and WASP− mice are shown. Gates used to quantify “biotin-high” reticulated platelets (top right), “biotin-high” nonreticulated platelets (lower right), and biotin+ platelets (lower two quadrangles) are shown. (B) Mean values for “biotin high” nonreticulated platelets and reticulated platelets are shown. (C) Mean values for reticulated platelets. Best fit rate constants are −0.057 [WASP(−)] and −0.059 (WT); mean value is shown. All error bars are standard errors.
Consumption of ex vivo–labeled platelets
To assess whether platelet consumption is affected by WASP deficiency, we labeled WT and WASP(−) platelets with the sulfhydryl-reactive fluorescein derivative CMFDA, then injected them via tail vein into WT and WASP(−) recipients. We quantified the fraction of each recipient’s platelets that were CMFDA-positive at 5 minutes after injection (via small volume retro-orbital bleed and subsequent flow cytometry), and at subsequent daily intervals. Results (Fig. 2A) demonstrate a significantly more rapid, and qualitatively more exponential, consumption of WASP(−) platelets in WASP(−) recipients than of WT platelets in WT recipients. Best fit linear and exponential curves are shown in the Figure.
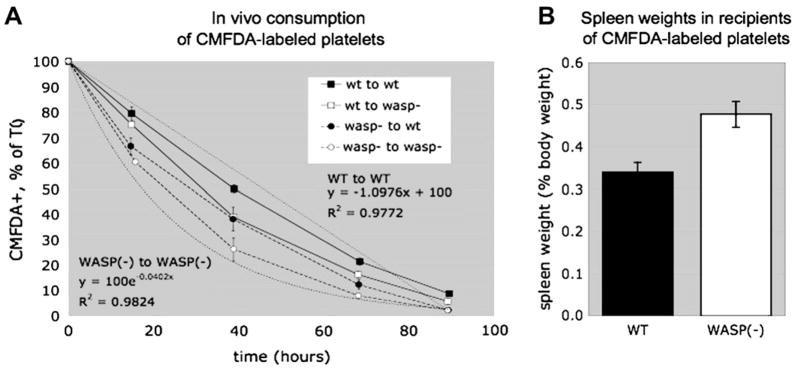
Figure 2.
Effects of WASP(−) deficiency on platelet consumption. (A) In vivo consumption of CMFDA-labeled platelets. Platelets were prepared from wild-type (WT) (C57Bl/6J, 14–24 weeks) and WASP(−) (N8, 23–24 weeks) donors, labeled with CMFDA, and injected via tail vein into WT (C57Bl/6J, 11 weeks) or WASP(−)(N8, 9–12 weeks) recipients. After retro-orbital bleed, the fraction of CMFDA+ circulating platelets was assessed by flow cytometry at 5 minutes (time zero) postinjection and at the indicated times. Mean values normalized to time zero are shown for each cohort of three recipients. (B) Spleen weights of the recipients shown in Figure 1B were assessed 1 week after the end of the experiment.
The data shows that WASP(−) platelets are also consumed more rapidly than WT platelets in WT recipients. However, WT platelets are consumed more rapidly in WASP(−) recipients as well. The latter would be expected in association with splenomegaly of any origin, and (consistent with our previous observations [26]) the WASP(−) recipients in this experiment had large spleens (Fig. 2B).
Consumption of in vivo–labeled platelets
To confirm our results with ex vivo–labeled platelets, we labeled WT and WASP(−) platelets in vivo via tail vein injection of sulfo-NHS-biotin, and followed the extent of labeling over time via binding of streptavidin-PE to subsequent blood samples. To distinguish between the turnover rate of mature and RP, we simultaneously assessed thiazole orange staining. The results, shown for two of the recipients in Figure 3A, demonstrate the expected rapid loss of biotinylation in the rapidly turning over RP fractions of both genotypes, and a slower loss of biotinylation of mature platelets. In between the populations seen at the beginning and end of the study, however, a substantial number of platelets showing intermediate levels of biotinylation are seen. Using the gates shown in Figure 3A, we quantified the total biotinylated platelets (the two lower gates) and the most highly biotinylated platelets (the lower right hand gate). Results (Fig. 3B) show that the consumption rates of the “biotin high” populations appear similar to what we saw with ex vivo CMFDA labeling, while the consumption of all of the biotinylated platelets (“biotin+”) is sigmoidal. The sigmoidal curves are most likely due to concurrent production of biotinylated platelets from in vivo biotinylated megakaryocytes (see Discussion). WT and WASP(−) RP (Fig. 3C) show an indistinguishable exponential consumption rate, implying that the reduced level of these platelets in WASP(−) mice is due to a reduced production rate. The population showing unusually high thiazole orange uptake at late time points in the WASP(−) specimens is not evident in unmanipulated WASP(−) mice, and we have not verified its identity by immunophenotyping.
Effects of splenectomy on platelet kinetics
Because WAS patients show marked improvement in their platelet counts after splenectomy, we asked whether splenectomy improves or normalizes platelet counts in WASP(−) mice. Although platelet counts improved significantly, they did not reach the same levels as those of splenectomized WT mice (Fig. 4A). Because splenic extramedullary thrombopoiesis is markedly increased in WASP(−) mice, we asked whether splenectomy might result in a net reduction in platelet production. Figure 4B shows that splenectomy does not reduce the absolute numbers of RP in either WTor WASP(−) mice. Interpretation of this is, again, subject to some caveats (see Discussion). To verify that splenectomy does not normalize the consumption rate of WASP(−) platelets, we injected CMFDA-labeled WASP(−) platelets into splenectomized WASP(−) recipients, and found (again) a significant increase in platelet consumption rate relative to WT controls (Fig. 4C). Platelet size is not affected by WASP deficiency in mice, and we have seen no evidence of a significant effect of splenectomy on platelet size in either WT or WASP(−) mice (data not shown).
Figure 4.
Effects of splenectomy on platelet kinetics. (A) Platelet counts after splenectomy. WASP(−) (B6, N3 to N4, 9 to 13 weeks of age) and wild-type (WT) littermates underwent splenectomy. Retro-orbital bleeds were performed at the indicated times, and platelet counts were assessed via a HemaVet analyzer. (B) Effect of splenectomy on reticulated platelet counts. WASP(−) (B6, N8, 12–15 weeks of age) underwent splenectomy. Four weeks later reticulated platelet counts were performed, n = 5 (WT), n = 7 [WASP(−)]. (C) Effect of splenectomy on platelet consumption rate. Five weeks after splenectomy, mice from (B) received CMFDA-labeled platelets. WT platelets were injected into WT recipients (n = 3), and WASP(−) platelets into WASP(−) recipients (n = 4). Platelet consumption was followed as in Figure 1. All error bars in the figure are standard errors.
Rapid platelet consumption associated with anti-platelet antibody in a WASP(−) mouse
CMFDA-labeled WT platelets, when introduced into a WASP(−) mouse with an unusually low platelet count (recipient 2), were consumed extremely rapidly (Fig. 5A and B). (The thrombocytopenia of recipient 2 was obvious during routine evaluation of the whole blood cell and platelet populations (forward vs side scatter), and this was not seen in any of the recipients shown in Fig. 2A.) Recipient 2 was in turn found to have a markedly increased percentage (27.3%) of RP (Fig. 5C).
Using an FITC-labeled polyclonal anti-mouse IgG + M reagent, we were unable to detect increased mouse antibodies on the surface of recipient 2’s platelets (data not shown). We were able to detect binding of 6A6 (mouse anti-platelet) antibody to WT platelets using the same assay. However, we found that after brief exposure of target WT platelets to paraformaldehyde, we could detect 6A6 added to mouse serum at significantly lower concentrations (data not shown). We then used this method to assay for antiplatelet antibody in the sera of recipient’s 1, 2, and 3. Figure 5D shows that by this method, serum from recipient 2 contained significant levels of detectable antiplatelet antibody. Sera from recipient 1, and from a WT mouse that had also served as a platelet recipient, did not, nor did serum from recipient 3 (data not shown). Serum from WASP(−) mouse 4911, one of the unmanipulated mice found in Figure 3 to have a high percentage of RP (8.73%, platelets 449 K/uL, 3 weeks prior to the antibody assay), also showed antiplatelet antibodies.
Megakaryopoiesis in WASP(−) mice
Reduced absolute RP counts in WASP(−) mice could be due to reduced or abnormal megakaryopoiesis. We counted megakaryocytes in bone marrow aspirates from WASP(−) mice using forward and side scatter in combination with CD42B expression, and found elevated numbers of megakaryocytes (Fig. 6A). Additionally, the ploidy distribution of these megakaryocytes was not significantly different from that of WT mice (Fig. 6B).
Figure 6.
Platelet production parameters in WASP(−) mice. (A,B) Flow cytometric analysis. Bone marrow cells from WASP(−) (B6) mice (n = 3, N4, 41 weeks) and wild-type littermates (n = 3, 34 weeks) were analyzed as described in Materials and Methods.
Ex vivo phagocytosis of opsonized WASP(−) platelets
Ex vivo uptake of CMFDA-labeled platelets by bone marrow derived macrophages can be quantified by flow cytometry, and distinguished from adsorption by the lack of binding of PE-labeled anti-CD41 to the macrophages. Increased phagocytosis of opsonized CD47(−/−) platelets has been demonstrated by others with this method [32]. Figure 7A shows that after binding anti-CD61 antibody, WASP(−) platelets are phagocytosed more rapidly than WT platelets, and at a rate comparable to that seen with CD47(−/−) platelets. While WASP(−) platelets show a similarly increased uptake when opsonized with 6A6 antibody, they are phagocytosed less rapidly than similarly opsonized CD47(−/−) platelets (Fig. 7B). Because the anti-CD61 antibody we used is of the IgG1 class, while 6A6 is of the IgG2a class, we tested whether a form of 6A6 engineered to contain IgG1-determining constant domains [28] might also demonstrate an enhanced ability to induce phagocytosis of WASP(−) platelets. Figure 7C shows that when opsonized with this antibody, WASP(−) platelets are not taken up significantly faster than WT platelets, while phagocytosis of CD47(−/−) platelets is still elevated, but less so than is the case when 6A6-IgG2a is used (Fig. 6B).
Figure 7.
Ex vivo phagocytosis of opsonized WASP(−) platelets: (A) Opsonization with anti-CD61 antibody. Platelets from the three sources shown were labeled with CMFDA, opsonized with anti-CD61 antibody at the concentrations shown, then exposed to murine bone marrow–derived macrophages. Macrophages were removed from the wells, exposed to phycoerythrin (PE)-labeled anti-CD41 antibody, and analyzed by flow cytometry. Cells positive for CMFDA and negative for PE were inferred to have ingested platelets, and were quantified as a percentage of total cells (CMFDA-positive, PE-positive cells were assumed to represent adsorption). Platelets exposed to 150 ng isotype (IgG1) control antibody are shown as the “0” points. Each point represents the mean of duplicate assays except WASP(−), 150 ng (1 assay). (B) Opsonization with 6A6 antibody. Platelets from the sources shown were exposed to 15 ng per million platelets of 6A6 antibody or isotype (IgG2) control. Phagocytosis assays were performed as in (A). Individual assay points are shown. (C) Opsonization with 6A6-IgG1 antibody. Assays were performed as described in (B), using 15 ng per million platelets of antibody or isotype (IgG1) control. Means of triplicate assays (for isotype controls shown at “0,” duplicates) are shown. (D) WASP(−), CD47(−/−) platelets. Assays were performed as described in (A). Individual assay points are shown, two per condition.
WASP deficiency in macrophages has been reported to inhibit phagocytosis. This finding would appear to be at odds with the more rapid consumption of WASP(−) platelets in WASP(−) vs WT recipients shown in Figure 2. To look at this more closely, we evaluated the ability of WT and WASP(−) bone marrow–derived macrophages to take up opsonized WT platelets ex vivo. Representative results with 6A6 antibody are shown in Figure 8A. We performed this experiment three times with this antibody and three times with anti-CD61 antibody. For each amount of opsonizing antibody used, we calculated the ratio of WASP(−) to WT macrophage phagocytosis. The results, shown in Figure 8B, show no evidence of reduced phagocytic activity of WASP(−) macrophages in this context.
Figure 8.
Effects of macrophage WASP deficiency on platelet phagocytosis. (A) Phagocytosis of wild-type (WT) platelets opsonized with 6A6 antibody. WASP(−) or WT bone marrow–derived macrophages were exposed to WT platelets that had been opsonized with the indicated amounts of 6A6 antibody. Duplicate assays were performed as in Figure 7; individual assay results are shown. The “zero” points show phagocytosis of platelets exposed to 15 ng per million platelets of an isotype control antibody. WASP(−) results are offset slightly for clarity. (B) Summarized phagocytosis data from multiple studies. The study in (A) was performed two more times, and phagocytosis by WASP(−) macrophages (means of duplicate assays) was normalized to the means of duplicate WT macrophage assays performed in parallel. Results are shown in white symbols, with quantities of antibody (ng per million platelets) indicated at right. A similar set of three experiments with anti-CD61 opsonized platelets is also shown (black symbols). CMFDA gMFI values for the platelets used in these three experiments are shown in Figure 9B.
The amount of phagocytosis of WT platelets seen in Figure 8A is substantially greater than that seen for the comparably opsonized WT platelets in Figure 7B. This is due to an approximately 7-fold higher CMFDA geometric mean fluorescence intensity (gMFI) of the platelets used in Figure 8A. This enhances the sensitivity of the assay, as shown in Figure 9A, but does not significantly affect the relative phagocytic activities of WT and WASP(−) macrophages exposed to platelets opsonized with 6A6 (Fig. 9B) or with anti-CD61 (data not shown). For experiments involving comparisons of the phagocytosis of different platelet preparations, comparable platelet CMFDA gMFI values were verified prior to the phagocytosis assay.
Figure 9.
(A) WT platelets were labeled with increasing concentrations of CMFDA to generate preparations with the indicated gMFI values (platelet gMFI minus that of untreated control platelets). Opsonization and phagocytosis by bone marrow derived macrophages were as described in Materials and Methods. Points shown are means of triplicate measurements. Error bars are standard deviations (too small to be seen for some of the points). (B) Phagocytosis of 6A6-opsonized WT platelets (values shown are ng antibody per million platelets) by WASP(−) vs. WT macrophages is shown as a function of platelet CMFDA gMFI (minus negative control gMFI). Data is from the experiments shown in figure 8B. Each point is the mean of duplicate assays.
To test whether platelet WASP deficiency might inhibit amplification of signaling between platelet CD47 and it’s macrophage ligand (SIRP-α, also called SHPS-1), we generated mice deficient for both CD47 and WASP. Our prediction was that lack of both proteins might have no greater effect than lack of either. While the “double knockouts” showed no reduction in platelet counts below that seen in WASP(−) mice (Fig. 1A), their anti-CD61 opsonized platelets were taken up much more rapidly than either those of CD47(−/−) or WASP(−) mice (Fig. 7D).
Increased in vivo consumption of opsonized WASP(−) platelets
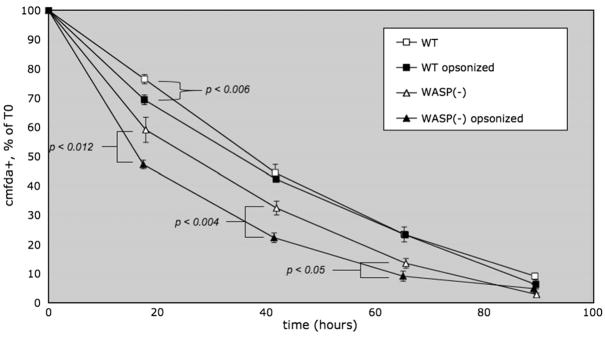
To test whether the increased ex vivo phagocytosis we have seen could play a role in the rapid in vivo consumption of WASP(−) platelets, we evaluated the consumption rates of CMFDA-labeled untreated, or anti-CD61 opsonized, platelets in WT recipients. Figure 10 shows that although opsonization increased the consumption rate of WT platelets slightly, it had a greater effect on WASP(−) platelets.
Figure 10.
Increased in vivo consumption of antibody opsonized WASP(−) platelets in wild-type (WT) recipients. Pooled WT or WASP(−) platelets were CMFDA-labeled. Opsonized (150 ng per million platelets anti-CD61 antibody) or untreated platelets were injected into WT recipients, and consumption was followed as in Figure 1. Error bars are standard errors. n = 6 per time point. p values: Student’s one-tailed t-test.
Normal protein/antigen expression levels on WASP(−) platelets
Platelet activation, as assessed by expression of CD62P (P-selectin), was not significantly enhanced in WASP(−) platelets after binding of anti-CD61 antibody (Fig. 11A). Phosphatidyl serine expression was not increased at baseline, and was increased to the same extent in WASP(−) vs WT platelets after 2-hour incubation in HBSS (Fig. 11B). CD61, CD47, and 6A6 antigen expression were comparable in WT and WASP(−) platelets, and 6A6 antigen expression was not affected by CD47 deficiency (Fig. 11C). BCLXL levels are normal in WASP(−) platelets (Fig. 12).
Figure 11.
Normal expression of relevant platelet antigens. (A) CD62P (P-selectin) levels were assessed without (solid gray) or after (black lines) binding of anti-CD61 antibody (150 ng per million platelets). As a positive control (at right), platelets were exposed to 0.1 U thrombin (black line) or not exposed (solid gray). (B) Phosphatidyl serine (Annexin-V). Wild-type (WT) or WASP(−) platelets were exposed to Cy-5.5–labeled Annexin-V (black lines) or not (solid gray). As a positive control, platelets were left for 24 hours in HBSS (pH 6.8) buffer, then exposed to Annexin-V (black lines) or not (solid gray). (C) WT (solid gray), WASP(−), and CD47(−/−) (black lines) platelets were exposed to the labeled antibodies shown.
Figure 12.

Normal expression of BCLXL. BCLXL protein levels were quantified by Western blotting of lysates from WASP(−) and wild-type (WT) platelets.
Discussion
Flow cytometric platelet counts of WASP(−) (C57BNl/6J, N8) mice suggest a more significant thrombocytopenia than we and others have previously reported. Significantly, platelet counts in WASP(−) mice were reduced by only 29% on the 129SvEv background [24], and by approximately 40% after the mice were crossed for two to four generations onto the C57Bl/6J background (Fig. 1C; Strom et al. [35]). Our current finding of an approximately 50% reduction in platelet count is likely due to genetic modifiers and not to the counting method, as the latter has been shown to correlate well with commercial impedance or optical systems [36].
After ex vivo labeling with CMFDA, we find an increased in vivo consumption rate of WASP(−) platelets, and consumption kinetics more consistent with an exponential than a linear process. We infer that consumption is increased two- to fourfold, as the best fit curves shown in Figure 1A suggest a fourfold increase, while a more conservative interpretation of the rate during the first 24 hours [~20% loss for WT, ~40% for WASP(−)] suggests a twofold increase. Some of this is due to features intrinsic to the platelets, as it is seen when they are injected into WT mice, while some of it is due to extrinsic features that impact on WT platelets injected into WASP(−) mice.
Consistent with this conclusion, removal of the extrinsic factor of splenomegaly is insufficient to fully normalize platelet counts in WASP(−)mice (Fig. 1C). Because the spleens of WASP(−) mice show extensive extramedullary hematopoiesis [26], we asked whether splenectomy might actually reduce platelet production in these mice. Reticulated platelet counts show no evidence of this, although their interpretation is problematic. Platelet consumption rates remain increased after splenectomy of WASP(−) mice, suggesting that rapid clearance of WASP(−) platelets is not a function of splenic phagocytes only.
Our in vivo biotinylation study demonstrates a spectrum of biotinylation levels for both mature and RP during the course of the study. A “biotin high” initial population gives way to an intermediate population before a nonbiotinylated population accumulates. We used a higher dose of sulfo-NHS-biotin than most others have reported [37,38], and in our hands this leads to greater separation of the intermediate and “biotin high” populations than does a lower dose (data not shown). If the intermediate population arose due to gradual loss of biotin from the surface of circulating platelets, then platelet consumption would be represented by the “biotin+” curves in Figure 3B. But the plateaus these curves demonstrate are not consistent with any plausible model of platelet population turnover. It seems likely that the intermediate levels of biotinylation are due instead to production of platelets from biotinylated megakaryocytes, as has been suspected by other users of this technique [37,39]. Accumulation of significant amounts of biotinylated RP at 24 hours after labeling (Fig. 3A) is also more consistent with in vivo labeling than with loss of biotin from circulating RP, because most of the latter would be expected to lose their “reticulated” status in that time period. It follows that only the loss of the “biotin high” population is a direct measure of platelet turnover. The resultant consumption rates (Figs. 3B and C) corroborate those measured via ex vivo CMFDA labeling. The kinetics of the remaining populations, while potentially of interest, are difficult to interpret because they could reflect changes in both production and destruction rates.
Our finding of a low absolute RP count in WASP(−) mice, and a normal consumption rate, indicates that RP production is impaired. The rate of RP consumption we observed (5.8%/hour, corresponding to a “lifespan,” or time period in which the equivalent of the population is consumed, of 17 hours) is somewhat faster than the 1.4-day lifespan estimated by others [37,40]. The difference may be due to use of linear interpolations in the latter studies. In conjunction with increased bone marrow megakaryocytes, normal megakaryopoiesis as assessed by ploidy distribution, and increased extramedullary hematopoiesis [26], our results suggest that WASP deficiency impairs thrombopoiesis. This would be consistent with related studies in both murine and clinical WAS [15,17,41]. There are, however, significant caveats associated with this interpretation. One is that it is inconsistent with our observed platelet counts and platelet consumption rates. A two- to fourfold increase in platelet consumption and an approximately twofold reduction in platelet production cannot yield the roughly 50% reduction in platelet count that we see. Another stoichiometric problem becomes evident during evaluation of the hypothesis that all mature platelets are generated initially as RP. Table 1 demonstrates that the number of RP lost (presumably due to maturation) per hour is well below the number needed to offset mature platelet consumption. This suggests that most recently produced platelets are not reticulated. We have not measured their production rate directly.
Table 1.
Platelet and reticulated platelet (RP) turnover stoichiometry
| WT | WASP(−) | |
|---|---|---|
| RP fractional loss/h | 0.058 | 0.058 |
| Absolute RP (K/uL) | 69.6 | 32.3 |
| RP loss rate (K/uL/h) | 4.0 | 1.9 |
| Platelet fractional loss/h | 0.011 | 0.033 |
| Absolute platelets (K/uL) | 1230 | 578 |
| RP (%) | 5.98 | 5.76 |
| Absolute nonreticulated platelets (K/uL) | 1156 | 545 |
| Nonreticulated platelet loss rate (K/uL/h) | 12.7 | 18.0 |
Fractional reticulated platelet (RP) loss rates per hour are from Figure 2B. Absolute RP values are from Figure 3C.
Wild-type (WT) platelet fractional loss rate is from Figure 1A (WT). Wiskott-Aldrich syndrome protein (WASP)(−) platelet fractional loss rate is the mean of a two- to fourfold increase over WT (see Discussion).
Absolute platelet counts are from Figure 3A.
RP values (%) are from Figure 3B.
A significant proportion of WASP(−) mice show an elevated fraction of RP, usually in the context of a more significant thrombocytopenia (Fig. 1B). Given the reduced number of RP and their normal consumption rate, the increased RP fraction could be due to particularly increased consumption of non-RP. In support of this, we found a marked increase in consumption of WT platelets introduced into one such mouse (Fig. 5A). Antiplatelet antibodies could explain this. We detected such antibodies in this recipient, using formaldehyde-treated WT platelets as targets (Fig. 5D). This method is thought to improve detection of platelet bound antibody by inhibiting secretion of a protease [42,43], although there are few published reports of its use [44,45]. It might also be expected to inhibit the reported ability of platelets to clear surface antibodies [46,47]. It is conceivable that increased consumption in this case was a consequence of the thrombocytopenia, because rapid platelet clearance in severe (<50 K/uL) clinical thrombocytopenia has been attributed to the predominance of a rapid clearance mechanism associated with platelet activation and/or aggregation [48]. That mechanism is unlikely in this case because the recipient had >100 K/uL platelets.
It will require a more extended survey of control and WASP(−) mice to determine what fraction of the latter show antiplatelet antibodies. But our results suggest that antiplatelet antibodies could play a key role in the development of thrombocytopenia in WAS patients. In support of this, we find that WASP(−) platelets opsonized with anti-CD61 antibody are more susceptible than WT platelets to ex vivo phagocytosis, and are comparable to CD47(−/−) platelets in this regard. Their in vivo consumption rate is also more accelerated by this antibody than is that of WT platelets. Anti-CD61 antibodies are of pathophysiologic relevance because they are often detected in immune thrombocytopenic purpura (reviewed by McMillan [49]). Our results suggest that WASP(−) mice may be both more prone to develop antiplatelet antibodies and more susceptible to their effects.
Increased sensitivity of WASP(−) platelets to the prophagocytic effects of antiplatelet antibodies of low affinity or low concentration could conceivably result in either a chronic or an episodic thrombocytopenia. Human platelets normally demonstrate a low level of bound antibodies [50], a finding we have verified in mice by comparing WT platelets to those from uMT(−/−) mice (data not shown). This level of surface antibody, which presumably does not lead to significant levels of phagocytosis of WT platelets, could lead to increased in vivo clearance of murine WASP(−) platelets via a modest increase in FcR-mediated phagocytosis. This might be expected to cause a chronic thrombocytopenia.
Alternatively, if increased platelet consumption were to result in increased host antigen presentation, the process could become self-reinforcing through the production of still more platelet-reactive antibodies (whether of high or low affinity). Clearance of antibody-opsonized platelets could, in turn, be augmented by concomitant infection, as has been demonstrated in a mouse model [51]. These mechanisms could result in the type of episodic thrombocytopenia seen in some WAS patients at presentation [9] and in others after splenectomy [10]. They could also contribute to the chronic thrombocytopenia of WAS patients, given that the latter show increased levels of platelet associated antibodies (seen in 13 of 14 cases in one study [52], and corroborated in several other reports [23,53,54]). Augmented host antigen presentation has also been suggested to underlie the development of autoimmune hemolytic anemia in the context of CD47 deficiency [55]. It may not be a coincidence that autoimmune hemolytic anemia is the most common autoimmune condition seen in WAS patients.
Our finding of normal phagocytosis of antibody opsonized WT platelets by WASP(−) macrophages contrasts with reports of impaired phagocytosis of opsonized particles, opsonized bacteria, or apoptotic cells by human WASP(−) macrophages [56], impaired phagocytic cup formation [57], and impaired uptake of opsonized sheep red blood cells and apoptotic Jurkat cells by WASP-deficient murine macrophages [58]. Possible reasons for our divergent results include the substrates themselves (platelets), and several additional differences between our studies and those cited, such as the genetic background of the donor mice, the sources of opsonizing antibodies, and the use of flow cytometric vs microscopic readouts.
We do not know the biochemical mechanism responsible for increased phagocytosis of opsonized WASP(−) platelets. We have ruled out abnormal expression of the host antigens recognized by the opsonizing antibodies we have used; platelet activation by the antibodies; increased surface exposure of phosphatidyl serine; and reduced levels of Bcl-xL. The relatively reduced phagocytosis of 6A6-opsonized WASP(−) vs CD47(−/−) platelets suggested that WASP(−) platelets could be particularly susceptible to phagocytosis induced by IgG1 antibodies (such as the hamster anti-CD61 we used) but not IgG2a (such as 6A6), as antibodies of these two classes have substantially different effects on the Fc receptors with which they interact [28]. The low susceptibility of 6A6-IgG1 opsonized WASP(−) platelets to phagocytosis argues against this model. A role for platelet WASP in amplifying signaling between CD47 and its macrophage ligand (Sirp-α, also termed SHPS-1) could be envisioned given WASP’s similar role in T-cell receptor and integrin signaling [5,59]. If this were the case, removing CD47 from WASP(−) platelets should have little if any effect on their susceptibility to phagocytosis. The substantial increase in phagocytosis we see using opsonized platelets lacking both proteins argues against this model, although an expected further reduction in the platelet counts of the “double knockouts” is not evident (Fig. 1A). The additive effect of the two knockouts suggests that CD47 and WASP inhibit platelet phagocytosis by two independent mechanisms. We are currently investigating whether the distribution or clearance of antigen/antibody complexes on WASP(−) platelets differs from WT.
Acknowledgments
We thank David A. Wilcox, Medical College of Wisconsin, for advice on platelet preparations; Peter Murray, St. Jude Children’s Research Hospital, for reagents; and Roland Lang, Institute of Medical Microbiology, Immunology and Hygiene, Technical University Munich, Munich, Germany, for advice on cell culture. We also thank Shirley Steward, St. Jude Children’s Research Hospital, and Nancy Appling, Department of Surgery, Memphis VA Medical Center, for technical assistance.
This work was funded by National Institutes of Health, National Heart, Lung, and Blood Institute award 1 K08 HL72865-01A1; The US Department of Veterans Affairs Biomedical Laboratory Research and Development Service, Merit Review system; Research Inc., a nonprofit corporation associated with the Memphis VA Medical Center (Memphis, TN); the Department of Pathology, University of Tennessee Health Sciences Center, Memphis, TN; and the American Lebanese Syrian Associated Charities. A.H. was supported by a scholarship from the Egyptian Government.
References
- 1.Wiskott A. Familiarer, angeborener morbus werlhofii. Monatsschrift fur kinderheilkunde. 1936;68:212–216. [Google Scholar]
- 2.Aldrich R, Steinberg AG, Campbell DC. Pedigree demonstrating a sex-linked recessive condition characterized by draining ears, eczematoid dermatitis and bloody diarrhea. Pediatrics. 1954;13:133–139. [PubMed] [Google Scholar]
- 3.She HY, Rockow S, Tang J, et al. Wiskott-Aldrich syndrome protein is associated with the adapter protein Grb2 and the epidermal growth factor receptor in living cells. Mol Biol Cell. 1997;8:1709–1721. doi: 10.1091/mbc.8.9.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Page Y, Demay F, Salbert G. A neural-specific splicing event generates an active form of the Wiskott-Aldrich syndrome protein. EMBO Rep. 2004;5:895–900. doi: 10.1038/sj.embor.7400239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badour K, Zhang J, Siminovitch KA. Involvement of the Wiskott-Aldrich syndrome protein and other actin regulatory adaptors in T cell activation. Semin Immunol. 2004;16:395–407. doi: 10.1016/j.smim.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Burns S, Cory GO, Vainchenker W, Thrasher AJ. Mechanisms of WASp-mediated hematologic and immunologic disease. Blood. 2004;104:3454–3462. doi: 10.1182/blood-2004-04-1678. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan KE, Mullen CA, Blaese RM, Winkelstein JA. A multiinstitutional survey of the Wiskott-Aldrich syndrome. J Pediatr. 1994;125:876–885. doi: 10.1016/s0022-3476(05)82002-5. [DOI] [PubMed] [Google Scholar]
- 8.Jin Y, Mazza C, Christie JR, et al. Mutations of the Wiskott-Aldrich Syndrome Protein (WASP): hotspots, effect on transcription, and translation and phenotype/genotype correlation. Blood. 2004;104:4010–4019. doi: 10.1182/blood-2003-05-1592. [DOI] [PubMed] [Google Scholar]
- 9.Notarangelo LD, Mazza C, Giliani S, et al. Missense mutations of the WASP gene cause intermittent X-linked thrombocytopenia. Blood. 2002;99:2268–2269. doi: 10.1182/blood.v99.6.2268. [DOI] [PubMed] [Google Scholar]
- 10.Mullen C, Anderson KD, Blaese RM. Splenectomy and/or bone marrow transplantation in the management of the Wiskott-Aldrich syndrome: long-term followup of 52 cases. Blood. 1993;82:2961–2966. [PubMed] [Google Scholar]
- 11.Schurman SH, Candotti F. Autoimmunity in Wiskott-Aldrich syndrome. Curr Opin Rheumatol. 2003;15:446–453. doi: 10.1097/00002281-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Baldini MG. Nature of the platelet defect in the Wiskott-Aldrich syndrome. Ann N Y Acad Sci. 1972;201:437–444. doi: 10.1111/j.1749-6632.1972.tb16316.x. [DOI] [PubMed] [Google Scholar]
- 13.Grottum KA, Hovig T, Holmsen H, Abrahamsen AF, Jeremic M, Seip M. Wiskott-Aldrich syndrome: qualitative platelet defects and short platelet survival. Br J Haematol. 1969;17:373–388. doi: 10.1111/j.1365-2141.1969.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 14.Murphy S, Oski FA, Naiman JL, Lusch CJ, Goldberg S, Gardner FH. Platelet size and kinetics in hereditary and acquired thrombocytopenia. N Engl J Med. 1972;286:499–504. doi: 10.1056/NEJM197203092861001. [DOI] [PubMed] [Google Scholar]
- 15.Ochs HD, Slichter SJ, Harker LA, Von Behrens WE, Clark RA, Wedgwood RJ. The Wiskott-Aldrich syndrome: studies of lymphocytes, granulocytes, and platelets. Blood. 1980;55:243–252. [PubMed] [Google Scholar]
- 16.Kajiwara M, Nonoyama S, Eguchi M, et al. WASP is involved in proliferation and differentiation of human haemopoietic progenitors in vitro. Br J Haematol. 1999;107:254–262. doi: 10.1046/j.1365-2141.1999.01694.x. [DOI] [PubMed] [Google Scholar]
- 17.Sabri S, Foudi A, Boukour S, et al. Deficiency in the Wiskott-Aldrich protein induces premature proplatelet formation and platelet production in the bone marrow compartment. Blood. 2006;108:134–140. doi: 10.1182/blood-2005-03-1219. [DOI] [PubMed] [Google Scholar]
- 18.Haddad E, Cramer E, Riviere C, et al. The thrombocytopenia of Wiskott Aldrich syndrome is not related to a defect in proplatelet formation. Blood. 1999;94:509–518. [PubMed] [Google Scholar]
- 19.Shcherbina A, Rosen FS, Remold-O’Donnell E. Pathological events in platelets of Wiskott-Aldrich syndrome patients. Br J Haematol. 1999;106:875–883. doi: 10.1046/j.1365-2141.1999.01637.x. [DOI] [PubMed] [Google Scholar]
- 20.Gross BS, Wilde JI, Quek L, Chapel H, Nelson DL, Watson SP. Regulation and function of WASp in platelets by the collagen receptor, glycoprotein VI. Blood. 1999;94:4166–4176. [PubMed] [Google Scholar]
- 21.Rengan R, Ochs HD, Sweet LI, et al. Actin cytoskeletal function is spared, but apoptosis is increased, in WAS patient hematopoietic cells. Blood. 2000;95:1283–1292. [PubMed] [Google Scholar]
- 22.Falet H, Hoffmeister KM, Neujahr R, Hartwig JH. Normal Arp2/3 complex activation in platelets lacking WASp. Blood. 2002;100:2113–2122. [PubMed] [Google Scholar]
- 23.Semple JW, Siminovitch KA, Mody M, et al. Flow cytometric analysis of platelets from children with the Wiskott-Aldrich syndrome reveals defects in platelet development, activation and structure. Br J Haematol. 1997;97:747–754. doi: 10.1046/j.1365-2141.1997.1132938.x. [DOI] [PubMed] [Google Scholar]
- 24.Snapper SB, Rosen FS, Mizoguchi E, et al. Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity. 1998;9:81–91. doi: 10.1016/s1074-7613(00)80590-7. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Shehabeldin A, da Cruz LA, et al. Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott-Aldrich syndrome protein-deficient lymphocytes. J Exp Med. 1999;190:1329–1342. doi: 10.1084/jem.190.9.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andreansky S, Liu CH, Turner SJ, et al. WASP- mice Exhibit Defective Immune Responses to Influenza A Virus, Streptococcus pneumoniae, and Mycobacterium bovis BCG. Exp Hematol. 2005;33:443–451. doi: 10.1016/j.exphem.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Mizutani H, Engelman RW, Kurata Y, Ikehara S, Good RA. Development and characterization of monoclonal antiplatelet autoantibodies from autoimmune thrombocytopenic purpuraprone (NZW x BXSB) F1 mice. Blood. 1993;82:837–844. [PubMed] [Google Scholar]
- 28.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 29.Manning KL, McDonald TP. C3H mice have larger spleens, lower platelet counts, and shorter platelet lifespans than C57BL mice: an animal model for the study of hypersplenism. Exp Hematol. 1997;25:1019–1024. [PubMed] [Google Scholar]
- 30.Arnold JT, Daw NC, Stenberg PE, Jayawardene D, Srivastava DK, Jackson CW. A single injection of pegylated murine megakaryocyte growth and development factor (MGDF) into mice is sufficient to produce a profound stimulation of megakaryocyte frequency, size, and ploidization. Blood. 1997;89:823–833. [PubMed] [Google Scholar]
- 31.Alugupalli KR, Michelson AD, Barnard MR, Leong JM. Serial determinations of platelet counts in mice by flow cytometry. Thromb Haemost. 2001;86:668–671. [PubMed] [Google Scholar]
- 32.Olsson M, Bruhns P, Frazier WA, Ravetch JV, Oldenborg PA. Platelet homeostasis is regulated by platelet expression of CD47 under normal conditions and in passive immune thrombocytopenia. Blood. 2005;105:3577–3582. doi: 10.1182/blood-2004-08-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strom T, Turner SJ, Lang R, et al. Wiskott-Aldrich Syndrome (WAS) knockout mice can be phenotypically corrected by transplantation of syngeneic hematopoietic stem cells transduced with a WASP-expressing retroviral vector. Mol Ther. 2002;5:S23. [Google Scholar]
- 34.Ault KA, Rinder HM, Mitchell J, Carmody MB, Vary CP, Hillman RS. The significance of platelets with increased RNA content (reticulated platelets). A measure of the rate of thrombopoiesis. Am J Clin Pathol. 1992;98:637–646. doi: 10.1093/ajcp/98.6.637. [DOI] [PubMed] [Google Scholar]
- 35.Strom TS, Li X, Cunningham JM, Nienhuis AW. Correction of the murine Wiskott-Aldrich syndrome phenotype by hematopoietic stem cell transplantation. Blood. 2002;99:4626–4628. doi: 10.1182/blood-2001-12-0319. [DOI] [PubMed] [Google Scholar]
- 36.Harrison P, Horton A, Grant D, Briggs C, MacHin S. Immunoplatelet counting: a proposed new reference procedure. Br J Haematol. 2000;108:228–235. doi: 10.1046/j.1365-2141.2000.01846.x. [DOI] [PubMed] [Google Scholar]
- 37.Ault KA, Knowles C. In vivo biotinylation demonstrates that reticulated platelets are the youngest platelets in circulation. Exp Hematol. 1995;23:996–1001. [PubMed] [Google Scholar]
- 38.Berger G, Hartwell DW, Wagner DD. P-Selectin and platelet clearance. Blood. 1998;92:4446–4452. [PubMed] [Google Scholar]
- 39.Dale GL, Friese P, Hynes LA, Burstein SA. Demonstration that thiazole-orange-positive platelets in the dog are less than 24 hours old. Blood. 1995;85:1822–1825. [PubMed] [Google Scholar]
- 40.Robinson M, MacHin S, Mackie I, Harrison P. In vivo biotinylation studies: specificity of labelling of reticulated platelets by thiazole orange and mepacrine. Br J Haematol. 2000;108:859–864. doi: 10.1046/j.1365-2141.2000.01939.x. [DOI] [PubMed] [Google Scholar]
- 41.Schulze H, Korpal M, Hurov J, et al. Characterization of the megakaryocyte demarcation membrane system and its role in thrombopoiesis. Blood. 2006;107:3868–3875. doi: 10.1182/blood-2005-07-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adelman B, Carlson P, Handin RI. Evaluation of platelet surface antigens by fluorescence flow cytometry. Methods Enzymol. 1992;215:420–427. doi: 10.1016/0076-6879(92)15082-n. [DOI] [PubMed] [Google Scholar]
- 43.Phillips DR, Jakabova M. Ca2+-dependent protease in human platelets. Specific cleavage of platelet polypeptides in the presence of added Ca2+ J Biol Chem. 1977;252:5602–5605. [PubMed] [Google Scholar]
- 44.Oyaizu N, Yasumizu R, Miyama-Inaba M, et al. (NZW x BXSB)F1 mouse. A new animal model of idiopathic thrombocytopenic purpura. J Exp Med. 1988;167:2017–2022. doi: 10.1084/jem.167.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizumoto Y, Fujimura Y, Nishikawa K, et al. Flow cytometric analysis of anti-platelet antibodies in patients with chronic idiopathic thrombocytopenic purpura (ITP) using acid-treated, formalin-fixed platelets. Am J Hematol. 1991;37:274–276. doi: 10.1002/ajh.2830370413. [DOI] [PubMed] [Google Scholar]
- 46.Santoso S, Kiefel V, Mueller-Eckhardt C. Redistribution of platelet glycoproteins induced by allo- and autoantibodies. Thromb Haemost. 1987;58:866–871. [PubMed] [Google Scholar]
- 47.Santoso S, Zimmermann U, Neppert J, Mueller-Eckhardt C. Receptor patching and capping of platelet membranes induced by monoclonal antibodies. Blood. 1986;67:343–349. [PubMed] [Google Scholar]
- 48.Hanson SR, Slichter SJ. Platelet kinetics in patients with bone marrow hypoplasia: evidence for a fixed platelet requirement. Blood. 1985;66:1105–1109. [PubMed] [Google Scholar]
- 49.McMillan R. Antiplatelet antibodies in chronic adult immune thrombocytopenic purpura: assays and epitopes. J Pediatr Hematol Oncol. 2003;25(Suppl 1):S57–S61. doi: 10.1097/00043426-200312001-00013. [DOI] [PubMed] [Google Scholar]
- 50.George JN. Platelet immunoglobulin G: its significance for the evaluation of thrombocytopenia and for understanding the origin of alpha-granule proteins. Blood. 1990;76:859–870. [PubMed] [Google Scholar]
- 51.Musaji A, Cormont F, Thirion G, Cambiaso CL, Coutelier JP. Exacerbation of autoantibody-mediated thrombocytopenic purpura by infection with mouse viruses. Blood. 2004;104:2102–2106. doi: 10.1182/blood-2004-01-0310. [DOI] [PubMed] [Google Scholar]
- 52.Corash L, Shafer B, Blaese RM. Platelet-associated immunoglobulin, platelet size, and the effect of splenectomy in the Wiskott-Aldrich syndrome. Blood. 1985;65:1439–1443. [PubMed] [Google Scholar]
- 53.Kanegane H, Nomura K, Miyawaki T, et al. X-linked thrombocytopenia identified by flow cytometric demonstration of defective Wiskott-Aldrich syndrome protein in lymphocytes. Blood. 2000;95:1110–1111. [PubMed] [Google Scholar]
- 54.Litzman J, Jones A, Hann I, Chapel H, Strobel S, Morgan G. Intravenous immunoglobulin, splenectomy, and antibiotic prophylaxis in Wiskott-Aldrich syndrome. Arch Dis Child. 1996;75:436–439. doi: 10.1136/adc.75.5.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oldenborg PA, Gresham HD, Chen Y, Izui S, Lindberg FP. Lethal autoimmune hemolytic anemia in CD47-deficient nonobese diabetic (NOD) mice. Blood. 2002;99:3500–3504. doi: 10.1182/blood.v99.10.3500. [DOI] [PubMed] [Google Scholar]
- 56.Lorenzi R, Brickell PM, Katz DR, Kinnon C, Thrasher AJ. Wiskott-Aldrich syndrome protein is necessary for efficient IgG-mediated phagocytosis. Blood. 2000;95:2943–2946. [PubMed] [Google Scholar]
- 57.Tsuboi S, Meerloo J. Wiskott-Aldrich syndrome protein is a key regulator of the phagocytic cup formation in macrophages. J Biol Chem. 2007;282:34194–34203. doi: 10.1074/jbc.M705999200. [DOI] [PubMed] [Google Scholar]
- 58.Leverrier Y, Lorenzi R, Blundell MP, et al. Cutting edge: the Wiskott-Aldrich syndrome protein is required for efficient phagocytosis of apoptotic cells. J Immunol. 2001;166:4831–4834. doi: 10.4049/jimmunol.166.8.4831. [DOI] [PubMed] [Google Scholar]
- 59.Zhang H, Schaff UY, Green CE, et al. Impaired integrin-dependent function in Wiskott-Aldrich syndrome protein-deficient murine and human neutrophils. Immunity. 2006;25:285–295. doi: 10.1016/j.immuni.2006.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]