Abstract
The quantity of total dietary zinc (Zn) and phytate are the principal determinants of the quantity of absorbed Zn. Recent estimates of Dietary Reference Intakes (DRI) for Zn by the Institute of Medicine (IOM) were based on data from low-phytate or phytate-free diets. The objective of this project was to estimate the effects of increasing quantities of dietary phytate on these DRI. We used a trivariate model of the quantity of Zn absorbed as a function of dietary Zn and phytate with updated parameters to estimate the phytate effect on the Estimated Average Requirement (EAR) and Recommended Daily Allowance for Zn for both men and women. The EAR predicted from the model at 0 phytate was very close to the EAR of the IOM. The addition of 1000 mg phytate doubled the EAR and adding 2000 mg phytate tripled the EAR. The model also predicted that the EAR for men and women could not be attained with phytate:Zn molar ratios > 11:1 and 15:1, respectively. The phytate effect on upper limits (UL) was predicted by first estimating the quantity of absorbed Zn corresponding to the UL of 40 mg for phytate-free diets, which is 6.4 mg Zn/d. Extrapolation of the model suggested, for example, that with 900 mg/d phytate, 100 mg dietary Zn is required to attain 6.4 mg absorbed Zn/d. Experimental studies with higher Zn intakes are required to test these predictions.
Introduction
In the Institute of Medicine's (IOM)3 2001 report (1) that included the Dietary Reference Intakes (DRI) for zinc (Zn), these estimates of the DRI were based on data derived from low-phytate or phytate-free meals. The inhibitory role of dietary inositol hexa- and penta-phosphate, commonly referred to as phytate, was recognized appropriately in that publication. However, at that time, the data were considered too limited to estimate the quantitative effect of different levels of phytate on DRI for Zn.
Since that time, at least 2 models of the effect of phytate on Zn absorption have been published, one a purely mathematical model (2), the other a physiologically based mathematical model based on the inhibitory effect of phytate on Zn absorption (3). Subsequent to its publication, the physiological model has been the subject of continuing development and evaluation, most notably the refinement of parameter estimates resulting from fitting additional data. The objective of this project was to use this model with the updated parameter values to predict the effect of dietary phytate on the Estimated Average Requirement (EAR) and, hence, the recommended daily allowance (RDA) for Zn in adults of both genders. In addition, extrapolation of the model has been utilized to achieve a tentative prediction of the effect of dietary phytate on the upper limit (UL) for Zn in adults.
Materials and Methods
The mathematical model used is that reported by Miller et al. (3). The model is based on the accepted view that Zn absorption is a carrier-mediated process, phytate inhibits absorption by binding with Zn in the gut to form an insoluble complex, and that dietary Zn and phytate are the primary dietary factors determining Zn absorption. It was derived from a basic conception of the Zn-transport protein and Zn-phytate binding processes in the intestine, using the law of mass action and occupancy theory from pharmacokinetic-pharmacodynamic analysis. The model is trivariate, modeling Zn absorption as a function of both dietary Zn and phytate and has 3 parameters: AMAX, KR, and KP, which are the maximum possible absorbed Zn and the Zn-transport receptor and Zn-phytate equilibrium dissociation constants, respectively. Variables and parameters are in units of mmol/d, although values are routinely converted to mg/d, as in this article.4 The derivation of the model assumes that the binding reactions are reversible and in equilibrium, transport receptors have a single Zn binding site and each phytate molecule binds 1 Zn ion. Other dietary factors also thought to influence Zn absorption, e.g. calcium and protein, were investigated using the model and no effect was discerned. The model's primary application thus far has been as a predictive tool, usually to predict Zn absorption from diets or, as reported here, to predict combinations of dietary Zn and phytate that result in given levels of Zn absorption.
Using nonlinear regression analysis, the model was originally fit to 21 data from isotope studies (in adults) of Zn absorption from total daily diets for which Zn and phytate content was reported. As suitable additional data become available, they have been incorporated into the data set to which the model is fit to further evaluate model validity, extend the range of the data when possible, and to increase confidence in the parameter estimates. Since the publication of the model, 12 new data from 4 studies (4–7) have been added. All the new data are from whole-day isotope studies of adults and meet the other criteria previously outlined (3). Each datum was the mean of data from between 7 and 21 subjects. As is the case with the original data, dietary phytate values are derived by analyses in some studies and calculated from food composition databases in others.
Using the model with the new parameter estimates, the combination of dietary Zn and phytate values predicted to be necessary to attain daily Zn absorption levels matching the physiological requirements were calculated. The equations for the resulting relationships between dietary Zn and phytate were derived and plotted to assess the effect of dietary phytate on EAR. The physiologic requirement (i.e. the quantity of absorbed Zn required to balance excretion of endogenous Zn) for adult males utilized in this project (3.8 mg Zn/d) is that calculated by the IOM (1). The figure used for adult women is that from the IOM (3.3 mg Zn/d), corrected for an error of + 0.1 mg Zn/d in the estimate of menstrual Zn losses averaged over the month. Therefore, the figure used here is 3.2 mg Zn/d. In addition, the model was used to investigate the effect of increasing phytate:Zn molar ratios on the potential for achieving the gender-specific EAR of the IOM (1).
To predict how the UL for Zn in adults could reasonably be adjusted to take into account the effect of dietary phytate, the first step was to use the model to estimate the absorbed Zn from 40 mg dietary Zn, i.e. the UL of the IOM (1,3). This calculation was made on the basis of the UL being determined from data for which there was no phytate effect (1). The 2nd step was to then use the model to predict the effect of progressively increasing dietary phytate on the dietary Zn that is required to attain this estimated quantity of absorbed Zn.
Results
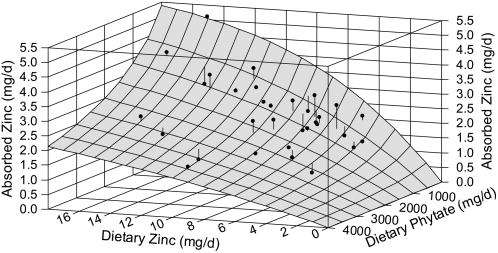
With the additional data, one of the original points that now has an externally studentized residual of 4.4 has been removed as an outlier. Parameter estimates and 95% CI resulting from fitting the model to the remaining 32 data points are listed in Table 1. The R2, quantifying the goodness of fit, is 0.86. A comparison with the values originally published (3) finds that the new parameter values are lower, CI are notably smaller, and quality of the fit is improved. A 3-dimensional graph of the data and fitted model surface using the updated data set is provided in Figure 1.
TABLE 1.
Summary of parameter values for trivariate model
| Parameters | Estimated value | 95% CI |
|---|---|---|
| AMAX,mmol/d (mg/d) | 0.11 (7.2) | 0.07–0.15 (4.5–9.9) |
| KP,mmol/d (mg/d) | 0.77 (50) | 0.04–1.5 (2.7–98) |
| KR,mmol/d (mg/d) | 0.065 (4.3) | −0.01–0.14 (−0.5–9.1) |
FIGURE 1 .
Three-dimensional graph of the trivariate model of absorbed Zn (mg/d) as a function of dietary Zn and dietary phytate. Also shown are the 32 mean data from 15 total diet absorption studies to which the model was fit using nonlinear regression analysis. Stems on data symbols show residuals, i.e. deviation of data from fitted model. Graph positioned with origin (0, 0, 0) at right rear to provide clearer perspective.
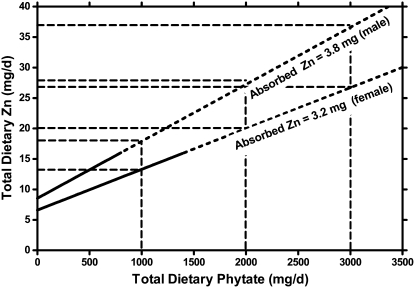
The model predicts that the quantity of dietary Zn required to achieve absorption of the physiologic requirements increases linearly with increasing dietary phytate (Fig. 2). The y-intercepts are ∼8.5 mg dietary Zn for men and 6.6 mg for women, close to the EAR of the IOM (9.4 and 6.8 mg Zn/d) and the slopes of these relationships are 9.3 and 6.5 mg dietary Zn/1000 mg dietary phytate, respectively. As a result, adding the first 1000 mg/d dietary phytate approximately doubled the EAR for both men and women and adding 2000 mg phytate triples the EAR (Fig. 2). Representative examples of predicted increases in the EAR and RDA for Zn with increasing dietary phytate are given in Table 2.
FIGURE 2 .
Model predicted effect of increasing total dietary phytate on the total dietary Zn required to achieve the absorption of the physiologic requirements of 3.8 and 3.2 mg Zn/d for adult men and women, respectively. These values are derived from the IOM (1) with minor adjustment for adult women. The dotted part of the lines showing the predicted relationships between dietary Zn and phytate indicate extrapolation beyond the current experimental data. The y-intercepts of these lines indicate the EAR at 0 dietary phytate. Similarly, the y-intercepts of the dashed lines show the predicted EAR for dietary phytate levels of 1000, 2000, and 3000 mg/d for both genders.
TABLE 2.
Representative examples of the effect of dietary phytate on the EAR and RDA for men and women1
| Men |
Women |
|||
|---|---|---|---|---|
| Phytate | EAR | RDA2 | EAR | RDA |
| mg/d | ||||
| 0 | 9 | 11 | 7 | 8 |
| 1000 | 18 | 22 | 13 | 16 |
| 2000 | 27 | 32 | 20 | 24 |
| 3000 | 37 | 44 | 27 | 32 |
Data derived from Figure 2.
RDA of IOM are 11 and 8 mg Zn/d for men and women, respectively.
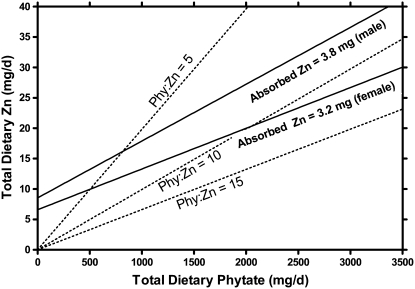
The model predicts that the ranges of phytate:Zn molar ratios over which the absorption of Zn is adequate to meet physiologic requirements are quite limited (Fig. 3). With a phytate:Zn molar ratio of 5:1, the physiological requirements could be attained for men but required a minimum dietary Zn of 16 mg/d. The EAR for men could not be attained with a phytate:Zn ratio of 11:1. For women, the EAR could be attained with a phytate:Zn ratio of 10:1, but this required a dietary Zn intake of 20 mg/d. The EAR for women could not be attained with a phytate:Zn ratio of 15:1.
FIGURE 3 .
Model predictions of the relationship between dietary phytate:Zn molar ratios and the potential for achieving the physiological requirements determined by the IOM (1), with minor correction for physiological requirements for women. Selected phytate:Zn molar ratios indicated with dotted lines.
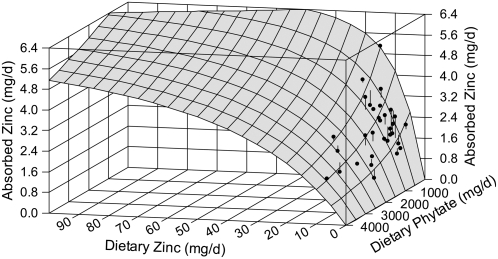
The UL for adults (1) is 40 mg Zn/d. The model predicts that the quantity of Zn absorbed from 40 mg dietary Zn at 0 phytate intake is 6.4 mg Zn/d. Even at 0 dietary phytate, this calculation required extrapolation of the model to higher levels of dietary Zn than are available from the experimental data available in developing the model. Assuming the validity of the model in the extrapolated region, the model predicts, for example, that 100 mg Zn is required to achieve absorption of 6.4 mg Zn/d with a dietary phytate of 900 mg/d (Fig. 4).
FIGURE 4 .
Three-dimensional graph of model surface and data with dietary Zn extrapolated to 100 mg Zn/d. The upper end of the range of Zn absorption shown, 6.4 mg/d, corresponds to the absorption from the UL for adults. Thus, any dietary Zn and phytate combination in the ranges covered by the model surface is predicted to result in absorption less than the predicted safe limit of 6.4 mg/d.
Discussion
Phytate is present in virtually all plant foods, with especially high concentrations in cereal grains and legumes. In populations that are dependent on unrefined grains as a major food staple, daily phytate intakes are frequently as high as 2500 mg for women and higher for men.
The phytate intake data available for inclusion in this model cover a range that addressed the majority of dietary phytate intakes globally, although it does not cover the most extreme intakes by adult men. In contrast, the range of Zn intake data are limited. The model has, therefore, been extrapolated beyond maximal measured levels of dietary Zn. There are, then, 2 kinds of uncertainty associated with the model predictions: the validity of the model beyond the range of the data fitted and the uncertainty in the parameter estimates. We feel there is little need for concern regarding the former, because there is no known physiologic reason for a deviation from the behavior exhibited by the model as diet increases beyond current experimental data limits. The uncertainty of the parameter values does, of course, affect confidence in the predictions, but this cannot be easily quantified. While the standard errors and CI of the parameter estimates are available, meaningful CI for the predictions cannot be calculated without taking into account the physiologically possible relationships of the parameters, about which we have insufficient information.
The predicted effects of phytate on DRI are severe and one question that demands attention is whether there might be adaptation to the long-term or habitual high-phytate intakes resulting in more favorable absorption of Zn than indicated by this model. One theoretical possibility is the secretion of endogenous phytases. However, secretion of endogenous phytases into the intestinal lumen has not been observed in humans and there is no evidence of hydrolysis of phytate in the gut lumen resulting from a diet habitually high in phytate. A 2nd possibility is a greater-than-expected upregulation of Zn absorption beyond that associated with intakes of bioavailable Zn that are insufficient to meet physiologic Zn requirements. The data contributing to the trivariate model were derived from projects having a wide range of study designs, including a variety of habitual diets and a wide range of duration of the phytate-containing study diets prior to measuring Zn absorption. This range included several studies in which Zn absorption was measured after 2 d or less on the study diet. Although upregulation (8) or downregulation (9,10) occurs quickly after reducing or increasing, respectively, the intake of bioavailable Zn, this raises the question whether there was time for regulation to occur in response to a change in the quantity of bioavailable Zn in the study diet. Accordingly, the model was also fit to only the 20 data sets for which the subjects were on the study diet for at least 1 wk prior to absorption measurements. Regression analysis of these data produced parameter estimates very similar (varying no more than one-quarter of the SEE) to those from analysis of all the data, thus giving no indication that the shorter-term studies had failed to provide sufficient time for adaptation to occur. This is consistent with the current concept that recent intake rather than long-term intake of Zn affects fractional Zn absorption (11–13).
Although the magnitude of the estimated increase in UL with increasing dietary phytate may appear surprising, absorption of Zn via the enterocyte is a saturable process (14) and the relationship between absorbed Zn and diet Zn is most appropriately described by saturation response modeling (10) (Fig. 1). This model, even within the range of current experimental data, makes evident the diminishing rate of increase in the quantity of Zn absorbed with increasing diet Zn, with absorbed Zn approaching a maximum value (AMAX) of 7.2 mg Zn/d at an extremely high diet Zn level. The relatively modest 6.4 mg absorbed Zn/d predicted for a diet Zn of 40 mg Zn/d is, therefore, not unexpected. Figure 1 also illustrates the large predicted effect of phytate on absorbed Zn at any given level of diet Zn within the range of experimental data.
This is not the first occasion in which dietary phytate has been taken into consideration in estimating Zn requirements. Notably, this was done as part of very detailed estimates of Zn requirements by the WHO in 1996 (15). These estimates continue to provide a cornerstone for the Recommended Nutrient Intakes for Zn of WHO/FAO today (16). However, it is now widely accepted that the underlying estimates of physiologic requirements, although painstakingly and otherwise laudably determined, appear to be flawed, resulting in remarkably low and what are now considered erroneous estimates of physiologic requirements (1,2). Moreover, the phytate effect was based on multiple single meal studies which, for the same dietary intakes of phytate and Zn, give a distinctly lower figure for absorbed Zn than do total diet studies (17).
A notable omission from this article is any data for young children, the population group at greatest recognized risk of morbidity/mortality from inadequate Zn intake. There are published results of saturation response modeling of the quantity of Zn absorbed as a function of diet Zn in infants not yet receiving complementary foods (18) and in toddlers whose phytate intake was too low to demonstrate a phytate effect (19). There are, however, no experimental data for very young children that include absorbed Zn, diet Zn, and a range of diet phytate. This is an important target for future research.
Subject to confirmation with studies that include much higher levels of Zn intake, the results of this modeling serve to emphasize the predicted magnitude of the effect of dietary phytate on dietary Zn requirements. This increase is linear with no threshold for the inhibitory effect to become discernible. Utilizing the model to examine absorbed Zn as a function of dietary phytate:Zn molar ratios further emphasizes the potential benefits of either phytate reduction or/and Zn fortification of food staples/diets that are habitually high in phytate.
Supported by The International Atomic Energy Agency Research Contract 11069/RO, HarvestPlus 8030, K24 RR018357-01 and MRDDR Center Grant P30 HD004024.
Author disclosures: K. Hambidge, L. Miller, J. Westcott, and N. Krebs, no conflicts of interest.
Abbreviations used: AMAX, maximal possible absorbed zinc from all meals during one day; DRI, Dietary Reference Intake; EAR, estimated average requirement; IOM, Institute of Medicine; KP, zinc-phytate equilibrium constant; KR, zinc-transport receptor equilibrium dissociation constant; RDA, recommended daily allowance; UL, upper limit.
1 mmol zinc = 65.4 mg; 1 mmol phytate = 660 mg.
References
- 1.Food and Nutrition Board, Institute of Medicine. Dietary reference intakes for vitamin A, vitamin K, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. Washington, DC: National Academy Press; 2001.
- 2.International Zinc Nutrition Consultative Group. Assessment of the risk of zinc status in populations and options for the control of zinc deficiency. Boston: International Nutrition Foundation for United Nations University Press; 2004. [PubMed]
- 3.Miller LV, Krebs NF, Hambidge KM. A mathematical model of zinc absorption in humans as a function of dietary zinc and phytate. J Nutr. 2007;137:135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hambidge KM, Rosado JL, Miller LV, Hotz C, Westcott JE, Garcia OP, Gonzalez K, Ortiz-Monasterio I, Pfeiffer W, et al. Absorption of zinc (Zn) from high Zn and control wheat. FASEB J. 2008;22:149.5. [Google Scholar]
- 5.Kim J, Paik HY, Joung H, Woodhouse LR, Li S, King JC. Effect of dietary phytate on zinc homeostasis in young and elderly Korean women. J Am Coll Nutr. 2007;26:1–9. [DOI] [PubMed] [Google Scholar]
- 6.Kristensen MB, Hels O, Morberg CM, Marving J, Bugel S, Tetens I. Total zinc absorption in young women, but not fractional zinc absorption, differs between vegetarian and meat-based diets with equal phytic acid content. Br J Nutr. 2006;95:963–7. [DOI] [PubMed] [Google Scholar]
- 7.Sheng XY, Hambidge KM, Miller LV, Bailey K, Gibson RS, Westcott JE, Lei S, Grunwald G, Krebs NF. Major parameters of zinc homeostasis in adult women with a wide range of habitual zinc intake. FASEB J. 2008;22:697.1. [Google Scholar]
- 8.Weaver BP, Dufner-Beattie J, Kambe T, Andrews GK. Novel zinc-responsive post-transcriptional mechanisms reciprocally regulate expression of the mouse Slc39a4 and Slc39a5 zinc transporters (Zip4 and Zip5). Biol Chem. 2007;388:1301–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem. 2006;281:24085–9. [DOI] [PubMed] [Google Scholar]
- 10.Hambidge KM, Miller LV, Tran CD, Krebs NF. Measurements of zinc absorption: application and interpretation in studies designed to improve human zinc nutriture. Int J Vitam Nutr Res. 2005;75:385–93. [DOI] [PubMed] [Google Scholar]
- 11.Johnson PE, Hunt CD, Milne DB, Mullen LK. Homeostatic control of zinc metabolism in men: zinc excretion and balance in men fed diets low in zinc. Am J Clin Nutr. 1993;57:557–65. [DOI] [PubMed] [Google Scholar]
- 12.Krebs NF, Hambidge KM. Zinc metabolism and homeostasis: the application of tracer techniques to human zinc physiology. Biometals. 2001;14:397–412. [DOI] [PubMed] [Google Scholar]
- 13.Chung CS, Stookey J, Dare D, Welch R, Nguyen TQ, Roehl R, Peerson JM, King JC, Brown KH. Current dietary zinc intake has a greater effect on fractional zinc absorption than does longer term zinc consumption in healthy adult men. Am J Clin Nutr. 2008;87:1224–9. [DOI] [PubMed] [Google Scholar]
- 14.Steel L, Cousins RJ. Kinetics of zinc absorption by luminally and vascularly perfused rat intestine. Am J Physiol. 1985;248:G46–53. [DOI] [PubMed] [Google Scholar]
- 15.WHO. Trace elements in human nutrition and health. Geneva: WHO; 1996.
- 16.WHO/FAO. Vitamin and mineral requirements in human nutrition. 2nd ed. Geneva: WHO, FAO; 2004.
- 17.Hambidge KM, Miller LV, Westcott JE, Krebs NF. Modeling zinc (Zn) absorption (AZ) from single test meals (SM) as a function of dietary zinc (DZ) and phytate (DP). FASEB J. 2008;22:697.4. [Google Scholar]
- 18.Hambidge KM, Krebs NF, Westcott JE, Miller LV. Changes in zinc absorption during development. J Pediatr. 2006;149:S64–8. [DOI] [PubMed] [Google Scholar]
- 19.Sheng XY, Hambidge KM, Zhu XX, Ni JX, Bailey KB, Gibson RS, Krebs NF. Major variables of zinc homeostasis in Chinese toddlers. Am J Clin Nutr. 2006;84:389–94. [DOI] [PubMed] [Google Scholar]