Abstract
Zinc is essential for normal erythroid cell functions and therefore intracellular zinc homeostasis during erythroid differentiation is tightly regulated. However, a characterization of zinc transporters in erythrocytes has not been conducted. The membrane fraction of mature mouse RBC was screened for zinc transporter expression using western analysis as a first step in the characterization process. ZnT1, Zip8, and Zip10 were detected among the 12 transporter proteins tested. We examined expression of these zinc transporters during erythropoietin (EPO)-induced differentiation of splenic erythroid progenitor cells into reticulocytes. Both Zip8 and Zip10 mRNA increased by 2–6 h after addition of EPO to the cells. In contrast, maximal RNA levels for the zinc transporter ZnT1 and erythroid δ-aminolevulinic acid synthase were only produced by 24 h after EPO. We confirmed these changes in transcript abundance by western analysis. Dietary zinc status influences zinc-dependent functions of RBC. To determine whether the identified zinc transporters respond to dietary zinc status, mice were fed a zinc-deficient or control diet. Incorporation of 65Zn into erythrocytes in vitro was significantly increased in cells from the zinc-deficient mice. Western analysis and densitometry revealed that erythrocyte Zip10 was upregulated and ZnT1 was downregulated in the zinc-depleted mice. Zip8 was not affected by restricted zinc intake. Collectively, these data suggest that the zinc transporters ZnT1, Zip8, and Zip10 are important for zinc homeostasis in erythrocytes and that ZnT1 and Zip10 respond to the dietary zinc supply.
Introduction
Differential expression of zinc transporters is an important component for the regulatory mechanism of zinc homeostasis. There are 2 distinct gene families of zinc transporters, 10 ZnT (Slc30a) and 14 Zip (Slc39a) transporter genes (1,2). ZnT proteins facilitate the removal of cytosolic free zinc either by exporting zinc through the plasma membrane or by sequestering zinc in vesicles, whereas the Zip transporters function in an opposite manner as a pathway for zinc influx through the plasma membrane or from vesicles.
The zinc concentration is ∼15 times greater in mature RBC than in plasma (3). More than 90% of RBC zinc functions as a component essential for the activity of zinc metalloenzymes, particularly carbonic anhydrase and Cu2+/Zn2+-superoxide dismutase (4). Some zinc may be bound to metallothionein (MT)4 (5,6). Calculations of zinc recycling through the human erythron suggest there is an overall turnover for this pool of between 0.12 and 0.25 mg zinc/d (7). This estimate is based on a zinc concentration of 20–40 μg zinc/g hemoglobin, a RBC zinc pool of 15–30 mg, and an RBC turnover rate of 120 d.
Various routes of zinc influx into circulating RBC have been reported. Mechanisms suggested involve Cl−/HCO3– anion exchanger activity, neutral complex formation with thiocyanate or salicylate ions, and chelation by amino acids (8–10). A calcium-dependent zinc efflux by a Cu2+/Zn2+ exchanger has been considered as the mechanism for the cellular zinc export from circulating RBC (11). These mechanisms were proposed before the identification of any zinc transporter proteins. Therefore, reconsideration of the possible transport mechanisms is necessary. Additionally, there have been animal and human studies with zinc-deficient subjects implying expression of zinc-responsive intrinsic factors that influence the RBC zinc transport system (12–15). In those studies, RBC from zinc-deficient subjects consistently demonstrated higher 65Zn uptake in vitro. Unfortunately, various forms of stress that produce hypozincemia also increase 65Zn uptake kinetics by RBC. This diminishes the latter as a diagnostic tool for assessment of zinc status (13,15). Although the zinc uptake rate is likely to be influenced by zinc transporter expression, there have been no reports related to the identification of erythroid zinc transporters.
Proteins involved in zinc metabolism and function in the enucleated mature RBC are remnants from earlier developmental stages where gene expression and protein production were active. A study showing increased zinc uptake by the bone marrow during induced erythropoiesis in zinc deficiency supports the necessity of an adequate amount of zinc during erythroid differentiation (16). In addition, a well-studied role of zinc in erythroid differentiation is its incorporation into zinc finger transcription factors essential for the expression of proteins involved in events of terminal erythroid maturation (17,18). Zinc status/supplementation may influence hemoglobin production (19). In contrast, there have been reports of sideroblastic anemia caused by zinc intoxication (20,21). This may be due to the interference of excessive free zinc ions with incorporation of ferrous ions into protoporphyrin during heme biosynthesis (22). Consequently, it seems critical for the intracellular zinc level to be tightly regulated during late-stage erythroid differentiation so that adverse effects introduced by an inadequate or excessive zinc supply can be avoided.
The major aim of this study was to determine which zinc transporters may be involved in the erythroid zinc trafficking system. Our results define that the zinc transporters found in circulating erythrocytes include ZnT1, Zip8, and Zip10. We then identify the temporal trends in expression of those genes in differentiating erythroid progenitor cells and their responsiveness to the dietary zinc supply.
Materials and Methods
Mice and diets.
Young male mice of the CD-1 strain (Charles River) were housed and fed as previously described (23,24). The mice were fed a commercial diet (Teklad 8604, Harlan) and received conventional husbandry. For some experiments, the mice were fed the AIN76 diet (Research Diets) formulated with egg white rather than casein and containing <1 mg zinc/kg diet or 30 mg zinc/kg diet and were given deionized drinking water (23). In this case, the plasma zinc concentrations and small intestinal MT-1 mRNA levels were analyzed as positive controls for induction of zinc deficiency by the zinc-restricted diet (25). These methods were approved by the University of Florida Institutional Animal Care and Use Committee.
Preparation of RBC membranes.
Whole blood was collected from anesthetized mice by cardiac puncture and placed into EDTA-treated tubes for most experiments. Three volumes (vol) of wash buffer [5 mmol/L Na2HPO4 (pH 7.4), 0.15 mol/L NaCl] was added. After centrifugation at 2000 × g; 10 min at 4°C, the RBC-enriched pellet was washed extensively with 2 vol of wash buffer. In all cases, the presence of RBC was confirmed by phase contrast microscopy. Isolated RBC were lysed by washing with 3 vol of a hypotonic lysis buffer [5 mmol/L Na2HPO4 (pH 7.4) containing protease inhibitor cocktail (P2714; Sigma)]. After centrifugation at 12,000 × g; 10 min at 4°C, RBC membranes were separated from the supernatant and the residual debris pellet. The cell lysis step was repeated with the membrane pellet until it lost its red color (5–7 times). Each final membrane fraction was suspended in 5 mmol/L Tris-HCl, 0.5% Triton X-100 with the protease inhibitor cocktail and protein concentrations were determined colorimetrically (Bio-Rad) (26). In the mice fed controlled amounts of zinc, heparin-treated tubes were utilized to obtain plasma from whole blood to measure the zinc concentration by atomic absorption spectrophotometry.
Primary erythroid progenitor cells.
Primary erythroid progenitor cells were prepared from spleens of phenylhydrazine (PHZ)-treated anemic mice. CD-1 mice were injected with PHZ (60 mg/kg body weight; Sigma) in saline, intraperitoneally, on d 1 and 2. On d 5, the PHZ-treated mice were killed and the spleen was collected. Splenocyte suspensions were prepared by mincing the spleen, placing the fragments in α minimum essential medium (AMEM; Mediatech) containing 10% fetal bovine serum and antibiotic/antimycotic solution (Sigma), and passing the disrupted spleen through nylon mesh. Viability of the isolated splenocytes was determined with Trypan Blue (Sigma).
To induce further differentiation of the late-stage erythroid progenitors, the splenocytes at 5.0 × 109 cells/L of the above supplemented AMEM were treated with 5 kIU/L of recombinant human erythropoietin (EPO; ProSpec-Tany) and then incubated for up to 48 h at 37°C in 5% CO2. Differentiation was confirmed by comparing the hemoglobin content of cells collected at 0 and 48 h after EPO treatment using o-dianisidine staining and light microscopy (27).
RNA isolation and quantitative real-time-PCR.
Erythroid cells were harvested at various time points up to 48 h. After centrifugation at 400 × g; 5 min at 4°C, cells were lysed with TRIzol reagent (Invitrogen) for total RNA isolation. Total RNA was treated with DNase I (Ambion) to avoid any DNA contamination and the concentrations were determined spectrophotometrically.
Relative mRNA levels of the erythroid δ-aminolevulinic acid synthase (ALAS-2) and specific zinc transporter genes were measured by quantitative real-time PCR (qPCR). All qPCR assays were conducted with SYBR Green (Applied Biosystems) and cDNA templates generated by using the High-Capacity cDNA Archive method (Applied Biosystems). Primers for the PCR application of ALAS-2, Zip8, and Zip10 cDNA were designed with PRIMER EXPRESS V2.0 (Applied Biosystems) (Supplemental Table 1). We have reported other primer sequences previously (25). The specificity of each primer pair was confirmed by melting curve analyses and the relative quantity was determined by normalization with respective 18S rRNA values.
Protein isolation.
Erythroid splenocytes were treated and collected as above. Total membrane fractions of the cells were prepared by utilizing a commercial membrane protein extraction method (BioVision). Cell pellets (∼1.0 × 108 cells) were homogenized as recommended by the manufacturer and the final cellular membrane fraction was placed in 50 μL of solubilization buffer [5 mmol/L Tris-HCl, 0.5% Triton X-100 with protease inhibitor cocktail (BioVision)] and stored at −80°C until used. The protein concentrations were determined as above.
Affinity-purified antibodies.
Rabbits were injected with respective antigenic peptides for production of polyclonal antibodies against numerous murine Zip and ZnT transporters as previously described (28,29). The amino acid sequences used are presented in Supplemental Table 2. Briefly, total IgG was prepared from serum using the PROSEP-A procedure (Millipore). Affinity chromatography of each antibody was as previously described (28,29)
Western analysis.
Protein preparations (40 μg) were denatured in an equal amount of Laemmli buffer for 15 min at 55°C and separated by SDS-PAGE (10% acrylamide). For the assessment of glycosylation, protein samples (20 μg) were digested with 10 μL PNGase F (New England Bio Labs) for 2 h at 37°C prior to denaturation. Detection of zinc transporters by western analysis was as previously described (27). Equal protein loading was confirmed by Ponceau Red staining. Specificity of the primary antibody was determined by preincubation with the respective peptide (250 mg/L peptide). Blots of erythroid splenocyte membranes were stripped (Restore PLUS; Pierce) and then reprobed with an anti-Na+/K+-ATPase α1 monoclonal antibody (Santa Cruz Biotechnology) and enhanced chemiluminescence-peroxidase labeled anti-mouse antibody (Santa Cruz Biotechnology) as primary and secondary antibody, respectively.
Immunocytochemistry.
Purified RBC were incubated in wells of a Lab-Tek chambered cover glass (Nunc), pretreated with 0.01% solution of poly-l-lysine (Sigma), for 1 h. Attached cells were fixed and permeabilized by 4% paraformaldehyde in 0.1 mol/L PBS for 30 min and 0.1% Triton X-100, 1% bovine serum albumin in PBS for 10 min, respectively. After an extensive wash with PBS, the cellular localization of ZnT1, Zip8, or Zip10 was visualized by immunolabeling and fluorescence microscopy as described earlier (25,26).
Measurement of erythrocyte zinc uptake.
Erythrocytes were isolated from heparinized whole blood as above. RBC were resuspended to 7% with prewarmed serum-free DMEM (Mediatech) and incubated under reciprocal shaking at 37°C for 1 h. After centrifugation at 300 × g for 10 min, packed RBC were added into ice-cold DMEM containing 45 μCi/L (1 Ci = 37 GBq) 65Zn (Oak Ridge National Laboratory) and 7.5 μmol/L ZnCl2 with the same 7% ratio of cells to medium. Aliquots of each cell suspension were incubated at 37°C for designated times. After removal of the zinc-supplemented medium, cells were washed 3 times with ice-cold 10 mmol/L HEPES-buffered saline containing 10 mmol/L EDTA and the final cell pellets were lysed in 0.2% SDS and 0.2 mol/L NaOH. 65Zn was measured with a γ-ray spectrometer (Packard) and total zinc accumulation was calculated (26).
Statistical analysis.
Data are expressed as means ± SD of biological replicates. In experiments with erythroid splenocytes, data were analyzed by 2-way ANOVA with time and EPO treatment as independent variables (Prism 5, GraphPad Software). Differences between each treatment group were determined using the Bonferroni post-test. For comparison between the dietary groups in the controlled zinc intake study, Student's t test was conducted (InStat, GraphPad Software). The level of significance was set at P < 0.05 for all analyses.
Results
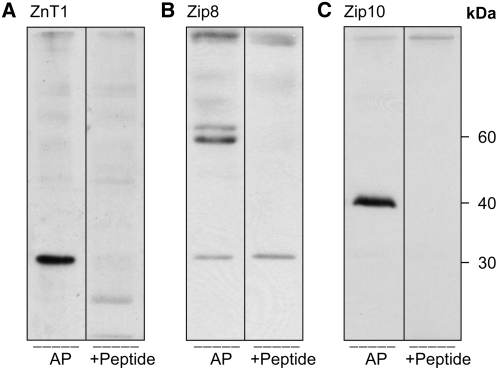
Zinc transporters expressed in circulating mature erythrocytes were identified at the protein level. Membrane fractions (erythrocyte ghosts) were prepared directly from blood of adult mice. Purity of the RBC population was confirmed by light microscopy. Of the ZnT proteins tested (ZnT1, ZnT2, ZnT4, ZnT5, and ZnT6), ZnT1 was the only ZnT detected in the erythrocyte membrane fractions (Fig. 1A). As the estimated molecular mass at 30 kDa was inconsistent with that reported with this antibody in our previous studies (28,29), the specificity of this affinity-purified antibody to ZnT1 was further evaluated by peptide competition. The signal on the blot was competed out when the antibody was preincubated with the ZnT1 peptide (Fig. 1A). Among the Zip proteins screened (Zip1, Zip2, Zip3, Zip4, Zip8, Zip10, and Zip14), only Zip8 and Zip10 were detected (Fig. 1B,C). The rabbit Zip8 antibody produced a major band of immunoreactivity at 60 kDa (Fig. 1B). A very faint band was observed at ∼30 kDa. Upon peptide competition, the 60-kDa band disappeared, suggesting specificity for Zip8. Zip10 had the expected band size of 40 kDa shown by others (30). Peptide competition showed this single prominent signal was eliminated by preincubation with the Zip10 peptide (Fig. 1C).
FIGURE 1 .
Western analysis of zinc transporters in RBC membranes. Erythrocytes were lysed by hypotonic buffer and the plasma membrane fraction was separated by SDS-PAGE. Among the transporters tested, only ZnT1 (A), Zip8 (B), and Zip10 (C) were detected. Specificity of signals was determined by preexposing the respective antibodies to their corresponding peptide.
The localization of these zinc transporters to plasma membrane of the RBC was further confirmed by immunocytochemistry. When cells were labeled by either anti-ZnT1, -Zip8, or -Zip10 antibodies, positive immunoreactivity was observed throughout the cells but with higher intensity around the plasma membrane fraction of each cell (Fig. 2A–C). In contrast, no fluorescence was detected when the anti-zinc transporter antibodies were excluded during preparation (Fig. 2D).
FIGURE 2 .
Immunofluorescence detection of zinc transporters in RBC. Erythrocytes were placed on poly-l-lysine–coated coverslips and fixed by 4% paraformaldehyde. After permeabilization, cells were treated with anti-ZnT1 (A), anti-Zip8 (B), or anti-Zip10 (C) AP. Cellular localization of each transporter was visualized by immunofluorescence microscopy after incubation with a secondary Alexa Fluor 594-conjugated anti-rabbit IgG antibody. (D) No signals were detected in the absence of a primary antibody.
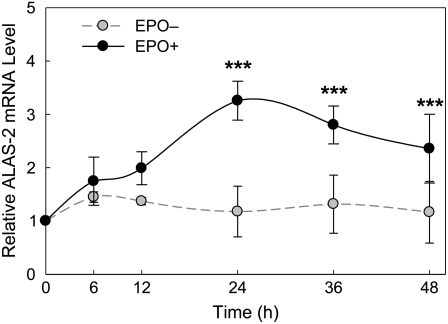
The transporter proteins in circulating RBC are remnants of the protein components synthesized in differentiating erythroid progenitor cells. To evaluate the expression of the ZnT and Zip transporters during cell maturation, we utilized an in vitro model of late-stage erythroid progenitors, splenocytes prepared from PHZ-treated anemic mice exhibiting splenomegaly. An increase in the number of hemoglobin-positive cells determined with o-dianisidine was observed in the cell population incubated with EPO for 48 h (data not shown). Transcript abundance of an EPO-dependent erythroid-specific gene, ALAS-2, was also measured during the 48 h time course as a marker of terminal erythroid differentiation. Values were normalized to 18S rRNA and 0 h of cells cultured in the presence or absence of EPO. Maximum relative ALAS-2 mRNA abundance was observed at 24 h, with a 2-fold increase after EPO stimulation (Fig. 3). This temporal pattern of ALAS-2 expression in the EPO-treated cells is consistent with that reported by Hodges et al. (31).
FIGURE 3 .
Relative ALAS-2 mRNA abundance in EPO-treated and -deprived cells. Splenocytes were collected from spleens of PHZ-injected mice and pooled for culture in each experiment. Cells were placed in AMEM containing 10% fetal bovine serum with or without recombinant EPO (5 IU/mL). qPCR assays were performed using total RNA. Values at each time point are relative to the basal levels at 0 h and were normalized to 18S rRNA. Data are means ± SD of 4 independent experiments, n = 4. ***Different from EPO deprived (EPO–) cells, P < 0.001.
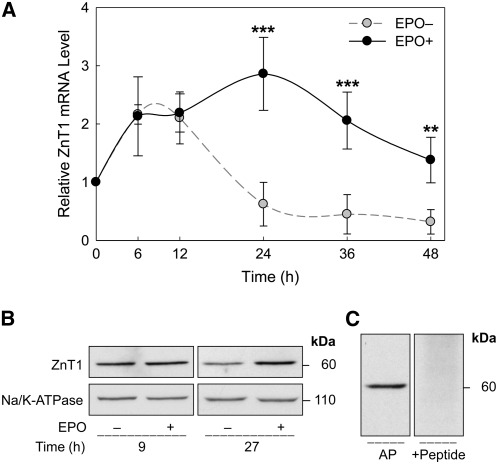
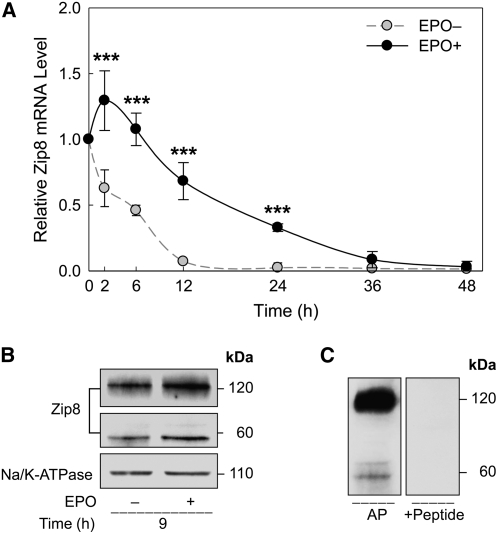
We further investigated the expression of transporter genes during late-stage erythroid differentiation. ZnT1 mRNA during cell differentiation was similar to that of ALAS-2 mRNA. The change in ZnT1 transcript levels was observed by 24 h. The mRNA abundance of ZnT1 in differentiating cells was ∼2-fold higher than the 0-h basal levels in EPO-deprived cells (Fig. 4A). Zip8 was the least responsive to EPO treatment among the 3 transporter genes examined. After an initial (2 h) increase after EPO was added, Zip8 mRNA levels steadily declined (Fig. 5A). Zip10 mRNA levels in differentiating cells showed less than a 1-fold increase after 6 h of incubation with EPO (Fig. 6A). However, as for Zip8, the Zip10 transcript levels were not sustained and decreased to <50% of the basal (0 h) levels after 24 h of EPO treatment. Cells deprived of EPO showed a steady decrease in both Zip8 and Zip10 mRNA levels throughout the 48-h period.
FIGURE 4 .
Regulation of ZnT1 expression during terminal erythroid differentiation. (A) Relative ZnT1 mRNA abundance in erythroid progenitor cells was measured after being cultured in the conditions described in Figure 3. Data are means ± SD, n = 4 (independent experiments). **Different from EPO deprived (EPO–) cells, P < 0.01; ***, P < 0.001. (B) EPO-dependent ZnT1 protein expression was analyzed by western analysis. Total cellular membrane fractions were isolated and proteins were separated by SDS-PAGE. The relative densitometric value of ZnT1 in the EPO-treated cells was 2.3-fold of the control level after 27 h of incubation. Densitometric values of Na+/K+-ATPase levels were used for normalization. (C) Specificity of the 60-kDa band was determined by preincubating the respective antibody with a ZnT1 peptide solution.
FIGURE 5 .
Regulation of Zip8 expression during terminal erythroid differentiation. (A) Relative Zip8 mRNA abundance in erythroid progenitor cells were measured after being cultured in the conditions described in Figure 3. Data are means ± SD, n = 4 (independent experiments). ***Different from EPO deprived (EPO–) cells, P < 0.001. (B) Western analysis for detection of the EPO-dependent Zip8 protein expression was conducted as in Figure 4B. Relative densitometric values of the 60-kDa and 120-kDa bands for Zip8 in the EPO-treated cells were 1.5-fold of their respective control levels after 9 h of incubation. (C) Specificity of the 60-kDa and 120-kDa bands was determined by preincubating the respective antibody with a Zip8 peptide solution.
FIGURE 6 .
Regulation of Zip10 expression during terminal erythroid differentiation. (A) Relative Zip10 mRNA abundance in erythroid progenitor cells was measured after being cultured in the conditions described in Figure 3. Data are means ± SD, n = 4 (independent experiments). *Different from EPO deprived (EPO–) cells, P < 0.05; ***, P < 0.001. (B) Western analysis for detection of the EPO-dependent Zip10 protein expression was conducted as in Figure 4B. The relative densitometric value of Zip10 in the EPO-treated cells was 2.5-fold of the control level after 9 h of incubation. (C) Specificity of the 40-kDa band was determined by preincubating the respective antibody with a Zip10 peptide solution.
Effects of EPO induction on protein levels were determined by western analyses using total erythroid cell membrane preparations. Abundance of ZnT1 protein was greater at the 27-h time point in EPO-mediated differentiation (Fig. 4B). Peptide competition supports specificity of the 60-kDa band (Fig. 4C). The apparent discrepancy between the molecular mass estimates of ZnT1 in RBC ghosts vs. erythroid progenitor cells is discussed below. Zip8 protein levels (9 h) were greater with EPO treatment (Fig. 5B). The induction of Zip10 expression by EPO was observed in differentiating erythroid splenocytes at 9 h (Fig. 6B). Peptide competition also supports the specificity of protein bands for Zip8 and Zip10 (Figs. 5C and 6C).
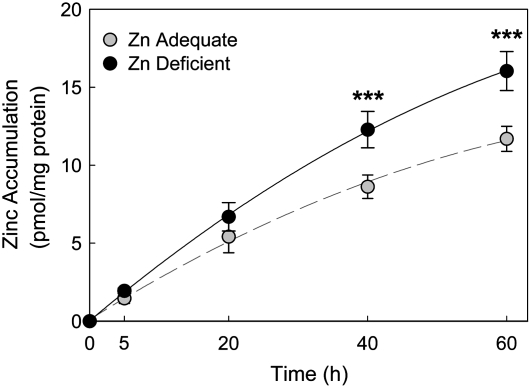
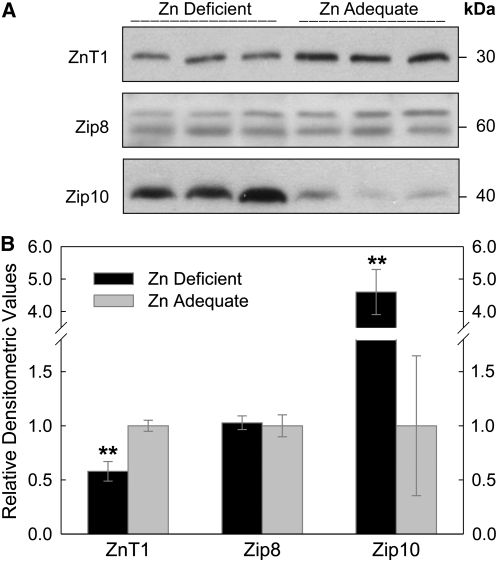
Having identified those zinc transporters that are highly expressed in RBC, we next evaluated their responsiveness to dietary zinc restriction. After the mice were fed the zinc-deficient diet for 3 wk, RBC were isolated and their ability to accumulate 65Zn in vitro was assessed. The RBC population of zinc-deficient mice had a greater (P < 0.001) 65Zn uptake (16.0 ± 1.2 pmol/mg protein) than in controls (11.7 ± 0.8 pmol/mg protein) during the 60-min incubation period (Fig. 7). Western analysis demonstrated that both ZnT1 and Zip10 are zinc responsive to zinc depletion, but in contrast, Zip8 is not influenced by zinc depletion (Fig. 8A). Densitometric values indicated ZnT1 protein decreased by 40%, whereas that for Zip10 increased >350% in mice fed the zinc-restricted diet, and confirmed that Zip8 was not responsive to the dietary zinc supply (Fig. 8B). The plasma zinc and intestinal MT-1 mRNA levels were reduced in all mice fed the zinc-deficient diet and served as positive controls for zinc-deficient conditions (Supplemental Fig. 1).
FIGURE 7 .
Effect of dietary zinc status on erythrocyte zinc transport. Mice were fed a zinc-deficient or control diet for 21 d. Zinc accumulation in RBC in vitro was calculated from 65Zn uptake measured by γ-ray spectrometry. Isolated RBC were incubated in DMEM containing 65ZnCl2 at 37°C. Zinc uptake reactions were stopped by storage in ice. Cells were washed with HEPES-buffered saline containing 10 mmol/L EDTA. 65Zn accumulation was expressed as pmol/mg protein. Data are means ± SD, n = 3. ***Different from zinc adequate, P < 0.001.
FIGURE 8 .
Effect of dietary zinc status on protein levels of RBC zinc transporters. Whole blood was collected from mice fed a zinc-deficient or control diet for 21 d. Erythrocyte membrane fractions were prepared as previously described. (A) Western analyses for ZnT1, Zip8, and Zip10 were conducted and (B) relative abundance of each transporter was determined by densitometric analysis. Data are expressed as mean ± SD, n = 3. **Different from zinc adequate, P < 0.01.
Discussion
Even though differential activities of the erythroid zinc trafficking system have been shown by certain conditions such as zinc deficiency or bicarbonate deprivation (9,10,12–14), no studies have defined the presence of zinc transporters in circulating RBC, with 1 exception. That occurred during the screening of 340 RBC membrane proteins identified by MS-based proteomics (32). ZnT1 was 1 of 47 transporter proteins detected in this analysis of mature human RBC. Consequently, with this dearth of information, the purpose of this study was to determine which transporters are expressed in mature RBC. Our strategy was to use a battery of 12 well-characterized antibodies to gain an initial understanding of which ZnT and Zip transporter proteins may function in the adult murine erythrocyte. Our finding that ZnT1, Zip8, and Zip10 are primarily localized to the plasma membrane fits with the proteomic analysis of RBC membrane proteins cited above (32). In addition, our localization is comparable to those of erythroid glucose transporters and endothelial-type nitric oxide synthase to the plasma membrane of RBC reported by others (33,34).
A unique characteristic of circulating erythrocytes is their enucleated nature. Consequently, transporters that function in mature cells are remnants formed during preceding developmental stages, i.e. erythropoiesis. Splenocytes from PHZ-treated animals most accurately represent the physiological aspects of in vivo erythroid progenitor cell differentiation (33). EPO acts as a key factor for the initiation of further differentiation of late-stage erythroid progenitor cells into reticulocytes in vivo or in vitro (35,36). The actions of EPO in RBC protein production during terminal erythroid differentiation can be categorized into 2 general aspects: the introduction of synthesis of specific proteins and the enhancement of an ongoing production of proteins initiated at a developmental stage prior to terminal erythroid differentiation (37). Our results indicate that basal levels of transporter mRNA expressed prior to the in vitro EPO induction could not be sustained when further erythroid differentiation was discontinued. Therefore, zinc transporter protein abundance in mature RBC is the result of EPO-mediated enhancement of transporter expression initiated prior to the terminal erythroid developmental stage. In comparison, the ALAS-2 mRNA levels in non-EPO–treated cells were sustained at the basal (0 h) level. The temporal increase of ALAS-2 expression induced by EPO during terminal erythroid differentiation was clearly evident in our experiments.
The hierarchical precedence of EPO-dependent expression of the 2 zinc importers, Zip8 and Zip10, to that of the exporter, ZnT1, is in agreement with the zinc expenditure trend during terminal erythroid differentiation. Thus, the early EPO responsiveness of Zip8 and Zip10 gene expression may be associated with an increased requirement of zinc supply based on metabolic use during these events. However, after these cells reach the very late stage of terminal erythropoiesis, metabolic needs of zinc decrease and, additionally, free zinc ions can adversely affect heme biosynthesis by interfering with incorporation of ferrous iron into protoporphyrin (22,38). Thus, the later EPO-dependent expression of ZnT1 could be a strategic mechanism for differentiating progenitor cells to remove excessive free zinc ions and, consequently, ensure the normal hemoglobin biosynthesis at the final step of RBC maturation.
The temporal trends in EPO-dependent expression of each transporter gene were confirmed in protein levels as well. Molecular masses of Zip10 in the erythroid progenitor cells corresponded to those determined in mature RBC. The migration of Zip8 during PAGE is in agreement with the findings of others (39) and was consistently observed at 60 kDa in both RBC and erythroid progenitor cells. However, the presence of a larger band size of Zip8 (∼120 kDa) was also observed in the erythroid progenitor cells. Glycosylation of mouse Zip8 protein has been shown by previous studies (40,41). In addition, cell type- and stage-dependent glycosylation activities in differentiating cells have been reported (42,43). Thus, as we have shown here using the glycosidase, PNGaseF (Supplemental Fig. 2), the 120-kDa band of Zip8 is reduced, suggesting that it is likely a product of glycosylation activity that may cease upon the termination of erythropoiesis. Of relevance is that substantial glycosylation has been observed with aquaporin 9, the major glycerol transport protein in mouse erythrocytes (44). Even though the predicted molecular mass of ZnT1 calculated from the amino acid composition is 55 kDa, the estimated molecular mass of ZnT1 in RBC was ∼30 kDa. Compared with that found with total cell lysates of erythroid progenitors (∼30 kDa; data not shown), the molecular size of ZnT1 detected in the total cellular membrane fraction (∼60 kDa) is closer to the predicted size of ZnT1. Our previous experience with this antibody for ZnT1 detection in intestinal and liver cells produced apparent molecular masses of 42 and 36 kDa, respectively (28). Thus, the discrepancy in the aberrant migration of ZnT1 in this study might have been introduced by variations related to the preparation procedures of each protein sample and/or cell development and type.
The zinc responsiveness and bicarbonate dependency of the zinc transport across the erythrocyte membrane has been well-characterized through various animal and human studies (9,10,12–15). However, the molecular mechanisms of these phenomena have not been clarified. Even though the enucleated nature of mature RBC and the limitations in current biochemical methodologies for functional assays do not allow the direct assessment of a specific transporter function in these cells, the following evidence strongly implicates ZnT1, Zip8, and Zip10 involvement in zinc trafficking across the erythrocyte plasma membrane. Here, we have shown ZnT1 and Zip10 are regulated in a zinc-dependent manner but in opposite modes. Our results from the controlled dietary zinc intake study confirm the differential expression pattern of these transporters and correspond well to the 65Zn accumulation levels of RBC in vitro. Thus, we suggest that the modulated zinc uptake rate of RBC during zinc deficiency may be associated with upregulation of Zip10 and the downregulation of ZnT1 during preceding erythroid developmental stages. In a recent study, the stimulatory effects of bicarbonate on the Zip8 transport activity for either zinc or cadmium have been shown by Zip8-transfected MDCK cells (41). The observation of bicarbonate dependency of Zip8 activities suggests it is the transporter mediating the bicarbonate-dependent zinc uptake by erythrocytes (9,10).
In this report, we have identified EPO- and zinc-mediated mechanisms as modes of regulation for ZnT1 and Zip10 in erythroid cells. At the present time, we cannot determine whether these regulatory mechanisms are independent or work in concert during terminal erythroid differentiation. However, it is of interest that the DNA binding of metal-response element binding transcription factor 1 (MTF-1) has been associated with the regulatory mechanism of both of these transporter genes, albeit in an opposite manner (45,46). We also found that the temporal pattern of MTF-1 mRNA expression follows that of ZnT1 while opposing that of Zip10 mRNA abundance, particularly during the late stage of EPO-induced differentiation (data not shown). In addition, we have previously reported that the induction of bone marrow MT by zinc, which is also mediated by MTF-1, is dependent on stimulation of erythropoiesis (16). Although the effects of EPO on MTF-1 in erythroid progenitor cells need to be further explored, we suggest that EPO-dependent expression of MTF-1 may be involved in the regulation of Zip10 and ZnT1 expression during erythroid maturation and zinc deficiency.
Plasmodia, the protozoan responsible for malaria, accumulate large quantities of zinc while in the host RBC (47,48). The proliferative stage of this parasite occurs in host erythrocytes, which provide nutrients and metabolites (49). Our delineation of Zip8 and Zip10 as major transporters of zinc accumulation by RBC suggest they provide the route by which the malarial parasite acquires its large complement of zinc. Restriction of zinc acquisition via changes in transport may represent a therapeutic target (49). Also relevant to RBC-related diseases is that erythrocyte Zip10 upregulates during zinc deficiency as shown in our studies. Consequently, infectious disease concurrent with zinc deficiency may provide the pathogen additional availability of this essential nutrient. Such a scenario could have a negative impact on morbidity, particularly in the blood stage of malaria infection when the parasite is very metabolically active (44,50).
Overall, Zip8, Zip10, and ZnT1 have been identified as erythroid zinc transporters and EPO-mediated expression of these transporters was confirmed in differentiating erythroid progenitor cells. Furthermore, ZnT1 and Zip10 expression is responsive to dietary zinc restriction but with opposing modes of the response. Several suggestions for further approaches, particularly, with clinical perspectives can be derived from these results. Specifically, the differential expression of Zip10 and ZnT1 in RBC could be targets for the assessment of dietary zinc status and therapeutic actions against diseases involving erythrocytes.
Supplementary Material
Acknowledgments
We thank Shou-Mei Chang for suggestions of techniques for protein isolation and immunohistochemistry and Liang Guo for helpful discussions.
Supported by NIH grant DK 31127, by funds from the Boston Family Endowment (to R.J.C.), and by College of Agricultural and Life Sciences Alumni Awards (to M.-S.R. and L.A.L.).
Author disclosures: M.-S. Ryu, L. A. Lichten, J. P. Liuzzi, and R. J. Cousins, no conflicts of interest.
Supplemental Tables 1 and 2 and Supplemental Figures 1 and 2 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: ALAS-2, erythroid δ-aminolevulinic acid synthase; AMEM, α minimum essential medium; AP, affinity-purified IgG; EPO, erythropoietin; MT, metallothionein; MTF-1, metal-response element binding transcription factor 1; PHZ, phenylhydrazine; qPCR, quantitative real-time PCR; vol, volume.
References
- 1.Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutr. 2004;24:151–72. [DOI] [PubMed] [Google Scholar]
- 2.Eide DJ. Zinc transporters and the cellular trafficking of zinc. Biochim Biophys Acta. 2006;1763:711–22. [DOI] [PubMed] [Google Scholar]
- 3.Milne DB, Ralston NV, Wallwork JC. Zinc content of cellular components of blood: methods for cell separation and analysis evaluated. Clin Chem. 1985;31:65–9. [PubMed] [Google Scholar]
- 4.Ohno H, Doi R, Yamamura K, Yamashita K, Iizuka S, Taniguchi N. A study of zinc distribution in erythrocytes of normal humans. Blut. 1985;50:113–6. [DOI] [PubMed] [Google Scholar]
- 5.Robertson A, Morrison JN, Wood AM, Bremner I. Effects of iron deficiency on metallothionein-I concentrations in blood and tissues of rats. J Nutr. 1989;119:439–45. [DOI] [PubMed] [Google Scholar]
- 6.Grider A, Bailey LB, Cousins RJ. Erythrocyte metallothionein as an index of zinc status in humans. Proc Natl Acad Sci USA. 1990;87:1259–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King J, Cousins RJ. Zinc. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Modern nutrition in health and disease. 10th ed. Baltimore: Lippincott Williams and Wilkins; 2006. p. 271–85.
- 8.Horn NM, Thomas AL, Tompkins JD. The effect of histidine and cysteine on zinc influx into rat and human erythrocytes. J Physiol. 1995;489:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalfakakou V, Simons TJ. Anionic mechanisms of zinc uptake across the human red cell membrane. J Physiol. 1990;421:485–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savigni DL, Morgan EH. Transport mechanisms for iron and other transition metals in rat and rabbit erythroid cells. J Physiol. 1998;508:837–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simons TJ. Calcium-dependent zinc efflux in human red blood cells. J Membr Biol. 1991;123:73–82. [DOI] [PubMed] [Google Scholar]
- 12.De Kok J, Van Der SC, Veldhuizen M, Wolterbeek HT. The uptake of zinc by erythrocytes under near-physiological conditions. Biol Trace Elem Res. 1993;38:13–26. [DOI] [PubMed] [Google Scholar]
- 13.Naber TH, van den Hamer CJ, van den Broek WJ, van Tongeren JH. Zinc uptake by blood cells of rats in zinc deficiency and inflammation. Biol Trace Elem Res. 1992;35:137–52. [DOI] [PubMed] [Google Scholar]
- 14.Van Wouwe JP, Veldhuizen M, De Goeij JJ, Van den Hamer CJ. Laboratory assessment of early dietary, subclinical zinc deficiency: a model study on weaning rats. Pediatr Res. 1991;29:391–5. [DOI] [PubMed] [Google Scholar]
- 15.Chesters JK, Will M. The assessment of zinc status of an animal from the uptake of 65Zn by the cells of whole blood in vitro. Br J Nutr. 1978;39:297–306. [DOI] [PubMed] [Google Scholar]
- 16.Huber KL, Cousins RJ. Zinc metabolism and metallothionein expression in bone marrow during erythropoiesis. Am J Physiol. 1993;264:E770–5. [DOI] [PubMed] [Google Scholar]
- 17.Perkins A. Erythroid Kruppel like factor: from fishing expedition to gourmet meal. Int J Biochem Cell Biol. 1999;31:1175–92. [DOI] [PubMed] [Google Scholar]
- 18.Trainor CD, Ghirlando R, Simpson MA. GATA zinc finger interactions modulate DNA binding and transactivation. J Biol Chem. 2000;275:28157–66. [DOI] [PubMed] [Google Scholar]
- 19.Nishiyama S, Irisa K, Matsubasa T, Higashi A, Matsuda I. Zinc status relates to hematological deficits in middle-aged women. J Am Coll Nutr. 1998;17:291–5. [DOI] [PubMed] [Google Scholar]
- 20.Forman WB, Sheehan D, Cappelli S, Coffman B. Zinc abuse: an unsuspected cause of sideroblastic anemia. West J Med. 1990;152:190–2. [PMC free article] [PubMed] [Google Scholar]
- 21.Fiske DN, McCoy HE III, Kitchens CS. Zinc-induced sideroblastic anemia: report of a case, review of the literature, and description of the hematologic syndrome. Am J Hematol. 1994;46:147–50. [DOI] [PubMed] [Google Scholar]
- 22.Bloomer JR, Reuter RJ, Morton KO, Wehner JM. Enzymatic formation of zinc-protoporphyrin by rat liver and its potential effect on hepatic heme metabolism. Gastroenterology. 1983;85:663–8. [PubMed] [Google Scholar]
- 23.Moore JB, Blanchard RK, McCormack WT, Cousins RJ. cDNA array analysis identifies thymic LCK as upregulated in moderate murine zinc deficiency before T-lymphocyte population changes. J Nutr. 2001;131:3189–96. [DOI] [PubMed] [Google Scholar]
- 24.Moore JB, Blanchard RK, Cousins RJ. Dietary zinc modulates gene expression in murine thymus: results from a comprehensive differential display screening. Proc Natl Acad Sci USA. 2003;100:3883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liuzzi JP, Bobo JA, Lichten LA, Samuelson DA, Cousins RJ. Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis. Proc Natl Acad Sci USA. 2004;101:14355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, Ganz T, Cousins RJ. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci USA. 2005;102:6843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kakeda M, Kyuno J, Kato T, Nishikawa M, Asashima M. Role of the thrombopoietin (TPO)/Mpl system: c-Mpl-like molecule/TPO signaling enhances early hematopoiesis in Xenopus laevis. Dev Growth Differ. 2002;44:63–75. [DOI] [PubMed] [Google Scholar]
- 28.McMahon RJ, Cousins RJ. Regulation of the zinc transporter ZnT-1 by dietary zinc. Proc Natl Acad Sci USA. 1998;95:4841–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liuzzi JP, Bobo JA, Cui L, McMahon RJ, Cousins RJ. Zinc transporters 1, 2 and 4 are differentially expressed and localized in rats during pregnancy and lactation. J Nutr. 2003;133:342–51. [DOI] [PubMed] [Google Scholar]
- 30.Kaler P, Prasad R. Molecular cloning and functional characterization of novel zinc transporter rZip10 (Slc39a10) involved in zinc uptake across rat renal brush-border membrane. Am J Physiol Renal Physiol. 2007;292:F217–29. [DOI] [PubMed] [Google Scholar]
- 31.Hodges VM, Winter PC, Lappin TR. Erythroblasts from friend virus infected- and phenylhydrazine-treated mice accurately model erythroid differentiation. Br J Haematol. 1999;106:325–34. [DOI] [PubMed] [Google Scholar]
- 32.Pasini EM, Kirkegaard M, Mortensen P, Lutz HU, Thomas AW, Mann M. In-depth analysis of the membrane and cytosolic proteome of red blood cells. Blood. 2006;108:791–801. [DOI] [PubMed] [Google Scholar]
- 33.Concha II, Velásquez FV, Martínez JM, Angulo C, Droppelmann A, Reyes AM, Slebe JC, Vera JC, Golde DW. Human erythrocytes express GLUT5 and transport fructose. Blood. 1997;89:4190–5. [PubMed] [Google Scholar]
- 34.Kleinbongard P, Schulz R, Rassaf T, Lauer T, Dejam A, Jax T, Kumara I, Gharini P, Kabanova S, et al. Red blood cells express a functional endothelial nitric oxide synthase. Blood. 2006;107:2943–51. [DOI] [PubMed] [Google Scholar]
- 35.Krantz SB. Erythropoietin. Blood. 1991;77:419–34. [PubMed] [Google Scholar]
- 36.Kaushansky K. Lineage-specific hematopoietic growth factors. N Engl J Med. 2006;354:2034–45. [DOI] [PubMed] [Google Scholar]
- 37.Koury MJ, Bondurant MC, Mueller TJ. The role of erythropoietin in the production of principal erythrocyte proteins other than hemoglobin during terminal erythroid differentiation. J Cell Physiol. 1986;126:259–65. [DOI] [PubMed] [Google Scholar]
- 38.Welch JJ, Watts JA, Vakoc CR, Yao Y, Wang H, Hardison RC, Blobel GA, Chodosh LA, Weiss MJ. Global regulation of erythroid gene expression by transcription factor GATA-1. Blood. 2004;104:3136–47. [DOI] [PubMed] [Google Scholar]
- 39.Dalton TP, He L, Wang B, Miller ML, Jin L, Stringer KF, Chang X, Baxter CS, Nebert DW. Identification of mouse SLC39A8 as the transporter responsible for cadmium-induced toxicity in the testis. Proc Natl Acad Sci USA. 2005;102:3401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M, Nebert DW. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Mol Pharmacol. 2006;70:171–80. [DOI] [PubMed] [Google Scholar]
- 41.Liu Z, Li H, Soleimani M, Girijashanker K, Reed JM, He L, Dalton TP, Nebert DW. Cd2+ versus Zn2+ uptake by the ZIP8 HCO3–dependent symporter: kinetics, electrogenicity and trafficking. Biochem Biophys Res Commun. 2008;365:814–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carlsson SR, Sasaki H, Fukuda M. Structural variations of O-linked oligosaccharides present in leukosialin isolated from erythroid, myeloid, and T-lymphoid cell lines. J Biol Chem. 1986;261:12787–95. [PubMed] [Google Scholar]
- 43.Bettaieb A, Farace F, Mitjavila MT, Mishal Z, Dokhelar MC, Tursz T, Breton-Gorius J, Vainchenker W, Kieffer N. Use of a monoclonal antibody (GA3) to demonstrate lineage restricted O-glycosylation on leukosialin during terminal erythroid differentiation. Blood. 1988;71:1226–33. [PubMed] [Google Scholar]
- 44.Liu Y, Promeneur D, Rojek A, Kumar N, Frøkiaer J, Nielsen S, King LS, Agre P, Carbrey JM. Aquaporin 9 is the major pathway for glycerol uptake by mouse erythrocytes, with implications for malarial virulence. Proc Natl Acad Sci USA. 2007;104:12560–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langmade SJ, Ravindra R, Daniels PJ, Andrews GK. The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. J Biol Chem. 2000;275:34803–9. [DOI] [PubMed] [Google Scholar]
- 46.Wimmer U, Wang Y, Georgiev O, Schaffner W. Two major branches of anti-cadmium defense in the mouse: MTF-1/metallothioneins and glutathione. Nucleic Acids Res. 2005;33:5715–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ginsburg H, Gorodetsky R, Krugliak M. The status of zinc in malaria (Plasmodium falciparum) infected human red blood cells: stage dependent accumulation, compartmentation and effect of dipicolinate. Biochim Biophys Acta. 1986;886:337–44. [DOI] [PubMed] [Google Scholar]
- 48.Hiremath GS, Sullivan DJ Jr, Tripathi AK, Black RE, Sazawal S. Effect of Plasmodium falciparum parasitemia on erythrocyte zinc protoporphyrin. Clin Chem. 2006;52:778–9. [DOI] [PubMed] [Google Scholar]
- 49.Kirk K, Saliba KJ. Targeting nutrient uptake mechanisms in Plasmodium. Curr Drug Targets. 2007;8:75–88. [DOI] [PubMed] [Google Scholar]
- 50.Beitz E. Jammed traffic impedes parasite growth. Proc Natl Acad Sci USA. 2007;104:13855–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.