Abstract
Energy restriction (ER) without malnutrition extends lifespan in mice and postpones age-related changes in immunity. However, we have previously shown that aged (22 mo old) ER mice exhibit increased mortality, impaired viral clearance, and reduced natural killer (NK) cell cytotoxicity following influenza infection compared with aged mice that consumed food ad libitum (AL). To determine whether the detrimental effects of ER in response to influenza infection occur independently of advanced age, young adult (6 mo) male C57BL/6 mice consuming an AL or ER diet were infected with influenza A virus (H1N1, PR8). Young adult ER mice exhibited increased mortality (P < 0.05) and weight loss (P < 0.01) in response to infection. ER mice exhibited decreased total (P < 0.001) and NK1.1+ lymphocytes (P < 0.05) in lung and reduced influenza-induced NK cell cytotoxicity in both lung (P < 0.01) and spleen (P < 0.05). Importantly, the mRNA expression of interferon (IFN)α/β (P < 0.05) was also reduced in the lungs of ER mice in response to infection, and in vitro stimulation of NK cells from ER mice with type I IFN resulted in cytotoxicity comparable to that in NK cells from AL mice. In contrast, NK cell activation was enhanced in ER mice, determined as an increase in the percentage of NK cells expressing B220 (P < 0.001) and increased intracellular production of IFNγ (P < 0.01). These data describe an age-independent and detrimental effect of ER on the innate immune response to influenza infection and suggest that a decrease in NK cell number and alterations in the NK cell-activating environment may contribute to decreased innate immunity in ER mice.
Introduction
The study of aging in multiple species has revealed that dietary energy restriction (ER),5 also referred to as caloric restriction, is the only known intervention capable of extending maximal lifespan (1–4). Extension of both median and maximal lifespan in rodents by ER without malnutrition was first demonstrated by McCay et al. (5) in 1935. Since then, diets restricting energy by 30–70% have been shown to increase median and maximal lifespan by up to ∼65 and 50%, respectively, compared with mice consuming food ad libitum (AL) (6). ER has also been shown to reduce the incidence of spontaneous tumors and cancers in rodents, suggesting positive effects on immune function (7–9). ER is now generally acknowledged to delay the development of immunity, as well as to preserve various aspects of immune function with advanced age, including T cell proliferation, cytokine production, and natural killer (NK) cell and cytotoxic T lymphocyte activities (10–16).
Improvement in general indices of immune responsiveness prompted the examination of the effects of ER on age-related changes in the response to antigen-specific stimulation, such as influenza. Rita Effros and colleagues (7) demonstrated positive effects of ER on cell-mediated and antibody responses of aged mice to influenza vaccination, relative to aged AL mice. Importantly, live virus was given intraperitoneally, a protocol that induces immunization, and influenza-specific responses were assessed in the spleen. However, the effects of ER on age-related changes in the immune response to immunization may not necessarily reflect those seen during a primary virus infection, particularly at the site of infection, the lung. Thus, although the preponderance of evidence suggests that ER maintains immune function at an advanced age, the effect of ER on the immune response to a primary virus infection has not been adequately considered.
Our laboratory has previously observed an increase in the severity of influenza infection in aged ER mice following intranasal (i.n.) inoculation, which produces infection in the lung (17). Aged ER mice exhibited reduced influenza-induced NK cell cytotoxicity, as well as increased lung virus. However, because the study did not include young ER mice, it could not be determined whether ER alone or ER in combination with advanced age accounted for the inability to mount an effective innate immune response against influenza virus infection. Therefore, in the current study, young AL and ER mice were challenged i.n. with influenza virus to determine the effects of ER alone, independent of advanced age, on the innate immune response to influenza virus infection.
Materials and Methods
Mice and diets.
The protocol was approved by the Drexel University Institutional Animal Care and Use Committee. Specific pathogen-free young adult (6 mo) male C57BL/6 mice were purchased from the National Institute on Aging colony maintained by Charles River Laboratories. ER mice from the colony are weaned and fed an increasingly restricted diet beginning at age 14 wk and reaching 40% ER at age 17 wk, according to published protocols (18). Mice achieve energy balance within 30 d, comparable to ∼2.5 y in humans, such that 6-mo-old ER mice are weight stable (1). Mice were housed in micro-isolator cages in the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited barrier facility at Drexel University and acclimated for at least 1 wk before use, during which time mice were weighed daily to monitor energy balance. The AL mice consumed a mean of 4.3 g of an NIH-31 diet daily, providing a mean energy intake of 72.3 kJ (Table 1). The ER mice were fed 2.7 g of an NIH-31/NIA-fortified diet, providing 44.6 kJ. As a result, ER mice were maintained on a diet sufficient in micronutrients but restricted in total energy intake by ∼40%. As expected, AL mice weighed more than ER mice at baseline (means ± SEM, 23.8g ± 0.2 vs. 17.7g ± 0.3; n = 15 mice per group, t test; P < 0.0001).
TABLE 1.
Nutrient composition of animal diets1
| NIH-31 | NIH-31/NIA fortified | |
|---|---|---|
| Protein, g/kg | 184.20 | 187.40 |
| Fat, g/kg | 44.70 | 44.10 |
| Fiber, g/kg | 40.50 | 45.80 |
| Gross energy, kJ/g | 16.82 | 16.53 |
| Ash, g/kg | 66.40 | 65.10 |
| Nitrogen-free extract, g/kg | 559.10 | 550.40 |
| Amino acids, g/kg | ||
| Arginine | 10.60 | 11.00 |
| Methionine | 3.90 | 3.60 |
| Histidine | 4.10 | 4.20 |
| Leucine | 16.10 | 15.00 |
| Lysine | 9.50 | 9.60 |
| Tryptophan | 2.40 | 2.20 |
| Valine | 9.60 | 8.80 |
| Cystine | 2.80 | 2.60 |
| Isoleucine | 9.00 | 7.60 |
| Threonine | 7.10 | 7.10 |
| Phenylalanine | 9.20 | — |
| Tyrosine | 7.00 | — |
| Phenylalanine + tyrosine | — | 15.30 |
| Vitamins | ||
| Retinyl acetate, mg/kg | — | 13.93 |
| 3-hydroxy retinol, mg/kg | 10.57 | — |
| Cholecalciferol, μg/kg | 10.48 | 17.50 |
| dl-α-Tocopheryl acetate, mg/kg | 38.30 | 52.15 |
| Choline, mg/g | 1.96 | 2.60 |
| Niacin, mg/kg | 92.20 | 116.16 |
| Pantothenic acid, mg/kg | 39.50 | 55.07 |
| Pyridoxine, mg/kg | 10.20 | 13.16 |
| Riboflavin, mg/kg | 7.80 | 11.04 |
| Thiamine, mg/kg | 77.30 | 123.44 |
| Menadione, mg/kg | 22.00 | 111.01 |
| Folic acid, mg/kg | 1.70 | 2.13 |
| Biotin, mg/kg | 0.13 | 0.38 |
| Cobalamin, μg/kg | 53.00 | 93.80 |
| Minerals | ||
| Calcium, g/kg | 10.60 | 10.30 |
| Phosphorus, g/kg | 9.20 | 9.30 |
| Sodium, g/kg | 2.60 | 3.00 |
| Chlorine, g/kg | 4.20 | 4.80 |
| Potassium, g/kg | 5.90 | 5.90 |
| Magnesium, g/kg | 2.00 | 2.00 |
| Iron, mg/kg | 300.20 | 336.41 |
| Manganese, mg/kg | 152.80 | 156.01 |
| Zinc, mg/kg | 50.40 | 48.41 |
| Copper, mg/kg | 13.20 | 13.28 |
| Iodine, mg/kg | 1.94 | 2.01 |
| Cobalt, mg/kg | 0.53 | 0.53 |
| Selenium, mg/kg | — | 0.30 |
Provided by the National Institute on Aging and Harlan Teklad. Ingredients: ground wheat, ground corn, ground oats, wheat middlings, fish meal, soybean meal, corn gluten meal, dehydrated alfalfa meal, soybean oil, dicalcium phosphate, brewers dried yeast, salt, calcium carbonate, choline chloride, menadione sodium bisulfite complex, thiamine mononitrate, calcium pantothenate, tocopheryl acetate, retinyl acetate, riboflavin, Cobalamin supplement, niacin, cholecalciferol supplement, pyridoxine HCl, folic acid, biotin, magnesium oxide, ferrous sulfate, manganous oxide, copper sulfate, zinc oxide, calcium iodate, and cobalt carbonate.
Influenza infection.
Mouse-adapted influenza A/Puerto Rico/8/34 (H1N1, PR8) was propagated in specific pathogen-free eggs (B&E Eggs) and cell-free supernatants were stored at −36°C until use. At baseline (d 0), mice were anesthetized by intraperitoneal injection with Avertin (2,2,2-tribromoethanol, Sigma) and infected i.n. with 104× the 50% tissue culture infectious dose (TCID50), also calculated as 100 hemagglutinating units, of PR8 in saline. All mice were weighed daily to monitor their ability to control infection.
Lymphocyte isolation.
The isolation of mononuclear cells from spleens and lungs has been described in detail (19). A lobe of each lung was frozen in TRI Reagent (Molecular Research Center) and saved for RNA extraction. Following Collagenase A (Roche) digestion of lung tissue, supernatants were aliquoted and stored at −36°C for analyses of lung virus titers and cytokine production. Cell suspensions from spleens and lungs were layered on Histopaque-1083 (Sigma) for density gradient centrifugation and cells from each tissue were resuspended at appropriate concentrations for either NK cell cytotoxicity assay or antibody staining for analysis by flow cytometry.
NK cell cytotoxicity.
A standard 51Cr-release assay with YAC-1 target cells was employed to assess NK cytotoxicity (19,20). Briefly, YAC-1 cells were incubated with Na51CrO4 (PerkinElmer) and plated in triplicate with lung and spleen cell preparations from uninfected and infected mice at an effector:target (E:T) ratio of 50:1. Following a 4-h incubation at 37°C with 5% CO2, supernatants were harvested onto UniFilter microplates (PerkinElmer) and radioactivity was quantitated by a γ-counter (Packard TopCount) and reported as counts per minute (CPM). Spontaneous release was determined in medium alone and maximum release in 5% Triton X-100 (Sigma). Spontaneous release was <10% of maximum release. Percent killing was then calculated as follows:
 |
Alternatively, to determine the effects of in vitro stimulation with type I interferon (IFN), cells were obtained from uninfected AL and ER mice and precultured with 104 units of type I IFN per 106 cells for 4 h prior to culture with target cells at the specified E:T ratio.
Flow cytometry.
Cells from lungs were resuspended in PBS containing various combinations of the following fluorochrome-conjugated antibodies (eBioscience) at concentrations ranging from 1:100 to 1:300: CD4 (fluorescein isothiocyanate), CD8 (PE-Cy5 or APC), NK1.1 (PE or PE-Cy7), CD11b (fluorescein isothiocyanate), B220 (APC), and IFNγ (APC). Cells were incubated in staining cocktails on ice in the dark for 30 min. Intracellular staining was performed after surface staining utilizing reagents provided in the Fixation and Permeabilization kit (eBioscience). Briefly, cells were resuspended in fixation buffer (containing 4% paraformaldehyde), incubated for 20 min on ice, and washed in 1× permeabilization buffer (containing 0.1% saponin and 0.09% sodium azide). Cells were incubated in the appropriate intracellular staining cocktails on ice in the dark for 30 min. Samples were then acquired on a FACSCanto flow cytometer (Becton Dickinson) and analyzed using FlowJo software (Tree Star).
Lung virus titer by Madin-Darby canine kidney assay.
Supernatants from lung homogenates were serially diluted and used to infect Madin-Darby canine kidney cells, as previously described (19). Virus titers were then determined based on the presence or absence of hemagglutination of chicken RBC (B&E Eggs) and reported as TCID50.
Cytokine analysis by real-time quantitative PCR.
Changes in the expression of IFNα/β and interleukin (IL)-12 (p40) due to infection were determined using real-time quantitative PCR (RT-qPCR) (21). Briefly, RNA was isolated from harvested lung tissues using a QIAamp viral RNA kit and reverse transcribed using an Omniscript RT kit, according to the manufacturer's instructions (Qiagen). RT reactions were primed with 1 μmol/L random hexamers and incubated at 42°C for 60 min, heated at 95°C for 5 min, and cooled to 4°C in a 2720 Thermal Cycler (Applied Biosystems). The PCR mixtures contained 2 μL of cDNA, 1× Taqman Universal Master mix (Applied Biosystems), 900 nmol/L of each primer, and 100 nmol/L of probe, and brought to a final volume of 25 μL in nuclease-free water (Gibco). Forward and reverse primers and probe, tagged with (6-carboxyfluorescencein) reporter dye on the 5′ end and (6-carboxytetramethylrhodamine) quencher dye on the 3′ end, were purchased from IDT. Because IFNα contains >20 isoforms, primer and probe sequences were chosen based on a published consensus (21). The levels of mRNA for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were also determined for each sample and were used to normalize gene expression during influenza infection. Primers and probe sequences were as follows: IFNα, forward primer: 5′ TGC AAC CCT CCT AGA CTC ATT CT 3′, IFNα, reverse primer: 5′ CCA GCA GGG CGT CTT CCT 3′, IFNα, probe: 5′ CTG CAT CAG ACA GCC TTG CAG GTC ATT 3′; IFNβ, forward primer: 5′ TGA ATG GAA AGA TCA ACC TCA CCT A 3′, IFNβ, reverse primer: 5′ CTC TTC TGC ATC TTC TCC GTC A 3′, IFNβ, probe: 5′ AGG GCG GAC TTC AAG ATC CCT ATG GA 3′; IL-12 (p40), forward primer: 5′ AGC TAA CCA TCT CCT GGT TTG C 3′, IL-12 (p40), reverse primer: 5′ CCA CCT CTA CAA CAT AAA CGT CTT TC 3′, IL-12, (p40), probe: 5′ TGC TGG TGT CTC CAC TCA TGG CCA 3′; GAPDH, forward primer: 5′ GCA GTG GCA AAA GTG GAG ATT G 3′, GAPDH, reverse primer: 5′ CCA TTC TCG GCC TTA CTG T 3′, GAPDH, probe: 5′ TGA CTC CAC TCA CGG CAA ATT CAA CG 3′.
Data were expressed in each group as a fold induction relative to d 0, calculated as follows, where CT refers to the threshold count:
 |
Cytokine analysis by ELISA.
Cytokines, including IL-12 (p40) and IFNγ in lungs, were quantitated by ELISA according to the manufacturer's protocols (eBioscience). The concentration of each cytokine was determined using a standard curve.
Lung pathology.
Pathology was assessed in the same lungs obtained for the assessment of virus titer. A portion of each lung was removed, formalin fixed, and paraffin embedded. Serial sections were cut to a 4-μm thickness and mounted onto glass slides. Tissues were then stained with hematoxylin (Harleco), rinsed, and counterstained with eosin-Y (1% alcoholic; Harleco). Pathology was assessed using a semiquantitative scale from 0 (no pathology) to 4 (100% pathology), as previously described (22,23), and as cellular infiltration using Image J software (Sun Microsystems). A minimum of 3 slides were prepared from each lung tissue and the slides were analyzed without knowledge of the treatments in triplicate. All scores were combined for final analysis.
Statistics.
Survival data were analyzed using the Kaplan-Meier test with censoring using Prism 5 software (GraphPad). All other statistical analyses were performed using SigmaStat (Systat). Values in the text are means ± SEM and animal numbers are as indicated. We have previously found that these animal numbers (n = 15 for survival and n = 4–8 for other variables) are required to detect significant differences in young adult mice. Baseline body weights were analyzed by Student's t test, while the effects of time and diet on all other variables were determined between and within groups by 2-way ANOVA with Holm-Sidak pairwise multiple comparisons. Significance was accepted at P < 0.05.
Results
Decreased survival in young adult ER mice following influenza virus infection.
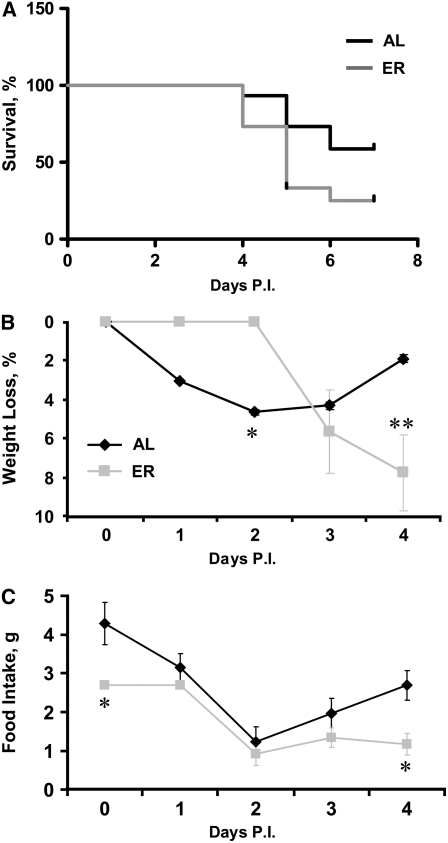
Young adult AL and ER mice were infected i.n. with 104 TCID50 (100 HAU) of PR8 and monitored for 7 d postinfection. ER mice exhibited increased mortality by d 7 (P < 0.05), with a median survival of 5 d (Fig. 1A).
FIGURE 1 .
Percent survival (A), weight loss (B), and food intake (C) in young adult AL and ER mice during influenza infection. Values are means, n = 15 (A) or 8 (B,C). In A, groups differ at d 7, P < 0.05. In B and C, asterisks indicate that the groups differ at that time: *P < 0.05, **P < 0.01.
Increased weight loss and anorexia in ER mice during influenza virus infection.
Following infection, AL mice began to lose body weight immediately and lost more weight as a percentage of baseline than age-matched ER mice at d 2 (Fig. 1B; P < 0.05). In contrast, ER mice maintained a constant body weight through d 2; however, by d 4, AL mice began to recover weight, whereas ER mice demonstrated increased weight loss as both a percentage (Fig. 1B; P < 0.01) and total amount lost (AL, 0.5 ± 0.1 g vs. ER, 1.3 ± 0.3 g; P < 0.05).
ER mice were fed a controlled, 40%-restricted diet at baseline (Fig. 1C; P < 0.05). Following infection, both AL and ER mice exhibited decreased food intake through d 2 (P < 0.01). The AL mice then began to recover, consistent with the recovery of body weight, whereas ER mice continued to consume ∼60% less food than they did at baseline. As a result, ER mice consumed less food than AL mice at d 4 (P < 0.05).
Increased lung virus in ER mice.
Virus was detected at d 1 in the lungs of ER mice, but not until d 2 in AL mice (Table 2). This early increase in lung virus was confirmed by RT-qPCR analysis of M1 expression, a conserved virus protein (data not shown). Lung virus titers were increased at d 3 in both AL and ER mice (P < 0.05) but did not differ between diet groups.
TABLE 2.
Lung virus titers in AL and ER mice at baseline and during influenza infection1
| d 0 | d 1 | d 2 | d 3 | d 4 | |||||
|---|---|---|---|---|---|---|---|---|---|
| TCID50/mL, log10 | |||||||||
| AL | ND2 | ND | 6.50 ± 0.5a | 8.34 ± 1.0b | 6.75 ± 0.6a | ||||
| ER | ND | 7.69 ± 0.2a | 8.31 ± 0.2ab | 9.16 ± 0.2b | 6.75 ± 0.6ab | ||||
Values are means ± SEM, n = 4. Means in a row with superscripts without a common letter differ, P < 0.05.
ND, Not detectable.
Increased lung pathology in ER mice.
Increased lung pathology is indicative of inflammation and increased mortality in influenza-infected mice (22,24). Cellular infiltration increased from baseline in the lungs of ER mice at d 3 (Table 3; P < 0.05). Analysis by flow cytometry indicated an increased percentage in high side-scatter, nonlymphocyte events in lungs from ER mice (52.8 ± 2.2%) compared with those of AL mice (27.2 ± 2.1%) at d 3 (P < 0.001). These events may represent a number of inflammatory cell types, such as neutrophils or macrophages. Indeed, the percentage and number of CD11b+ cells in lungs of ER mice increased over baseline at d 3 but not in those of AL mice (data not shown). AL and ER did not differ in the percentage or number of CD11b+ cells in the lungs at any time point (data not shown).
TABLE 3.
Lung pathology in AL and ER mice at baseline and during influenza infection1
| d 0 | d 3 | d 4 | ||||
|---|---|---|---|---|---|---|
| Lung pathology scores | ||||||
| AL | 0.4 ± 0.1 | 1.0 ± 0.4 | 0.8 ± 0.2 | |||
| ER | 0.8 ± 0.3 | 2.3 ± 0.4 | 2.2 ± 0.5 | |||
| Cellular infiltration, n | ||||||
| AL | 1048 ± 39 | 1164 ± 105 | 838 ± 110 | |||
| ER | 1057 ± 116a | 1535 ± 88b | 1160 ± 117ab | |||
Values are means ± SEM, n = 4. Means in a row with superscripts without a common letter differ, P < 0.05.
Impaired influenza-induced NK cell cytotoxicity in ER mice.
Influenza-induced NK cell cytotoxicity was elevated in young AL mice compared with ER mice at d 1 in lung (Table 4; P < 0.01) and at d 2 in spleen (P < 0.05). Percent killing did not significantly differ between groups at baseline.
TABLE 4.
Influenza-induced NK cell cytotoxicity in the lungs and spleens of AL and ER mice at baseline and during influenza infection1
| d 0 | d 1 | d 2 | d 3 | ||||
|---|---|---|---|---|---|---|---|
| Lung | % cytotoxicity | ||||||
| AL | 2.46 ± 1.75a | 12.71 ± 5.17b** | 2.74 ± 0.39a | 2.98 ± 0.20a | |||
| ER | 0.26 ± 0.22 | 0.75 ± 0.31 | 2.07 ± 0.31 | 0.39 ± 0.13 | |||
| Spleen | |||||||
| AL | 2.48 ± 0.48a | 2.69 ± 1.13a | 6.22 ± 1.01b* | 4.18 ± 0.18ab | |||
| ER | 1.17 ± 0.44 | 0.48 ± 0.26 | 1.78 ± 0.58 | 2.27 ± 0.60 | |||
Values are means ± SEM, n = 4. Means in a row with superscripts without a common letter differ, P < 0.05. Asterisks indicate difference between groups at that time: *P < 0.05, **P < 0.01.
Decreased total and NK1.1+ lymphocytes in the lungs and spleens of ER mice.
The percentage (Table 5; P < 0.001) and number (P < 0.01) of total lymphocytes, as well as the number of NK1.1+ lymphocytes (P < 0.05), were reduced in the lungs of ER mice compared with AL controls at baseline. At various time points during infection, ER mice had a lower percentage and number of total and NK1.1+ lymphocytes in lungs than did controls.
TABLE 5.
Percent and number of total and NK1.1+ lymphocytes in the lungs of AL and ER mice at baseline and during influenza infection1
| d 0 | d 1 | d 2 | d 3 | |||
|---|---|---|---|---|---|---|
| Total lymphocytes | % | |||||
| AL | 62.0 ± 2.7bc | 48.3 ± 3.3a | 53.9 ± 4.4ab | 65.3 ± 2.6c | ||
| ER | 36.1 ± 1.7*** | 39.7 ± 1.2 | 35.6 ± 2.1*** | 30.7 ± 1.5*** | ||
| NK1.1+ | ||||||
| AL | 11.5 ± 1.6 | 15.4 ± 1.1 | 12.7 ± 2.3 | 13.8 ± 3.0 | ||
| ER | 10.0 ± 1.3 | 6.5 ± 0.5* | 5.8 ± 0.7* | 4.7 ± 0.7* | ||
| Total lymphocytes | n x 10−4 | |||||
| AL | 38.6 ± 10.9 | 23.7 ± 5.5 | 18.6 ± 2.1 | 30.1 ± 9.5 | ||
| ER | 4.3 ± 2.0** | 19.6 ± 2.8 | 8.4 ± 2.4 | 10.0 ± 0.6* | ||
| NK1.1+ | ||||||
| AL | 4.6 ± 1.8 | 3.8 ± 1.1 | 2.5 ± 0.7 | 3.3 ± 0.3 | ||
| ER | 0.4 ± 0.1* | 1.3 ± 0.2* | 0.4 ± 0.1 | 0.5 ± 0.1* | ||
Values are means ± SEM, n = 4. Means in a row with superscripts without a common letter differ, P < 0.05. Asterisks indicate difference between groups at that time: *P < 0.05, **P < 0.01, ***P < 0.001.
Decreased IFNα/β expression in the lungs of ER mice.
At baseline, IFNα/β expression in the lungs did not differ between AL and ER mice. In contrast, however, the expressions of both IFNα (Fig. 2A; P < 0.01) and IFNβ (P < 0.05) were lower in the lungs of ER mice than in AL controls at d 2. IL-12 mRNA expression and protein production did not differ in the lungs of AL and ER mice (data not shown). Importantly, following in vitro stimulation with type I IFN, pulmonary and splenic NK cells from ER mice exhibited an intermediate induction in cytotoxicity compared with unstimulated cells and NK cells from AL controls (Fig. 2B).
FIGURE 2 .
IFNα (upper panel) and IFNβ (lower panel) expression (A) in the lungs of AL and ER mice during influenza infection. Type I IFN-induced NK cell cytotoxicity at specified in vitro E:T ratios (B). Values are means ± SEM, n = 4. Asterisks indicate different from ER at that time: *P < 0.05, **P < 0.01.
Increased percentage of NK1.1+ cells expressing B220 and producing IFNγ in the lungs of ER mice.
The percentage of NK1.1+ lymphocytes expressing B220 (CD45R) increased in ER mice in response to infection and compared with AL controls at d 3 (Table 6; P < 0.001). Similarly, the mean fluorescence intensity of B220 on the NK cell surface, which provides additional information regarding the number of surface antigens expressed per NK cell, also increased in ER mice in response to infection and compared with AL controls at d 2 and d 3 (data not shown).
TABLE 6.
Percent of activated NK1.1+ lymphocytes in the lungs of AL and ER mice at baseline and during influenza infection1
| d 0 | d 1 | d 2 | d 3 | d 4 | ||||
|---|---|---|---|---|---|---|---|---|
| B220+ (surface) | % | |||||||
| AL | 4.7 ± 0.5 | 4.5 ± 0.1 | 7.3 ± 2.6 | 7.7 ± 2.9 | 6.8 ± 2.1 | |||
| ER | 3.2 ± 0.5a | 10.3 ± 3.3a | 19.6 ± 2.2b | 23.8 ± 2.6b*** | 11.2 ± 3.1a | |||
| IFNγ+ (intracellular) | ||||||||
| AL | 0.5 ± 0.2 | 5.7 ± 2.8 | 3.5 ± 2.0 | 2.8 ± 1.2 | 1.9 ± 1.2 | |||
| ER | 1.0 ± 0.5a | 7.6 ± 2.5a | 14.2 ± 1.2b** | 25.1 ± 3.3c*** | 23.7 ± 0.3c*** | |||
Values are means ± SEM, n = 4. Means in a row with superscripts without a common letter differ, P < 0.05. Asterisks indicate difference between groups at that time: **P < 0.01, ***P < 0.001.
The percentage of NK1.1+ cells producing IFNγ in the lungs of ER mice was elevated compared with AL mice from d 2 to 4 (Table 6; P < 0.01). Further, ER mice exhibited an increase in the percentage of NK cells producing IFNγ that was 20- to 25-fold of baseline. In contrast, AL mice demonstrated an induction of IFNγ production by NK cells in the lungs at d 1 that was 10-fold of d 0. We have consistently observed this approximate increase in the percentage of NK1.1+ lymphocytes producing IFNγ in the lungs of AL mice in response to infection with this dose of influenza virus. The increased production of IFNγ by ER mice was confirmed in lung homogenates by ELISA (data not shown). The number of NK1.1+ cells expressing B220 and producing IFNγ did not differ between AL and ER mice at any time (data not shown).
Discussion
The purpose of this study was to determine the effects of ER, independent of advanced age, on the innate immune response to primary influenza infection. Young adult ER mice exhibited increased mortality, weight loss, lung virus titers, and lung pathology in response to influenza infection, as well as an inability to mount an effective influenza virus-induced NK cell cytotoxic response. These data clearly demonstrate an age-independent defect in NK cell function and an increase in the severity of influenza infection in young adult ER mice compared with age-matched AL controls.
Additional information on the effects of ER on NK cell cytotoxicity is limited, but an early study demonstrated lower basal NK cell cytotoxicity and higher PolyI:C-stimulated NK cell cytotoxicity in splenocytes from aged ER mice compared with aged AL mice (16). The effects of ER on NK cell cytotoxicity, however, have not been studied in young mice or in response to infection. Our data demonstrate a defect in influenza-induced NK cell cytotoxicity in young adult ER mice that is associated with a more severe infection.
The assay to determine NK cell cytotoxicity utilizes a fixed ratio of effector cells from a mixed lymphocyte sample to YAC-1 target cells, so a decrease in influenza-induced NK cell cytotoxicity can reflect either a decrease in activity or a relative decrease in the percentage or total number of NK cells in the sample. Therefore, it is important to consider NK cell cytotoxicity during an infection in the context of changes in the percentage and number of NK cells within the lymphocyte population of the lungs. Whereas findings in nonhuman primates have been contradictory to date, studies in aged ER rodents have shown a clear decrease in the total number of lymphocytes in spleens but no change in total circulating peripheral blood mononuclear cells (12). To our knowledge, total lymphocytes in young adult ER mice have not been examined compared with young adult AL mice, nor have differences in the pulmonary lymphocyte compartment been considered in response to infection. We observed a consistent decrease in total lymphocytes in the lungs and spleens of young adult ER mice, both at baseline and in response to infection. The percentage of NK cells did not differ in the lungs of AL and ER mice at baseline; however, with a decrease in total lymphocytes, the absolute number of NK cells in the lungs of ER mice was lower than that of AL at baseline. Further, both the percentage and number of NK cells in the lungs of ER mice were lower compared with AL mice during infection. Importantly, lytic efficiency, calculated as the number of target cells killed per NK cell in the in vitro assessment of cytotoxicity, did not differ between lungs from AL mice at d 1 and those from ER mice at d 2. These data suggest that the lack of influenza-induced NK cell cytotoxicity in ER mice may be explained in part by a relative decrease in the percentage and number of NK cells in the lungs of ER mice compared with AL controls. Therefore, it was important to consider other aspects of NK cell activation and function.
The endogenous production of IFNα/β is critically important to host defense to viruses, including influenza, as IFNα/β activates NK cells, induces an antiviral state, inhibits virus replication, and increases major histocompatibility complex class I expression and antigen presentation in all cells (25,26). Importantly, the induction of NK cell-mediated cytolysis by type I IFN has been demonstrated (27). Therefore, IFNα/β gene expression was assessed by RT-qPCR in the lungs of AL and ER mice during primary influenza infection. The expression of IFNα/β was reduced in the lungs of young ER mice at d 2. These data have clear implications for the ability of ER mice to mount an antiviral response to influenza infection in the lung, as well as to induce NK cell cytotoxicity. Following in vitro stimulation with type I IFN, pulmonary and splenic NK cells from ER mice exhibited an intermediate induction in cytotoxicity compared with NK cells from AL controls. Importantly, these data confirm that NK cells from ER mice maintain some ability to become activated and suggest that alterations in the NK cell-stimulating environment may contribute to the reduced NK cell cytotoxicity in ER mice.
In addition to type I IFN, IL-12 (NK stimulating factor) is involved in the induction of NK cell effector functions. The cytokine IL-12 is produced by macrophages and dendritic cells in response to virus infections and contributes to peak IFNγ production by NK cells, thus providing an important link to adaptive immunity (26,28,29). The in vitro production of IL-12 by DCs from young (8 wk old) ER mice was recently reported to be lower than IL-12 production by DCs from AL mice (30). However, in our study, the in vivo expression of IL-12 was maintained in ER mice during infection. Consistent with this observation, ER mice exhibited increased NK cell activation, determined as increased B220 surface expression, as well as a dramatic increase in the intracellular production of IFNγ. The surface expression of B220 (CD45R) is associated with IFNγ production and is a marker of nonmajor histocompatibility complex restricted killing (31,32).
In conclusion, ER increased the severity of influenza infection in young adult mice and induced differential effects on NK cell cytotoxicity and activation. Further, decreased NK cell number and IFNα/β expression in ER mice may contribute to this impaired cytotoxic defense. The observed increase in IFNγ production may represent a compensatory mechanism in which ER mice attempt to stimulate adaptive immunity after innate immunity has failed to control the infection. However, ER mice exhibited increased mortality, suggesting that compensatory events, if in place, may have been detrimental or insufficient to alter the course of the infection.
Acknowledgments
We thank Jeffrey Gerbino, Yuvraj Parmar, and Karna Sura for laboratory assistance.
Supported by NIH Academic Research Enhancement Award (R15), AG029637-01.
Author disclosures: B. W. Ritz, I. Aktan, S. Nogusa, and E. M. Gardner, no conflicts of interest.
Abbreviations used: AL, ad libitum; CPM, counts per minute; ER, energy restriction; E:T, effector:target ratio; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IFN, interferon; IL, interleukin; i.n., intranasal; NK, natural killer; RT-qPCR, real-time quantitative PCR; TCID50, 50% tissue culture infectious dose.
References
- 1.Speakman JR, Hambly C. Starving for life: what animal studies can and cannot tell us about the use of caloric restriction to prolong human lifespan. J Nutr. 2007;137:1078–86. [DOI] [PubMed] [Google Scholar]
- 2.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–22. [DOI] [PubMed] [Google Scholar]
- 3.Yamaza H, Chiba T, Higami Y, Shimokawa I. Lifespan extension by caloric restriction: an aspect of energy metabolism. Microsc Res Tech. 2002;59:325–30. [DOI] [PubMed] [Google Scholar]
- 4.Wanagat J, Allison DB, Weindruch R. Caloric intake and aging: mechanisms in rodents and a study in nonhuman primates. Toxicol Sci. 1999;52S:35–40. [DOI] [PubMed] [Google Scholar]
- 5.McCay CM, Cromwell MF, Maynard LA. The effect of retarded growth upon the length of the lifespan and ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 6.Barger JL, Walford RL, Weindruch R. The retardation of aging by caloric restriction: its significance in the transgenic era. Exp Gerontol. 2003;38:1343–51. [DOI] [PubMed] [Google Scholar]
- 7.Effros RB, Walford RL, Weindruch R, Mitcheltree C. Influences of dietary restriction on immunity to influenza in aged mice. J Gerontol. 1991;46:B142–7. [DOI] [PubMed] [Google Scholar]
- 8.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116:641–54. [DOI] [PubMed] [Google Scholar]
- 9.Weindruch R. Dietary restriction, tumors, and aging in rodents. J Gerontol. 1989;44:67–71. [DOI] [PubMed] [Google Scholar]
- 10.Ritz BW, Gardner EM. Malnutrition and energy restriction differentially affect viral immunity. J Nutr. 2006;136:1141–4. [DOI] [PubMed] [Google Scholar]
- 11.Messaoudi I, Warner J, Fischer M, Park B, Hill B, Mattison J, Lane MA, Roth GS, Ingram DK, et al. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc Natl Acad Sci USA. 2006;103:19448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikolich-Zugich J, Messaoudi I. Mice and flies and monkeys too: caloric restriction rejuvenates the aging immune system of non-human primates. Exp Gerontol. 2005;40:884–93. [DOI] [PubMed] [Google Scholar]
- 13.Pahlavani MA. Caloric restriction and immunosenescence: a current perspective. Front Biosci. 2000;5:D580–7. [DOI] [PubMed] [Google Scholar]
- 14.Weindruch R, Gottesman SRS, Walford RL. Modification of age-related immune decline in mice dietarily restricted from or after midadulthood. Proc Natl Acad Sci USA. 1982;79:898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkatraman J, Fernandes G. Modulation of age-related alterations in membrane composition and receptor-associated immune functions by food restriction in Fischer 344 rats. Mech Ageing Dev. 1992;63:27–44. [DOI] [PubMed] [Google Scholar]
- 16.Weindruch R, Devens BH, Raff HV, Walford RL. Influence of dietary restriction and aging on natural killer cell activity in mice. J Immunol. 1983;130:993–6. [PubMed] [Google Scholar]
- 17.Gardner EM. Caloric restriction decreases survival of aged mice in response to primary influenza infection. J Gerontol A Biol Sci Med Sci. 2005;60:688–94. [DOI] [PubMed] [Google Scholar]
- 18.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the biomarkers of aging program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–501. [DOI] [PubMed] [Google Scholar]
- 19.Ritz BW, Nogusa S, Ackerman EA, Gardner EM. Supplementation with active hexose correlated compound increases the innate immune response of young mice to primary influenza infection. J Nutr. 2006;136:2868–73. [DOI] [PubMed] [Google Scholar]
- 20.Plett PA, Gardner EM, Murasko DM. Age-related changes in interferon-α/β receptor expression, binding, and induction of apoptosis in natural killer cells from C57BL/6 mice. Mech Ageing Dev. 2000;118:129–44. [DOI] [PubMed] [Google Scholar]
- 21.Hunzeker J, Padgett DA, Sheridan PA, Dhabhar FS, Sheridan JF. Modulation of natural killer cell cytotoxicity by restraint stress during an influenza A/PR8 infection in mice. Brain Behav Immun. 2004;18:526–35. [DOI] [PubMed] [Google Scholar]
- 22.Smith AG, Sheridan PA, Harp JB, Beck MA. Diet-induced obese mice have increased mortality and altered immune responses when infected with influenza virus. J Nutr. 2007;137:1236–43. [DOI] [PubMed] [Google Scholar]
- 23.Nelson HK, Shi Q, Van Dael P, Schiffrin EJ, Blum S, Barclay D, Levander OA, Beck MA. Host nutritional selenium status as a driving force for influenza virus mutations. FASEB J. 2001;15:1846–8. [PubMed] [Google Scholar]
- 24.Bender BS, Taylor SF, Zander DS, Cottey R. Pulmonary immune response of young and aged mice after influenza challenge. J Lab Clin Med. 1995;126:169–77. [PubMed] [Google Scholar]
- 25.Garcia-Sastre A, Biron CA. Type 1 interferons and the virus-host defense: a lesson in détente. Science. 2006;312:879–82. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen KB, Salazar-Mather TP, Dalod MY, Van Deusen JB, Wei XQ, Liew FY, Caligiuri MA, Durbin JE, Biron CA. Coordinated and distinct roles for IFNalpha/beta, IL-12, and IL-15 regulation of NK cell response to viral infection. J Immunol. 2002;169:4279–87. [DOI] [PubMed] [Google Scholar]
- 27.Liang S, Wei H, Sun R, Tian Z. IFNα regulates NK cell cytotoxicity through STAT1 pathway. Cytokine. 2003;23:190–9. [DOI] [PubMed] [Google Scholar]
- 28.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. [DOI] [PubMed] [Google Scholar]
- 29.Cousens LP, Orange JS, Su HC, Biron CA. Interferon-alpha/beta inhibition of interleukin 12 and interferon-gamma production in vitro and endogenously during viral infection. Proc Natl Acad Sci USA. 1997;94:634–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niiya T, Akbar SM, Yoshida O, Miyake T, Matsuura B, Murakami H, Abe M, Hiasa Y, Onji M. Impaired dendritic cell function resulting from chronic undernutrition disrupts the antigen-specific immune response in mice. J Nutr. 2007;137:671–5. [DOI] [PubMed] [Google Scholar]
- 31.Kassim SH, Rajasagi NK, Zhao X, Chervenak R, Jennings SR. In vivo ablation of CD11c-positive dendritic cells increases susceptibility of herpes simplex virus type 1 infection and dimishes NK and T-cell responses. J Virol. 2006;80:3985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ballas ZK, Rasmussen W. Lymphokine-activated killer (LAK) cells. IV. Characterization of murine LAK effector subpopulations. J Immunol. 1990;144:386–95. [PubMed] [Google Scholar]