Abstract
Introduction:
Changes to cardiac matrix are central to ventricular remodelling after acute MI and matrix metalloproteinase expression is implicated in this process. We investigated the temporal profile of MMP-3 and its relationship to LV dysfunction and prognosis following AMI.
Methods:
We studied 382 patients with AMI. Plasma MMP-3 was measured at 0-12, 12-24 and 24hr periods after symptoms. LV function (LVEF) was assessed by echocardiography pre-discharge and at a median of 148days and clinical end-points at a median of 313days.
Results:
MMP-3 peaked prior to discharge thus pre-discharge levels were used in analyses. MMP-3 was associated with patient age (p<0.001), creatinine (p<0.001) was higher in males (p<0.001) and hypertensives (p<0.001). MMP-3 inversely correlation with LVEF at follow up (p=0.043), was higher in subjects with LVEF <40% (p=0.017) and in subjects with increasing EDV (p=0.017) or ESV (p=0.007) compared to those in whom volumes fell between visits. In the 58 patients reaching end point of death or heart failure MMP-3 was higher (p<0.001). On Kaplan-Meier analysis subjects with levels above optimum cut off identified via ROC curves were more likely to suffer a clinical event (p=0.037).
Conclusion:
MMP-3 is associated with left ventricular dysfunction, adverse left ventricular remodelling and prognosis after AMI.
Introduction
The cardiac extracellular matrix (ECM), the connective tissue scaffold on which cellular elements are arranged, plays a vital role in the maintenance of myocardial structure and function, particularly that of the left ventricle (LV). Physiological integrity of ECM structure is largely under the control of the matrix metalloproteinase MMP family of endopeptidases, activity of which maintain a balance between connective tissue synthesis and degradation. Altered MMP expression and activity under pathological conditions may lead to a situation favouring proteolysis. The result is adverse ventricular remodelling, leading to LV dilatation, loss of contractile function and progressive clinical heart failure. The severity of this process of LV remodelling is linked intimately to adverse prognosis 1,2.
The MMP's are zinc dependent endopeptidases with varying substrate specificity and the capacity to degrade many components of the cardiac ECM. Stromelysin-1 (MMP-3) is a 45 kDa protein with a wide range of connective tissue substrates, including gelatin types I, III, IV and V, collagen types III, IV, IX and X. In addition MMP-3 may regulate the activity of other MMP enzymes 3. Myocardial MMP-3 expression is up-regulated soon after experimental AMI in animals (Ducharme et al 2000; Romanic, Burns-Kurtis et al. 2001 ) and remains so for several days 4. In man circulating MMP-3 concentrations are higher 3 months, compared to 48 hours, after AMI (Samnegard A, et al 2006) and are influenced by MMP-3 gene promoter polymorphisms (Samnegard A, at al 2005). Taken together, these features implicate MMP-3 as a plausible candidate in adverse cardiac remodelling. However few studies to date have explored this area.
We (Squire, Evans et al. 2004; Kelly, Cockerill et al. 2007) and others (Webb, Bonnema et al. 2006) have demonstrated distinct profiles of circulating plasma MMP-2 and MMP-9 concentrations, and differing relationships with LV dysfunction, after AMI in man. 5-7No previous study has investigated the possible association of circulating MMP-3 with LV remodelling, or with prognosis, after AMI in man. The aim of this study was to investigate the temporal profile of circulating MMP-3 following AMI in man, and any relationships with LV dysfunction, remodelling and prognosis in this setting.
Methods
Study Population and design
We conducted a prospective, cohort study. We enrolled 382 patients with AMI admitted to the Coronary Care unit (CCU) of our hospital between 1st September 2004 and 28th February 2006. The diagnosis was based on symptoms consistent with AMI in conjunction with appropriate, dynamic ECG changes (ST segment elevation, STEMI, 81.2%) or ST segment / T wave changes (NSTEMI, 18.8%) and elevation in plasma markers of myocardial necrosis (creatine kinase or troponin I).
The pre-defined primary outcome measure was the composite of all-cause mortality or heart failure episode during follow-up (median 313, range 1-619, days). Heart failure episode was defined as an unplanned hospital admission for which the primary reason was clinical heart failure requiring high dose diuretic, intravenous nitrate or inotropic support. Secondary outcomes were echocardiographic measures of LV function, dimensions and remodelling. Clinical endpoints were identified through the hospital patient tracking system, with review of medical records for each recorded endpoint. At the end of the study further checks were made by telephone contact with all surviving patients.
Venous blood was sampled at 0-12hrs, 12-24hrs and subsequent 24hr periods during the index admission for the assay of plasma MMP-3 concentration. All patients underwent echocardiographic examination immediately prior to discharge and at a median of 148 (range 90-378) days after AMI. Venous blood was also obtained at follow up visit for further MMP-3 analysis. The local research ethics review committee approved the study and all patients gave written consent to participation. The conduct of the study was in keeping with the declaration of Helsinki.
Laboratory methods
Plasma MMP-3 was measured using a commercially available kit (R&D systems-Fluorokine multianalyte profiling, human MMP base kit. Cat No.LMP000). The lower limit of detection was 55.0 pg/ml. Interassay coefficient of variation was <10%. Presented MMP-3 concentrations are the mean of duplicate assays.
Echocardiographic assessment
Echocardiographic assessment was carried out immediately prior to discharge and at follow-up, by a single operator (DK) using a Sonos 5500 or IE33 scanner. Left ventricular end systolic volume (LVESV), LV end diastolic volume (LVEDV) and LV ejection fraction (LVEF) were estimated using the bi-planar modified Simpson's rule from apical 2 and 4 chamber views. Left ventricular wall motion index score (WMIS) was measured using a standard 16 segment model from parasternal long and short axis and apical 2- and 4-chamber views. Each LV segment is scored as 0-hyperkinetic, 1-normal, 2-hypokineic, 3-akinetic, 4-dyskinetic. The total divided by the number of segments analysed gives an overall score with higher values indicating more impaired LV function.
The degree of ventricular remodelling after AMI was assessed from the change in LVEDV (ΔEDV) and LVESV (ΔESV) between pre-discharge and follow-up examinations, expressed as a percentage of the pre-discharge measurement. When assessing remodelling, we considered data pertaining only to patients with adequate echocardiographic examinations both pre-discharge and at follow-up. Intra-observer variation, assessed in a subset of the cohort (N=45; Mean ± SD) was 0.36% ± 1.75 for WMIS, 5.2% ± 3.9 for EDV, 6.0% ± 6.6 for ESV and 6.7% ± 7.6 for LVEF.
Statistical Analysis
For all variables with non-Gaussian distribution (MMP-3, CK, TnI, WMIS), log-transformed values were used in analyses. Differences in MMP-3 between time periods was analysed using ANOVA followed by Tukey t-test where appropriate. The association of MMP-3 levels with categorical variables was assessed using paired t-test, or Mann-Whitney U test for non-normally distributed variables, and with continuous variables using Pearson correlation coefficient. Differences between groups experiencing or not experiencing each clinical endpoint were assessed using χ2 analysis for categorical variables and Mann-Whitney U-test for continuous variables. Optimal prognostic thresholds of MMP-3 for the prediction of death or heart failure were derived from the Receiver Operator Characteristic (ROC) curve. For all analyses, statistical significance was ascribed at p<0.05. All statistical analyses were carried out using SPSS version 12. The authors had full access to the data, accept responsibility for their validity, and have read and agreed to the manuscript as submitted
Results
Admission demographic features of the study population are shown in Table 1. Approximately 75% of the patients were male, ST-elevation was evident on the admission ECG in over 80%, and median creatine kinase was 1099 I.U. Three hundred and ten patients presented with STEMI, of whom 189 (61%) received thrombolytic therapy. No patient received primary percutaneous revascularisation. No patient was lost to follow-up over a median of 313 (range 1-619) days. For patients alive at the end of the study, minimum follow-up was 113 days with a range of 113-619.
Table 1.
Population demographics at admisssion
CK=Creatine Kinase; NR = Normal Range; ACE-I=Angiotensin Converting Enzyme Inhibitor
| Median | Range | |
|---|---|---|
| Age (yrs) | 64 | 24-91 |
| CK (I.U., NR 0-200) | 1099 | 41-7384 |
| Troponin I (NR <0.06) | 10.7 | 0.06-150 |
| Number (%) | ||
| Male | 284 (74.3) | |
| STEMI | 310 (81.2) | |
| Anterior Territory | 157 (41.1) | |
| Thrombolysis | 189 (49.5) | |
| Current Smoker | 140 (36.6) | |
| Diabetes | 63 (16.5) | |
| Hypertension | 157 (41.1) | |
| Previous MI | 38 (9.9) | |
| Previous Revascularisation | 5 (1.3) | |
| Medications | ||
| Admission | Discharge | |
| Aspirin | 77 (20.2) | 340 (89.0) |
| Clopidogrel | 14 (3.7) | 89 (23.3) |
| Beta Blocker | 78 (20.4) | 341 (89.3) |
| ACE-I/ARB | 87 (22.8) | 337 (88.2) |
| Statin | 78 (20.4) | 363 (95.0) |
| Furosemide | 39 (10.2) | 53 (13.90) |
Temporal Profile of MMP-3
Circulating MMP-3 concentration increased steadily between admission and discharge, with statistically significant differences between time periods (ANOVA, p<0.001)(Figure 1). Compared to 0-12 hours, plasma MMP-3 concentrations were higher at 24-48hrs (p=0.07), 48-72hrs (p=0.003) and 72-96hrs (p=0.005). In view of this temporal profile, we used pre-discharge levels in all further analyses. We also investigated the effects of the degree of changes (ΔMMP-3) between admission and discharge.
Figure 1.
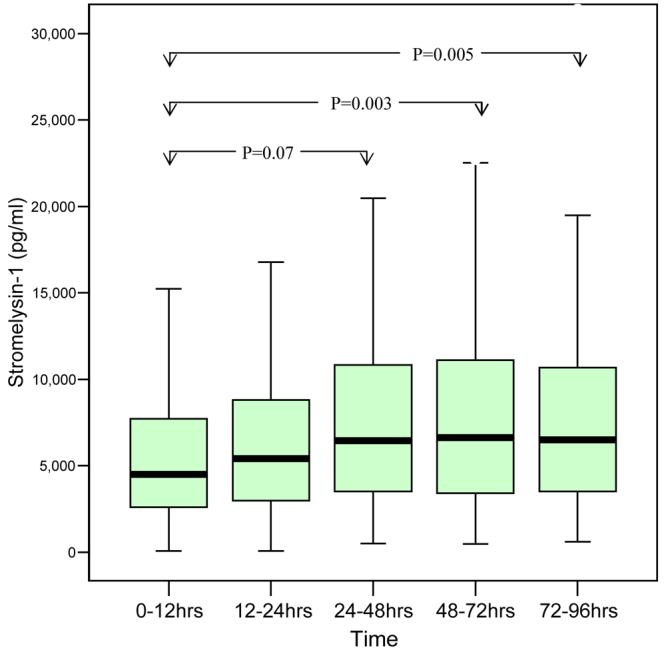
Temporal profile of Stromelysin-1 (MMP-3)
MMP-3 at follow up visit was reduced being significantly lower than pre-discharge levels (Median 3825.8 v 5787.8, p=0.003).
Predictors of plasma MMP-3
Pre-discharge plasma MMP-3 concentration was associated with patient age (r=0.201, p<0.001), and creatinine (r=0.238, p<0.001) and was higher in males compared to females (7581.1 v 4687.8pg/ml, p<0.001). There was a relatively weak but statistically significant, association with peripheral neutrophil count (r=0.155, p=0.003). MMP-3 was higher in subjects with a history of hypertension (8064.4 v 5752.5 pg/ml, p=0.001) and in subjects prescribed beta-blocker (8953.9 v 6130.2 pg/ml, p=0.002), or ACE-inhibitor/ARB (8071.6 v 6123.1 pg/ml, p=0.006) prior to admission. Plasma MMP-3 was similar between STEMI v NSTEMI (6590.1 v 7003.4 pg/ml, p=0.121) or between anterior versus inferior AMI (6938.6 v 6590.0 pg/ml, p=0.793). There was no association with either creatine kinase (r=0.056, p=0.297) or troponin I (r=0.065, p=0.253) concentration. On multi-variable analysis, plasma MMP-3 levels retained direct association with patient age (p=0.003), creatinine (p=0.03) and male sex (p=0.003).
Echocardiographic Assessment
There was no association between pre-discharge MMP-3 and pre-discharge echocardiographic markers of LV volume or function. However MMP-3 measured pre-discharge correlated weakly with greater LV volumes (EDV r=0.130, p=0.025; ESV r=0.144, p=0.013) and lower LVEF (r=−0.118, p=0.043) at follow-up. In subjects with echocardiographic evidence of LV impairment at follow-up (LVEF<40%), plasma MMP-3 was higher compared to those with preserved LV function (8071.0 v 6010.0 pg/ml, p=0.017) at this time. Plasma MMP-3 concentration correlated directly with the magnitude of the change in LV systolic volume (ΔESV r=0.123, p=0.041) between pre-discharge and follow-up echocardiographic assessment. Further to this, in subjects in whom either ΔEDV or ΔESV increased between discharge and follow up, MMP-3 was higher compared to those in whom these measurements showed a reduction (ΔEDV 7269.3 v 5821.8 pg/ml, p=0.017; ΔESV 7521.1 v 5539.4 pg/ml, p=0.007)(Figure 2).
Figure 2.
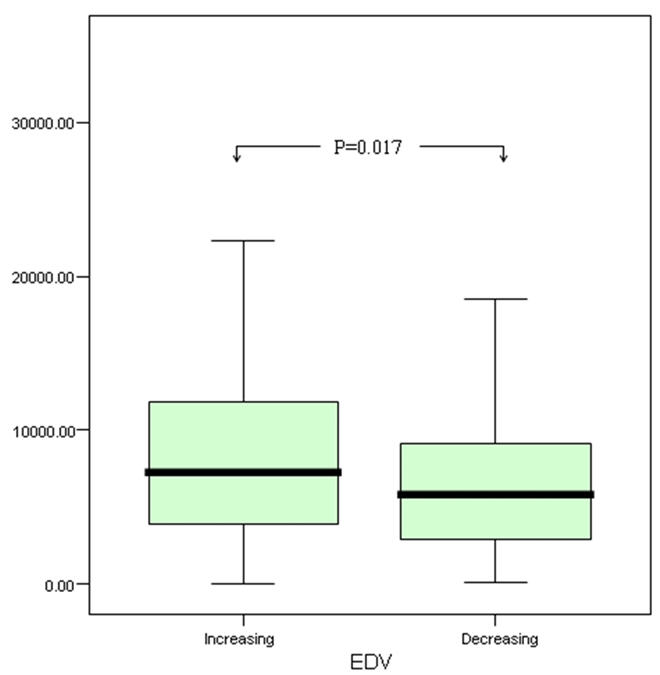
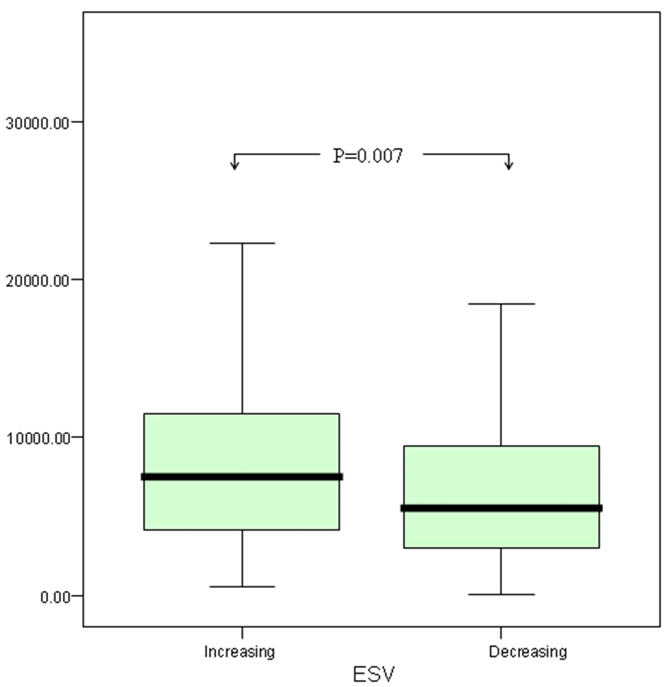
Comparison of Stromelysin-1 (MMP-3) levels between those with increasing versus decreasing EDV (above) and ESV (below)
The degree of change of MMP-3 (ΔMMP-3) was associated with the extent of LV impairment with direct correlation with WMIS (r= 0.174, p=0.002) and inverse correlation with LVEF (r= −0.179, p=0.002) prior to discharge and at follow up (r=0.138, p=0.023 & r=−0.196, p=0.001 respectively). There was no association between ΔMMP-3 and the degree of LV remodelling.
MMP-3 measured at follow up showed inverse correlation with LV volumes at that time (EDV, r= −0.238, p<0.001, ESV −0.153, p=0.014). There was no association with LV function or previous remodelling.
Clinical Endpoints
During follow up 33 (8.6 %) patients died and 46 (12.0 %) experienced a heart failure episode. Plasma MMP-3 was higher in the 58 patients (15.2 %) patients reaching the combined end point of death or heart failure episode, and was higher in patients reaching the individual components of the composite end-point (Table 2). There was no association of MMP-3 with the occurrence of reinfarction, which occurred in 42 (11.0 %) patients. In addition there was no association between our end-points and ΔMMP-3.
Table 2.
Stromelysin-1 (MMP-3) levels in relation to the occurrence of clinical endpoints.
| Endpoint | MMP-3 (pg/ml) median [range] | ||
|---|---|---|---|
| Event | No event | p-value | |
| Death/heart failure (N=58) | 9141.2 | 6230.2 | 0.001 |
| Death (N=33) | 9076.0 | 6550.1 | 0.033 |
| Heart failure (N=46) | 8068.0 | 6505.8 | 0.033 |
| Reinfarction (N=42) | 6941.6 | 6630.8 | 0.923 |
We used the ROC curve (Area under curve=0.636, p<0.001) to identify the cut off point for the optimum combination of sensitivity and specificity for MMP-3 (7371.5pg/ml) in the prediction of the primary endpoint. Kaplan-Meier survival curves indicated increased risk of death or heart failure (OR=1.816, 95% CI 1.03-3.2, p=0.037) for patients in whom MMP-3 was above 7371.5 pg/ml, with divergence continuing for approximately 100 days after AMI (Fig 3).
Figure. 3.
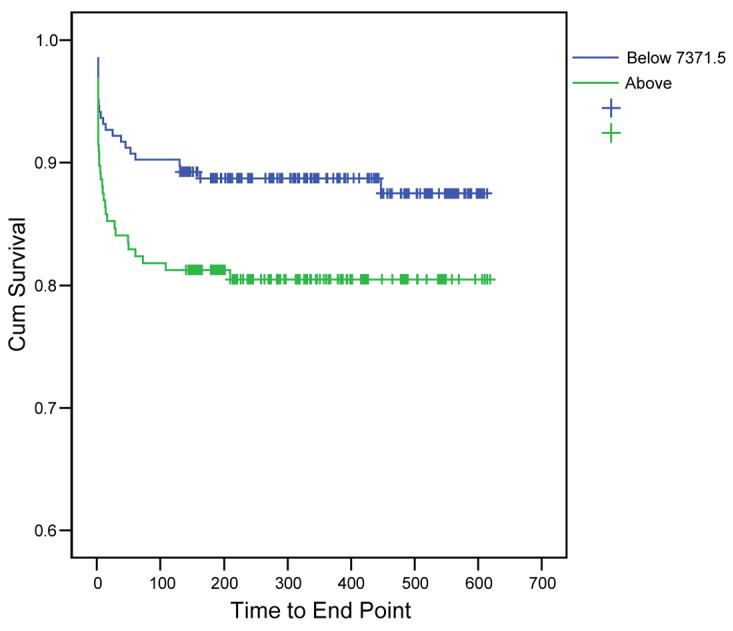
Kaplan- Meier Survival curves for the occurrence of primary outcome (death or heart failure) according to Stromelysin-1 (MMP-3) above versus below cut off of 7371pg/ml
Discussion
The present study is the first extensive examination human study of the relationship of MMP-3 with LV remodelling and prognosis after AMI. We report several novel observations of potential clinical relevance. First we observed steadily increasing plasma MMP-3 concentrations in the first few days after AMI. Second, circulating MMP-3 concentration prior to discharge showed direct association with LV function and volume measured in the subsequent follow up period. Moreover, we observed association between circulating MMP-3 and the degree of LV remodelling occurring after AMI, as assessed echocardiographically. Finally, and in keeping with the relationship with remodelling, we observed association between higher MMP-3 and adverse outcome post AMI.
Adverse LV remodelling is one of the most prognostically significant consequences of AMI 1,2. The observed association between plasma MMP-3 and adverse outcome in our study is supported by those between plasma MMP-3 and adverse LV remodelling. While the current report is the first to link circulating MMP-3 with remodelling and with outcome in man, experimental studies support our findings. Circulating MMP-3 concentrations are increased in animal models of ischaemic injury or hypoxia 4. In rats with progressive decompensated heart failure, MMP-3 is similarly upregulated 3, and there is an association between LV remodelling and MMP-3 in pigs with tachycardia induced cardiomyopathy 8. The wide substrate specificity of MMP-3 for elements of the cardiac ECM, and the possible role for MMP-3 in the regulation of activity of other MMP entities, also support a possible pathophysiological role for MMP-3 in the process of LV remodelling. In this context we observed consistent associations for higher circulating MMP3 in the days following AMI with a number of echocardiographic measures of adverse LV remodelling and dysfunction. In keeping with these observations, MMP-3 concentration was also linked to greater risk of death or heart failure, lending strong support to the possible involvement of MMP-3 in adverse LV remodelling after AMI. The apparent lack of correlation in our study between MMP-3 and markers of the extent of myocardial necrosis, namely CK and Troponin I, may support the assertion that MMP-3 is an indicator of the remodelling which follows infarction, rather than simply a marker of the infarction itself.
Our observations may be viewed in the context of previous reports considering MMP-3 in patients with impaired LV function. Higher MMP-3 has been reported in subjects with non-ischaemic compared to ischaemic cardiomyopathy 9. In addition the MMP-3 5A/6A polymorphism may have prognostic implications in subjects with non-ischaemic, but not ischaemic, cardiomyopathy 10. Finally, MMP-2 but not MMP-3 has been reported to predict prognosis in patients with congestive cardiac failure 11. A number of factors are likely to explain these apparently discrepant findings, such as methodological and cohort differences. One potentially important factor is the possibility of survivor bias in studies of CHF, in whom patients with low, prognostically favourable MMP concentrations may be over represented. None of this detracts from the internal consistency of our findings, which are reinforced by our observations regarding prognosis.
Experimental studies using broad spectrum MMP inhibition support a central role for the MMP enzymes in LV remodelling. Broad spectrum MMP inhibition in-vivo attenuates LV dilatation and dysfunction in a variety of animal models of experimental AMI 13 and chronic heart failure 15. Selective MMP inhibition also attenuates LV remodelling in experimental studies 16.
On this background, MMP inhibition has been postulated as a potential therapeutic intervention in patients with AMI. In the only clinical study to date of MMP inhibition as a potential therapeutic intervention, the PREMIER trial, the broad spectrum inhibitor PG-116800 failed to attenuate adverse LV remodelling after AMI17. In this trial, PG-116800 was administered for 90 days with the first dose given on average >48 hours after the onset of AMI. We suggest that elucidation of the temporal profile of plasma profiles of individual MMP enzymes, and their relationship with LV remodelling and outcome, may guide and assist the development of therapeutic MMP manipulation. We previously described distinct temporal profiles of circulating MMP-2 and MMP-9 following AMI in man 7. While circulating MMP-2 showed little change over the first few days after AMI, plasma concentrations of MMP-9 were highest within 12 hours of the event, falling to a lower, pre-discharge plateau thereafter. Higher MMP-9 concentrations within 12 hours of the index AMI showed association with adverse remodelling and outcome 7. Moreover, in contrast to the adverse prognosis of higher MMP-9 in this early period after AMI, higher pre-discharge levels of MMP-9 were associated with preservation of LV function and lesser adverse remodelling 7.
The current report adds information regarding MMP-3 to these findings. We observed a temporal profile of plasma MMP-3 different to that of either MMP-2 or MMP-9 after AMI 7. Moreover, the current report presents data indicating a link with adverse LV remodelling and outcome for higher circulating MMP-3 levels in the pre-discharge period, in contrast to the observed findings with MMP-9 7. The demonstration of unique temporal profiles of a variety of MMP enzymes, together with individual patterns of association with measures of LV remodelling and prognosis, suggest that manipulation of specific MMP activity may therefore have a temporal window in which beneficial effects may be observed. Our interesting observation between MMP-3 measured at follow up and an inverse correlation with LV volumes at that time may again add additional weight to this argument.
A number of additional observations from the current study are worthy of comment. We observed higher MMP-3 concentrations in patients with a history of hypertension and in subjects prescribed anti-hypertensive therapy. Such findings may be related to prior LV remodelling associated with left ventricular hypertrophy (LVH). Indeed higher plasma MMP-3 has been described in subjects with LVH secondary to hypertension 18 and in subjects with aortic stenosis, again potentially secondary to LVH 19. Gene expression of MMP-3 falls after surgical correction of aortic stenosis with associated regression of LVH 20.
While these observations suggest pre-existing LV remodelling may contribute to higher MMP-3 concentrations, this is unlikely to be the sole, or even the main, explanation for differences in MMP-3 in our study. The observed correlation with circulating neutrophil counts suggests that MMP-3 is released as part of the inflammatory response to AMI. In this context, our study lacks information regarding any possible relationship between MMP-3 and more sensitive markers of the inflammatory response such as CRP. Moreover, MMP-3 concentration predicted the degree of LV dysfunction and remodelling after discharge, rather than during the early period after AMI.
Study Limitations
Our study has several limitations. The results of our single centre study should be confirmed or refuted in other populations. However our population was unselected, representative of that seen in routine practice, and managed according to contemporary guidelines. Differences in drug therapy may have influenced our observations. However drug treatment after admission was relatively uniform, the vast majority of patients receiving anti-platelet therapy, beta-blocker, renin-angiotensin system inhibition and lipid-lowering therapy.
As discussed above, prior events may have influenced the observed MMP-3 profile. A proportion of patients died prior to, failed to attend for, follow-up echocardiographic assessment. However with regard to LV remodelling, we considered echocardiographic parameters only for those patients with both pre-discharge and follow-up examinations. Moreover, as patients with the most extensive, early remodelling are likely to be among non-survivors, our data are likely to underestimate the strength of the relationship between MMP-3 and remodelling. We recognise that, while statistically significant, the associations between MMP-3 and parameters of LV function, volume and remodelling were in some cases relatively weak.
In summary we have shown a relationship between the degree of MMP-3 release and subsequent LV dysfunction and remodelling following AMI in man. Circulating MMP-3 concentrations in the immediate post MI period are linked with adverse prognosis. MMP-3 may be a potential prognostic marker or therapeutic target after AMI.
References
- 1.White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 2.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–72. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 3.Mori S, Gibson G, McTiernan CF. Differential expression of MMPs and TIMPs in moderate and severe heart failure in a transgenic model. J Card Fail. 2006;12:314–25. doi: 10.1016/j.cardfail.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Romanic AM, Burns-Kurtis CL, Gout B, Berrebi-Bertrand I, Ohlstein EH. Matrix metalloproteinase expression in cardiac myocytes following myocardial infarction in the rabbit. Life Sci. 2001;68:799–814. doi: 10.1016/s0024-3205(00)00982-6. [DOI] [PubMed] [Google Scholar]
- 5.Webb CS, Bonnema DD, Ahmed SH, Leonardi AH, McClure CD, Clark LL, Stroud RE, Corn WC, Finklea L, Zile MR, Spinale FG. Specific temporal profile of matrix metalloproteinase release occurs in patients after myocardial infarction: relation to left ventricular remodeling. Circulation. 2006;114:1020–7. doi: 10.1161/CIRCULATIONAHA.105.600353. [DOI] [PubMed] [Google Scholar]
- 6.Squire IB, Evans J, Ng LL, Loftus IM, Thompson MM. Plasma MMP-9 and MMP-2 following acute myocardial infarction in man: correlation with echocardiographic and neurohumoral parameters of left ventricular dysfunction. J Card Fail. 2004;10:328–33. doi: 10.1016/j.cardfail.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Kelly D, Cockerill G, Ng LL, Thompson M, Khan S, Samani NJ, Squire IB. Plasma matrix metalloproteinase-9 and left ventricular remodelling after acute myocardial infarction in man: a prospective cohort study. Eur Heart J. 2007;28:711–8. doi: 10.1093/eurheartj/ehm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spinale FG, Coker ML, Thomas CV, Walker JD, Mukherjee R, Hebbar L. Time-dependent changes in matrix metalloproteinase activity and expression during the progression of congestive heart failure: relation to ventricular and myocyte function. Circ Res. 1998;82:482–95. doi: 10.1161/01.res.82.4.482. [DOI] [PubMed] [Google Scholar]
- 9.Tziakas DN, Chalikias GK, Papaioakeim M, Hatzinikolaou EI, Stakos DA, Tentes IK, Papanas N, Kortsaris A, Maltezos E, Hatseras DI. Comparison of levels of matrix metalloproteinase-2 and -3 in patients with ischemic cardiomyopathy versus nonischemic cardiomyopathy. Am J Cardiol. 2005;96:1449–51. doi: 10.1016/j.amjcard.2005.06.096. [DOI] [PubMed] [Google Scholar]
- 10.Mizon-Gerard F, de Groote P, Lamblin N, Hermant X, Dallongeville J, Amouyel P, Bauters C, Helbecque N. Prognostic impact of matrix metalloproteinase gene polymorphisms in patients with heart failure according to the aetiology of left ventricular systolic dysfunction. Eur Heart J. 2004;25:688–93. doi: 10.1016/j.ehj.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 11.George J, Patal S, Wexler D, Roth A, Sheps D, Keren G. Circulating matrix metalloproteinase-2 but not matrix metalloproteinase-3, matrix metalloproteinase-9, or tissue inhibitor of metalloproteinase-1 predicts outcome in patients with congestive heart failure. Am Heart J. 2005;150:484–7. doi: 10.1016/j.ahj.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Rohde LE, Ducharme A, Arroyo LH, Aikawa M, Sukhova GH, Lopez-Anaya A, McClure KF, Mitchell PG, Libby P, Lee RT. Matrix metalloproteinase inhibition attenuates early left ventricular enlargement after experimental myocardial infarction in mice. Circulation. 1999;99:3063–70. doi: 10.1161/01.cir.99.23.3063. [DOI] [PubMed] [Google Scholar]
- 13.Mukherjee R, Brinsa TA, Dowdy KB, Scott AA, Baskin JM, Deschamps AM, Lowry AS, Escobar GP, Lucas DG, Yarbrough WM, Zile MR, Spinale FG. Myocardial infarct expansion and matrix metalloproteinase inhibition. Circulation. 2003;107:618–25. doi: 10.1161/01.cir.0000046449.36178.00. [DOI] [PubMed] [Google Scholar]
- 14.Spinale FG, Coker ML, Krombach SR, Mukherjee R, Hallak H, Houck WV, Clair MJ, Kribbs SB, Johnson LL, Peterson JT, Zile MR. Matrix metalloproteinase inhibition during the development of congestive heart failure : effects on left ventricular dimensions and function. Circ Res. 1999;85:364–76. doi: 10.1161/01.res.85.4.364. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Simon H, Bocan TM, Peterson JT. MMP/TIMP expression in spontaneously hypertensive heart failure rats: the effect of ACE- and MMP-inhibition. Cardiovasc Res. 2000;46:298–306. doi: 10.1016/s0008-6363(00)00028-6. [DOI] [PubMed] [Google Scholar]
- 16.Lindsey ML, Gannon J, Aikawa M, Schoen FJ, Rabkin E, Lopresti-Morrow L, Crawford J, Black S, Libby P, Mitchell PG, Lee RT. Selective matrix metalloproteinase inhibition reduces left ventricular remodeling but does not inhibit angiogenesis after myocardial infarction. Circulation. 2002;105:753–8. doi: 10.1161/hc0602.103674. [DOI] [PubMed] [Google Scholar]
- 17.Hudson MP, Armstrong PW, Ruzyllo W, Brum J, Cusmano L, Krzeski P, Lyon R, Quinones M, Theroux P, Sydlowski D, Kim HE, Garcia MJ, Jaeber WA, Weaver WD. Effects of selective matrix metalloproteinase inhibitor (PG-116800) to prevent ventricular remodelling after myocardial infarction. Results of the PREMIER trial. J.Am.Coll.Cardiol. 2006;48:15–20. doi: 10.1016/j.jacc.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 18.Saglam M, Karakaya O, Esen AM, Barutcu I, Dogan S, Karavelioglu Y, Karapinar H, Akgun T, Esen O, Ozdemir N, Turkmen S, Kaymaz C. Contribution of plasma matrix metalloproteinases to development of left ventricular hypertrophy and diastolic dysfunction in hypertensive subjects. Tohoku J Exp Med. 2006;208:117–22. doi: 10.1620/tjem.208.117. [DOI] [PubMed] [Google Scholar]
- 19.Fielitz J, Leuschner M, Zurbrugg HR, Hannack B, Pregla R, Hetzer R, Regitz-Zagrosek V. Regulation of matrix metalloproteinases and their inhibitors in the left ventricular myocardium of patients with aortic stenosis. J Mol Med. 2004;82:809–20. doi: 10.1007/s00109-004-0606-4. [DOI] [PubMed] [Google Scholar]
- 20.Walther T, Schubert A, Falk V, Binner C, Kanev A, Bleiziffer S, Walther C, Doll N, Autschbach R, Mohr FW. Regression of left ventricular hypertrophy after surgical therapy for aortic stenosis is associated with changes in extracellular matrix gene expression. Circulation. 2001;104:I54–8. doi: 10.1161/hc37t1.094777. [DOI] [PubMed] [Google Scholar]


