Abstract
It has been suggested that NR2B-containing NMDA receptors have a selective tendency to promote pro-death signalling and synaptic depression, compared to the survival promoting, synapse potentiating properties of NR2A-containing NMDA receptors. A preferential localization of NR2A-containing NMDA receptors at the synapse in maturing neurons could thus explain differences in synaptic vs. extrasynaptic NMDA receptor signalling.
We have investigated whether NMDA receptors can mediate signalling to survival, death, and synaptic potentiation, in neurons at a developmental stage prior to significant NR2A expression and subunit-specific differences between synaptic and extrasynaptic NMDA receptors. We show that in developing hippocampal neurons, the progressive reduction in sensitivity of NMDA receptor currents to the NR2B antagonist ifenprodil applies to both synaptic and extrasynaptic locations. However, the reduction is less acute in extrasynaptic currents, indicating that NR2A does partition preferentially, but not exclusively, into synaptic locations at DIV>12. We then studied NMDA receptor signalling at DIV10, when both synaptic and extrasynaptic NMDA receptors are both overwhelmingly and equally NR2B-dominated. To analyse pro-survival signalling we studied the influence of synaptic NMDA receptor activity on staurosporine-induced apoptosis. Blockade of spontaneous NMDAR activity with MK-801, or ifenprodil exacerbated the apoptotic insult. Furthermore, MK-801 and ifenprodil both antagonized neuroprotection promoted by enhancing synaptic activity. Pro-death signalling induced by a toxic dose of NMDA is also blocked by NR2B-specific antagonists. Using a cell culture model of synaptic NMDA receptor-dependent synaptic potentiation, we find that this is mediated exclusively by NR2B-containing NMDARs, as implicated by NR2B-specific antagonists and the use of selective vs. non-selective doses of the NR2A-preferring antagonist NVP-AAM077.
Therefore, within a single neuron, NR2B-NMDA receptors are able to mediate both survival and death signalling, as well as model of NMDA receptor-dependent synaptic potentiation. In this instance, subunit differences cannot account for the dichotomous nature of NMDA receptor signalling.
Keywords: Apoptosis, necrosis, extrasynaptic, neuroprotection, NR2A
Introduction
NMDARs (N-methyl-D-aspartate (NMDA) receptors) are ionotropic receptors gated by the neurotransmitter glutamate, that play an important role in the physiology and pathophysiology of the central nervous system (CNS) (Waxman and Lynch, 2005). Most NMDARs in the mammalian CNS are comprised of two NR1 subunits and two NR2 subunits. There are four types of NR2 subunit (NR2A-D) which contain the binding site for glutamate, confer on the NMDAR distinct biophysical and pharmacological properties and potentially their ability to interact with different intracellular signalling molecules (Cull-Candy and Leszkiewicz, 2004, Erreger et al., 2004, Chen and Wyllie, 2006, Kohr, 2006).
NMDAR activity has the potential to promote survival or death in CNS neurons (Papadia and Hardingham, 2007). The magnitude of activation, be it intensity or duration, is very important in determining the nature of the response to an episode of NMDAR activity. The classical bell-shaped curve model of the neuronal response to NMDA or glutamate contends that intermediate, physiological levels of NMDAR activity are necessary for neuroprotection whereas too little or too much NMDAR activity promotes cell death or vulnerability to trauma (Lipton and Nakanishi, 1999). Aside from stimulus intensity, the location of the NMDAR may also profoundly affect the signals that emanate from the NMDAR. Developing neurons have sizeable pools of NMDARs at extrasynaptic, as well as synaptic locations, which signal very differently. Ca2+ influx that is dependent on intense synaptic NMDAR activation is well tolerated by cells whereas activation of extrasynaptic NMDARs, either on their own or accompanied by synaptic NMDAR activation, causes a loss of mitochondrial membrane potential and cell death (Hardingham et al., 2002, Leveille et al., 2005, Zhang et al., 2007). Differential synaptic vs. extrasynaptic NMDAR effects also extend to other signal pathways. While synaptic NMDAR activity strongly induces CREB-dependent gene expression, extrasynaptic NMDARs are coupled to a CREB shut-off pathway (Hardingham et al., 2002) in a developmentally regulated manner (Hardingham and Bading, 2002). It has also been shown that there is opposing regulation of the ERK1/2 pathway by synaptic and extrasynaptic NMDARs in hippocampal neurons: Synaptic NMDARs activate the ERK pathway whereas extrasynaptic NMDARs evoke ERK inactivation (Ivanov et al., 2006). Extrasynaptic NMDARs have also been implicated specifically in promoting long term depression while synaptic NMDARs are responsible for long term potentiation (Massey et al., 2004).
The differences in synaptic vs. extrasynaptic signalling could be conceivably down to three factors. Firstly, synaptic and extrasynaptic NMDARs could be coupled to different signalling pathways, either physically or functionally due to their location. Secondly, differences in signalling could be due to the way in which these distinct pools are activated: brief saturating activation by trans-synaptic glutamate release (synaptic NMDARs) vs. chronic low level activation by bath/ambient glutamate (extrasynaptic NMDARs). Thirdly, differences could be a by-product of differences in the location of NR2B vs. NR2A-containing NMDARs (NR2A-NMDARs). NR2A expression in the rodent CNS begins 6-10 days post-natally (Sheng et al., 1994, Zhong et al., 1994). NR2A becomes incorporated into synaptic NMDARs, by a mechanism involving the cytoplasmic C-terminus (Steigerwald et al., 2000), (but see (Thomas et al., 2006)). NR2A may thus become enriched at synapses compared to extrasynaptic locations. Liu et al. reported that NR2A-containing NMDARs promote survival and NR2B-NMDARs promote death independent of their location (Liu et al., 2007). Therefore, synaptic NMDARs may selectively promote survival due to the fact that they are enriched in NR2A-NMDARs. However, von Engelhardt et al. found that pro-death NMDAR signalling can be mediated by NR2A-NMDARs (von Engelhardt et al., 2007), indicating that the subunit is not important in determining excitotoxicity. Moreover, the concept that NR2A partitions near-exclusively into synaptic locations has been challenged recently by findings that NR2A can end up at extrasynaptic locations in cultured neurons (Thomas et al., 2006) and that the subunit compositions of synaptic and extrasynaptic NMDARs are similar in 3 week old acute hippocampal slices (Harris and Pettit, 2007).
The concept of NR2 subunit-specific signalling also an ongoing area of interest within the synaptic plasticity field. It has been proposed that NR2A-containing NMDARs are preferentially involved in potentiation of synapses, while NR2B-containing NMDARs play a role principally in depression (Liu et al., 2004, Massey et al., 2004, Bartlett et al., 2007). However, this is controversial: other studies have claimed that NR2A-containing NMDARs are not essential for induction of NMDAR-dependent LTP, and that NR2B-containing NMDARs can mediate it equally well (Berberich et al., 2005, Weitlauf et al., 2005, Zhao et al., 2005, Berberich et al., 2007, Le Roux et al., 2007).
In this study we have assessed the capacity of the NMDAR to support dichotomous survival/death signalling as well as synaptic potentiation in hippocampal neurons at a developmental stage (DIV7-11) where NR2A expression is a very minor component of either synaptic or extrasynaptic NMDARs. We find that NR2B-NMDARs can mediate both excitotoxic effects as well as pro-survival synaptic NMDARs signalling. Moreover, NR2B-NMDARs mediate synaptic NMDAR-dependent changes in a model of synaptic potentiation. Thus, synaptic potentiation can be mediated solely by NR2B-NMDARs, and dichotomous NMDAR signalling to survival/death exists in hippocampal neurons at a developmental stage where subunit differences cannot offer an explanation.
Experimental procedures
Hippocampal cultures, stimulation, and the induction of apoptosis/necrosis
Hippocampal neurons were cultured as described (Bading and Greenberg, 1991) except that growth medium was supplemented with B27 (Invitrogen). All experiments were performed after a culturing period of >7 days during by which time hippocampal neurons have developed a rich network of processes, express functional NMDA-type and AMPA/kainate-type glutamate receptors, and form synaptic contacts (Hardingham et al., 2001, Hardingham and Bading, 2002, Hardingham et al., 2002). Prior to the stimulation of neurons or the addition of excitotoxic doses of NMDA or apoptosis inducers, neurons were placed in a non-trophic medium by transferring them from growth medium to a medium containing 10% MEM (Invitrogen), 90% Salt-Glucose-Glycine (SGG) medium ((Bading et al., 1993), SGG: 114 mM NaCl, 0.219 % NaHCO3, 5.292 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM HEPES, 1 mM glycine, 30 mM glucose, 0.5 mM sodium pyruvate, 0.1 % Phenol Red; osmolarity 325mosm/l). Bursts of action potential firing were induced by treatment of cultured hippocampal neurons with 50 μM bicuculline; bursts are associated with NMDAR-dependent intracellular Ca2+ transients (Hardingham et al., 2001). For inducing excitotoxic cell death, neurons were exposed to NMDA (50 μM) in our standard trophically deprived medium (see above) for 1 hr, after which neurons were washed once and returned to fresh medium. Neurons that die in response to exposure to excitotoxic levels of glutamate exhibit swollen cell bodies and pyknotic nuclei with small irregular chromatin clumps, a characteristic of necrotic cell death as opposed to apoptotic-like death ((Fujikawa et al., 2000), see (Hardingham et al., 2002) for example pictures). Cell death was determined 24 h later by counting the number of DAPI-stained pyknotic nuclei as a percentage of the total. Staurosporine exposure (24 h) was also used to induce apoptosis (50-100 nM). Morphologically, trophically-deprived and staurosporine-treated neurons show typical signs of apoptotic-like cell death (shrunken cell body and large round chromatin clumps). Staurosporine activates caspases and death is blocked by pan-caspase inhibitors (Hardingham, unpublished). Examples of pictures of apoptotic nuclei of trophically deprived and staurosporine-treated neurons are shown here and in previous studies (Hardingham et al., 2002, Papadia et al., 2005). Neurons were fixed and subjected to DAPI staining and cell death quantified by counting (blind) the number of apoptotic nuclei as a percentage of the total. MK-801 was from Tocris Bioscience, ifenprodil from Merck Biosciences. NVP-AAM077 was a gift from Dr Y.P. Auberson (Novartis Institutes for Biomedical Research, Basel, Switzerland).
Electrophysiological recording and analysis
Coverslips containing hippocampal neurons were transferred to a recording chamber perfused with an external recording solution composed of (in mM): 152 NaCl, 2.8 KCl, 10 HEPES, 2 CaCl2, 10 glucose, 0.1 glycine and 0.02 strychnine, pH 7.3 (320-330 mOsm). Patch pipettes were made from thick-walled borosilicate glass (Harvard Apparatus, Kent, UK) and filled with a K-gluconate-based internal solution containing (in mM): 155 K-gluconate, 2 MgCl2, 10 Na-HEPES, 10 Na-PiCreatine, 2 Mg2-ATP and 0.3 Na3-GTP, pH 7.3 (300 mOsm). Electrode tips were fire-polished for a final resistance ranging between 5-10 MΩ. For experiments requiring a high signal-to-noise ratio, electrodes were coated with Sylgard 184 resin (Dow Corning, Midland, MI). Currents were recorded at room temperature (21 ± 2°C) using an Axopatch-1C amplifier (Molecular Device, Union City, CA) and stored on digital audio tape. Data was subsequently digitized and analyzed using WinEDR v6.1 software (John Dempster, University of Strathclyde, UK). Hippocampal neurons were voltage-clamped at -70 mV, and recordings were rejected if the holding current was greater than -100 pA or if the series resistance drifted by more than 20% of its initial value (<35 MΩ).
Whole-cell NMDAR-mediated currents were measured in external recording solution supplemented with 0.3 μM tetrodotoxin (TTX) and 50 μM picrotoxin (PTX, both from Tocris Bioscience, Bristol, UK), flowing in the recording chamber at a rate of 3-5 ml/min. NMDAR-mediated currents were elicited by switching from the external recording solution line to one containing 150 μM NMDA for 5-10 sec, until the current reached a steady-state, then back to the agonist-free solution. Because cells were difficult to maintain after too many agonist applications, each NMDA application was followed by at least 1 min washout and limited to 2-3 repeats. NMDAR antagonists NVP-AAM077 and ifenprodil (Tocris Bioscience or Merck) were bath-applied for 3 min before current measurements. All currents were normalized to their respective initial whole-cell agonist-elicited current and are shown in percentage of basal. Applying increasing concentrations of NVP-AAM077 to inhibit of glutamate (3 mM) evoked currents allowed us to determine an IC50 value for NVP-AAM077 (Frizelle et al., 2006). For these experiments the external recording solution was supplemented with CNQX (10 mM) to block glutamate-evoked activation of AMPA and kainate receptors.
Analysis of extrasynaptic NMDAR currents
To block synaptically located NMDARs, we used a quantal activation-mediated blockade by MK-801 (Nakayama et al., 2005). In Mg2+-free external recording solution containing TTX, release of glutamate into the synaptic cleft can occur only via spontaneous (action potential independent) release of synaptic vesicles (packets) of glutamate. In the added presence of MK-801, an irreversible (in our experimental time-frame) open-channel blocker MK-801, only the NMDARs experiencing this localized release, therefore defined as “synaptic”, were antagonized by MK-801. Following MK-801 application to allow sufficient block of synaptic NMDARs and a 3 min washout of MK-801, only extrasynaptic NMDAR contributed to subsequent whole-cell NMDAR-mediated currents. When using ifenprodil, because of the difficult washout of the drug and the possibility that it might cause an underestimation of the NR2B-containing synaptic NMDAR population, the drug was never applied before the quantal block protocol. Thus, ifenprodil sensitivity of “whole-cell” and “extrasynaptic” fractions was compared in an unpaired manner. The extrasynaptic NMDA currents measured were normalized to their pre-MK-801 currents and are shown as percentage of basal.
Model of NMDAR-dependent synaptic potentiation
To potentiate miniature excitatory postsynaptic currents (mEPSCs) frequency, 50 μM bicuculline was added to culture medium for 15 min, which was then replaced with drug-free medium for approximately 1 h before electrophysiological recordings. By adding GABAA receptor antagonist bicuculline, tonic inhibition on the neuronal network is relieved, inducing synchronous bursting of neurons which triggers a long-lasting increase in mEPSC frequency, likely due to conversion of silent synapses into functional ones (Arnold et al., 2005). Following the waiting period, hippocampal neurons were transferred into the recording chamber filled with an external recording solution composed of (in mM): 150 NaCl, 2.8 KCl, 10 HEPES, 2 CaCl2, 1 MgCl2 and 10 glucose, pH 7.3 (320-330 mOsm).. mEPSCs were recorded for 5-10 min (minimum of 200 events) from neurons clamped at −70 mV. Data was analyzed offline with MiniAnalysis software (Synaptosoft, Fort Lee, NJ) as described (Baxter and Wyllie, 2006). mEPSCs were manually selected with a minimum amplitude threshold of 6 pA (approximately 2 times the baseline noise level). For the experiments using NMDAR antagonists, drugs were pre-applied 20 min before the bicuculline stimulation.
Results
DIV 12-18 hippocampal neurons contain a lower proportion of NR2B-NMDARs than DIV 7-11 neurons
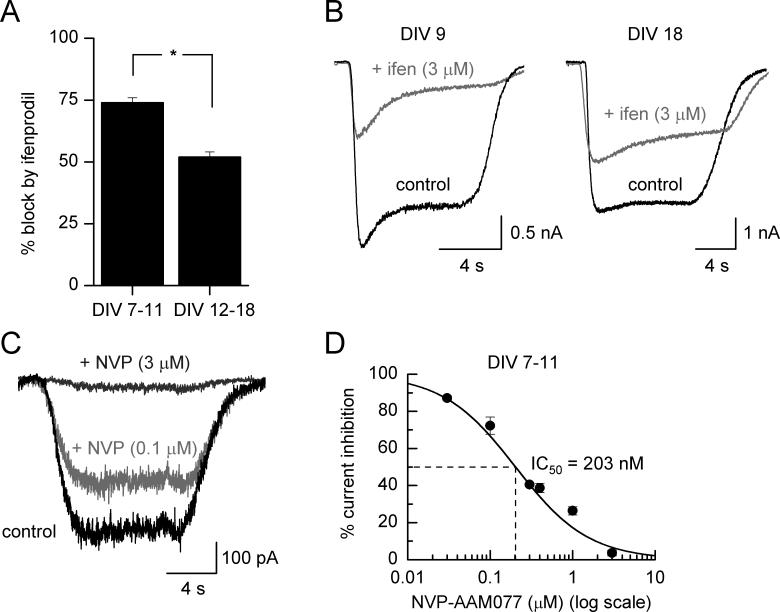
We first investigated the sensitivity of glutamate-evoked whole-cell NMDAR currents to the NR2B-selective antagonist ifenprodil (Williams, 1993) at different developmental stages. As expected, we observed a large increase in the magnitude of NMDAR currents as the neurons progress through in vitro development (data not shown). However, the sensitivity of this current to ifenprodil remains very high with currents being blocked by 74 ± 2 % (n=21) up to DIV11 (Fig. 1a,b). Ifenprodil is an incomplete antagonist: it blocks pure NR1/NR2B NMDARs by approximately 80 % (Williams, 1993, Tovar and Westbrook, 1999, Frizelle et al., 2006) and so the degree of blockade that we see here is indicative of a near-pure population of NR2B-NMDARs. To confirm this further we determined the sensitivity of our whole-cell currents to the competitive NMDAR antagonist NVP-AAM077 (Auberson et al., 2002, Liu et al., 2004). In recombinant NR2A- or NR2B-containing NMDARs this antagonist blocks glutamate-evoked currents with IC50 values of 31 nM and 215 nM respectively (Frizelle et al., 2006) when glutamate is used at its EC50 concentration for each NMDAR subtype (see (Wyllie and Chen, 2007) for a discussion of this point). We find that at DIV 7-11 NMDAR-mediated currents evoked by glutamate at its EC50 concentration (3 mM) are blocked (Fig. 1c) in a manner that is consistent with a near pure NR2B-NMDAR population with the IC50 for NVP-AAM077 block being 203 nM (Fig. 1d). In agreement with previous studies (Kew et al., 1998, Tovar and Westbrook, 1999) ifenprodil sensitivity of NMDAR currents is lower in older neurons: sensitivity is only around 50% in DIV12-18 neurons (Fig. 1a,b), indicative of increased expression of ifenprodil-insensitive NR2A-NMDARs.
Fig. 1. DIV 12-18 hippocampal neurons contain a lower proportion of NR2B-NMDARs than DIV 7-11 neurons.
A) Comparison of ifenprodil sensitivity of whole-cell NMDAR currents (evoked by 150 μM NMDA) in DIV 7-11 neurons (n =21) with DIV 12-18 neurons (n=19). B) Examples of traces used to generate the data in (A). C) example NMDAR-mediated whole-cell currents recorded from a DIV 9 neuron in the absence and presence of NVP-AAM077. D) Mean inhibition curve used to determine the IC50 of NVP-AAM077 acting at NMDAR currents of DIV 7-11 neurons. The value obtained (203 nM) is consistent with a near-pure NR2B-NMDAR population. For each data point measurements were made from 3-7 cells (DIV 7-11). * p<0.001 2-tailed unpaired T-test.
Developmental loss of ifenprodil sensitivity of NMDAR currents is not solely restricted to synaptic locations
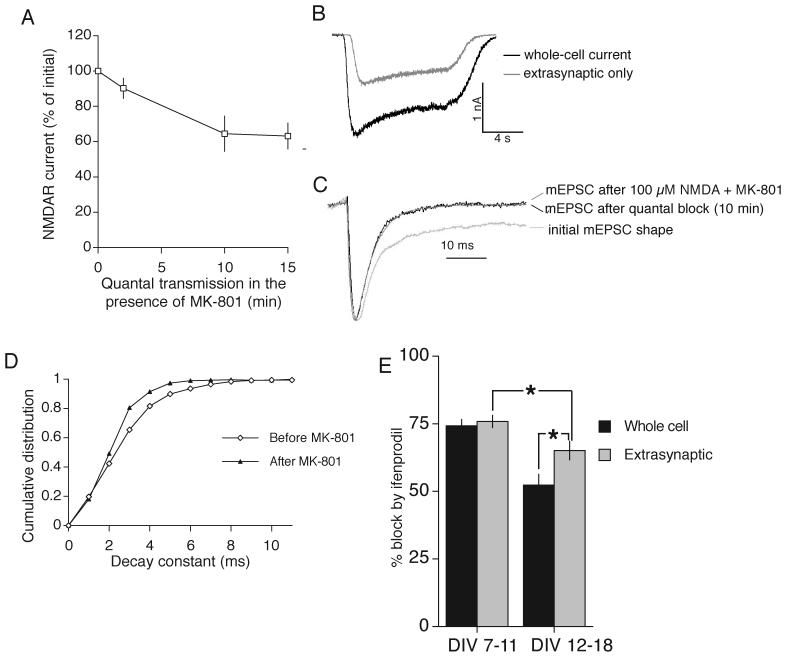
We next sought to determine whether this loss of ifenprodil sensitivity applied equally to synaptic and extrasynaptic currents, or whether extrasynaptic currents remained essentially NR2B-containing. This would tell us whether NR2A was partitioning selectively into synaptic locations. To analyze extrasynaptic NMDAR currents we used an established method for selectively and irreversibly blocking synaptic NMDARs (Nakayama et al., 2005) whereupon bath application of agonist is then used to activate extrasynaptic NMDARs. Following the measurement of whole-cell NMDAR currents, neurons were placed in TTX and Mg2+-free solution, in the presence of MK-801. Under these conditions, spontaneous release of quanta of glutamate activate synaptic NMDARs, which are then blocked by the open channel blocker MK-801. Comparing NMDAR currents before and after this treatment revealed that open-channel NMDAR blockade under spontaneous quantal neurotransmitter release is progressive, plateaus after 10 min and goes no further (Fig. 2a,b). This remaining current, undiminished by this protocol for blocking synaptic NMDARs, is extrasynaptic. To demonstrate conclusively that all synaptic NMDARs are blocked by this protocol we analyzed the shape of mEPSCs before and after MK-801 blockade of NMDARs that had been activated by spontaneous quantal neurotransmitter release (see methods). There is a clear change in the decay kinetics, indicative of a loss of the NMDAR component of the current (Fig. 2c,d). To confirm that this loss is complete, we compared the kinetics of these mEPSCs with those where all NMDARs are blocked by a high agonist application (100 μM NMDA) in the presence of MK-801. Under these conditions, the mEPSC kinetics are identical to those subjected to NMDAR blockade under spontaneous quantal release for 10 min (Fig. 2c), showing that all synaptic NMDARs are indeed blocked.
Fig. 2. Developmental loss of ifenprodil sensitivity of NMDAR currents is not solely restricted to synaptic locations.
A) Loss of whole-cell current due to MK-801 exposure under quantal transmission plateaus after 10 min (n=3). Neurons were placed under voltage clamp and whole-cell NMDAR-mediated currents were measured. Neurons were then placed in Mg2+-free external recording solution containing TTX and MK-801 for the indicated times, to allow open-channel blockade of synaptic NMDARs following their activation by quantal release of glutamate. B) Example of a whole-cell current trace (DIV 9) before and after 10 min application of MK-801 when NMDARs are activated only by spontaneous release of glutamate (denoted as “quantal block”). C,D) Confirmation that all synaptic NMDARs are blocked by this procedure. C) Examples of mEPSC shape before and after 10 min of MK-801 block of NMDARs activated by spontaneous transmitter release. Also for comparison is a mEPSC recorded at the end of the experiment where all NMDARs were blocked by addition of a high concentration of agonist in the presence of MK-801. D) Example of a cumulative distribution curve of the decay constant of mEPSCs recorded from a cell before (n=185) and after (n=157) 10 min of “quantal block” to illustrate the change in decay kinetics. E) Comparison of the developmental loss of ifenprodil (3 μM) sensitivity of whole cell and extrasynaptic NMDAR currents. Whole cell currents of DIV 7-11 neurons (n=21) and DIV 12-18 neurons (n=19) analysed. Extrasynaptic currents of DIV 7-11 neurons (n=21) and DIV 12-18 neurons (n=17) analysed. * p<0.05 2-tailed unpaired T-test.
Not surprisingly we found that at DIV7-11, ifenprodil exerted a near-maximal block on extrasynaptic currents (77 ± 2 %, n=18), just as it did to whole-cell currents (Fig. 2e). At DIV12-18, however, the blockade of extrasynaptic NMDAR currents was significantly lower (65 ± 3 %, n=16, p < 0.01 Fig. 2e), indicating that some NR2A-NMDARs are incorporated into extrasynaptic sites, as has been shown previously (Thomas et al., 2006). Importantly, however, the ifenprodil sensitivity of extrasynaptic NMDAR currents was still significantly greater than the sensitivity of whole-cell currents (Fig. 2e), indicating that NR2A-NMDARs are preferentially incorporated into synaptic sites.
NR2B-NMDARs can mediate both pro-death and pro-survival NMDAR signalling
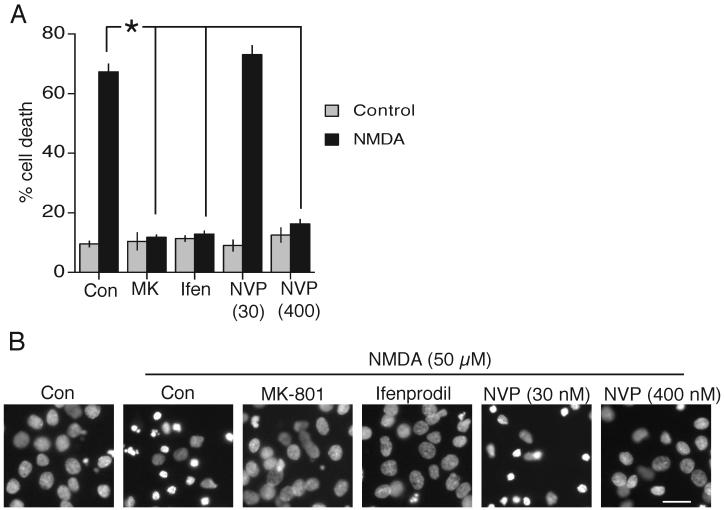
Given that both synaptic and extrasynaptic NMDAR currents at DIV7-11 are essentially NR2B-NMDAR-mediated, we wanted to determine whether NR2B-NMDARs were able to mediate both survival and death signalling within a single neuronal type. Experiments were carried out at DIV8-11. We first looked at pro-death signalling from the NMDAR, induced by the bath application of a toxic dose of NMDA (50 μM). Both MK-801 and ifenprodil prevented excitotoxic cell death (Fig. 3a,b). Furthermore, we tested the effect of NR2A-antagonizing levels of NVP-AAM077 (30 nM) which inhibits approximately 70 % of NR2A-mediated currents and 10 % NR2B-mediated currents evoked by 50 μM NMDA (Frizelle et al., 2006). 30 nM NVP-AAM077 did not impact on cell death. However, a concentration that inhibits over 70 % of NR2B-mediated currents in addition to NR2A-containing NMDARs (400 nM, (Frizelle et al., 2006)) was neuroprotective (Fig. 3a,b). We next sought to determine whether synaptic NMDAR-dependent neuroprotection is achievable in DIV9 hippocampal neurons, and whether it is mediated by NR2B--NMDARs.
Fig. 3. NR2B-NMDARs can mediate pro-death NMDAR signalling.
A,B) A) Neurons were pre-treated with the indicated antagonists prior to exposure to 50 μM NMDA for 1 h. Neurons were then returned to NMDA-free medium and death assessed after 24 h. MK-801 (10 μM), ifenprodil (3 μM), NVP-AAM077 (30 nM and 400 nM). B) Example pictures (scale bar 20 μm). * p<0.05 2-tailed paired T-test.
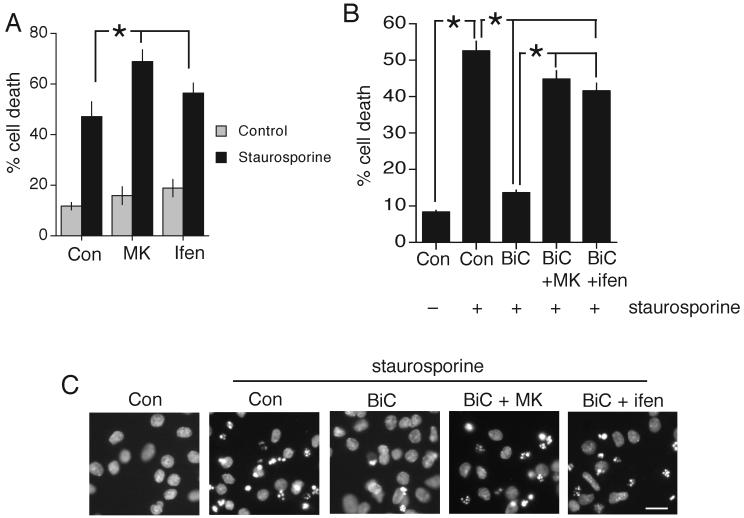
We then used the established model of staurosporine-induced apoptosis and assessed the influence of synaptic NMDAR activity on vulnerability to this insult. We found that blockade of spontaneous NMDAR activity with MK-801 and NR2B-NMDAR activity with ifenprodil exacerbated staurosporine-induced neuronal death (Fig. 4a). Thus, spontaneous NR2B-NMDAR activity is clearly exerting a protective effect in this model of cell death. In addition to inhibiting spontaneous NMDAR activity with antagonists, synaptic NMDAR activity can be enhanced by dis-inhibiting the neuronal network. Synaptic activity was initiated by treating rat cortical neurons with the GABAA receptor blocker bicuculline (Hardingham et al., 2001). This stimulation protocol induces bursts of action potentials which are associated with NMDAR-dependent Ca2+ transients (Hardingham and Bading, 2002). This enhanced activity promotes strong protection in the face of the apoptotic insult (Fig. 4b,c) and moreover both MK-801 and ifenprodil reduce the protection afforded. Note that bicuculline treatment still has a small protective effect in the presence of ifenprodil (Fig. 4b), potentially due to the fact that it does not block NR2B-NMDARs completely. In conclusion, NR2B-NMDARs are able to signal to neuroprotection as well as neuronal death.
Fig. 4. NR2B-NMDARs can mediate pro-survival NMDAR signalling.
A) Blockade of spontaneous NR2B-NMDAR activity exacerbates staurosporine-induced apoptosis. Neurons treated with MK-801 (10 μM) or ifenprodil (3 μM) for 16 h prior to exposure to staurosporine (50 nM) for 24 h. B) Neurons treated where indicated with bicuculline (50 μM) in the presence or absence of the indicated antagonists for 16 h prior to exposure to staurosporine (100 nM) for 24 h. C) Example pictures from (B) * p<0.05 2-tailed paired T-test.
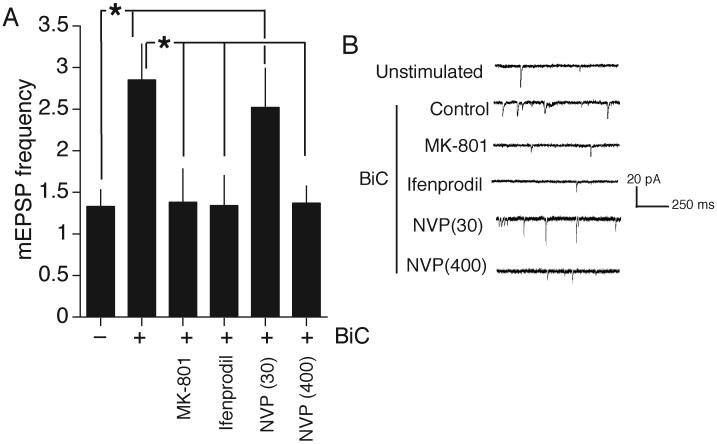
NR2B-NMDARs can mediate synaptic activity-dependent potentiation of mEPSC frequency
It has been suggested that NR2B-NMDARs couple selectively to synaptic depression, and not potentiation (Liu et al., 2004, Massey et al., 2004). We wanted to determine whether NMDAR-dependent synaptic potentiation was achievable in our cultures at this developmental stage. We employed a model of plasticity (Arnold et al., 2005) in which a brief period of bicuculline-induced bursting causes an NMDAR-dependent increase in mEPSC frequency, attributed to AMPA receptor insertion and unsilencing of synapses (Lu et al., 2001, Abegg et al., 2004, Baxter and Wyllie, 2006). We found that this model of potentiation is very robust in DIV 8-11 hippocampal neurons (Fig. 5a,b). Moreover, MK-801 and ifenprodil blocked the increase in mEPSC frequency, demonstrating that this potentiation process is NR2B-dependent (Fig. 5a,b). In addition, a concentration of NVP-AAM077 (30 nM) predicted to impair activation of synaptic NR2A-NMDARs but not NR2B-NMDARs (Frizelle et al., 2006) failed to block potentiation, while a non-discriminating concentration (400 nM) did block potentiation (Fig. 5a,b).
Fig. 5. NR2B-NMDARs mediate activity-dependent potentiation of mEPSC frequency.
A) Neurons pre-treated with antagonists as indicated and in the presence of these compounds were treated with medium ± bicuculline for 15 min, then allowed to settle for 30 min. mEPSCs were then recorded for 5-10 min (minimum 300 events) and the frequency calculated (n=8-10 for each condition). MK-801 (10 μM), ifenprodil (3 μM), NVP-AAM077 (30 nM and 400 nM) B) Examples of traces used to generate the data shown in (A).
Discussion
We have shown that in developing hippocampal neurons, NR2B-NMDARs are capable of mediating antagonistic signalling to survival/death as well as synaptic potentiation, depending on the stimulus employed. This indicates that in immature hippocampal neurons at least, the subunit composition of the NMDAR cannot account for dichotomous NMDAR signalling.
Signalling to survival and death by the NMDAR
Despite an overwhelming body of evidence from animal studies implicating NMDAR activity in neuronal loss following ischemia, the many clinical trials of different NMDAR antagonists for stroke have failed due to poor tolerance and efficacy (Ikonomidou and Turski, 2002, Muir, 2006). The fact that the NMDAR plays a central role in synaptic plasticity and transmission, and learning and cognition accounts for the undesired psychomimetic and CNS-adverse effects of antagonists (Muir, 2006). However, trial design may have been erring too far on the side of caution in seeking to avoid psychosis and other CNS-adverse effects, when these side-effects are on-target and not off-target effects. Other issues cloud a clear assessment of NMDAR antagonists, such as numbers of patients within the trials and time taken to administrate the drug. With many large pharmaceutical companies having shied away from NMDAR antagonists, these issues may not be resolved any time soon.
Nevertheless, the growing body of evidence that physiological synaptic NMDAR activity exerts a neuroprotective effect (Ikonomidou and Turski, 2002, Hardingham and Bading, 2003, Hetman and Kharebava, 2006) has led to suggestions that it may play a role in promoting recovery and preventing delayed neuronal loss in the penumbra (Albers et al., 2001, Ikonomidou and Turski, 2002). Thus, global NMDAR antagonists may block NMDAR-activated pro-death signals triggered in response to an ischemic challenge, but interfere with some recovery or preconditioning processes in the penumbra. The anti-excitotoxic effects of NMDAR antagonists have never been in question, but until relatively recently the pro-survival role of the NMDAR was not known and so antagonists were not tested in contexts that would expose their harmful effects. In treating disorders associated with pro-death NMDAR signalling, it may be desirable to block pro-death signalling, without affecting pro-survival signalling or synaptic plasticity.
A specific role for NR2B-NMDARs in promoting cell death would enable this particular subtype of NMDAR to be targeted without impairing pro-survival signalling, using the highly selective antagonists available. However, our observations suggest that NR2B-NMDARs are capable of promoting both survival and death signalling. Moreover, in more mature neurons (DIV21) NR2A-NMDARs were recently shown to be capable of mediating excitotoxicity as well as protective signalling (von Engelhardt et al., 2007). Taken together, these studies indicate that NR2B-NMDARs and NR2A-NMDARs are both capable of mediating survival and death signalling. Thus, the specific ability of NR2B-NMDARs and NR2A-NMDARs to promote death and survival respectively as described recently (Liu et al., 2007) may not apply in all neuronal types at all developmental stages, and so NR2B-specific antagonism may not be an optimal anti-excitotoxic strategy. Furthermore, the recent observation that synaptic and extrasynaptic NMDARs have similar NR2B components in 3 week old acute hippocampal slices (Harris and Pettit, 2007) suggests that NR2B-selective antagonists may not be successful even in selectively targetting extrasynaptic NMDARs in vivo. An added complication is the fact that ifenprodil and related NR2B antagonists actually potentiate NR2B-NMDAR currents when agonist concentrations are low, and so may boost extrasynaptic currents under conditions of low-to-modest ambient glutamate (Neyton and Paoletti, 2006). Another pharmacological approach to sparing trans-synaptic NMDAR signalling while blocking the effects of excitotoxic doses of elevated glutamate in the extracellular medium could involve low-affinity uncompetitive antagonists such as memantine. Its uncompetitive nature results in very effective blockade of excessive chronic NMDAR activity caused by high ambient levels of glutamate or NMDA (Chen and Lipton, 2006). However, due to its fast off rate, memantine will not substantially interfere with normal synaptic NMDAR activity by accumulating in the channel (Chen et al., 1998, Chen and Lipton, 2006, Wrighton et al., 2007), in contrast to MK-801, which is a uncompetitive antagonist/open channel blocker with an extremely slow off-rate. As a drug that antagonizes the NMDAR preferentially under pathological rather than physiological scenarios, it has the advantage of potentially protecting all neurons regardless of the subunit composition of their NMDARs.
Subunit-dependence of the directionality of synaptic plasticity?
The NR2 composition of the NMDAR may play an important role in the directionality of synaptic plasticity. It has been proposed that NR2A-containing NMDARs are preferentially involved in potentiation of synapses, while NR2B-containing NMDARs play a role mainly in depression (Liu et al., 2004, Massey et al., 2004, Bartlett et al., 2007). However, other studies have claimed that NR2A-containing NMDARs are not essential for induction of NMDAR-dependent LTP, and that NR2B-containing NMDARs can mediate it equally well (Berberich et al., 2005, Weitlauf et al., 2005, Zhao et al., 2005, Berberich et al., 2007, Le Roux et al., 2007).
One potential reason for this controversy lies in the use of NVP-AAM077 which has been reported to selectively block NR2A-NMDARs (Auberson et al., 2002) and which has resulted in it being used to implicate NR2A-NMDARs in various processes. However, later studies have shown this antagonist to be less select than originally thought (Berberich et al., 2005, Frizelle et al., 2006, Neyton and Paoletti, 2006). Moreover NVP-AAM077 was shown to inhibit NMDAR-dependent LTP even in NR2A -/- mice and attenuate NMDAR currents mediated by NR2B-containing receptors in neurons (Weitlauf et al., 2005). Indeed at the concentrations most commonly used (300 – 400 nM) NVP-AAM077 will block, substantially, synaptic activation of both NR2A- and NR2B containing NMDARs since its slow dissociation rate constant will mean that on a synaptic timescale glutamate and NVP-AAM077 binding cannot reach equilibrium (Frizelle et al., 2006, Wyllie and Chen, 2007). Nevertheless, application of low concentrations (30 nM or less) can be used to provide some NR2A-selective antagonism and implicate NR2A specifically in synaptic potentiation (Frizelle et al., 2006, Bartlett et al., 2007).
Another potential reason for disagreement lies in the fact that the expression of NR2A is developmentally regulated, starting around P6-P10 (Sheng et al., 1994, Zhong et al., 1994). NR2A subunits contribute increasingly to synaptic NMDAR currents (Stocca and Vicini, 1998, Tovar and Westbrook, 1999) and so the developmental stage could have a significant bearing on the involvement of NR2A- vs. NR2B-NMDARs in a process (e.g. LTP) independently of any subunit-specific signalling requirements (e.g. see (Le Roux et al., 2007)). Our studies indicate that in immature hippocampal cultures, NR2B-NMDARs are capable of mediating activity-dependent potentiation of synaptic connections, as measured by analysing mEPSC frequency.
Pattern of NMDA receptor activity vs subtype of NMDA receptor?
In this study we show that when bicuculline-induced AP bursting activates NR2B-NMDARs trans-synaptically, neuroprotective signalling is induced, as is synaptic potentiation. In contrast, extended bath-activation of all (synaptic and extrasynaptic) NMDARs promotes cell death, also via NR2B-NMDAR activation. Furthermore, we find that brief bath application of NMDA triggers synaptic depression: spontaneous mEPSC frequency is lowered 30 min after brief NMDA exposure (30 μM for 4 min, Martel, Wyllie and Hardingham, unpublished observations). The opposing effects of bath activating all (synaptic and extrasynaptic) NMDARs and trans-synaptically activating synaptic NMDARs is evident in neurons expressing essentially only NR2B-NMDARs. This demonstrates that in this particular instance, consequences of NMDAR signalling is determined by the type of stimulus, and not the subunit composition. Quite what it is about the stimuli which trigger the different effects is not addressed here, but previous work has demonstrated a dominant effect of extrasynaptic NMDARs in promoting neuronal death (Hardingham et al., 2002) and synaptic depression (Massey et al., 2004), which would explain the observations in this study. It will be interesting to see how synaptic and extrasynaptic NMDARs are coupled to different signalling pathways. It could be that they are coupled differently (either physically/functionally) as a result of their differing location. Another contributing factor could be the way in which these distinct pools are activated: brief saturating activation in the case of trans-synaptic activation of synaptic NMDARs vs. chronic low level activation of extrasynaptic NMDARs by bath/ambient glutamate. Differences in the properties of intracellular Ca2+ transients evoked by these different stimuli could differentially affect signalling, even if the overall Ca2+ load were similar.
Acknowledgements
We are grateful to Dr Y.P. Auberson (Novartis Institutes for Biomedical Research, Basel, Switzerland) for the kind gift if NVP-AAM077. This work is supported by the Royal Society, the Wellcome Trust and the European Commission.
References
- Abegg MH, Savic N, Ehrengruber MU, McKinney RA, Gahwiler BH. Epileptiform activity in rat hippocampus strengthens excitatory synapses. J Physiol. 2004;554:439–448. doi: 10.1113/jphysiol.2003.052662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers GW, Goldstein LB, Hall D, Lesko LM. Aptiganel hydrochloride in acute ischemic stroke: a randomized controlled trial. Jama. 2001;286:2673–2682. doi: 10.1001/jama.286.21.2673. [DOI] [PubMed] [Google Scholar]
- Arnold FJ, Hofmann F, Bengtson CP, Wittmann M, Vanhoutte P, Bading H. Microelectrode array recordings of cultured hippocampal networks reveal a simple model for transcription and protein synthesis-dependent plasticity. J Physiol. 2005;564:3–19. doi: 10.1113/jphysiol.2004.077446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auberson YP, Allgeier H, Bischoff S, Lingenhoehl K, Moretti R, Schmutz M. 5-Phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg Med Chem Lett. 2002;12:1099–1102. doi: 10.1016/s0960-894x(02)00074-4. [DOI] [PubMed] [Google Scholar]
- Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993;260:181–186. doi: 10.1126/science.8097060. [DOI] [PubMed] [Google Scholar]
- Bading H, Greenberg ME. Stimulation of protein tyrosine phosphorylation by NMDA receptor activation. Science. 1991;253:912–914. doi: 10.1126/science.1715095. [DOI] [PubMed] [Google Scholar]
- Bartlett TE, Bannister NJ, Collett VJ, Dargan SL, Massey PV, Bortolotto ZA, Fitzjohn SM, Bashir ZI, Collingridge GL, Lodge D. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology. 2007;52:60–70. doi: 10.1016/j.neuropharm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Baxter AW, Wyllie DJ. Phosphatidylinositol 3 kinase activation and AMPA receptor subunit trafficking underlie the potentiation of miniature EPSC amplitudes triggered by the activation of L-type calcium channels. J Neurosci. 2006;26:5456–5469. doi: 10.1523/JNEUROSCI.4101-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberich S, Jensen V, Hvalby O, Seeburg PH, Kohr G. The role of NMDAR subtypes and charge transfer during hippocampal LTP induction. Neuropharmacology. 2007;52:77–86. doi: 10.1016/j.neuropharm.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Berberich S, Punnakkal P, Jensen V, Pawlak V, Seeburg PH, Hvalby O, Kohr G. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci. 2005;25:6907–6910. doi: 10.1523/JNEUROSCI.1905-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HS, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97:1611–1626. doi: 10.1111/j.1471-4159.2006.03991.x. [DOI] [PubMed] [Google Scholar]
- Chen HS, Wang YF, Rayudu PV, Edgecomb P, Neill JC, Segal MM, Lipton SA, Jensen FE. Neuroprotective concentrations of the N-methyl-D-aspartate open-channel blocker memantine are effective without cytoplasmic vacuolation following post-ischemic administration and do not block maze learning or long-term potentiation. Neuroscience. 1998;86:1121–1132. doi: 10.1016/s0306-4522(98)00163-8. [DOI] [PubMed] [Google Scholar]
- Chen PE, Wyllie DJ. Pharmacological insights obtained from structure-function studies of ionotropic glutamate receptors. Br J Pharmacol. 2006;147:839–853. doi: 10.1038/sj.bjp.0706689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Erreger K, Chen PE, Wyllie DJ, Traynelis SF. Glutamate receptor gating. Crit Rev Neurobiol. 2004;16:187–224. doi: 10.1615/critrevneurobiol.v16.i3.10. [DOI] [PubMed] [Google Scholar]
- Frizelle PA, Chen PE, Wyllie DJA. Equilibrium constants for NVP-AAM077 acting at recombinant NR1/NR2A and NR1/NR2B NMDA receptors: implications for studies of synaptic transmission. Mol Pharmacol. 2006;70:1022–1032. doi: 10.1124/mol.106.024042. [DOI] [PubMed] [Google Scholar]
- Fujikawa DG, Shinmei SS, Cai B. Kainic acid-induced seizures produce necrotic, not apoptotic, neurons with internucleosomal DNA cleavage: implications for programmed cell death mechanisms. Neuroscience. 2000;98:41–53. doi: 10.1016/s0306-4522(00)00085-3. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJ, Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat Neurosci. 2001;4:261–267. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Coupling of extrasynaptic NMDA receptors to a CREB shut-off pathway is developmentally regulated. Biochim Biophys Acta. 2002;1600:148–153. doi: 10.1016/s1570-9639(02)00455-7. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. The Yin and Yang of NMDA receptor signalling. Trends Neurosci. 2003;26:81–89. doi: 10.1016/S0166-2236(02)00040-1. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Harris AZ, Pettit DL. Extrasynaptic and synaptic NMDA receptors form stable and uniform pools in rat hippocampal slices. J Physiol. 2007;584:509–519. doi: 10.1113/jphysiol.2007.137679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetman M, Kharebava G. Survival signaling pathways activated by NMDA receptors. Curr Top Med Chem. 2006;6:787–799. doi: 10.2174/156802606777057553. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? The Lancet Neurology. 2002;1:383–386. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, Medina I. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the ERK activity in cultured rat hippocampal neurons. J Physiol. 2006;572(3):789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew JN, Richards JG, Mutel V, Kemp JA. Developmental changes in NMDA receptor glycine affinity and ifenprodil sensitivity reveal three distinct populations of NMDA receptors in individual rat cortical neurons. J Neurosci. 1998;18:1935–1943. doi: 10.1523/JNEUROSCI.18-06-01935.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohr G. NMDA receptor function: subunit composition versus spatial distribution. Cell Tissue Res. 2006;326:439–446. doi: 10.1007/s00441-006-0273-6. [DOI] [PubMed] [Google Scholar]
- Le Roux N, Amar M, Moreau A, Fossier P. Involvement of NR2A- or NR2B-containing N-methyl-D-aspartate receptors in the potentiation of cortical layer 5 pyramidal neurone inputs depends on the developmental stage. Eur J Neurosci. 2007;26:289–301. doi: 10.1111/j.1460-9568.2007.05671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveille F, Nicole O, Buisson A. Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: 2005. Cellular location of NMDA receptors influences their implication in excitotoxic injury. Online Program No. 946.2.2005. [Google Scholar]
- Lipton SA, Nakanishi N. Shakespeare in Love - with NMDA receptors? Nature Medicine. 1999;5:270–271. doi: 10.1038/6481. [DOI] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27:2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir KW. Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists. Curr Opin Pharmacol. 2006;6:53–60. doi: 10.1016/j.coph.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Kiyosue K, Taguchi T. Diminished neuronal activity increases neuron-neuron connectivity underlying silent synapse formation and the rapid conversion of silent to functional synapses. J Neurosci. 2005;25:4040–4051. doi: 10.1523/JNEUROSCI.4115-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci. 2006;26:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadia S, Hardingham GE. The Dichotomy of NMDA Receptor Signaling. Neuroscientist. 2007 doi: 10.1177/10738584070130060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadia S, Stevenson P, Hardingham NR, Bading H, Hardingham GE. Nuclear Ca2+ and the cAMP response element-binding protein family mediate a late phase of activity-dependent neuroprotection. J Neurosci. 2005;25:4279–4287. doi: 10.1523/JNEUROSCI.5019-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Steigerwald F, Schulz TW, Schenker LT, Kennedy MB, Seeburg PH, Kohr G. C-Terminal truncation of NR2A subunits impairs synaptic but not extrasynaptic localization of NMDA receptors. J Neurosci. 2000;20:4573–4581. doi: 10.1523/JNEUROSCI.20-12-04573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocca G, Vicini S. Increased contribution of NR2A subunit to synaptic NMDA receptors in developing rat cortical neurons. J Physiol. 1998;507(Pt 1):13–24. doi: 10.1111/j.1469-7793.1998.013bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol. 2006;95:1727–1734. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. The Journal of Neuroscience. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt J, Coserea I, Pawlak V, Fuchs EC, Kohr G, Seeburg PH, Monyer H. Excitotoxicity in vitro by NR2A- and NR2B-containing NMDA receptors. Neuropharmacology. 2007;53:10–17. doi: 10.1016/j.neuropharm.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37–49. doi: 10.1177/1073858404269012. [DOI] [PubMed] [Google Scholar]
- Weitlauf C, Honse Y, Auberson YP, Mishina M, Lovinger DM, Winder DG. Activation of NR2A-containing NMDA receptors is not obligatory for NMDA receptor-dependent long-term potentiation. J Neurosci. 2005;25:8386–8390. doi: 10.1523/JNEUROSCI.2388-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Wrighton D, Baker E, Chen P, Wyllie D. Mg2+ and memantine block of rat recombinant NMDA receptors containing chimaeric NR2A/2D subunits expressed in Xenopus laevis oocytes. J Physiol. doi: 10.1113/jphysiol.2007.143164. DOI 10.1113/jphysiol.2007.143164.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie DJ, Chen PE. Taking the time to study competitive antagonism. Br J Pharmacol. 2007;150:541–551. doi: 10.1038/sj.bjp.0706997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SJ, Steijaert MN, Lau D, Schutz G, Delucinge-Vivier C, Descombes P, Bading H. Decoding NMDA Receptor Signaling: Identification of Genomic Programs Specifying Neuronal Survival and Death. Neuron. 2007;53:549–562. doi: 10.1016/j.neuron.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, Kaang BK, Zhuo M. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47:859–872. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Zhong J, Russell SL, Pritchett DB, Molinoff PB, Williams K. Expression of mRNAs encoding subunits of the N-methyl-D-aspartate receptor in cultured cortical neurons. Mol Pharmacol. 1994;45:846–853. [PubMed] [Google Scholar]