Abstract
Pre-eclampsia is a pregnancy-related condition characterized by hypertension, proteinuria and endothelial dysfunction. VEGF165b, formed by alternative splicing of VEGF (vascular endothelial growth factor) pre-mRNA, inhibits VEGF165-mediated vasodilation and angiogenesis, but has not been quantified in pregnancy. ELISAs were used to measure means±S.E.M. plasma VEGF165b, sEng (soluble endoglin) and sFlt-1 (soluble fms-like tyrosine kinase-1). At 12 weeks of gestation, the plasma VEGF165b concentration was significantly up-regulated in plasma from women who maintained normal blood pressure throughout their pregnancy (normotensive group, 4.90±1.6 ng/ml; P<0.01, as determined using a Mann-Whitney U test) compared with non-pregnant women (0.40±0.22 ng/ml). In contrast, in patients who later developed pre-eclampsia, VEGF165b levels were lower than in the normotensive group (0.467±0.209 ng/ml), but were no greater than non-pregnant women. At term, plasma VEGF165b concentrations were greater than normal in both pre-eclamptic (3.75±2.24 ng/ml) and normotensive (10.58 ng/ml±3.74 ng/ml; P>0.1 compared with pre-eclampsia) pregnancies. Patients with a lower than median plasma VEGF165b at 12 weeks had elevated sFlt-1 and sEng pre-delivery. Concentrations of sFlt-1 (1.20±0.07 and 1.27±0.18 ng/ml) and sEng (4.4±0.18 and 4.1±0.5 ng/ml) were similar at 12 weeks of gestation in the normotensive and pre-eclamptic groups respectively. Plasma VEGF165b levels were elevated in pregnancy, but this increase is delayed in women that subsequently develop pre-eclampsia. In conclusion, low VEGF165b may therefore be a clinically useful first trimester plasma marker for increased risk of pre-eclampsia.
Keywords: angiogenesis, plasma marker, pre-eclampsia, splice variant, vascular endothelial growth factor165b (VEGF165b), vascular permeability
Abbreviations: AUC, area under the curve; BP, blood pressure; CV, coefficient of variation; EIA, enzyme immunoassay; cEIA, competitive EIA; Flt-1, fms-like tyrosine kinase-1; ROC, receiver operating characteristic; sEng, soluble endoglin; sFlt-1, soluble Flt-1; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor
INTRODUCTION
Pre-eclampsia, the pregnancy-related disease of hypertension, proteinuria and oedema, is responsible for approx. 12% of the world's annual 514000 maternal deaths [1]. Aside from maternal and fetal death, the condition may also result in intra-uterine growth restriction, seizures (eclampsia), renal or liver failure, and placental abruption. Despite much investigation, the pathological processes underlying this disease are still largely undiscovered. Previous investigations have focussed on defective placental implantation as an important aetiological factor, with the resulting release of placentally derived circulating factors, which cause endothelial dysfunction [2–4]. At the microvascular level, there is a state of vasoconstriction from smooth muscle contraction, increased vascular permeability and anti-angiogenesis [5], which correspond to the clinical findings of high BP (blood pressure), oedema and a characteristically small placenta at delivery of the baby.
The VEGF (vascular endothelial growth factor) family is thought to be one of the important molecular systems involved in the pathogenesis of pre-eclampsia. Conventional VEGF, also known as VEGF-A, is made up of six different isoforms formed from alternative exon splicing, resulting in proteins of varying amino acid length, termed VEGFxxx. VEGF165 is the most common isoform of VEGFxxx, and consists of 165 amino acids. VEGF165 acts via its receptor VEGFR-2 to increase vascular permeability, vasodilation and angiogenesis [6]. Endogenous alternative splicing of the VEGFR results in soluble VEGFR-1 {also known as sFlt-1 [soluble Flt-1 (fms-like tyrosine kinase-1)]}, which binds to VEGF and inhibits its function [6]. High levels of sFlt-1 have been documented in pre-eclampsia [7].
VEGF levels in pre-eclampsia have been measured by a number of techniques, with conflicting results according to the technique used. When measured by commercial sandwich ELISAs, which have been proposed to measure only the free unbound forms of VEGF, levels appear to be reduced in pre-eclampsia [8,9]. When measured by RIA or cEIA [competitive EIA (enzyme immunoassay)], VEGF levels have been shown to increase substantially [10]. This discrepancy has been proposed to be due to these latter two methods not being affected by circulating binding proteins [10,11].
In 2002, an alternative family of VEGF-A isoforms were identified, termed VEGFxxxb. These are the same size as conventional VEGF-A, but are alternatively spliced in exon 8 [12]. This alternative splice site selection results in an alternate six amino-acid C-terminus, which affects the property of the isoforms. VEGF165b is the most widely studied of these isoforms. VEGF165b has been shown to inhibit the effects of VEGF165 by binding to its principal receptor VEGFR-2 and preventing it from exerting its physiological effects, such as endothelial cell proliferation and migration. VEGF165b also binds to and activates Flt-1 (VEGFR-1), resulting in a transient increase in capillary hydraulic conductivity, but no sustained increase in permeability, in contrast with VEGF165 [13]. A previous study [14] of VEGF165b in term placenta detected a decrease in VEGFxxxb expression in pre-eclamptic placenta compared with control placenta, and an uncoupling of the splicing link between VEGF165b and VEGF165. To determine whether VEGF165b may play a role in the pathogenesis of pre-eclampsia, we have investigated the expression of VEGF165b in maternal plasma from normotensive and pre-eclamptic pregnancies.
MATERIALS AND METHODS
Subjects
Pregnant subjects were recruited from St Michael's Maternity Hospital, Bristol, U.K. between June 2006 and December 2007. A total of 18 non-pregnant females, aged between 20 and 39 years, were recruited from the University of Bristol, Bristol, U.K. The protocol for the present study was granted ethical approval by Central and South Bristol Research Ethics Committee, and all subjects provided written informed consent. A total of 50 subjects were recruited from routine antenatal clinics in the first trimester of pregnancy. Subjects were aged between 17 and 42 years. Blood was taken from subjects for VEGF165b quantification at recruitment, and at a further three times at 28, 34 and 37 weeks of gestation. Pre-eclampsia was defined as a BP ≥140/90 mmHg on two or more occasions measured 6 h apart and ≥300 mg of proteinuria/24 h, in the absence of a urinary tract infection, occurring after 20 weeks of gestation. Five patients who later developed pre-eclampsia had further blood taken at disease diagnosis. Following venepuncture, blood was immediately centrifuged at 179 g for 10 min at 5 °C, the supernatant was removed and stored at −80 °C until protein quantification. Subjects also received fetal growth ultrasound scans at 28, 34 and 37 weeks of gestation to screen for intra-uterine growth restriction secondary to pre-eclampsia. During the study period, a further 20 patients who developed pre-eclampsia in the third trimester were recruited into the study at disease diagnosis, and received fetal growth scans at the point of recruitment into the study. In these cases, plasma from their first trimesters was obtained from aliquots of frozen plasma stored under the same standard blood storage conditions by the hospital's virology department.
Sample size was calculated to observe an 80% change in mean VEGF165b levels at P<0.05 with a power of >90% given an S.D. equivalent to the mean (calculated using G Power).
VEGF165b ELISA
The anti-VEGFxxxb antibody (MAB3045, clone 56/1; R&D Systems) was coated overnight on to the surface of a sterile Immulon-2HB 96-well plate at a concentration of 200 μg/ml. This antibody recognizes an epitope within a nine amino-acid sequence at the C-terminus of human VEGF165b. The plate was washed three times with 100 μl/well of PBS/0.05% Tween 20. The plate was blocked for 12 h with Superblock (250 μl/well; Pierce). Serial dilutions of recombinant VEGF165b standards (R&D Systems) diluted in PBS/1% (w/v) BSA up to a concentration of 16 ng/ml were then added to the wells in triplicate (200 μl/well). Plasma samples were also added in triplicate (200 μl/well). Plates were then incubated at room temperature (22–24 °C) with shaking for 2 h, and were then washed as above. Biotinylated anti-(human VEGF) affinity-purified polyclonal antibody (50 ng/ml; BAF293; R&D systems), as a detection reagent, was added (200 μl/well) and incubated at room temperature with shaking for 2 h with the plate protected from light. Following a further wash, 100 μl of HRP (horseradish peroxidase)-streptavidin diluted 1:200 in PBS was added for 20 min protected from light, and then substrates A and B (100 μl/well) were added following washing. After 25 min, the colour change was stopped on addition of 1 mol/l H2SO4 (50 μl/well), and the plates were read immediately at a wavelength of 450 nm using a plate photospectrometer (Dynex Technologies). Revelation Quicklink 4.25 software was used to construct a standard curve from mean absorbance values of VEGF165b standards, which enabled estimation of the VEGF165b concentration in plasma samples. VEGF165b sample concentrations were quantified at multiple different concentrations in triplicate to ensure values were in the range of the ELISA. Values are expressed as means±S.E.M.
This sandwich ELISA measures total circulating VEGF165b. It has been shown not to detect VEGF165, and sFlt-1 is known not to interfere due to the use of antibodies against the VEGF165b molecule with epitopes at different parts of the molecule [15]. The CVs (coefficients of variation) of this assay in quantifying VEGFxxxb was 17% for within-subject variation (samples taken at least a week apart), and 7% for within-sample variation, whereas the between-sample CV was >200%, indicating consistency of assay and a significant variation among the population. VEGF165b concentration in maternal plasma was quantified at 8–12, 28, 34 and 37 weeks of gestation in 45 normotensive subjects and four subjects recruited in the first trimester who later developed pre-eclampsia in the third trimester. The VEGF165b concentration was also quantified in 21 preeclamptic patients at 12 weeks of gestation and again in the third trimester at disease diagnosis. A similar version of this ELISA is now available as a DuoSet Kit from R&D Systems.
Endoglin and sFlt-1 ELISAs
ELISAs for sEng (soluble endoglin) and sFlt-1 were carried out on maternal plasma samples using commercial ELISA kits from R&D Systems (DNDG00 and DVR100B respectively), according to the manufacturer's instructions. Values are expressed as means±S.E.M.
Total VEGF ELISA and EIA
Total circulating VEGF was quantified by commercial ELISA (45-VEGFH-0111; Alpco Diagnostics) and by cEIA (QIA69; Calbiochem). The EIA measures both bound and free forms of VEGF. cEIAs for total VEGF quantification have not been commercially available since 2006 and we had access to only a single 96-well EIA. For this reason, total VEGF quantification was possible in only ten patients. For each plasma sample, the VEGF concentration was determined both by ELISA and EIA. Values are expressed as means±S.E.M.
RESULTS
During the study period, 100 patients were recruited: 25 patients had pre-eclampsia and 45 remained normotensive. Of the 30 recruits who were excluded from the study, five developed pregnancy-induced hypertension, one developed idiopathic fetal growth restriction, nine chose not to attend follow-up appointments due to social reasons, two experienced intra-uterine deaths at 21 and 28 weeks of gestation, three experienced pre-term labour in the absence of pre-eclampsia, and ten with pre-eclampsia had no first trimester blood sample available.
The mean maternal age within the normotensive (n=45) and pre-eclamptic (n=25) groups was 30±0.8 and 30±1.3 years respectively (Table 1). There were no differences in smoking status or ethnicity between the groups. Within the pre-eclamptic group, the mean gestational age at diagnosis was 34+5±0.6 weeks, the mean proteinuria was 1.3±0.17 g/24 h and the mean BP was 151/98±3.1/1.7 mmHg (Table 1). Mean birthweight within the pre-eclamptic and normotensive groups was 2513±166 and 3495±481 g respectively. Of the 25 pre-eclamptic patients, six developed early-onset pre-eclampsia (<34 weeks of gestation) and 12 developed pre-eclampsia between 34 and 37 weeks of gestation. The remaining seven patients developed pre-eclampsia at full term. Five of the 25 pre-eclamptic patients developed severe pre-eclampsia [according to the Royal College of Obstetricians and Gynaecologists criteria: systolic BP >169 mmHg or diastolic BP >109 mmHg with proteinuria >1 g/24 h; or the occurrence of HELLP (haemolysis, elevated liver enzymes and low platelet) syndrome]. Five of the 25 fetuses born to pre-eclamptic mothers had growth restriction (ultrasonically defined as estimated fetal weight <10th percentile for gestational age with further evidence of placental insufficiency, such as oligohydramnios or abnormal umbilical artery Dopplers) [16].
Table 1. Clinical characteristics of the study participants.
Values are means±S.E.M. NA, not applicable.
| Characteristic | Normotensive subjects (n=45) | Pre-eclamptic patients (n=25) |
|---|---|---|
| Maternal age (years) | 30±0.8 | 30±1.3 |
| Gestational age at diagnosis (weeks) | NA | 34+5±0.6 |
| Gestational age at birth (weeks) | 39+3±0.17 | 36+3±0.47 |
| Systolic BP (mmHg) | <140 | 151±3.1 |
| Diastolic BP (mmHg) | <90 | 98±1.7 |
| Proteinuria (g/24 h) | <0.3 | 1.3±0.17 |
| Primiparous (%) | 58 | 52 |
| Birthweight (g) | 3495±481 | 2513±166 |
| Platelet count (109/l) | 259±10 | 206±17 |
| Creatinine (mmol/l) | 60±1.3 | 79±2.5 |
Increased VEGF165b in pregnancy
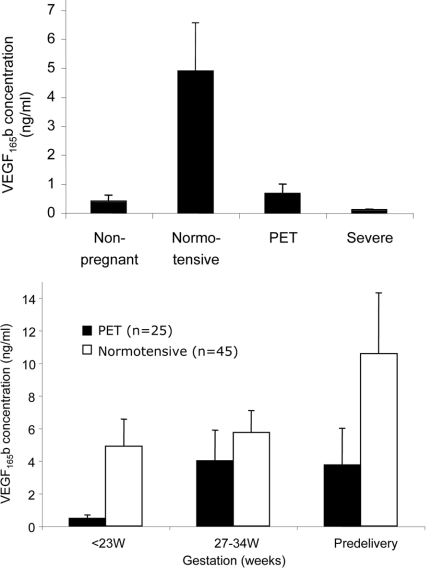
Plasma VEGF165b concentrations from non-pregnant women were 0.40±0.22 ng/ml. In the normotensive group, circulating plasma VEGF165b at 12 weeks of gestation was significantly increased (4.90±1.66 ng/ml; P<0.001, as determined using a Mann–Whitney U test; Figure 1, upper panel) and remained so throughout pregnancy.
Figure 1. Measurement of VEGF165b levels in human plasma.
(Upper panel) At 12 weeks of gestation, VEGF165b was increased in plasma from pregnant women who went on to have normotensive pregnancies (n=45) compared with non-pregnant women. This was not the case in patients who subsequently developed severe early-onset and non-severe pre-eclampsia (n=25; P=0.0003, as determined using a one-way ANOVA and Kruskal–Wallis test). Subgroup analysis of severe/early-onset pre-eclampsia patients (n=9) compared with normotensive subjects also showed that VEGF165b was significantly lowered (P=0.008, as determined using a Mann–Whitney U test). (Lower panel) VEGF165b levels in both pre-eclamptic patients and normotensive subjects were increased in the third trimester (P=0.0012, as determined using a Mann–Whitney U test). Values are means±S.E.M. PET, pre-eclampsia.
Reduced first trimester VEGF165b in patients who later develop pre-eclampsia
At 12 weeks of gestation, the plasma VEGF165b concentration was significantly lower in patients who later developed pre-eclampsia (0.467±0.209 ng/ml) compared with plasma from normotensive pregnancies (Figure 1, upper panel). When the severe early-onset pre-eclampsia subgroup was analysed, a low first trimester VEGF165b concentration was also predictive at 12 weeks (P=0.008, as determined using a Mann–Whitney U test). In contrast, at term there was no significant difference in plasma VEGF165b concentrations between pre-eclamptic (3.75±2.24 ng/ml) and normal (10.58±3.74 ng/ml) pregnancies (Figure 1, lower panel). Thus pre-eclampsia was associated with an 8±1.8-fold increase in plasma VEGF165b from the first trimester to pre-delivery compared with a 2±0.3-fold increase in normotensive plasma (P<0.0012, as determined using a Mann–Whitney U test).
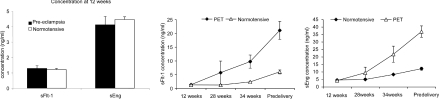
Patients with a lower than median plasma VEGF165b at 12 weeks had elevated sFlt-1 and sEng just before delivery. Concentrations of sFlt-1 and sEng were similar at 12 weeks of gestation in the normotensive and pre-eclamptic groups (Figure 2, left-hand panel). Therefore, at 12 weeks of gestation, neither sFlt-1 nor sEng were able to predict the onset of pre-eclampsia later in the pregnancy (see Figure 6 middle and right-hand panels). At disease diagnosis, however, both sFlt-1 (Figure 2, middle panel) and sEng (Figure 2, right-hand panel) were significantly up-regulated compared with normotensive subjects (P<0.001, as determined using a Mann–Whitney U test).
Figure 2. First trimester sFlt-1 and sEng do not predict an increased risk of pre-eclampsia.
(Left-hand panel) At 12 weeks of gestation, healthy subjects and subjects who later developed pre-eclampsia had similar levels of both sFlt-1 and sEng. Neither plasma marker was able to predict pre-eclampsia at 12 weeks of gestation. Pre-eclampsia was associated with an up-regulation of maternal plasma levels of sFlt-1 (middle panel) and sEng (right-hand panel) relative to first trimester levels. In normotensive pregnancies, plasma levels of both molecules increased with advancing gestational age by 2.8-fold (sEng) and 5.3-fold (sFlt-1). P<0.001, as determined using a Mann–Whitney U test. Values are means±S.E.M. PET, pre-eclampsia.
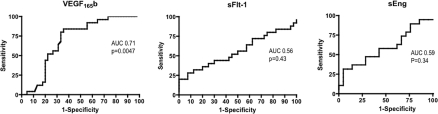
Figure 6. ROC curves for first trimester VEGF165b, sFlt-1 and sEng in the prediction of pre-eclampsia.
AUC was highest for VEGF165b [P=0.0047 compared with random (0.5)]. AUC for sEng and sFlt-1 were 0.59 (P=0.34) and 0.56 (P=0.43) respectively, and were not different from random (0.5).
VEGF165b predicts sFlt-1 and sEng
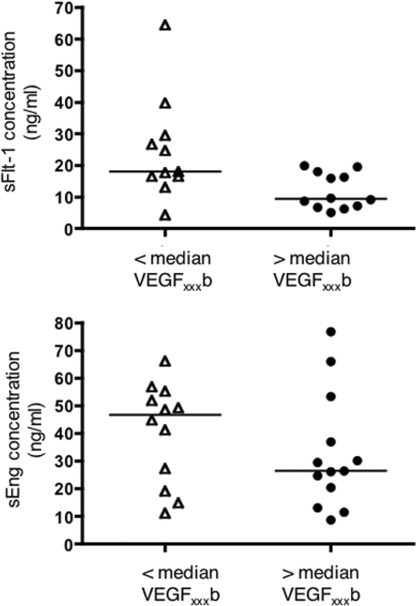
The reduced first trimester levels of VEGF165b were able to predict the elevated sFlt-1 which occurred with the onset of pre-eclampsia (Figure 3, upper panel; P=0.028, as determined using a Mann–Whitney U test); however, VEGF165b concentrations in the first trimester did not correlate with the elevated sEng of pre-eclampsia (Figure 3, lower panel).
Figure 3. Lack of up-regulation of VEGF165b in the first trimester is able to predict the elevated sFlt-1 concentration occurring with the onset of pre-eclampsia but not sEng.
For sFLt-1, P=0.028, as determined using a Mann–Whitney U test. However, first trimester VEGF165b does not correlate with sEng concentration at pre-eclampsia diagnosis.
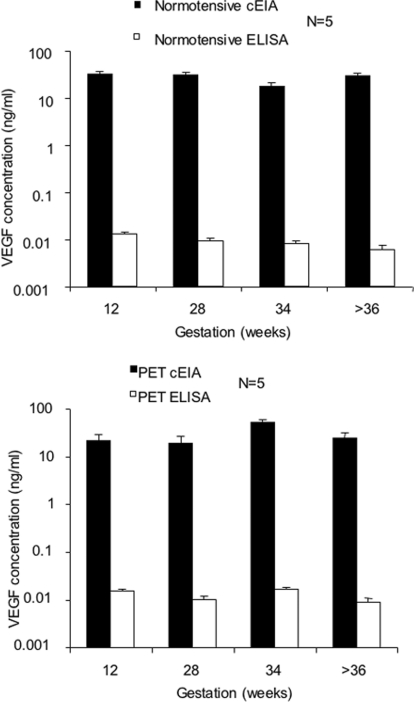
Commercial total VEGF ELISAs underestimate total VEGF levels
Total circulating VEGF was quantified in the same plasma samples both by commercial ELISA and EIA. When quantified by ELISA, VEGF concentrations were on average 2500-fold lower than when quantified by EIA (Figure 4; P<0.0001, as determined using a Mann–Whitney U test).
Figure 4. Total VEGF was quantified both by EIA and ELISA in maternal plasma from normotensive and pre-eclamptic pregnancies (n=10).
Detectable levels of VEGF were 2500-fold lower when measured by ELISA compared with EIA (P<0.0001 as determined using a Mann–Whitney U test). PET, pre-eclampsia. Values are means±S.E.M.
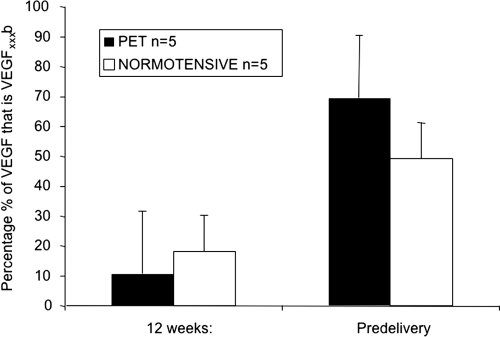
VEGF165b accounts for the majority of total circulating VEGF in the third trimester in pre-eclamptic pregnancy
In five patients from each group, we were able to quantify VEGF165b and total VEGF in the same samples. VEGF165b expression increased in both pre-eclampsia and normotensive pregnancy with increasing gestational age. At 12 weeks of gestation, VEGF165b accounted for 10.5±20% of total plasma VEGF in patients that went on to develop pre-eclampsia compared with 18.1±10% in control subjects (Figure 5). With the onset of pre-eclampsia, VEGF165b accounted for the majority of total circulating VEGF, comprising 69.3±21% of total plasma VEGF in the patient group and 49±12% in the control group.
Figure 5. Increase in VEGF levels observed during pregnancy are primarily due to increased VEGF165b.
At 12 weeks, only a small proportion of total VEGF (10–18%) was VEGF165b (n=10). In contrast, at term approx. 50% of the VEGF was VEGF165b in normotensive subjects, whereas in pre-eclampsia 70% of total VEGF was VEGF165b. Values are means±S.E.M. PET, pre-eclampsia.
VEGF165b levels at 12 weeks predict pre-eclampsia
To determine which of VEGF165b, sFlt1 and sEng are more accurate prognostic factors, ROC (receiver operating characteristic) curves were generated by calculating sensitivity (proportion of times that the test predicts pre-eclampsia) and specificity (proportion of times that the test excluded pre-eclampsia). Thus a high sensitivity value would include all patients, but if not discriminatory would provide a low specificity value (and would include false positives). Thus a non-discriminatory test would give a straight line with a slope of 1 and an AUC (area under the curve) of 0.5. A perfect discriminatory test would have an AUC of 1.0. As shown in Figure 6 (left-hand panel), VEGF165b levels have an AUC significantly greater than 0.5 in contrast with sFlt-1 (Figure 6, middle panel) and sEng (Figure 6, right-hand panel).
DISCUSSION
There have been a number of studies investigating the VEGF family of proteins in pre-eclampsia [4,17,18], which have suggested that they may play a role in its pathophysiology [19,20]. In the present study, the total VEGF levels measured by EIA are consistent with those measured previously using this assay methodology [11] and by those using an independent method, the RIA [10]. In contrast, the ELISA results in the present study from the same samples gave much lower readings, consistent with previous ELISA reports of plasma VEGF [21]. These experiments therefore highlight the discrepancy reported previously between measurements of total circulating VEGF in plasma by commercial ELISAs compared with cEIA or RIA [22]. The antibodies used in the ELISA are two monoclonals raised against the VEGF peptide sequence and thus may be raised against a similar or identical epitope. The ELISA appears to yield artificially low results, presumably as VEGF is bound by agents in plasma which prevent its detection by both antibodies simultaneously. sFlt-1 does not affect this ELISA when given as a recombinant protein [15], but the effect of endoglin or other plasma constituents have not been tested. The discrepancy was particularly striking after measurement of VEGF165b levels, using an ELISA that detects plasma VEGF165b using two antibodies that have epitopes on completely separate parts of the antigen (VEGF) molecule. It is therefore rather disturbing that the cEIA is no longer commercially available and was withdrawn from sale by all known suppliers between 2006 and 2007.
Of the VEGF family, VEGF165, the most widely studied form [6], is known to increase vascular leakage, induce vasodilation and promote angiogenesis. Although this isoform is up-regulated in pre-eclampsia, its metabolic activities may be blocked by other proteins which bind to VEGF and inhibit its function. sFlt-1 and sEng both bind to VEGF and prevent it from exerting its physiological effects [23]. sFlt-1 is an anti-angiogenic molecule that is able to induce a pre-eclamptic-like syndrome of hypertension and proteinuria when administered to pregnant rats [7]. sEng is an anti-angiogenic protein that inhibits TGF (transforming growth factor) β1 and β3 signalling and increases the severity of pre-eclampsia occurring in pregnant rats treated with sFlt-1 [24]. However, neither molecule can be used clinically as a first trimester marker of pre-eclampsia as sFlt-1 levels are observed to increase only 5 weeks before the onset of the clinical disease [25], and sEng concentrations become elevated at 17 weeks of gestation [23].
In 2002, VEGF165b was identified in normal renal cortex and was subsequently shown to be present in many different tissues, and forms the majority of VEGF in tissues such as human colon [15] and vitreous [26]. VEGF165b is relatively down-regulated in many conditions, including prostate, renal, bowel and skin cancers [12,15,25,27,28], diabetic retinopathy [26], Denys–Drash Syndrome [29] and in the placenta of patients with pre-eclampsia [14]. The mechanisms underlying these changes in expression are still under investigation, but the reduction is associated with excess angiogenesis. VEGF165b has been shown to be anti-angiogenic in animal models of VEGF165-induced blood vessel growth in the cornea [30], mouse subcutaneous tissue [31] and rat mesentery [27], and inhibits physiological [32] and pathological [15,30,32] angiogenesis. Studies have also shown that VEGF165b transiently, but not chronically, increases hydraulic conductivity [13].
The results shown in the present study indicate that VEGF165b fails to be up-regulated in the first trimester in those pregnancies that will later be complicated by pre-eclampsia. It can be concluded that VEGF165b may be a clinically useful first trimester marker for increased pre-eclampsia risk, providing for instance a guide to commencement of first trimester oral aspirin therapy, as this decreases the incidence of pre-eclampsia by 15% [33].
It is not clear what mediates the up-regulation of VEGF165b in early pregnancy or what prevents it in women who will develop pre-eclampsia, and further work must be done to investigate this finding. The failure of up-regulation may be reflective of the aetiology or could be contributory to the subsequent pre-eclampsia. For instance, in the first trimester, the reduced anti-angiogenic VEGF165b compared with normal pregnancy may reflect a maternal vasculature response to try and correct the defective implantation processes underlying the disease, or the failure to up-regulate VEGF165b may contribute to defective implantation.
Acknowledgments
We would like to thank the women who donated their time and their plasma to this study.
FUNDING
This work was supported by the British Heart Foundation [grant numbers FS/05/100, BS06/005]; and the Wellcome Trust [grant number 74702].
References
- 1.Khan K. S., Wojdyla D., Say L., Gülmezoglu A. M., Van Look P. F. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–1074. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Y., Damsky C. H., Fisher S. J. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J. Clin. Invest. 1997;99:2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maynard S. E., Venkatesha S., Thadhani R., Karumanchi S. A. Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr. Res. 2005;57:1R–7R. doi: 10.1203/01.PDR.0000159567.85157.B7. [DOI] [PubMed] [Google Scholar]
- 4.Levine R. J., Maynard S. E., Qian C., et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 5.Baumwell S., Karumanchi S. A. Pre-eclampsia: clinical manifestations and molecular mechanisms. Nephron Clin. Pract. 2007;106:c72–c81. doi: 10.1159/000101801. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr. Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 7.Maynard S. E., Min J. Y., Merchan J., et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Livingston J. C., Chin R., Haddad B., McKinney E. T., Ahokas R., Sibai B. M. Reductions of vascular endothelial growth factor and placental growth factor concentrations in severe preeclampsia. Am. J. Obstet. Gynecol. 2000;183:1554–1557. doi: 10.1067/mob.2000.108022. [DOI] [PubMed] [Google Scholar]
- 9.Lyall F., Young A., Boswell F., Kingdom J. C., Greer I. A. Placental expression of vascular endothelial growth factor in placentae from pregnancies complicated by pre-eclampsia and intrauterine growth restriction does not support placental hypoxia at delivery. Placenta. 1997;18:269–276. doi: 10.1016/s0143-4004(97)80061-6. [DOI] [PubMed] [Google Scholar]
- 10.McKeeman G. C., Ardill J. E., Caldwell C. M., Hunter A. J., McClure N. Soluble vascular endothelial growth factor receptor-1 (sFlt-1) is increased throughout gestation in patients who have preeclampsia develop. Am. J. Obstet. Gynecol. 2004;191:1240–1246. doi: 10.1016/j.ajog.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Lee E. S., Oh M. J., Jung J. W., et al. The levels of circulating vascular endothelial growth factor and soluble Flt-1 in pregnancies complicated by preeclampsia. J. Korean Med. Sci. 2007;22:94–98. doi: 10.3346/jkms.2007.22.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bates D. O., Cui T. G., Doughty J. M., et al. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62:4123–4131. [PubMed] [Google Scholar]
- 13.Glass C. A., Harper S. J., Bates D. O. The anti-angiogenic VEGF isoform VEGF165b transiently increases hydraulic conductivity, probably through VEGF receptor 1 in vivo. J. Physiol. 2006;572:243–257. doi: 10.1113/jphysiol.2005.103127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bates D. O., MacMillan P. P., Manjaly J. G., et al. The endogenous anti-angiogenic family of splice variants of VEGF, VEGFxxxb, are down-regulated in pre-eclamptic placentae at term. Clin. Sci. 2006;110:575–585. doi: 10.1042/CS20050292. [DOI] [PubMed] [Google Scholar]
- 15.Varey A. H., Rennel E. S., Qiu Y., et al. VEGF165b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br. J. Cancer. 2008;98:1366–1379. doi: 10.1038/sj.bjc.6604308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alberry M., Soothill P. W. Management of fetal growth restriction. Arch. Dis. Child. Fetal Neonatal Ed. 2007;92:F62–F67. doi: 10.1136/adc.2005.082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brockelsby J., Hayman R., Ahmed A., Warren A., Johnson I., Baker P. VEGF via VEGF receptor-1 (Flt-1) mimics preeclamptic plasma in inhibiting uterine blood vessel relaxation in pregnancy: implications in the pathogenesis of preeclampsia. Lab. Invest. 1999;79:1101–1111. [PubMed] [Google Scholar]
- 18.Sgambati E., Marini M., Zappoli Thyrion G. D., et al. VEGF expression in the placenta from pregnancies complicated by hypertensive disorders. Br. J. Obstet. Gynaecol. 2004;111:564–570. doi: 10.1111/j.1471-0528.2004.00143.x. [DOI] [PubMed] [Google Scholar]
- 19.Li Z., Zhang Y., Ying Ma J., et al. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension. 2007;50:686–692. doi: 10.1161/HYPERTENSIONAHA.107.092098. [DOI] [PubMed] [Google Scholar]
- 20.Murakami Y., Kobayashi T., Omatsu K., et al. Exogenous vascular endothelial growth factor can induce preeclampsia-like symptoms in pregnant mice. Semin. Thromb. Hemostasis. 2005;31:307–313. doi: 10.1055/s-2005-872437. [DOI] [PubMed] [Google Scholar]
- 21.Nadar S. K., Karalis I., Al Yemeni E., Blann A. D., Lip G. Y. Plasma markers of angiogenesis in pregnancy induced hypertension. Thromb. Haemostasis. 2005;94:1071–1076. doi: 10.1160/TH05-03-0167. [DOI] [PubMed] [Google Scholar]
- 22.Anthony F. W., Evans P. W., Wheeler T., Wood P. J. Variation in detection of VEGF in maternal serum by immunoassay and the possible influence of binding proteins. Ann. Clin. Biochem. 1997;34:276–280. doi: 10.1177/000456329703400309. [DOI] [PubMed] [Google Scholar]
- 23.Levine R. J., Lam C., Qian C., et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N. Engl. J. Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 24.Venkatesha S., Toporsian M., Lam C., et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 25.Levine R. J., Maynard S. E., Qian C., et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 26.Perrin R. M., Konopatskaya O., Qiu Y., Harper S., Bates D. O., Churchill A. J. Diabetic retinopathy is associated with a switch in splicing from anti- to pro-angiogenic isoforms of vascular endothelial growth factor. Diabetologia. 2005;48:2422–2427. doi: 10.1007/s00125-005-1951-8. [DOI] [PubMed] [Google Scholar]
- 27.Woolard J., Wang W. Y., Bevan H. S., et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64:7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- 28.Pritchard-Jones R. O., Dunn D. B., Qiu Y., et al. Expression of VEGFxxxb, the inhibitory isoforms of VEGF, in malignant melanoma. Br. J. Cancer. 2007;97:223–230. doi: 10.1038/sj.bjc.6603839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schumacher V. A., Jeruschke S., Eitner F., et al. Impaired glomerular maturation and lack of VEGF165b in Denys-Drash syndrome. J. Am. Soc. Nephrol. 2007;18:719–729. doi: 10.1681/ASN.2006020124. [DOI] [PubMed] [Google Scholar]
- 30.Konopatskaya O., Churchill A. J., Harper S. J., Bates D. O., Gardiner T. A. VEGF165b, an endogenous C-terminal splice variant of VEGF, inhibits retinal neovascularization in mice. Mol. Vis. 2006;12:626–632. [PubMed] [Google Scholar]
- 31.Rennel E., Waine E., Guan H., et al. The endogenous anti-angiogenic VEGF isoform, VEGF165b inhibits human tumour growth in mice. Br. J. Cancer. 2008;98:1250–1257. doi: 10.1038/sj.bjc.6604309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu Y., Bevan H., Weeraperuma S., et al. Mammary alveolar development during lactation is inhibited by the endogenous antiangiogenic growth factor isoform, VEGF165b. FASEB J. 2008;22:1104–1112. doi: 10.1096/fj.07-9718com. [DOI] [PubMed] [Google Scholar]
- 33.Duley L., Henderson-Smart D., Knight M., King J. Antiplatelet drugs for prevention of pre-eclampsia and its consequences: systematic review. Br. Med. J. 2001;322:329–333. doi: 10.1136/bmj.322.7282.329. [DOI] [PMC free article] [PubMed] [Google Scholar]