Abstract
Objective
Vascular endothelial growth factor (VEGF)-induced vascular permeability has been shown to be dependent on calcium influx, possibly through a transient receptor potential cation channel (TRPC)-mediated cation channel with properties of the TRPC3/6/7 subfamily. To investigate further the involvement of this subfamily, we determined the effects of dominant negative TRPC6 overexpression on VEGF-mediated changes of human microvascular endothelial cell (HMVEC) calcium, proliferation, migration, and sprouting.
Methods
Cytoplasmic calcium concentration was estimated by fura-2 fluorescence spectrophotometry, migration by Boyden chamber assay, sprouting by immunofluorescence imaging of stimulated endothelial cells, and proliferation by flow cytometry.
Results
Overexpression of a dominant negative TRPC6 construct in HMVECs inhibited the VEGF-mediated increases in cytosolic calcium, migration, sprouting, and proliferation. In contrast, overexpression of a wild-type TRPC6 construct increased the proliferation and migration of HMVECs.
Conclusions
TRPC6 is an obligatory component of cation channels required for the VEGF-mediated increase in cytosolic calcium and subsequent downstream signaling that leads to processes associated with angiogenesis.
Keywords: VEGF, TRPC6, calcium, angiogenesis
Blood-vessel growth and altered vascular permeability in many physiological and pathological conditions are regulated by the heparin-binding dimeric glycoprotein, vascular endothelial growth factor (VEGF). VEGF stimulates endothelial cell migration, tube formation, and proliferation, at least in part, through VEGF-receptor-mediated calcium influx into the endothelial cell. The mechanisms by which VEGF induces this calcium entry are not yet understood, although recent evidence suggests a role for the nonselective canonical transient receptor potential cation channels (TRPCs) [5, 12, 17]. TRPC channels form two subtypes, the TRP1/4/5 family of store-operated cation channels and the TRP3/6/7 family of receptor-operated channels [6]. TRPC1/4/5 channels generally require store depletion to activate channel opening, whereas TRPC3/6/7 channels can be activated by chemical mediators, such as the product of phospholipase C (PLC), diacylglycerol (DAG), or its analog, oleolyl acetyl glycerol (OAG) [11]. VEGF induces increases in intracellular calcium concentration in endothelial cells [4], and the VEGF-mediated vascular permeability and calcium entry in rat and frog microvessels has been shown to be dependent on calcium influx into the cell [2]. Recent studies have identified the TRPC3/6/7 subfamily of TRPC channels in microvascular endothelial cells [16, 17], and TRPC1/4/5 subfamily of TRPC channels in human umbilical vein endothelial cells [1, 12] as prime candidates for the VEGF-induced cation entry in human endothelial cells. TRPC proteins can form cation channels by heterotetrameric as well as homotetrameric interactions within each subfamily, thus TRPC3, -6, and -7 can form heterotetrameric or homotetrameric complexes with each other [11], and TRPC1, -4, and -5 can do the same with each other [15]. As the action of VEGF in vivo is primarily through the microvascular endothelium, we investigated the role of TRPC3/6/7 channel subfamily in VEGF-mediated calcium entry and cellular processes associated with angiogenesis by the overexpression of a dominant negative TRPC6 in human microvascular endothelial cells (HMVECs).
METHODS
Materials
All chemicals and solutions were obtained from Sigma, unless otherwise stated. HMVECs and endothelial cell growth medium (EBM-2) were obtained from Clonetech (Lonza, Switzerland. These cells were used within four passages from defrosting. TRPC6 cDNA was a kind gift of Thomas Gudermann (Phillipps-Universität, Marburg, Germany). The C terminal myc-tagged nonfunctional dominant negative TRPC6 was a kind gift of William Cole (University of Calgary, Alberta, Canada).
Generation of Recombinant Adenoviruses
Replication-deficient adenoviruses carrying human wild-type (WT) AdvTRPC6 (2795 bp, NM 004621) and dominant negative (DN) human TRPC6 (LFW678-680AAA) were constructed using the Qbiogene AdEasy Vector System (Qbiogene, Nottingham, UK). Briefly, full-length WT and DN TRPC6 cDNA were cloned into the pShuttle vector under the transcriptional control of cytomegalovirus (CMV) early gene promoter/enhancer. Using the CMV promoter primer and TRPC6-specific reverse primer, the generated clones were sequenced in both directions to confirm the correct sequence and in frame insert. pShuttle-TRPCs were linearized with Pme1 and cotransformed into Escherichia coli BJ5183 cells (Strategene, Agilent Technologies, Cheshire, UK) with an adenoviral backbone plasmid, pADEasy-1, using Electroporation (200 Ohms, 25 μF, 2.5 KV; BioRad, Laboratories Ltd, Herts, UK). Recombinants were generated by homologous recombination and selected for Kanomycin resistance. Recombination was confirmed by restriction analysis and polymerase chain reaction (PCR), using TRPC6-specific primers. The positive recombinants were transformed into DH5α for propagation and transducing packaging cells. Amplified plasmid was purified by using a Maxi-prep kit (Qiagene Ltd, West Sussex, UK) and linearized by using Pac1. HEK293T cells were transduced with linearized Adv-TRPC6s plasmids, using standard Lipofectamine-Opti-MEM (Invitrogen Ltd., Paisley, UK) reagents. Transfection was monitored by the presence of Adv-EGFP (a kind gift from J. Uney, University of Bristol). Over the following two to three weeks, viral plaque formation and amplification of virus was performed according to the manufacturer’s instructions. Briefly, plaques were selected and used to transduce HEK293T cells in six-well plates and the first generation of the virus was produced by three rounds of freeze/thaw (liquid nitrogen/37°C). Cell debris was removed by centrifugation and the supernatant stored at −80°C until use. The presence of recombinant Adv-TRPC6s was confirmed by PCR, using protease-K-treated viral supernatant as a template and TRPC6-specific primers. Subsequent passages of virus were generated by transducing HEK293T cells with 50% of previously amplified viral stock. Large-scale production of virus was performed in T75 flasks of HEK cells at 90% confluency. The virus was purified by double ultracentrifugation in saturated CsCl solution (Sorvall Discovery Ultracentrifuge 90S; Kendro, Dorset, UK) at 65,000 rpm. Virus was desalted by using a PD-10 column (Pharmacia, GE Healthcare Ltd, Bucks, UK). The tissue-culture infectious dose (TCID50) was determined according to the manufacturers’ instructions (Qbiogene) before the infection of the endothelial cells. Eight serial dilutions (in duplicate) of the virus were added to 104 HEK cells and after 10 days of incubation at 37°C, the cytopathic effect (CPE) in each well was checked under an inverted microscope. The ratio of positive wells per row was determined and TCID50 was calculated by using the Karber statistic method: T = 10 + d (s −0.5), where d = log of dilution and S = the sum of the ratios (Qbiogene).
Transduction of HMVEC
Cells were seeded onto coverslips in six-well plates to give approximately 80% confluency. Before infection, cells were washed three times with phosphate-buffered saline (PBS) and serum starved in Opti-MEM (Gibco, Invitrogen, Paisley, UK) for approximately two to four hours. Diluted viral solution (2 × 106/mL particles in 200 μL of PBS) was added directly to the cells and the plates were incubated for 15-20 minutes at 37°C. Serum-free medium was added to a volume of 2ml to the wells and, after incubation for a further four to six hours, the growth medium was replaced with viral solution and cells were cultured for 48 hours. In all infection procedures, cells were infected with Adv-EGFP to monitor the transfection efficiency.
Analysis of mRNA Encoding TRPC 1, TRPC Subfamily 3, 6, 7, and ß-actin by RT-PCR
RNA was isolated from cells by using an RNeasy kit (Qiagen). Complementary DNA was synthesized with the Promega reverse transcriptase (RT) kit. PCR amplification of cDNA encoding human TRPCs and ß-actin was performed for 30 cycles of 95°C for one minute, 55°C for two minutes, and 72°C for three minutes.
Western Blotting
Cells were lysed on ice in Triton-X lysis buffer (20 mM Tris, pH 7.5, 1.5% Triton-X, 150 mM NaCl2, 10% glycerol, 1 mM ethylenediaminetetraacetic acid [EDTA]) containing proteinase inhibitors. The samples were cleared by centrifugation at 13,000 rpm for three minutes at 4°C and the pellet discarded. Total protein was then quantified by using the Bradford assay (Bio-Rad), according to the manufacturer’s instructions. Protein samples were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and were transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked in 10% fat-free milk before incubation with antibodies, as described above. After incubation with horseradish-peroxidase-conjugated secondary antibodies (Santa Cruz, Insight Biotechnology Ltd, Middlesex, UK), bands were detected by using the SuperSignal West Femto Maximum Sensitivity Substrate (Pierce, Perbio Science Ltd, Northumberland, UK).
Cell Migration Assay
Transfected Ad-TRPC6, Ad-Null, WT-TRPC6, and untransfected HMVECs were serum starved in EBM-2 for four hours, resuspended at 10 × 104 cells/mL in EBM containing 0.1%, 5% fetal bovine serum (FBS), and 40 ng/mL of VEGF in 0.1% FBS and plated on the 5% collagen-A-coated millicell inserts (8-μm pore size; Millipore, Billerica, MA, USA) in 24-well plates. Cells were allowed to migrate overnight at 37°C. The inserts were washed with 2 × 2 times PBS and fixed with 100% (10 minutes), 70% (two minutes), and 50% (two minutes) methanol. After removing the membrane from the insert and wash in PBS, the cells were stained with hematoxylin for 30 minutes at room temperature. The membranes were washed at least 3 times with distilled water and mounted. The migrated cells were counted under the microscope (40× magnification). Each experimental sample was in quadruplicate and counted in 10 different views away from the insert edge.
Analysis of Cell Cycle by Flow Cytometry
HMVEC cells were transduced with Ad-TRPC6 constructs, as previously described. After 48 hours, the medium was replaced with basal medium supplemented with VEGF (40 ng/mL) and cells were incubated for 24 hours. To analyze cell-cycle progression, cells were collected by trypsinization (1 × 106 cells) and centrifuged at 2,000 rpm for five minutes. The cells were resuspended in 1 mL of cold 70% ethanol and stored at 4°C for at least 30 minutes. For propidium iodide (PI) staining, the pelleted fixed cells were washed three times with cold PBS and resuspended in 1 mL of PI solution (50 μg/mL) and 100 U RNAse A. Cells were stained for more than one hour at 4°C and then analyzed by flow cytometry to obtain a histogram of DNA content relative to the cell number. The percentage of cells in the S phase was determined by analyzing the data, using ModFitLT software (ModFitLT, BD Bioscience, Oxford, UK).
In Vitro “Angiogenesis” Assay
EC Matrix Gel Preparation
The gel solution was prepared according to the manufacturer’s instructions (Chemicon International, Temecula, California, USA). Thirty milliliters of gel solution was transferred to each well of precooled culture slides (BD Falcon, Bedford, MA, USA) and carefully spread into a thin layer with a pipette tip. Incubation of the slides at 37°C let the gels solidify. VEGF165 was added on one side of the gels to a 1-nM final concentration.
Confluent layers of transfected and untransfected HMVEC cells were serum starved for three hours with EBM-2. The cells were then trypsinized (0.05% trypsin-EDTA; Gibco) and resuspended in EBM-2. Ten thousand cells in 100 mL of EBM-2 were seeded onto the gels and incubated at 37°C. The structure formation was observed under an inverted light microscope (Leica, DM-RB Fluorescent Microscope, Wetzlar, Germany, 10× and 20× magnification) and the assay stopped after five hours. After the removal of the media, cell structures were fixed with 4% paraformaldehyde/PBS, pH 7.4, for five minutes and washed twice with PBS. For F-actin staining, cell structures were incubated for one hour with Alexa 488 phalloidin (dilution 1:200 in PBS/0.5% Triton; Molecular Probes, Eugene, Oregon, USA) and 10 minutes with Hoechst 33342 (5 mg/mL PBS/0.5% Triton) at room temperature. Gels were washed twice with PBS/0.5% Triton and twice with PBS and mounted with Vectashield (Vector Laboratories, Burlingame, California, USA). Images were taken on a Leica DM RB fluorescence microscope for structural analysis, counting sprouts in six to eight random frames
Cytosolic Calcium Concentration ([Ca2+]c) Measurements
Ad-dnTRPC6s and untransfected HMVEC cells on 22-mm glass coverslips were serum starved for two to four hours and incubated with Fura 2-AM (25 μM) and 0.006% pluronic (Molecular Probes, Leiden, Netherlands) for ∼60 minutes in serum-free EBM-2 media at 37°C. The coverslip was then placed in a coverslip holder and subsequently transferred to a perfusion chamber. The holder was mounted on a rig consisting of an inverted fluorescence microscope (DM IRB; Leica) equipped with a UV source (Cairn Instruments, World Precision Instruments, Leica, DM 1RB, Inverted Microscope, Wetzlar, Germany) with filters for excitation at 340 and 380 nm. Fast switching was achieved by using a rotary filter wheel at 50 Hz and a spectrophotometer for photometric measurement (Cairn Instruments). The spectrophotometer received emitted light via a 400-nm dichroic filter and a 510-530-nm barrier filter in front of the photometer. Powerlab Chart v5 software (AD Instrument Ltd, Oxfordshire, UK) was used for data acquisition and Microsoft Excel (Microsoft Office, USA) for analysis and graphical display.
Experiments were conducted in Krebs Ringer solution (150 mM NaCl, 6 mM KCl, 1 mM MgCl2, 5 mM CaCl2, 10 mM D-glucose, and 10 mM HEPES). Cells were superfused with Krebs Ringer solution for 10 minutes or until a steady baseline was achieved. Cells were then superfused with Krebs Ringer containing 40 ng/mL (1 nM) VEGF or 100 μM 1-oleyl-2-acetyl-sn-glycerol (OAG), shown in previous patch-clamp experiments to result in a delayed cation entry in HMVECs [5]. To ensure that the cells remained viable at the end of the experiment 10 μM of ionomycin was added and [Ca2+]c measured. Then, 5 mM of manganese chloride (MnCl2), in the continued presence of 10 μM of ionomycin was used to quench the Fura to determine the background (Ca2+-independent) fluorescence signal.
Emission fluorescence measurements (If) were taken four times a second. The ratio of the If measured during the 340-nm excitation to that during the 380-nm excitation (Rexp) is directly proportional to the Ca2+ concentration in the cells, where Rexp = (If340-B340)/(If380-B380). If340 is the If measured during excitation at 340 nm, If380 is the If measured during excitation at 380 nm, and B340 and B380 are the background If values measured during excitation at 340 and 380 nm, respectively (measured as the If after Mn2+quenching). To allow comparison between experiments, Rexp was normalized (giving the Rnorm) to the If340/If380 measured during the in vitro calibration in minimal [Ca2+] (Rmin).
The use of a ratiometric dye, such as Fura-2, eliminates errors introduced by small changes in fluorescent signal (due to, for example, changes in focal plane), which are independent of changes in [Ca2+]c. Data are expressed relative to the baseline for each cell population.
RESULTS
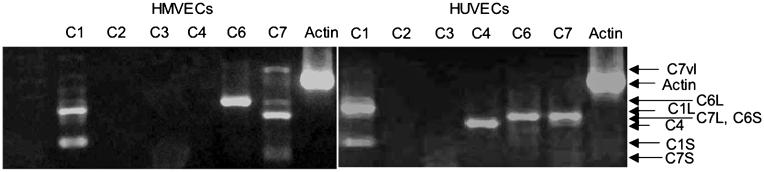
To determine the relative expression of TRPCs in HMVEC and HUVEC, RNA extracted from the two cell types was reverse transcribed and amplified by PCR, using primers for TRPC channels. Figure 1 shows that HUVECs and HMVECs differ in their relative TRPC channel expression. HMVECs expressed both splice isoforms of TRPC1 [20], all three TRPC7 isoforms [21], and a single form of TRPC6 (Figure 1A) of the same length as the full length cDNA control (not shown). HUVECS expressed the same isoforms of TRPC1 and TRPC7, but also expressed a single isoform of TRPC4, and TRPC6 primers gave a band of a different length from in HMVECs (Figure 1B). To determine the role of TRPC channels in HMVECs, adenoviruses were generated by expressing dominant negative TRPC6 (Ad-dnTRPC6) [13]. Figure 2 shows that the transduction of CHO cells with Ad-TRPC6 or Ad-dnTRPC6 resulted in the expression of both forms of the protein. Cells transduced with Ad-null, or untransduced cells, did not express TRPC6 protein. HMVECs transduced with the adenovirus resulted in the expression of the dnTRPC6 by Western blot, as evidenced by an increase in band density (Figure 2B). Expression of endogenous TRPC6 was confirmed by Western blot in HMVECs (Figure 2B). The dominant negative adenovirus was then used to transduce HMVECs and investigate cytosolic calcium concentration after being stimulated by TRPC activators. The DAG analog, OAG, is known to activate the TRPC3/6/7 family of TRP channels. Figure 3 shows that 100-μM OAG treatment of HMVECs resulted in a delayed, slow, but significant increase in intracellular calcium in both untransduced and vector-transduced cells. Delayed cation entry in response to OAG application is consistent with previous observations from HMVECs and vascular smooth muscle cells (for example, [5, 10]). In contrast, HMVECs transduced with the Ad-dnTRPC6 did not increase intracellular calcium in response to OAG. Figure 3B shows that the mean calcium concentration was significantly increased after OAG treatment in untransduced and wild-type TRPC6-expressing cells, but this increase was abolished by transduction with Ad-dnTRPC6. Figure 3C shows that there was a significant 70 ± 10% increase in the Rnorm in response to OAG in untransduced HMVECs (from 1.94 ± 0.11 to 3.35 ± 0.45; p < 0.01, paired t-test), and a 50 ± 23% increase in vector-transduced HMVECs (from 2.48 ± 0.39 to 3.77 ± 0.88; p = 0.06, paired t-test), but not in dominant negative transduced HMVECs (4 ± 3.3%, from 2.03 ± 0.28 to 2.06 ± 0.22; p > 0.1). These results show that the dn-TRPC6 adenovirus can inhibit OAG-sensitive cation channels required for calcium entry.
Figure 1.
Transient receptor potential cation channel (TRPC) mRNA expression of human umbilical vein endothelial cells (HUVECs) and microvascular endothelial cells (HMVECs). Expression of TRPC mRNA extracted from HUVEC and HMVEC was compared by RT-PCR. Expression of both the short (C1S) and long (C1L) isoforms of TRPC1 were seen in both cell types. Three isoforms of TRPC7 were seen in HMVEC, short (C7S), long (C7L), and very long (C7vl), whereas only one isoform (C7L) was seen in HUVECs. Two isoforms of TRPC6 were detected, a shorter isoform in HMVEC (C6S), and a longer isoform in HUVEC (C6L). A single isoform of RPC4 was seen in HUVECs only.
Figure 2.
Adenovirus transduction results in dnTRPC6 expression. (A) Chinese hamster ovary cells do not express TRPC6, but transduction resulted in the strong expression of dn-TRPC6. Transduction with wt-TRPC6 demonstrates specificity of the antibody. (B) Human microvascular endothelial cells transduced with dn-Ad-TRPC6 show increased TRPC6 protein compared with untransduced (no virus; both wild-type and dnTRPC6 are detected by this antibody).
Figure 3.
Oleolyl glycerol (OAG)-mediated increase in intracellular calcium in human microvascular endothelial cells (HMVECs) is inhibited by DN-TRPC6. HMVECs were transduced with adenoviruses generated by using the empty vector, dnTRPC6, or untransduced. Cells were then loaded with Fura-2, and calcium concentration was estimated from the fluorescence intensity of the dye at 340 nm excitation to that at 380 nm (Rnorm). (A) Whereas 100 μM of OAG induced an increase in Rnorm—indicating increased calcium—in control and vector-transduced cells, the OAG-mediated increase in calcium was blocked by the Ad-dnTRPC6. (B) OAG induced an increase in Rnorm with control and Adnull-transduced, but not with dnTRPC6-transduced, HMVECs. (C) The increase with OAG was significantly greater in AdNull-transduced than in dnTRPC6-transduced cells (* p = 0.06, ** p < 0.01 paired t-test, + p < 0.05 compared with dnTRPC6, Student Newman Keuls (SNK) post-hoc ANOVA).
To determine whether the VEGF-mediated cation entry was inhibited by dn-TRPC6, HMVECs were transduced with either the control vector or Ad-dnTRPC6. Whereas 1 nM of VEGF resulted in a significant increase in cytosolic calcium after five minutes incubation in vector-transduced HMVECs, this increase was not seen in Ad-dnTRPC6 transduced HMVECs (Figure 4A). Whereas VEGF increased the fluorescence ratio of Fura by 53 ± 13%, Ad-dnTRPC6 transduced HMVECs did not increase cytosolic calcium (10 ± 3.8% increase in ratio; p < 0.05, compared with control vector).
Figure 4.
Vascular endothelial growth factor (VEGF)-increased calcium is inhibited by DN-TRPC6. Human microvascular endothelial cells were transduced with either an adenovirus containing an empty vector (Ad-Null, grey) or dominant negative TRPC6 (dnTRPC6), loaded with Fura, and then treated with 1 nM of VEGF. * p < 0.05 compared with vector alone.
To determine whether the increase in calcium was necessary for processes that contribute toward the angiogenic properties of VEGF, we measured the effect of transducing HMVEC with dnTRPC6 on the migration of endothelial cells, using a modified Boyden chamber assay. Figure 5A shows that transduction of cells with either vector, WT-TRPC6 or dnTRPC6, did not affect migration in response to a minimal stimulus (0.1% serum). In contrast, HMVEC stimulation with VEGF resulted in a 96±7.5% increase in migration relative to 0.1%FCS. Both Ad-null treated (95 ± 21%) and Ad-wt-TRPC6 treated HMVECs (127 ± 11%) significantly migrated across the inserts. This migration was significantly inhibited by Ad-dn-TRPC6 transduction (16.9 ± 12%). To ensure that this was not a nonspecific effect, Figure 5C shows that whereas untransduced and vector transduction resulted in a VEGF-mediated migration that was 57 ± 6.7 and 59 ± 12%, respectively, of that induced by 10% FBS, dnTRPC6 transduced HMVECs treated with VEGF migrated at only 19 ± 12% of the FBS-mediated migration of those same Ad-dnTRPC6 transduced HMVECs. These results show that dn-TRPC6 inhibits VEGF-mediated migration specifically, indicating that cation entry through the TRPC3/6/7 family of channels is required for VEGF-mediated endothelial migration.
Figure 5.
Vascular endothelial growth factor (VEGF)-mediated migration of human microvascular endothelial cells (HMVECs) is inhibited by DN-TRPC6. Human dermal microvascular endothelial cells were treated with either an empty adenovirus vector (AdNull), adenovirus-expressing wild-type TRPC6 (Ad-wtTRPC6), or Ad-dnTRPC6. Migration was assessed in a Boyden chamber in response to 0.1% fetal calf serum (FCS), 1 nM VEGF in 0.1% FCS, or 10% FCS. (A) There was no difference in baseline migration (i.e., number of cells migrating across membrane without stimulation). (B) VEGF induced a significant increase in migration, which was substantially inhibited by Ad-dnTRPC6 (B; p < 0.001, ANOVA). To ensure that this was not a nonspecific effect, the increase in migration toward VEGF was expressed as a percentage of that toward FCS. Whereas VEGF induced a migration that was nearly as strong as that induced by 10% FCS, this was again significantly inhibited in the dnTRPC6 HMVECs, indicating that while FCS induced migration was not inhibited, VEGF-mediated migration was (C; p < 0.02, ANOVA). * p < 0.05, ** p < 0.01, *** p < 0.001, compared with dominant negative TRPC6, SNK.
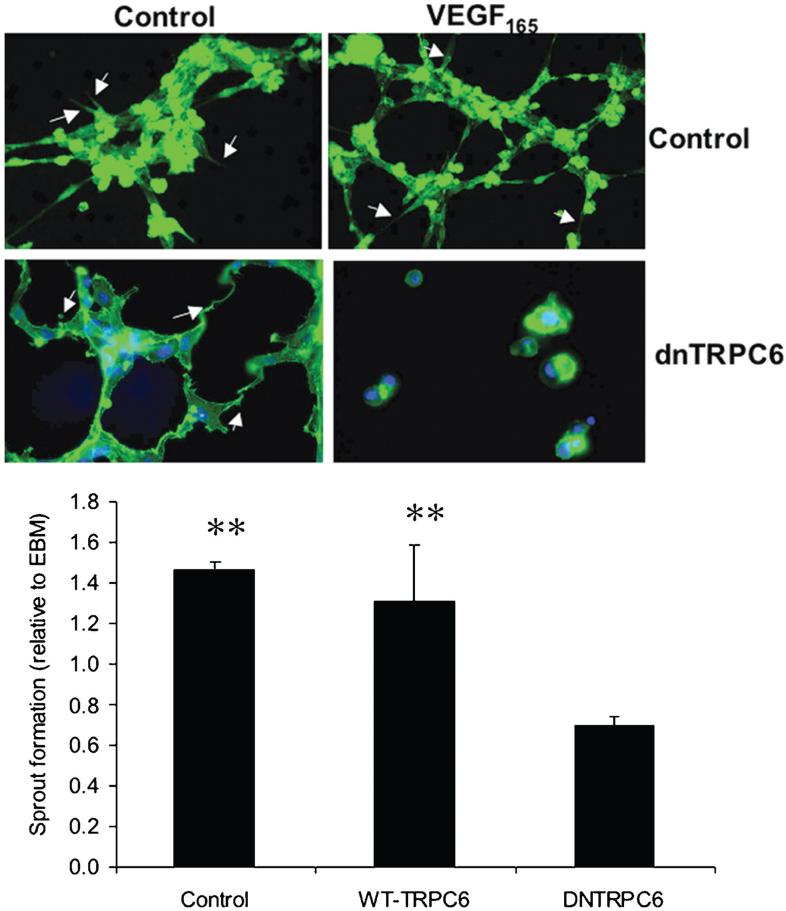
To determine whether this cation entry was also required for other processes contributing to angiogenesis, HMVECs were subjected to an in vitro angiogenesis assay, whereby HMVECs are grown on a matrigel matrix, and sprouting was determined in response to exogenous VEGF (Figure 6). Whereas VEGF induced sprouting in wild-type cells, HMVECs transduced with Ad-dnTRPC6 formed fewer sprouts in response to VEGF (69 ± 5% of untreated) than even control cells (146 ± 4% of untreated) or wild-type Ad-TRPC6 transduced cells (130 ± 28% of untreated). There was no difference in overall cell number at five hours.
Figure 6.
Vascular endothelial growth factor (VEGF)-mediated sprouting of human microvascular endothelial cells is inhibited by dnTRPC6. Human dermal microvascular endothelial cells were treated with Ad-dnTRPC6. Cells were exposed to EBM (negative control) or 1 nM VEGF165, fixed, and visualized with phalloidin Alexa 488 for actin five hours after seeding on a gel [Chemicon ECMatrix Angiogenesis Kit]. Number of sprouts were quantified and expressed relative to those formed with EBM alone. There was a dramatic reduction in VEGF-induced sprout formation detectable when cells were treated with dnTRPC6. ** p < 0.01, compared with dnTRPC6 one-way ANOVA SNK post-hoc test (N = 10 total).
To determine whether VEGF-induced endothelial proliferation was affected by dn-TRPC6, HMVECs were transduced with either Ad-null, Ad-wtTRPC6, or Ad-dn-TRPC6. The cells were then treated with VEGF, left for 24 hours, then fixed and permeabilized, stained with PI, and subjected to flow cytometry. Figure 7 shows that the proportion of cells in the S phase was significantly enhanced in Ad-Vector transduced HMVECs treated with 1 nM of VEGF from 1.75 ± 0.09 to 4.33 ± 0.3% (p < 0.05). When transduced with wt-TRPC6, this VEGF-mediated proliferation was significantly enhanced to 12.6 ± 1.2% (p < 0.001, compared with Ad-Null), but dn-TRPC6 reduced this down to 1.93 ± 0.15%, not significantly different from the untreated (1.75 ± 0.09%), but significantly lower than Ad-Null (p < 0.05, all analysis of variance [ANOVA]).
Figure 7.
Vascular endothelial growth factor (VEGF)-mediated proliferation of human microvascular endothelial cells is inhibited by dnTRPC6. Human dermal microvascular endothelial cells were treated with Ad-dnTRPC6, Ad-wtTRPC6, or Ad-Null. Cells were treated with 1 nM of VEGF for 48 hours, fixed, and stained with propidium iodide and subjected to flow cytometric analysis of DNA content. The proportion of cells with increased DNA content was calculated. Ad-Null treated cells contained significantly fewer cells than Ad-wtTRPC6 and significantly more cells than Ad-dnTRPC6 treated HMVECs. *** p < 0.001, * p < 0.05, compared with vector, SNK, one-way ANOVA.
DISCUSSION
The intracellular mechanisms through which VEGF induces angiogenesis are still to be understood. There have been a number of signal-transduction pathways implicated in the VEGF stimulation of endothelial cells, including calcium entry, calcium release, but also through the activation of many different serine/threonine or tyrosine kinases, including p42/p44 MAPK, src, PKC, etc. [3]. However, there is substantial evidence that implicates calcium entry as a key event in VEGF-mediated downstream events—specifically for permeability [2, 22]. The mechanisms through which calcium entry occurs are still controversial. Work from our group has consistently shown a role for the TRPC3/6/7 subfamily of proteins in the VEGF-mediated calcium response in microvascular endothelial cells. In vivo measurement of permeability and calcium concentrations after VEGF stimulation has shown that both are independent of calcium store release, as they are not inhibited by thapsigargin [18]. They are mimicked by OAG and can be potentiated by TRPC6 agonist flufenamic acid [17, 18]. We have shown, by whole-cell patch clamp of chinese hamster ovary cells overexpressing TRPC3 or TRPC6, that VEGF and OAG activate cation currents through heterologously expressed TRPC3 and TRPC6 channels [5]. Moreover, similar VEGF-activated cation currents to those carried by recombinant TRPC3/C6 could be recorded from HMVECs, in the presence of buffering of bulk-intracellular [Ca2+], suggesting that the VEGF-mediated cation entry occurs in these cells through a store-independent pathway [5]. The ionic current response to VEGF in HMVECs was found to be mimicked by OAG, stimulated by flufenamate (FFA), blocked by gadolinium, and exhibited a current-voltage relation and single-channel conductance properties consistent with TRPC6 or -3 (or possibly heteromeric channels involving both) [5]. This work demonstrates that VEGF-mediated cation entry is consistent with primary roles for TRPC6 or -C3. In contrast, there are reports that the inhibition of TRPC1 by neutralizing antibodies can inhibit VEGF-mediated cation entry into large-vessel endothelial cells (i.e., HUVECs) [12], and that HUVECs contain cation channels with single-channel conductance of ∼10pS—too large to be TRPC1 (1pS), but too small for TRPC6 (25-35 pS) [9]. To this end, it was interesting to note that the TRPC expression in HMVECs and HUVECs were substantially different, in that we did not see TRPC4 in HMVECs. TRPC channels are conventionally considered to form heterotetramers within the two subfamilies of channels. According to such a scheme, dnTRPC6 would be predicted to inhibit cation entry through either TRPC6 homotetramers, or through TRPC6/3/7 heterotetramers. Recent data, however, are suggestive of an additional layer of complexity: TRPC1, -3, and -7 have been proposed to be able to form native heteromeric receptor-operated channels in HEK293 cells [24], whereas stromal activating protein 1 (STIM1) has been suggested to facilitate heteromultimerization of TRPC3 with -1 and of -6 with -4 [23]. The absence of TRPC4 in HMVECs would argue against a heteromultimeric channel complex across subfamilies in this cell type involving TRPC4, though at present, we cannot rule out the possibility of heteromultimeric channels to which TRPC1 contributes. What is clear is that the results of the present experiments implicate TRPC6 as an obligatory component of the channels mediating cation entry in response to VEGF and OAG. Additional experiments employing a “knockdown” of other TRPC subunit candidates are now required to elucidate which may act as partners with TRPC6 in mediating OAG-VEGF responses. It is also worth noting that whereas a straightforward interpretation of our Ca data is that the rise in intracellular [Ca2+] in response to OAG and VEGF occurs directly through TRPC6-containing cation channels, in other cell types TRPC3 has been shown to form a signaling complex with the NCX1 isoform of the Na+−Ca2+ exchange (NCX) protein, with receptor or PLC activation leading to Ca2+ entry via the NCX, secondary to TRPC-mediated Na+-loading [7, 8, 19]. Future work is warranted to determine whether or not a TRPC-NCX complex exists in HMVECs and whether the NCX might contribute to the rise in intracellular [Ca2+] in response to VEGF and OAG.
In addition to the role of VEGF-mediated cation entry in the calcium response of VEGF, we show here, for the first time, that TRPC3/6/7 channels may be implicated in the angiogenesis response of VEGF. The inhibition of migration, proliferation, and sprout formation induced by VEGF by dn-TRPC6 was striking, particularly as it was specific to VEGF-mediated angiogenic responses (i.e., it did not affect serum mediated responses), and reduced VEGF-mediated proliferation to baseline values. These results suggest that antagonists to TRPC6 or other members of the subfamily may have antiangiogenic properties. The mechanisms through which this may occur are not known, but it is clear that eNOS activation, which is calcium dependent, is required for the mitogenesis of endothelial cells by VEGF [14].
CONCLUSIONS
In summary, we show here, for the first time, that calcium entry contingent upon channels involving TRPC6 is required for the VEGF-mediated calcium increase, migration, proliferation, and sprout formation of HMVECs, identifying a novel target for VEGF inhibitors.
ACKNOWLEDGMENTS
This work was funded by the Wellcome Trust (grant no. 77589, AMH-Z), The Richard Bright VEGF Research Trust (grant no. 07/PG01, CAG, AM), and the British Heart Foundation (BS/06/005, DOB; PG/07/026, DOB, JCH).
REFERENCES
- 1.Ahmmed GU, Malik AB. Functional role of TRPC channels in the regulation of endothelial permeability. Pflugers Arch. 2005;451:131–142. doi: 10.1007/s00424-005-1461-z. [DOI] [PubMed] [Google Scholar]
- 2.Bates DO, Curry FE. Vascular endothelial growth factor increases microvascular permeability via a Ca2+-dependent pathway. Am J Physiol. 1997;273:H687–H694. doi: 10.1152/ajpheart.1997.273.2.H687. [DOI] [PubMed] [Google Scholar]
- 3.Bates DO, Harper SJ. Regulation of vascular permeability by vascular endothelial growth factors. Vascul Pharmacol. 2002;39:225–37. doi: 10.1016/s1537-1891(03)00011-9. [DOI] [PubMed] [Google Scholar]
- 4.Brock TA, Dvorak HF, Senger DR. Tumor-secreted vascular permeability factor increases cytosolic Ca2+ and von Willebrand factor release in human endothelial cells. Am J Pathol. 1991;138:213–221. [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng HW, James AF, Foster RR, Hancox JC, Bates DO. VEGF activates receptor-operated cation channels in human microvascular endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:1768–1776. doi: 10.1161/01.ATV.0000231518.86795.0f. [DOI] [PubMed] [Google Scholar]
- 6.Clapham DE, Runnels LW, Strubing C. The Trp ion channel family. Nat Rev. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- 7.Eder P, Poteser M, Romanin C, Groschner K. Na(+) entry and modulation of Na+/Ca2+ exchange as a key mechanism of TRPC signaling. Pflugers Arch. 2005;451:99–104. doi: 10.1007/s00424-005-1434-2. [DOI] [PubMed] [Google Scholar]
- 8.Eder P, Probst D, Rosker C, Poteser M, Wolinski H, Kohlwein SD, Romanin C, Groschner K. Phospholipase C-dependent control of cardiac calcium homeostasis involves a TRPC3-NCX1 signaling complex. Cardiovasc Res. 2007;73:111–119. doi: 10.1016/j.cardiores.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Garnier-Raveaud S, Usson Y, Cand F, Robert-Nicoud M, Verdetti J, Faury G. Identification of membrane calcium channels essential for cytoplasmic and nuclear calcium elevations induced by vascular endothelial growth factor in human endothelial cells. Growth Fact. 2001;19:35–48. doi: 10.3109/08977190109001074. [DOI] [PubMed] [Google Scholar]
- 10.Hill AJ, Hinton JM, Cheng H, Gao Z, Bates DO, Hancox JC, Langton PD, James AF. A TRPC-like nonselective cation current activated by alpha 1-adrenoceptors in rat mesenteric artery smooth muscle cells. Cell Cal. 2006;40:29–40. doi: 10.1016/j.ceca.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 12.Jho D, Mehta D, Ahmmed G, Gao XP, Tiruppathi C, Broman M, Malik AB. Angiopoietin-1 opposes VEGF-induced increase in endothelial permeability by inhibiting TRPC1-dependent Ca2 influx. Circ Res. 2005;96:1282–1290. doi: 10.1161/01.RES.0000171894.03801.03. [DOI] [PubMed] [Google Scholar]
- 13.Maruyama Y, Nakanishi Y, Walsh EJ, Wilson DP, Welsh DG, Cole WC. Heteromultimeric TRPC6-TRPC7 channels contribute to arginine vasopressininduced cation current of A7r5 vascular smooth muscle cells. Circ Res. 2006;98:1520–1527. doi: 10.1161/01.RES.0000226495.34949.28. [DOI] [PubMed] [Google Scholar]
- 14.Morbidelli L, Chang CH, Douglas JG, Granger HJ, Ledda F, Ziche M. Nitric oxide mediates mitogenic effect of VEGF on coronary venular endothelium. Am J Physiol. 1996;270:H411–H415. doi: 10.1152/ajpheart.1996.270.1.H411. [DOI] [PubMed] [Google Scholar]
- 15.Nilius B, Droogmans G, Wondergem R. Transient receptor potential channels in endothelium: solving the calcium entry puzzle? Endothelium. 2003;10:5–15. doi: 10.1080/10623320303356. [DOI] [PubMed] [Google Scholar]
- 16.Paria BC, Vogel SM, Ahmmed GU, Alamgir S, Shroff J, Malik AB, Tiruppathi C. Tumor necrosis factor-alpha-induced TRPC1 expression amplifies store-operated Ca2+ influx and endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1303–L1313. doi: 10.1152/ajplung.00240.2004. [DOI] [PubMed] [Google Scholar]
- 17.Pocock TM, Foster RR, Bates DO. Evidence of arole for TRPC channels in VEGF-mediated increased vascular permeability in vivo. Am J Physiol Heart Circ. 2004;286:H1015–H1026. doi: 10.1152/ajpheart.00826.2003. [DOI] [PubMed] [Google Scholar]
- 18.Pocock TM, Williams B, Curry FE, Bates DO. VEGF and ATP act by different mechanisms to increase microvascular permeability and endothelial [Ca2+](i) Am J Physiol Heart Circ. 2000;279:H1625–H1634. doi: 10.1152/ajpheart.2000.279.4.H1625. [DOI] [PubMed] [Google Scholar]
- 19.Rosker C, Graziani A, Lukas M, Eder P, Zhu MX, Romanin C, Groschner K. Ca(2+) signaling by TRPC3 involves Na(+) entry and local coupling to the Na+/Ca2+ exchanger. J Biol Chem. 2004;279:13696–13704. doi: 10.1074/jbc.M308108200. [DOI] [PubMed] [Google Scholar]
- 20.Sakura H, Ashcroft FM. Identification of four trp1 gene variants murine pancreatic beta-cells. Diabetologia. 1997;40:528–532. doi: 10.1007/s001250050711. [DOI] [PubMed] [Google Scholar]
- 21.Walker RL, Hume JR, Horowitz B. Differential expression and alternative splicing of TRP channel genes in smooth muscles. Am J Physiol Cell Physiol. 2001;280:C1184–C1192. doi: 10.1152/ajpcell.2001.280.5.C1184. [DOI] [PubMed] [Google Scholar]
- 22.Wu HM, Yuan Y, Zawieja DC, Tinsley J, Granger HJ. Role of phospholipase C, protein kinase C, and calcium in VEGF-induced venular hyperpermeability. Am J Physiol. 1999;276:H535–H542. doi: 10.1152/ajpheart.1999.276.2.H535. [DOI] [PubMed] [Google Scholar]
- 23.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zagranichnaya TK, Wu X, Villereal ML. Endogenous TRPC1, TRPC3, and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. J Biol Chem. 2005;280:29559–29569. doi: 10.1074/jbc.M505842200. [DOI] [PubMed] [Google Scholar]