Abstract
Infection with Opisthorchis viverrini and its associated cholangiocarcinoma (CCA) is an underestimated problem in the Mekong region of Southeast Asia, despite the widespread use of praziquantel and health education measures for parasite control. Although data from Cambodia, Laos and Vietnam are rare, data from Thailand often show wide-ranging variability in epidemiological parameters, including human morbidity and the prevalence and incidence of CCA. The recent discovery of high levels of population genetic variability in O. viverrini in different wetlands in Thailand and Laos, which indicates the presence of sibling species, suggests that we have underestimated the complexity of this epidemiological situation. Future research should determine the relationship between the genetic variability of O. viverrini and patterns of opisthorchiasis-related disease.
Opisthorchis viverrini in Southeast Asia
The World Health Organization (WHO) estimates that approximately five million people with HIV live in the Asia-Pacific Region [1]. This can be compared with a conservative estimate of ten million people infected with the liver fluke Opisthorchis viverrini in Thailand and Laos alone [2]. People infected with Opisthorchis parasites are at risk of developing cholangiocarcinoma (CCA), and those who have advanced CCA will almost certainly die. As with HIV, infection with O. viverrini and the likelihood of developing CCA is related to culturally dependent behavioural patterns, which, if modified, can prevent infection [3]. Here, we briefly review epidemiological aspects of opisthorchiasis and CCA, and discuss these in relation to the recently published evidence that O. viverrini is a species complex.
O. viverrini: an underestimated parasite in world health
O. viverrini is an underestimated parasite in world health [4] because it is mistakenly thought to represent a limited, local problem in Southeast Asia, particularly in Thailand [5]. This is predominantly because of the fact that reasonably accurate spatial and temporal morbidity data and CCA incidence are available from Thailand but not from elsewhere within the Mekong region where O. viverrini occurs [6-9]. Although O. viverrini infection in humans is often asymptomatic, this parasite can be pathogenic in its own right and cause hepatobiliary diseases [10]. Its greatest impact, however, is as a direct risk factor for CCA of the bile duct [5,7], and O. viverrini has been recognized as a type-1 carcinogen since 1994 [11,12]. Thus, the infection is not immediately life threatening; cancer develops 30-40 years after infection, with death occurring within 3-6 months of diagnosis [5]. No pharmaceutical treatment is available, and surgery and supportive treatment are complicated [13] and often not accessible for victims in developing countries.
Economically, opisthorchiasis and CCA are estimated to cost US$120 million annually in medical care and lost wages in Thailand alone [14]. The cost to families and communities is enormous. CCA causes social, economic, community and family problems because it is more prevalent in males, the principal financial earners. Infection also occurs in young children, who are then at considerable risk of developing liver cancer when they are 30-40 years old, during their most productive years.
O. viverrini has a three-host life cycle (Figure 1). The first intermediate hosts are prosobranch snails, Bithynia spp. of the family Bithyiniidae [15]. At least 18 species of cyprinid fish act as second intermediate hosts, and carnivores (e.g. cats and dogs), but more specifically humans, are definitive hosts [6]. Humans become infected following the consumption of raw or insufficiently cooked fish containing metacercariae.
Figure 1.
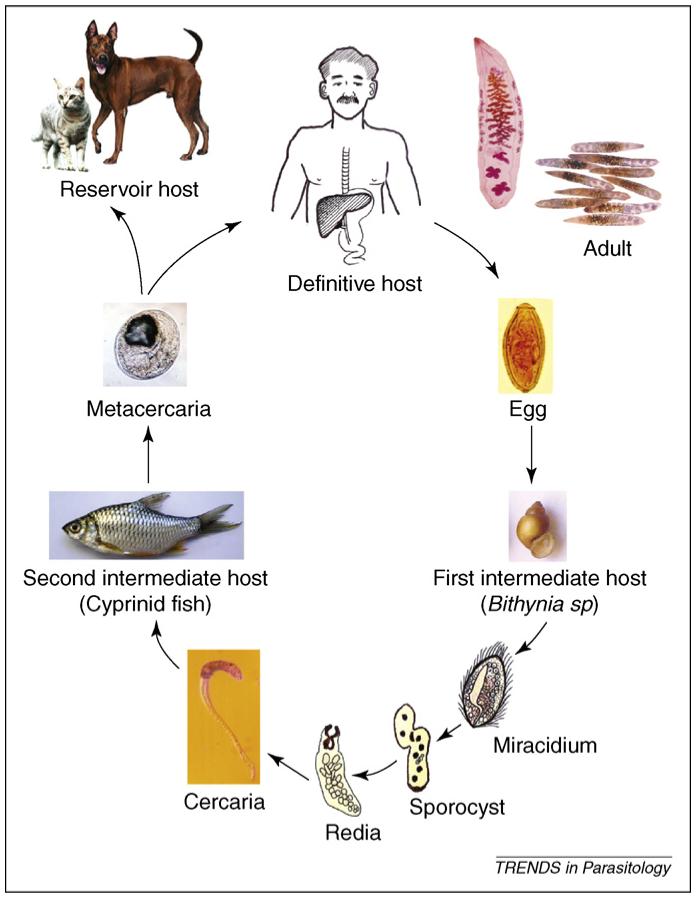
The life cycle of O. viverrini. Humans become infected by ingesting metacercariae in uncooked fish. The ingested metacercariae excyst in the duodenum and enter the bile duct, where they develop into sexually mature adult worms. Eggs are produced and discharged with bile fluid into the intestine and faeces. When eggs reach a body of freshwater and are ingested by an appropriate snail, miracidium hatch and develop into sporocysts and rediae. Different species of Bithynia snails serve as first intermediate hosts. The rediae gave rise to cercariae and, when exposed to appropriate cyprinid species of fish (the second intermediate hosts), the free-swimming cercariae penetrate into the tissues or skin of freshwater fish and become fully infective metacercariae, which completes the life cycle. Humans act as definite hosts, along with fish-eating carnivores (e.g. cats and dogs), which act as reservoir hosts.
The prevalence of O. viverrini in snails is generally low (<1%), whereas in cyprinid fish it is extremely high (90-95%) [2,16,17]. The parasite burden in fish is highly aggregated and the majority of infected fish harbour one to two metacercariae, so a repeated, low infectious dose is likely (P. Sithithaworn et al., unpublished).
Prevalence in humans
The most recent estimate of the number of people infected with O. viverrini in the Mekong region is eight million in Thailand and two million in Laos [2]. This is a substantial underestimate of its prevalence because no data are available for Cambodia or Vietnam, although opisthorchiasis in known to be common in parts of these countries [18,19]. In Thailand, an average of 9.6% of the population is infected [20], with the liver fluke being distributed mainly in the North (19.3% prevalence) and Northeast (15.7% prevalence) [2,21]. There has been a substantial decline in the prevalence of infection in Thailand from 34% (in 1992) to 10% (in 2002) [21], which has been attributed to intensive and continuous control activities. Disturbingly, substantial variation in prevalence ranging from 4% to 33% still remains in affected provinces [20]. Within Khon Kaen province (northeast Thailand), O. viverrini infection ranges from 2% to 71% between different districts [22]. Although data on the prevalence of infection are available, data on incidence are scant [23,24].
Incidence of CCA
CCA has caused millions of deaths in southeast Asia [25]. The most important risk factor for CCA is O. viverrini infection, with risk being dependent on intensity [26], previous or current exposure to infection and host genetic polymorphism [27]. A recent study detected residual O. viverrini DNA in both cancerous and non-cancerous tissue in CCA patients in Thailand [28]. By contrast, the risk factors for CCA in areas where liver flukes are not endemic include primary sclerosing cholangitis, hepatolithiasis and choledochal cysts [29]. Its incidence is 98 per 100 000 people in the Thai province of Khon Kaen, which is at least an order of magnitude higher than in areas outside of the Mekong region, even though CCA is becoming increasingly frequent in the Western world [30,31].
Because cancer registration is available in Thailand but not other countries in the Mekong region [32], a systematic investigation to estimate the socioeconomic impact of CCA is currently impossible [33]. Nevertheless, although <10% of people infected with O. viverrini develop CCA, a high proportion of the population are infected in many areas [8,22].
Importantly, the prevalence, intensity and medical significance of O. viverrini infection in humans varies geographically within the Mekong region, as do clinical symptoms and prevalence of CCA. Variation in the incidence of CCA in different communities in Thailand, however, is not correlated with the prevalence of O. viverrini [22].
Genetic and molecular characterization of O. viverrini
The study of the molecular genetics of O. viverrini has received considerable impetus from the recent publication that provides the first catalogue of expressed genes (∼14% of the entire transcriptome) for this species [34]. This advance will enhance our ability to conduct comprehensive molecular analyses of the systematics and population genetics of O. viverrini in a similar manner to that proven useful for other parasites, as has been the case for the parasitic protozoan Giardia intestinalis utilizing the genes encoding allozyme markers [35]. In addition, the possible role of genetic variation among O. viverrini populations in the local and regional variability in human disease, including CCA, has also begun to receive attention recently.
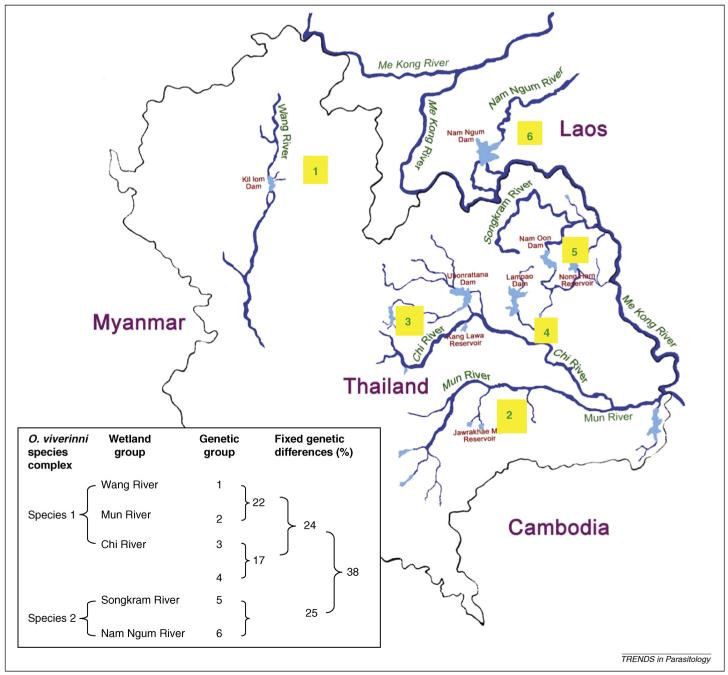
Analyses using random amplification of polymorphic DNA have detected genetic polymorphisms among O. viverrini isolates from Thailand and Laos [36]. This finding was supported by allozyme electrophoresis analyses at 32 enzyme loci [37-39]. Allozyme analyses have shown that O. viverrini is a species complex that consists of at least two, but possibly six, genetically distinct but morphologically similar, hence, ‘cryptic’ sibling species. The species are associated with different major wetland systems, including the Chi, Mun, Songkram and Wang Rivers in Thailand and the Nam Ngum River in Laos [39] (Figure 2).
Figure 2.
A summary of the systematics of the O. viverrini species complex. Depicted are the numbers of genetic groups (in yellow boxes) detected within each of the currently detected sibling species; the magnitude of fixed genetic differences between genetic groups; the levels of fixed genetic differences that define each species, and correlations of species and genetic groups with distinct wetlands after the application of the molecular genetic technique of allozyme electrophoresis. Fixed genetic differences occur when samples being compared do not have any alleles in common at the enzyme locus being examined. In the case of O. viverrini, 33 independent enzyme loci were examined [36]. The geographical distribution of O. viverrini includes Thailand, Laos, Cambodia, Vietnam and, possibly, Myanmar (no data are currently available).
The separation of O. viverrini into at least two sibling species is supported by preliminary evidence from other molecular and biological data (W. Saijuntha et al., unpublished) and coincides with the division of the intermediate host Bithynia siamensis goniomphalos into two major genetic groups [39]. The role that specific wetlands have between species and populations of Opisthorchis parasites in limiting gene flow requires investigation.
Current and future threats
The movement of people is a major component of globalization in Asia [40]. O. viverrini is regularly carried to regions outside of its normal distribution by tourists and Thai labourers [2,41]. Because infection is often asymptomatic and of a long duration, infected migrants are likely to remain undiagnosed and shed eggs for long time periods. However, the introduction of O. viverrini to human populations outside of the Mekong region is more likely to come from fish imports. Asia is the world’s major producer (90%) of aquaculture products and there has been an enormous recent expansion in aquaculture in O. viverrini endemic countries [25,42]. The increasing use of fish aquaculture, with high usage of cyprinid species [43], provides ample opportunities for species-switching by parasites, including those with complex life cycles [44]. The export and import of fish and fish products worldwide provides a transfer route for parasites [45], which in the case of O. viverrini are favoured by the fact that the metacercariae are resilient and can remain viable in fish muscle even if the fish is pickled or fermented [46].
The possible effects of global climate change on O. viverrini distribution are debatable. Regional projections for Southeast Asia indicate higher temperatures approaching the mean global increase with potentially higher rainfall depending on the pattern of tropical cyclones [47]. Increased temperature is likely to reduce the developmental time of immature stages and might potentially reduce cercarial host-searching time [48,49]. An increase in rainfall could lead to the extension of wetlands and, therefore, suitable new habitat, with an increased likelihood of parasitic gene flow between them through the expansion of snail and fish host distributions.
Prevention
The availability of praziquantel provides effective chemotherapy for Opisthorchis parasite infection [46]. There is, however, evidence that repeated infection and treatment could increase the risk of CCA [50,51]. Thus, a more basic approach involving prevention by reducing the consumption of infected fish is likely to have better long-term chances of success. It is difficult to achieve this goal, however, because it involves changing traditional patterns of eating behaviour. A control program for O. viverrini in Thailand operates through the primary care system and employs a selective treatment approach [21]; however, high transmission variation between communities is common [22]. Control activity should be targeted to communities with high O. viverrini intensities and areas with high risk of CCA [8]. Inflammatory biomarkers recently reported in opisthorchiasis and CCA are potentially useful tools for surveillance and control [52,53]. For adults, the campaign should emphasize a reduction in reinfection, and emphasis should be placed on health education for children. By building local knowledge of parasitic diseases and CCA into curricula, information could be systematically disseminated to schoolchildren.
A minimum requirement should be that an adequate health system be set up to enable appropriate surveillance work. This is particularly pertinent for Cambodia, Laos and Vietnam, which lag behind Thailand in the development of such systems.
The success of control programs is dependent on several epidemiological factors. First, humans seem to be the main, definitive hosts of the parasite, which is rarely found in animal reservoir hosts. Thus, by breaking the cycle at the human host, only a limited sylvatic input is likely. Second, population genetic data indicate that gene flow between areas is limited (W. Saijuntha, unpublished) and that once control measures have taken effect, the reintroduction of O. viverrini is likely to be slow if human movement is limited from areas where prevalence is still high. Here, it is important to understand more about the ecology of O. viverrini and whether disease characteristics are likely to change (i.e. towards higher virulence in a susceptible population) if new introductions occur.
Concluding remarks and future directions
Although our knowledge of the biology of O. viverrini is steadily increasing, fundamental studies on epidemiology and ecology, in addition to genomics and proteomics, are still needed. In addition, investigation of the molecular basis of fluke-induced CCA is likely to be of great importance in the development of biomarkers for the diagnosis of this devastating condition.
The scientific challenge of providing a comprehensive epidemiological understanding of the O. viverrini species complex remains immense. Finding substantial genetic variation among O. viverrini from different wetlands and among B. s. goniomphalos from different geographical areas provides a potential basis from which to investigate the epidemiological variability in opisthorchiasis and CCA. Furthermore, how abiotic and biotic characteristics of different wetlands influence the ecology and genetic variation within O. viverrini and its intermediate hosts needs to be explored, particularly in relation to future climate change. Understanding such relationships will have major implications for the surveillance, control and treatment of opisthorchiasis.
Acknowledgements
This research was supported by a Wellcome Trust Collaborative Research Initiative Grant, the Thailand Research Fund through the Royal Golden Jubilee PhD. Program, the European Commission through a TREMKIT project, the Faculty of Medicine, Khon Kaen University, Overseas Visiting Professor Program and the Deutsche Forschungsgemeinschaft (PE1611/1-1).
References
- 1.WHO . World Health Organisation-United Nations Program on HIV/AIDS. 2007. AIDS epidemic update. [Google Scholar]
- 2.Sithithaworn P, Haswell-Elkins M. Epidemiology of Opisthorchis viverrini. Acta Trop. 2003;88:187–194. doi: 10.1016/j.actatropica.2003.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Petney TN. Environmental, cultural and social changes and their influence on parasite infections. Int. J. Parasitol. 2001;31:919–932. doi: 10.1016/s0020-7519(01)00196-5. [DOI] [PubMed] [Google Scholar]
- 4.Hotez PJ, et al. Control of neglected tropical diseases. N. Engl. J. Med. 2007;357:1018–1027. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- 5.Sripa B, et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4:e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parkin DM, et al. Cholangiocarcinoma: epidemiology, mechanisms of carcinogenesis and prevention. Cancer Epidemiol. Biomarkers Prev. 1993;2:537–544. [PubMed] [Google Scholar]
- 7.Sithithaworn P, et al. Parasite-associated morbidity: liver fluke infection and bile duct cancer in northeast Thailand. Int. J. Parasitol. 1994;24:833–843. doi: 10.1016/0020-7519(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 8.Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Curr. Opin. Gastroenterol. 2008;24:349–356. doi: 10.1097/MOG.0b013e3282fbf9b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vatanasapt V, et al. Northeast Thailand: a region with high incidence of cholangiocarcinoma. Lancet. 1990;1:116–117. [Google Scholar]
- 10.Sripa B. Pathobiology of opisthorchiasis: an update. Acta Trop. 2003;88:209–220. doi: 10.1016/j.actatropica.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 11.IARC Infection with liver flukes (Opisthorchis viverrini, Opisthorchis felineus and Clonorchis sinensis) IARC Monogr. Eval. Carcinog. Risks Hum. 1994;61:121–175. [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer DA, Fried B. The role of helminth infections in carcinogenesis. Adv. Parasitol. 2007;65:239–296. doi: 10.1016/S0065-308X(07)65004-0. [DOI] [PubMed] [Google Scholar]
- 13.Patel T, Singh P. Cholangiocarcinoma: emerging approaches to a challenging cancer. Curr. Opin. Gastroenterol. 2007;23:317–323. doi: 10.1097/MOG.0b013e3280495451. [DOI] [PubMed] [Google Scholar]
- 14.Muller R. Worms and Human Disease. CABI publishing; 2002. [Google Scholar]
- 15.Kaewkes S. Taxonomy and biology of liver flukes. Acta Trop. 2003;88:177–186. doi: 10.1016/j.actatropica.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Brockelman WY, et al. Field studies on the transmission of the human liver fluke, Opisthorchis viverrini, in northeast Thailand: population changes of the snail intermediate host. Int. J. Parasitol. 1986;16:545–552. doi: 10.1016/0020-7519(86)90091-3. [DOI] [PubMed] [Google Scholar]
- 17.Vichasri S, et al. Opisthorchis viverrini: intensity and rates of infection in cyprinoid fish from an endemic focus in northeast Thailand. Southeast Asian J. Trop. Med. Public Health. 1982;13:138–141. [PubMed] [Google Scholar]
- 18.Sayasone S, et al. Epidemiology of Opisthorchis viverrini in a rural district of southern Lao PDR. Trans. R. Soc. Trop. Med. Hyg. 2007;101:40–47. doi: 10.1016/j.trstmh.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Sithithaworn P, et al. Epidemiology of food-borne trematodes and other parasite infections in a fishing community on the Nam Ngum reservoir, Lao PDR. Southeast Asian J. Trop. Med. Public Health. 2006;37:1083–1090. [PubMed] [Google Scholar]
- 20.Jongsuksuntigul P. Seminar in Parasitic Diseases in northeast Thailand. KlungnanaVitaya, Khon Kaen; Thailand: 2002. Parasitic disease in northeast Thailand. [Google Scholar]
- 21.Jongsuksuntigul P, Imsomboon T. Opisthorchiasis control in Thailand. Acta Trop. 2003;88:229–232. doi: 10.1016/j.actatropica.2003.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Sriamporn S, et al. Prevalence of Opisthorchis viverrini infection and incidence of cholangiocarcinoma in Khon Kaen, northeast Thailand. Trop. Med. Int. Health. 2004;9:588–594. doi: 10.1111/j.1365-3156.2004.01234.x. [DOI] [PubMed] [Google Scholar]
- 23.Saowakontha S, et al. Field trials in the control of Opisthorchis viverrini with an integrated programme in endemic areas of northeast Thailand. Parasitology. 1993;106:283–288. doi: 10.1017/s0031182000075107. [DOI] [PubMed] [Google Scholar]
- 24.Upatham ES, et al. Rate of re-infection by Opisthorchis viverrini in an endemic northeast Thai community after chemotherapy. Int. J. Parasitol. 1988;18:643–649. doi: 10.1016/0020-7519(88)90099-9. [DOI] [PubMed] [Google Scholar]
- 25.WHO . Report of joint WHO/FAO workshop on food-borne trematode infections in Asia. WHO Regional Office for the Western Pacific; 2004. pp. 1–58. [Google Scholar]
- 26.Haswell-Elkins MR, et al. Cross-sectional study of Opisthorchis viverrini infection and cholangiocarcinoma in communities within a high-risk area in northeast Thailand. Int. J. Cancer. 1994;59:505–509. doi: 10.1002/ijc.2910590412. [DOI] [PubMed] [Google Scholar]
- 27.Honjo S, et al. Genetic and environmental determinants of risk for cholangiocarcinoma via Opisthorchis viverrini in a densely infested area in Nakhon Phanom, northeast Thailand. Int. J. Cancer. 2005;117:854–860. doi: 10.1002/ijc.21146. [DOI] [PubMed] [Google Scholar]
- 28.Suksumek N, et al. TaqMan real-time PCR assay for specific detection of Opisthorchis viverrini DNA in Thai patients with hepatocellular carcinoma and cholangiocarcinoma. Exp. Parasitol. 2008;119:217–224. doi: 10.1016/j.exppara.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Yachimski P, Pratt DS. Cholangiocarcinoma: natural history, treatment, and strategies for surveillance in high-risk patients. J. Clin. Gastroenterol. 2008;42:178–190. doi: 10.1097/MCG.0b013e31806daf89. [DOI] [PubMed] [Google Scholar]
- 30.Khan SA, et al. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 31.Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology. 2004;66:167–179. doi: 10.1159/000077991. [DOI] [PubMed] [Google Scholar]
- 32.Khuhaprema T, et al. Cancer in Thailand. IV. Bangkok Medical Publisher; 2007. pp. 1998–2000. [Google Scholar]
- 33.Mathers CD, et al. Measuring the burden of neglected tropical diseases: the global burden of disease framework. PLoS Negl. Trop. Dis. 2007;1:e114. doi: 10.1371/journal.pntd.0000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laha T, et al. Gene discovery for the carcinogenic human liver fluke, Opisthorchis viverrini. BMC Genomics. 2007;8:189. doi: 10.1186/1471-2164-8-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monis PT, et al. Molecular systematics of the parasitic protozoan Giardia intestinalis. Mol. Biol. Evol. 1999;16:1135–1144. doi: 10.1093/oxfordjournals.molbev.a026204. [DOI] [PubMed] [Google Scholar]
- 36.Sithithaworn P, et al. Genetic variation in Opisthorchis viverrini (Trematoda: Opisthorchiidae) from northeast Thailand and Laos PDR based on random amplified polymorphic DNA analyses. Parasitol. Res. 2007;100:613–617. doi: 10.1007/s00436-006-0304-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saijuntha W, et al. Genetic markers for the identification and characterization of Opisthorchis viverrini, a medically important food borne trematode in southeast Asia. Acta Trop. 2006;100:246–251. doi: 10.1016/j.actatropica.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saijuntha W, et al. Enzyme markers to identify and characterize Opisthorchis viverrini in Thailand and Lao PDR. Southeast Asian J. Trop. Med. Public Health. 2006;37(Suppl 3):43–47. [PubMed] [Google Scholar]
- 39.Saijuntha W, et al. Evidence of a species complex within the food-borne trematode Opisthorchis viverrini and possible co-evolution with their first intermediate hosts. Int. J. Parasitol. 2007;37:695–703. doi: 10.1016/j.ijpara.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abella M. Labour migration in East Asian economics; Annual Bank Conference on Development Economics; 2004.pp. 1–19. [Google Scholar]
- 41.Cheng HS, Shieh YH. Investigation on subclinical aspects related to intestinal parasitic infections among Thai laborers in Taipei. J. Travel Med. 2000;7:319–324. doi: 10.2310/7060.2000.00086. [DOI] [PubMed] [Google Scholar]
- 42.Keiser J, Utzinger J. Emerging foodborne trematodiasis. Emerg. Infect. Dis. 2005;11:1507–1514. doi: 10.3201/eid1110.050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naylor RL, et al. Effect of aquaculture on world fish supplies. Nature. 2000;405:1017–1024. doi: 10.1038/35016500. [DOI] [PubMed] [Google Scholar]
- 44.Taraschewski H. Hosts and parasites as aliens. J. Helminthol. 2006;80:99–128. doi: 10.1079/joh2006364. [DOI] [PubMed] [Google Scholar]
- 45.Yossepowitch O, et al. Opisthorchiasis from imported raw fish. Emerg. Infect. Dis. 2004;10:2122–2126. doi: 10.3201/eid1012.040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.WHO Control of foodborne trematode infections. Report of a WHO Study Group. World Health Organ. Tech. Rep. Ser. 1995;849:1–157. [PubMed] [Google Scholar]
- 47.Christensen JH, et al. Mearns L, Menéndez CG, Räisänen J, Rinke A, Sarr A, Whetton P. Regional Climate Projections. Cambridge University Press; 2007. [Google Scholar]
- 48.Hudson PJ, et al. Climate disruption and parasite-host dynamics: patterns and processes associated with warming and the frequency of extreme climatic events. J. Helminthol. 2006;80:175–182. doi: 10.1079/joh2006357. [DOI] [PubMed] [Google Scholar]
- 49.Poulin R. Global warming and temperature-mediated increases in cercarial emergence in trematode parasites. Parasitology. 2006;132:143–151. doi: 10.1017/S0031182005008693. [DOI] [PubMed] [Google Scholar]
- 50.Pinlaor S, et al. Oxidative and nitrative stress in Opisthorchis viverrini-infected hamsters: an indirect effect after praziquantel treatment. Am. J. Trop. Med. Hyg. 2008;78:564–573. [PubMed] [Google Scholar]
- 51.Pinlaor S, et al. Hepatobiliary changes, antibody response, and alteration of liver enzymes in hamsters re-infected with Opisthorchis viverrini. Exp. Parasitol. 2004;108:32–39. doi: 10.1016/j.exppara.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Thanan R, et al. Urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine in patients with parasite infection and effect of antiparasitic drug in relation to cholangiocarcinogenesis. Cancer Epidemiol. Biomarkers Prev. 2008;17:518–524. doi: 10.1158/1055-9965.EPI-07-2717. [DOI] [PubMed] [Google Scholar]
- 53.Dechakhamphu S, et al. High excretion of etheno adducts in liver fluke-infected patients: protection by praziquantel against DNA damage. Cancer Epidemiol. Biomarkers Prev. 2008;17:1658–1664. doi: 10.1158/1055-9965.EPI-08-0191. [DOI] [PubMed] [Google Scholar]