Abstract
Hemispatial neglect is a common and disabling syndrome following unilateral brain damage, particularly to perisylvian right-hemisphere (RH) structures, in which behaviour and awareness appear biased away from the contralesional (typically left) side of space towards the ipsilesional side. Theoretical accounts in terms of hemispheric rivalry have speculated that the intact left hemisphere (LH) may become hyper-excitable after the RH lesion, due to release from rivalry with the damaged RH. We used a novel approach for assessing excitability within the intact LH of neglect patients, applying twin-coil TMS over that hemisphere to study the impact of a conditioning TMS pulse over left posterior parietal cortex (PPC), on motor evoked potentials (MEPs) in response to a left M1 pulse shortly after. We applied this paired-pulse, PPC-M1 TMS paradigm over the LH for 12 RH stroke patients with neglect, plus an age-matched group of 8 RH stroke patients without neglect, and 10 healthy controls. We found that excitability of the LH PPC-M1 system was pathologically increased in neglect patients. A follow-up found that 1 Hz repetitive TMS over the left PPC normalised this over-excitability, and also ameliorated neglect. Our results provide a new form of direct evidence for pathological over-excitability of the LH in the neglect syndrome, specifically here for left PPC influences on left M1, with implications for possible treatment.
Introduction
Hemispatial neglect is a common and disabling syndrome following unilateral brain damage, particularly to the right hemisphere (e.g. see Heilman,Watson & Valentstein, 2000; Karnath, Milner & Vallar, 2002; Driver, Vuilleumier & Husain, 2004, for reviews). In many cases neglect is associated with haemorrhagic or ischemic stroke to right perisylvian regions, often including the right inferior parietal lobe (IPL) and/or nearby temporo-parietal junction (e.g. Bowen et al., 1999; Bisiach et al., 1986; Karnath et al., 2002; Mort et al., 2003). Neglect is a multi-component syndrome that includes failures to acknowledge or explore stimuli towards the contralesional side of space (e.g. Heilman et al., 1987; Bisiach et al., 1986; Bisiach 1991; Vallar et al., 2003; Beschin and Robertson, 1987; Driver, Vuilleumier and Husain, 2004). Intentional neglect or directional hypokinesia is an additional aspect in some cases in which patients show difficulties in moving towards the contralesional hemispace (e.g. Mattingley et al., 1998), even if they have little or no weakness (e.g. Laplane and Degos, 1983).
One influential proposal about the mechanisms contributing to neglect has invoked hemispheric rivalry or competition (e.g. Kinsbourne, 1977, 1993, 1994). Normal individuals may have a dynamic balance between circuits in the two hemispheres, with appropriate activation of left-hemisphere (LH) structures tending to shift attention and spatial behaviour rightwards, but analogous activation of right-hemisphere (RH) structures tending to oppose or counterbalance this. From this perspective, the right-hemisphere lesions that typically induce left neglect may lead to pathological over-excitability of LH circuits, due to release from rivalry. While there have been many studies of neglect that have invoked this view or extended it (e.g. see Kinsbourne, 1977, 1993, 1994; Oliveri et al., 1999), there have been surprisingly few studies if any that have directly tested for hyper-excitability within the intact LH itself, for neglect patients after RH damage. Here we sought to address this by applying twin-coil, transcranial magnetic stimulation (TMS) methods to the intact LH of patients suffering from neglect after right-hemisphere strokes.
A further aspect of the rationale for our new study comes from recent anatomical and functional neuroimaging studies indicating that altered patterns of cortico-cortical connectivity or coupling may contribute to neglect. For instance, neglect symptoms can arise after damage to the superior and longitudinal fasciculi (e.g. Doricchi and Tomaiuolo, 2003; Thiebaut de Schotten et al., 2005; Bartolomeo et al., 2007). More generally, recent neglect research has begun to emphasize that changes in inter-regional influences, between remote parts of an interconnected network, may contribute to neglect and be pathologically altered by the typical right-hemisphere lesion (Corbetta et al., 2005; Thiebaut de Schotten et al., 2005; Bartolomeo, 2006; He et al., 2007), with changes in the interactions or balance of intact left parietal cortex relative to other areas..
In separate studies of neurologically healthy subjects, we recently introduced (Koch et al., 2007; Koch et al., 2008) a new method for studying functional influences from the posterior parietal cortex (PPC) upon ipsilateral motor cortex (M1), non-invasively in humans, via a twin-coil or ‘paired-pulse’ TMS paradigm. A conditioning TMS pulse is applied over PPC, shortly prior to a test pulse over the hand area of ipsilateral motor cortex (M1). The latter pulse evokes a small twitch in contralateral hand muscles that can be measured with surface EMG. In normals, when the interval between the PPC pulse and the M1 pulse is brief (around ∼4-6 ms), the EMG response triggered by the M1 pulse is enhanced (Koch et al., 2007), indicating that the PPC stimulation has a remote influence on M1. The timing and intensity of the effective paired pulses suggest that this effect involves cortico-cortico pathways between the two sites of stimulation. The site of the conditioning PPC pulse that led to the most pronounced impact on M1 (Koch et al., 2007; Koch et al., 2008) lay over the caudal part of the intraparietal sulcus (cIPS), presumably activating a pathway that may involves the superior longitudinal fasciculus (SLF).
The present study applied this recently introduced, PPC-M1 twin-coil TMS method, to neglect. Specifically, we applied it over the LH of patients with neglect after RH stroke, in order to provide a direct new test for the general idea that the LH may become hyper-excitable in such neglect patients, and more specifically to test whether the influence of left PPC on left M1 becomes over-excitable in this way. We examined PPC-M1 influences via paired-pulse TMS for the intact LH in a group of 12 sub-acute neglect patients with RH strokes, comparing them to an age-matched group of RH stroke patients without neglect, plus a group of healthy controls.
In a follow-up study, we also applied repetitive TMS (rTMS) over left PPC in a subset of patients, an intervention that has been reported to transiently ameliorate neglect (e.g. Brighina et al., 2003). We examined not only whether this intervention reduced neglect for our patients, but more specifically whether it normalised the hyper-excitable left PPC-M1 influences that we had uncovered in our first experiment here, as described below.
Materials and Methods
Subjects
Twenty consecutive patients with RH damage, as confirmed by radiological (CT or MRI) and clinical exam, entered the study. All had suffered an ischemic stroke. None had any history or evidence of dementia or psychiatric disorder. All were examined in the sub-acute phase on a rehabilitation ward, within 1-6 months from onset of symptoms. All were given a standard clinical neurological and neuropsychological examination to assess any sensory or motor deficits, language disturbances or cognitive impairment, and critically the presence or absence of left neglect. Diagnosis of visuomotor neglect was based on the following tests: copy a scene of two trees, a house, and a fence; line barrage and letter cancellation. In copying a scene, each stroke in the scene to be copied was counted as a point; each omitted stroke was scored as one error, and each distorted or misplaced stroke was scored as one-half of an error. Line barrage was tested by presenting 30 bars of 4 cm disposed with random orientation equally in the two sizes of a 257 × 364-mm sheet. The centre of the sheet was positioned at eye-level and aligned with the patient’s sagittal body plane. Total number of cancelled bars and omissions in each side of the sheets were calculated. The cancellation task consisted in an array of 90 randomly distributed letters (H) mixed with 180 distractors. The centre of the stimulus array was positioned at eye-level and aligned with the patient’s sagittal body plane. The patients were instructed to mark all the letters they could detect during a time period of 3 min. Total number of cancelled letters and omissions in each side of the sheets were calculated.
As a result of the latter assessment, twelve patients were included in the neglect-syndrome group while eight patients who did not show any symptom related to the neglect spectrum entered the NO-NEGLECT group (see Table 1). The two groups of patients did not differ in mean age, gender nor duration of illness.
Table 1. Clinical characteristics of neglect and non-neglect stroke patients participating in the study.
| Gender | Age (years) | Time since stroke (days) | Line barrage (total responses (L/R omissions)) | Letter cancellation (total responses (L/R omissions)) | |
|---|---|---|---|---|---|
| Neglect patients | |||||
| 1 | M | 64 | 172 | 15 (13/2) | 55 (35/10) |
| 2 | M | 79 | 121 | 5 (15/10) | 49 (41/0) |
| 3 | M | 38 | 58 | 25 (5/0) | 68 (22/0) |
| 4 | F | 71 | 32 | 24 (6/0) | 51 (28/11) |
| 5 | F | 74 | 36 | 28 (2/0) | 42 (37/11) |
| 6 | F | 57 | 108 | 26 (4/0) | 50 (32/8) |
| 7 | M | 54 | 41 | 29 (1/0) | 11 (40/29) |
| 8 | M | 49 | 37 | 13 (15/2) | 29 (45/16) |
| 9 | M | 65 | 125 | 26 (4/0) | 72 (12/6) |
| 10 | F | 76 | 68 | 19 (11/0) | 59 (28/13) |
| 11 | M | 60 | 159 | 22 (8/0) | 64 (19/7) |
| 12 | F | 71 | 142 | 18 (12/0) | 41 (39/10) |
| Mean | 63,2 | 91,6 | 20,8 | 49,3 | |
| SD | 12,2 | 51,9 | 7,1 | 17,1 | |
| Non Neglect patients | |||||
| 1 | M | 72 | 118 | 30 (0/0) | 90 (0/0) |
| 2 | F | 79 | 158 | 29 (1/0) | 90 (0/0) |
| 3 | F | 53 | 142 | 30 (0/0) | 84 (6/0) |
| 4 | F | 67 | 91 | 30 (0/0) | 90 (0/0) |
| 5 | M | 49 | 31 | 30 (0/0) | 90 (0/0) |
| 6 | M | 66 | 52 | 30 (0/0) | 90 (0/0) |
| 7 | M | 48 | 36 | 29 (1/0) | 90 (0/0) |
| 8 | F | 64 | 87 | 30 (0/0) | 88 (2/0) |
| Mean | 63,4 | 83,9 | 29,6 | 89,2 | |
| SD | 10,2 | 45,5 | 0,7 | 1,9 |
To provide an overview of brain lesions the damage evident in CT or MRI images was reconstructed for each patient and plotted using MRIcro software (www.sph.sc.edu/comd/rorden/mricro.html), using a graphics tablet (WACOM Intuos A6) by a neurologist who was blind to the TMS results and the clinical scores when plotting the lesions. A T1-weighted template comprising 12 axial slices was used to demarcate lesions for every patient.
A further group of ten age-matched healthy controls (five men and five women, 45-72 years old) participated for completeness.
All subjects were right-handed, according to the Edinburgh inventory (Oldfield, 1971). They all gave informed consent for participation in the study, and experimental procedures were approved by the local Ethics Committee and conducted in accordance with the Declaration of Helsinki.
Experimental procedures
Experiment 1: Twin-coil TMS test of PPC-M1 influences in the intact left hemisphere
Electromyographic (EMG) recordings were made from the first dorsal interosseous (FDI) muscles using 9-mm diameter, Ag-AgCl surface cup electrodes. The active electrode was placed over the muscle belly and the reference electrode over the metacarpophalangeal joint of the right index finger. Responses were amplified with a Digitimer D360 amplifier (Digitimer Ltd, Welwyn Garden City, Hertfordshire, UK), through filters set at 20 Hz and 2 kHz with a sampling rate of 5 kHz, then recorded by a computer using SIGNAL software (Cambridge Electronic Devices, Cambridge, UK). We used a paired-pulse TMS technique with two high-power Magstim 200 machines (Magstim Co., Whitland, Dyfed, UK). The magnetic stimulus had a nearly monophasic pulse configuration with a rise time of about 100 μs, decaying back to zero over about 0.8 ms.
As described in our recent study of normals (Koch et al., 2007; see also Koch et al., in press), the intensity of the test M1 TMS pulse (applied over left M1 here) was adjusted to evoke an MEP of approximately 1 mV peak-to-peak in the relaxed right FDI. The scalp location for the hand motor area of left M1 was defined as the point where stimulation evoked the largest MEP from the contralateral FDI muscle. The test stimulator was connected to a small custom figure-of-eight-shaped coil (external diameter 50 mm). In order to stimulate M1, the coil was always placed tangentially to the scalp at a 45 deg angle to the midline, in order to induce a posterior-anterior (PA) current flow across the central sulcus.
The conditioning stimulator for left PPC was connected to a larger figure-of-eight-shaped coil, 70 mm in external diameter. The coil position for left PPC TMS was then defined relative to the P3 position of the 10-20 EEG system (see also Koch et al., 2007, 2008). According to previous investigations, 3D MRI reconstruction for this site corresponds to over the inferior parietal lobule, close to the posterior part of the adjoining intraparietal sulcus (Rushworth and Taylor, 2006; Herwig et al., 2003; Koch et al., 2007). The centre of the conditioning left PPC coil was positioned tangentially to the skull, with the handle pointing downward and slightly medial (10°) in order to induce a posterior-anterior directed current in the underlying cortical tissue.
We defined the resting motor threshold (RMT) as the lowest intensity that evoked five small responses (about 50 μV) in the contralateral FDI muscle, for a series of ten stimuli applied over M1 when the subject kept the FDI muscles relaxed in both hands, in accord with the standard international procedure (Rossini et al., 1994). The intensity of the conditioning left PPC stimulus was adjusted to be either suprathreshold (110% RMT) or subthreshold (90% RMT) with respect to that RMT measure. For defining this, RMT was tested with the larger figure of eight coil over left M1, with posterior-anterior orientation.
Inter-stimulus-intervals (ISIs) between the conditioning (CS) and test (TS) pulses were 2, 4, 6, 8, 10, or 15 ms, selected equiprobably and randomly. In two separate blocks the intensity of CS was set either the 110% or 90% RMT. In each group, half of the subjects performed first the block in which the CS intensity was set at 90% RMT and the other half were tested first with the CS intensity=110% RMT. In each block seven conditions were randomly intermingled: TS alone (MEP) and CS1 + TS (conditioned MEP for each of six different ISIs). Twenty responses were collected for the test stimulus alone and ten responses for conditioned MEPs at each ISI (total number of trials: eighty). The inter-trial interval was set at 4 s (±10%), for a total duration of approximately five minutes. Measurements were made on each individual trial and the mean peak-to peak amplitude of the conditioned MEP was expressed as a percentage of the mean peak-to-peak amplitude of the unconditioned test pulse.
Experiment 2. Changes in left PPC-M1 influences and in neglect test after left PPC 1 Hz rTMS
In this experiment we compared possible changes in PPC-M1 influences for the left hemisphere before and one minute after the application of a single session of 1 Hz rTMS trains (600 pulses in total) over the left PPC in 10 patients from the NEGLECT group and 5 from the NO-NEGLECT group. This protocol is known to induce an inhibitory effect on the stimulated area lasting about fifteen minutes (Chen et al., 1997) and has been reported to induce improvement in neglect symptoms when applied over the left PPC (Brighina et al., 2003). The coil for rTMS was applied over the same site and with the same orientation as for the CS PPC pulses in experiment 1, and as repeated here for the twin-coil part of Experiment 2. A MagStim Rapid magnetic stimulator (Magstim, Whitland, UK), connected with a figure-of-eight coil of diameter 70 mm was used to deliver rTMS over the scalp site corresponding to the left PPC. We repeated the twin-coil PPC-M1 procedure over the left hemisphere as in experiment 1, both before and after the 1 Hz rTMS intervention.
In the neglect group we also assessed clinical neglect before and after the 1 Hz rTMS intervention, using 20 visual chimeric objects each of which had to be named and for which left neglect corresponds to failures to name the identity of the half-object shown on the left of the chimeric, despite naming that on the right. The materials and protocol for this followed Sarri et al. (2006), albeit without their prism manipulation (substituted for here with the 1Hz rTMS over left PPC). We choose this specific tasks since most of the patients were doing cognitive rehabilitation and already did learn more common procedures such clock drawing and line bisection. This evaluation was performed at least one week apart from the twin-coil PPC-M1 study.
Results
Experiment 1, hyperexcitability of left PPC-M1 in neglect after RH damage
Figure 1 summarises the lesion data, which are presented for completeness. As apparent in the top-row of Figure 1, the NEGLECT group typically had substantial lesions centred on right perisylvian structures, similar to many previous studies of neglect; while, also as expected, there was less overlap for the no neglect group.
Figure 1.
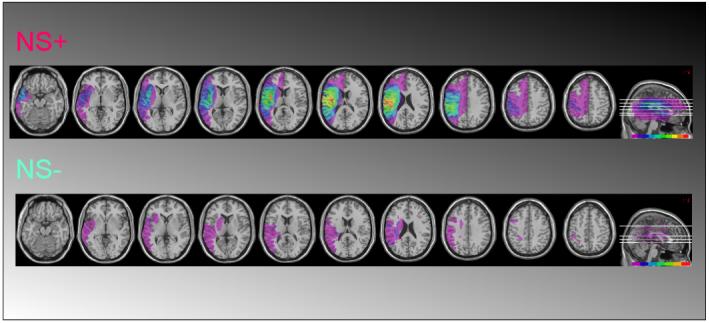
Summary of lesions and overlap in our sample of right-hemisphere stroke patients with neglect (NS+, top row) and those without neglect (NS-) participating in the study. The color-scale at bottom-right of each row indicates the proportion of patients (higher for colors further to the right in the scale).
In the NEGLECT patient group, RMT for left M1 (used to calibrate the intensity of the CS over PPC for the twin-coil experiment) was 37.4 ± 7.8% of maximal stimulator output (MSO). The intensity of TS over left M1 needed to produce the 1 mV MEP was 50.1 ± 11.8% MSO. The corresponding values for the NO-NEGLECT patient group were left M1 RMT = 36.3 ± 6.8%; TS = 48.3 ± 9.9% MSO (see Fig. 2). In the age-matched healthy control group the values were: RMT = 39.6 ± 7.8%; TS = 49.2 ± 8.4% MSO. The procedure was well tolerated by all subjects.
Figure 2.
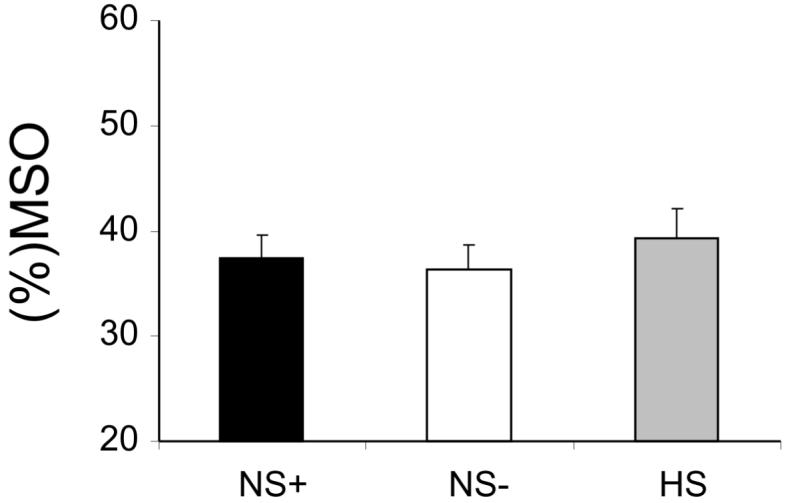
Resting Motor Thresholds (RMT) in the left motor cortex did not differ between the two groups of patients (NS+ and NS-) and the healthy control group (HS). Means and standard errors shown. MSO=maximal stimulator output.
Importantly, there were no significant differences between patient groups (see Fig. 2), nor against age-matched healthy controls, when comparing these M1 ‘threshold’ measures. This implies that excitability of motor cortex itself (left M1) was not pathologically increased for the intact hemisphere in the neglect group.
By contrast, the major new finding in Experiment 1 was that the strength of left PPC-MI functional connectivity did differ notably between NEGLECT patients versus the NO-NEGLECT and the HS groups (see Fig. 3). The effects of paired TMS over left PPC, on the size of MEP recorded from the contralateral FDI in response to left M1 TMS, were analyzed as the percentage of the mean peak-to-peak amplitude of the unconditioned test M1 pulse. Mean percentage values were analyzed in a mixed-design ANOVA, with group as a between-subjects factor (NEGLECT, NO-NEGLECT, or HS), plus conditioning intensity (110% or 90% RMT) and the ISI between left PPC and left M1 pulses (2, 4, 6. 8, 10, or 15 ms) as within-subjects factors.
Figure 3.
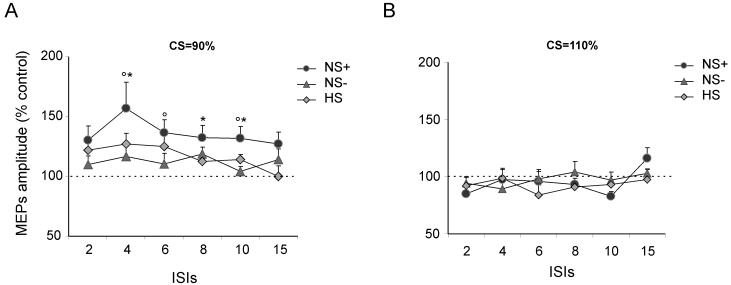
A conditioning TMS stimulus (CS) was applied at (A) 90% or (B) 110% of resting motor threshold (RMT) over left posterior parietal cortex (PPC) at a site corresponding to the inferior parietal lobe and angular gyrus near the caudal intraparietal sulcus, as different inter-stimulus-intervals (ISIs, shown across the x-axis of each graph) relative to a test stimulus (TS) pulse to left M1. The panels shows the left PPC-M1 effects (relative to an M1 pulse alone) obtained after PPC conditioning in the three different groups for Experiment 1. The intensity of the test stimulus (TS) was fixed to evoke an MEP of approximately 1 mV peak to peak in the relaxed left first dorsal interosseus (FDI). Pathologically increased left PPC-M1 effects were observed selectively in the neglect group at CS intensity of 90% RMT (see left graph, A). Errors bars indicate S.E.M.. * indicate significant differences in pairwise tests between NS+ and NS- groups; ° indicate significant differences at between NS+ and HS (i.e. healthy control subject) groups, all at p <0.05 or better).
In addition to main effects of group (F(2,27)=4.1, p<0.05) and intensity (F(1,27)=30.5, p<0.001) we found two-way interactions between group and intensity (F(2,27)=3.3, p<0.05) plus between intensity and ISI (F(5,135)=2.92, p<0.05) (Fig. 3). Post hoc t-tests with Bonferroni correction comparing the size of the left PPC-M1 influence between groups at different intensities and ISIs showed that NEGLECT patients in comparison with NO-NEGLECT patients had stronger PPC-M1 facilitation for CS intensity of 90% RMT at ISIs of 4 ms (p<0.05), 8 ms (p<0.05) and 10 ms (p<0.05). In comparison with healthy normals, NEGLECT patients analogously had stronger facilitation for CS intensity of 90% RMT, that reached significance at ISI s of 4 ms (p<0.05), 6 (p<0.05) and 10 ms (p<0.05). The NO-NEGLECT and healthy-control groups did not differ. In both groups the effects of PPC conditioning induced a peak facilitation of about 20%, as expected from our original study (Koch et al., 2007). There were no group differences at CS intensity of 110% (see Fig. 3b).
Thus, in comparison with RH stroke patients without neglect, and also against healthy controls, the neglect patients showed pathologically enhanced left PPC-M1 influences when the CS intensity was 90% RMT (but not at higher intensity). This specific outcome indicates hyper-excitability of left PPC-M1 influences in right-hemisphere-damaged patients with neglect (see Fig. 3). At this regard it is important to notice that not all the patients had the same trend of left PPC-M1 hyperexcitability. In fact in subsequent analyses, we found that the individual amount of facilitation induced by PPC conditioning at 90% RMT at ISI=4 ms correlated with number of left side omissions in the line barrage (r=0.69; p<0.05) and letter cancellation (r=0.63; p<0.05) task, showing that patients with worse visuospatial neglect had more increased connectivity (see Fig. 4).
Figure 4.
In patients with neglect the individual amount of facilitation induced by PPC conditioning at 90% RMT at ISI=4 ms correlated with number of left side omissions in the line barrage (r=0.69; p<0.05) and letter cancellation (r=0.63; p<0.05) task, showing that patients with worse visuospatial neglect had more increased connectivity.
Experiment 2. Changes in PPC-M1 influences and neglect following 1 Hz rTMS over left PPC
Our 1Hz rTMS protocol (of 600 pulses) was applied over the left PPC site for 10 patients from the NEGLECT group and 5 from the NO-NEGLECT group. In all these patients, our twin-coil PPC-M1 paradigm, with a single pulse applied at either site with ISIs of 2-15 msec between as before, was re-applied both before and one minute after the 1Hz rTMS intervention.
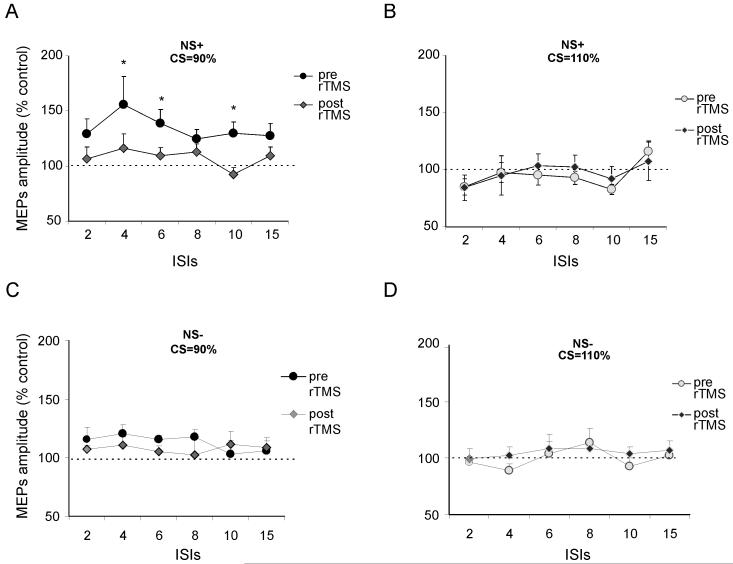
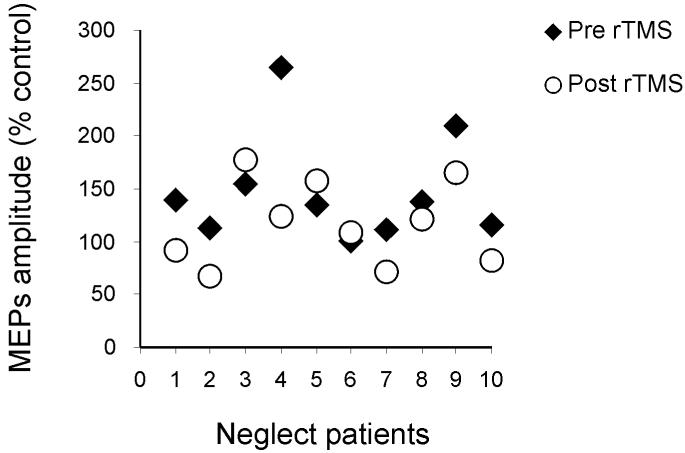
The rTMS intervention significantly changed the left PPC-M1 effects only for the NEGLECT group, and only at a conditioning intensity of 90% RMT (see Fig. 5). This was confirmed by a mixed-design four-way ANOVA, with group as a between-subjects factor (NEGLECT, NO-NEGLECT), plus treatment condition (before or after rTMS), conditioning intensity during twin-coil TMS (90% or 110% RMT), and PPC-M1 ISI (2, 4, 6, 8, 10, 15ms) as within-subject factor. In addition to main effects of intensity (F(1,9)=18.6, p<0.001) and two-way interactions for group x treatment condition (F(1,13)=3.3, p<0.05), group x intensity (F(1,13)=7.2, p<0.05), plus treatment x intensity (F(1,13)=6.6, p<0.05), we also found a triple interaction (F(1,13)=3.6, p<0.05). Subsequent post hoc t-tests confirmed that the size of the PPC-M1 effect was reduced after the 1 Hz rTMS treatment, only for the NEGLECT group, and for them only with the CS intensity of 90% RMT, and at the ISIs of 2,4,6 and 10 ms (all p<0.05); see Fig. 5A. Positive effects of treatment were detected in 7 out of 10 patients as showed by Figure 6 in which for each patient the individual peak of facilitation of PPC-M1 connectivity with CS at 90% RMT intensity and ISI=4 ms was plotted before and after rTMS procedure.
Figure 5.
The panel shows the changes induced by the left PPC 1 Hz rTMS treatment (600 stimuli at 90% RMT) on the left PPC-M1 connectivity effects. (A) The pathologically increased left PPC-M1 effects observed in the neglect group at intensity of 90% RMT (see also Experiment 1 and Figure 3) pre-rTMS were significantly reduced and indeed eliminated (relative to the NS- control group of patients) post rTMS in the NS+ group. (B) No significant changes were detected in the NS+ group at CS intensity of 110% RMT. (C and D) rTMS did not change the normal pattern for the NS-group at either intensity of CS. Errors bars indicate S.E.M.; * indicate p value <0.05 in paired comparison of the two scores within each graph at a given ISI.
Figure 6.
In neglect patients positive effects of treatment were detected in 7 out of 10 patients as showed by the graph in which for each patient the individual peak of facilitation of PPC-M1 connectivity with CS at 90% RMT and ISI=4 ms was plotted before and after rTMS procedure.
Another way of putting this outcome is that the 1 Hz rTMS over left PPC effectively ‘normalised’ the hyper-excitability of left PPC-M1 effects that was evident prior to the 1Hz rTMS treatment, only in the neglect group for CS intensity of 90% RMT. Direct comparison of the NEGLECT and NO-NEGLECT groups after the 1Hz rTMS treatment had been applied over left PPC confirmed that these patient groups no longer differed for left PPC-M1 effects after that treatment (all p’s=n.s). This implies that the 1Hz rTMS treatment can attenuate the pathological hyper-excitability for left PPC-M1 influences that we have uncovered for the first time. Finally, we also found that the single session of 1 Hz rTMS over left PPC not only normalized the abnormal left PPC-M1 influences, but was also able to improve visuospatial neglect, as assessed with the chimeric test taken from Sarri et al. (2006). 79.5% (± 7.5 %) of the left-sides of the chimeric objects were not named prior to the 1Hz rTMS treatment, but this improved to a reduced level of 65.6 % ± 8.8 % after the intervention (p<0.005). Visual neglect was not completely eliminated, and the improvement on chimerics did not correlate with the individual size of the left PPC-M1 normalisation revealed by the twin-coil TMS measure implemented before and after the 1Hz rTMS intervention. As noted in discussion, the relationship might potentially be stronger for more motoric aspects of neglect, as might be assessed in future research. Nevertheless, our results clearly show that 1hz rTMS over left PPC has potential to normalise hyper-excitable effects within the left hemisphere, as revealed here for the first time, and can also have some impact on clinical measures of neglect.
Discussion
The present findings used a new twin-coil TMS approach to show, for the first time, that even at rest, the functional influence or inferred ‘connection’ between left PPC-M1 is pathologically over-excitable in RH patients with neglect, than in RH patients without neglect, or healthy age matched controls. This provide a new and highly direct form of evidence for notions that some circuits in the LH may become disinhibited in RH neglect patients, presumably due to release from mutual inhibition due to the RH lesion of the neglect group. More specifically, we extended recent work in normals using the twin-coil TMS approach (Koch et al., 2007; Koch et al., 2008), that had uncovered PPC-M1 influences, to show that these specific influences become pathologically strong in the LH of neglect patients. We note that left M1 in itself did not seem hyper-excitable in the neglect group (see Fig 2), but rather it was the specific impact of left PPC upon left M1 that became exaggerated (see Fig 3). This indicates that rather than the LH as a whole becoming more excitable, specific influences from left PPC may do so, presumably in relation to right PPC damage. One possibility is that lesions in the right hemisphere may induce changes in the cortico-cortical excitability of the non-lesioned hemisphere trough a mechanism of reduced transcallosal inhibition.
As a further means of demonstrating the crucial role of left PPC, in a follow-up study (Experiment 2), we applied inhibitory 1 Hz rTMS over this side. In support of other work (e.g. Brighina et al., 2003) indicating that this may have potential for improving symptoms of neglect, we found that visual neglect for chimeric objects could be partially but significantly ameliorated by this intervention. But more specifically, we also found that the 1Hz rTMS intervention normalised the pathological hyper-excitability of left PPC-MI influences, indicating directly that it provides an effective means for eliminating over-excitability in LH circuits.
In recent experiments in normals (Koch et al., 2007, 2008), we demonstrated that PPC-M1 influences can relate to action planning of reaches in the contralateral direction, being enhanced in such a context. Invasive electrophysiological studies in monkeys have suggested roles for PPC is involved in converting target locations into motor intentions (e.g. see Cohen and Andersen, 2002 for review; see also Cavada and Goldman-Rakic, 1989; Johnson et al., 1996; Seltzer and Pandya, 1994). Moreover, parietal regions may over-represent contralateral workspace relative to ipsilateral workspace (e.g. Battaglia-Mayer et al., 2005). It has been suggested that, in humans, damage to such parietal representations might explain some directional motor aspects of neglect, which include right parietal patients being impaired at initiating reaches in the leftward direction, over and above any visual or attentional impairments for left targets (e.g. Heilman et al., 1985; Husain et al., 2000; Mattingley et al., 1998). In the light of this growing body of literature, the present hyper-excitability of parieto-motor influences that we found in the intact left hemisphere may contribute to the skewed spatiomotor plans behaviour that is so evidence in neglect patients. In this respect, it may be informative in future work to assess how the twin-coil PPC-M1 abnormalities for the left hemisphere in neglect patients may relate specifically to spatiomotor aspects of their clinical disorder (Heilman et al., 1985; Husain et al., 2000; Mattingley et al., 1998).
Finally, we found here that inhibitory low frequency 1 Hz rTMS applied over left PPC could attenuated the pathological over-excitability of parieto-motor circuits in neglect patients. This 1 Hz rTMS has also been shown to ameliorate neglect (Brighina et al., 2003), as we also found here for visual chimerics. Taken together, our findings provide a new and direct form of evidence for the idea (Kinsbourne, 1993; Oliveri et al., 1999) that unbalanced excitability of the two hemispheres, with hyper-excitability for some LH circuits, is an important contributor to neglect. We suggest that not only is damage in the extensive right-hemisphere, attention-related network an essential contributor to neglect (Mesulam, 1981; Corbetta et al., 2005; Thiebaut de Schotten et al., 2005; He et al. 2007), but also that consequent hyper-excitability of circuits in the intact hemisphere may make further contributions. At this regard, it is important to notice that the present experiments only address one out of many possible connections between parietal and frontal areas in the non-lesioned hemisphere. However, others could potentially be examined using combined TMS/EEG or fMRI methods such as Diffusion Tensor Imaging (DTI) to confirm this hypothesis and to better define which white matter fibers are associated with the observed neurophysiological changes.
In conclusion, these results suggest that the abnormal excitability of cortical networks in the unaffected hemisphere may be a crucial feature of neglect and reinforce the idea that interventional approaches that are directed to restore this imbalance may be useful to treat neglect symptoms.
REFERENCES
- Bartolomeo P, Thiebaut de Schotten M, Doricchi F. Left unilateral neglect as a disconnection syndrome. Cereb Cortex. 2007;17:2479–90. doi: 10.1093/cercor/bhl181. [DOI] [PubMed] [Google Scholar]
- Bartolomeo P. A parietofrontal network for spatial awareness in the right hemisphere of the human brain. Arch Neurol. 2006;63:1238–41. doi: 10.1001/archneur.63.9.1238. [DOI] [PubMed] [Google Scholar]
- Battaglia-Mayer A, Mascaro M, Brunamonti E, Caminiti R. The over-representation of contralateral space in parietal cortex: a positive image of directional motor components of neglect? Cereb Cortex. 2005;15:514–25. doi: 10.1093/cercor/bhh151. [DOI] [PubMed] [Google Scholar]
- Beschin N, Robertson IH. Personal versus extrapersonal neglect: a group study of their dissociation using a reliable clinical test. Cortex. 1997;33:379–84. doi: 10.1016/s0010-9452(08)70013-3. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Perani D, Vallar G, et al. Unilateral neglect: personal and extra-personal. Neuropsychologia. 1986;24:759–67. doi: 10.1016/0028-3932(86)90075-8. [DOI] [PubMed] [Google Scholar]
- Bisiach E. Extinction and neglect: same or different? In: Paillard J, editor. Brain and space. Oxford University Press; Oxford: 1991. pp. 251–7. [Google Scholar]
- Bowen A, McKenna K, Tallis RC. Reasons for variability in the reported rate of occurrence of unilateral spatial neglect after stroke. Stroke. 1999;30:1196–202. doi: 10.1161/01.str.30.6.1196. [DOI] [PubMed] [Google Scholar]
- Brighina F, Bisiach E, Oliveri M, Piazza A, La Bua V, Daniele O, Fierro B. 1 Hz repetitive transcranial magnetic stimulation of the unaffected hemisphere ameliorates contralesional visuospatial neglect in humans. Neurosci Lett. 2003;336:131–3. doi: 10.1016/s0304-3940(02)01283-1. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Cohen YE, Andersen RA. A common reference frame for movement plans in the posterior parietal cortex. Nat Rev Neurosci. 2002;3:553–62. doi: 10.1038/nrn873. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci. 2005;8:1603–10. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Tomaiuolo F. The anatomy of neglect without hemianopia: a key role for parietal-frontal disconnection? Neuroreport. 2003;14(17):2239–43. doi: 10.1097/00001756-200312020-00021. [DOI] [PubMed] [Google Scholar]
- Driver J, Vuilleumier P, Husain M. Spatial neglect and extinction. In: Gazzaniga M, editor. The new cognitive neurosciences III. MIT Press; Cambridge: 2004. pp. 589–606. 2004. [Google Scholar]
- Heilman KM, Bowers D, Coslett HB, Whelan H, Watson RT. Directional hypokinesia: prolonged reaction times for leftward movements in patients with right hemisphere lesions and neglect. Neurology. 1985;35:855–9. doi: 10.1212/wnl.35.6.855. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Valenstein E, Watson RT. Neglect and related disorders. Semin Neurol. 2000;20:463–70. doi: 10.1055/s-2000-13179. [DOI] [PubMed] [Google Scholar]
- Herwig U, Satrapi P, Schonfeldt-Lecuona C. Using the international 10-20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr. 2003;16:95–9. doi: 10.1023/b:brat.0000006333.93597.9d. [DOI] [PubMed] [Google Scholar]
- Husain M, Mattingley JB, Rorden C, Kennard C, Driver J. Distinguishing sensory and motor biases in parietal and frontal neglect. Brain. 2000;123:1643–59. doi: 10.1093/brain/123.8.1643. [DOI] [PubMed] [Google Scholar]
- Johnson PB, Ferraina S, Bianchi L, Caminiti R. Cortical networks for visual reaching: physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb Cortex. 1996;6:102–119. doi: 10.1093/cercor/6.2.102. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Milner AD, Vallar G, editors. The cognitive and neural bases of spatial neglect. Oxford University Press; Oxford: 2002. [Google Scholar]
- Karnath HO, Perenin MT. Cortical control of visually guided reaching: evidence from patients with optic ataxia. Cereb Cortex. 2005;15:1561–9. doi: 10.1093/cercor/bhi034. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. Hemi-neglect and hemisphere rivalry. Adv Neurol. 1977;18:41–9. [PubMed] [Google Scholar]
- Kinsbourne M. Orientational bias model of unilateral neglect: evidence from attentional gradients within hemispace. In: Robertson IH, Marshall JC, editors. Unilateral neglect: clinical and experimental studies. Lawrence Erlbaum; Hove (UK): 1993. pp. 63–86. [Google Scholar]
- Kinsbourne M. Mechanisms of neglect: implications for rehabilitation. Neuropsychol Rehabil. 1994;4:151–3. [Google Scholar]
- Koch G, Fernandez Del Olmo M, Cheeran B, Ruge D, Schippling S, Caltagirone C, Rothwell JC. Focal stimulation of the posterior parietal cortex increases the excitability of the ipsilateral motor cortex. J Neurosci. 2007;27:6815–22. doi: 10.1523/JNEUROSCI.0598-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Fernandez Del Olmo M, Cheeran B, Schippling S, Caltagirone C, Driver J, Rothwell JC. Functional interplay between posterior parietal and ipsilateral motor cortex revealed by twin-coil TMS during reach planning toward contralateral space. J Neurosci. 2008;28:5944–53. doi: 10.1523/JNEUROSCI.0957-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplane D, Degos JD. Motor neglect. J Neurol Neurosurg Psychiatry. 1983;46:152–8. doi: 10.1136/jnnp.46.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingley JB, Husain M, Rorden C, Kennard C, Driver J. Motor role of human inferior parietal lobe revealed in unilateral neglect patients. Nature. 1998;392:179–82. doi: 10.1038/32413. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann. Neurol. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H, Acuna C. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J Neurophysiol. 1975;38:871–908. doi: 10.1152/jn.1975.38.4.871. [DOI] [PubMed] [Google Scholar]
- Mort DJ, Malhotra P, Mannan SK, Rorden C, Pambakian A, Kennard C, Husain M. The anatomy of visual neglect. Brain. 2003;126:1986–1997. doi: 10.1093/brain/awg200. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Taylor PC. TMS in the parietal cortex: updating representations for attention and action. Neuropsychologia. 2006;44:2700–2716. doi: 10.1016/j.neuropsychologia.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Sarri M, Kalra L, Greenwood R, Driver J. Prism adaptation changes perceptual awareness for chimeric visual objects but not for chimeric faces in spatial neglect after right-hemisphere stroke. Neurocase. 2006;12:127–35. doi: 10.1080/13554790600598774. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Parietal, temporal, and occipital projections to cortex of the superior temporal sulcus in the rhesus monkey: a retrograde tracer study. J Comp Neurol. 1994;343:445–63. doi: 10.1002/cne.903430308. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature. 1997;386:167–70. doi: 10.1038/386167a0. [DOI] [PubMed] [Google Scholar]
- Tanne-Gariepy J, Rouiller EM, Boussaoud D. Parietal inputs to dorsal versus ventral premotor areas in the macaque monkey: evidence for largely segregated visuomotor pathways. Exp Brain Res. 2002;145:91–103. doi: 10.1007/s00221-002-1078-9. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Urbanski M, Duffau H, Volle E, Lévy R, Dubois B, Bartolomeo P. Direct evidence for a parietal-frontal pathway subserving spatial awareness in humans. Science. 2005 Sep 30;309(5744):2226–8. doi: 10.1126/science.1116251. [DOI] [PubMed] [Google Scholar]
- Vallar G, Bottini G, Paulesu E. Neglect syndromes: the role of the parietal cortex. Adv Neurol. 2003;93:293–319. [PubMed] [Google Scholar]