Abstract
Smoking behavior has been associated in two independent European cohorts with the most common Caucasian human leukocyte antigen (HLA) haplotype (A1-B8-DR3). We aimed to test whether polymorphic members of the two odorant receptor (OR) clusters within the extended HLA complex might be responsible for the observed association, by genotyping a cohort of Hungarian women in which the mentioned association had been found. One hundred and eighty HLA haplotypes from Centre d’Etude du Polymorphisme Humain families were analyzed in silico to identify single-nucleotide polymorphisms (SNPs) within OR genes that are in linkage disequilibrium with the A1-B8-DR3 haplotype, as well as with two other haplotypes indirectly linked to smoking behavior. A nonsynonymous SNP within the OR12D3 gene (rs3749971T) was found to be linked to the A1-B8-DR3 haplotype. This polymorphism leads to a 97Thr → Ile exchange that affects a putative ligand binding region of the OR12D3 protein. Smoking was found to be associated in the Hungarian cohort with the rs3749971T allele (p = 1.05×10−2), with higher significance than with A1-B8-DR3 (p = 2.38×10−2). Our results link smoking to a distinct OR allele, and demonstrate that the rs3749971T polymorphism is associated with the HLA haplotype-dependent differential recognition of cigarette smoke components, at least among Caucasian women.
Introduction
There were nearly 1.3 billion smokers worldwide in the year 2003, and this number is expected to rise to 1.7 billion (∼1.2 billion males and 500 million females) by 2025, with the number of female smokers contributing most to the increase (American Cancer Society, 2003). In nearly all investigated regions of the world, the ratio of female to male smokers among young people was found to be higher than the ratio among adults, suggesting a global trend for an increase in smoking habits among female adolescents and young women (Global Youth Tobacco Survey Collaborating Group, 2003). Smoking is associated with many serious health problems, including cancer of various organs, coronary artery disease, as well as several autoimmune disorders (Hegediüs et al., 2004; Klareskog et al., 2006; Warren et al., 2006; American Cancer Society, 2007; Koch et al., 2007; Hawkes, 2007), and it is thus considered a leading cause of death and disability worldwide. Although there is general agreement that nicotine is the core addictive component of cigarette smoke (Jarvis, 2004), there are hundreds of further substances that may influence the initiation and continuation of tobacco abuse (Baker et al., 2004), independent of nicotine (Franklin et al., 2007). Smoking is also modulated by genetic factors, as demonstrated by epidemiological and twin studies (Sullivan and Kendler, 1999; Li et al., 2003). In support, a haplotype of the major histocompatibility (human leukocyte antigen, HLA) complex was found to be associated with smoking behavior, stronger in women (odds ratio: 13.6) than in men (odds ratio: 2.79) (Füst et al., 2004). This haplotype, –HLA-A1-B8-DR3–, the most common among Caucasians (Alper et al., 2006), is also associated with autoimmune disorders, of which some are clearly connected with tobacco abuse, such as Graves’ ophthalmopathy (Weetman, 2000; Hunt et al., 2001; Hegediüs et al., 2004; Holm et al., 2005).
At least two further HLA haplotypes have also been linked to autoimmune diseases that are triggered or heavily influenced by tobacco smoking. The HLA-A3-B7-DR15 haplotype is overrepresented in individuals with multiple sclerosis (Dyment et al., 2004; Herrera et al., 2006), while HLA-DR “Shared Epitope” (SE) haplotypes are overrepresented in individuals who smoke, possess antibodies against citrullinated proteins, and suffer from rheumatoid arthritis (Klareskog et al., 2006; Linn-Rasker et al., 2006). SE haplotypes are characterized by the presence of the HLA-DRB1*01, -DRB1*04, or -DRB1*10 alleles.
The exceptionally strong linkage disequilibrium (LD) that is typical for certain haplotypes of the xHLA encompasses a chromosomal segment with a length of about 7 Mb between the gene HFE and loci within the HLA class II region (Horton et al., 2004). In case of the A1-B8-DR3 haplotype, LD is extreme over its entire length (Alper et al., 2006), suggesting that an allele of any genes within the region of LD could in principle predispose to smoking. Therefore, polymorphic members of the two odorant receptor (OR) gene clusters within the telomeric xHLA (Ehlers et al., 2000; Younger et al., 2001; Ziegler and Uchańska-Ziegler, 2006) must be considered as plausible candidate genes.
The Centre d’Etude du Polymorphisme Humain (CEPH) panel of families (Miretti et al., 2005) offers unique opportunities for genetic association studies comprising the HLA region. The samples analyzed here comprise 180 founder chromosome 6 that have already been analyzed with regard to their single-nucleotide polymorphism (SNP) alleles and tagSNPs (representative SNPs for a genomic region exhibiting high LD) (Miretti et al., 2005). Recently, the analysis has been extended to both OR gene clusters at the telomeric section of the xHLA, permitting the correlation with alleles within the HLA class I, II, and III regions (de Bakker et al., 2006).
The present study was conducted in two steps: (i) in silico, we aimed to identify SNP alleles within the two HLA-linked OR gene clusters that are characteristic for the HLA haplotypes mentioned before; and (ii) in vitro we wanted to test whether their possible association with tobacco abuse is stronger or weaker than the one observed with A1-B8-DR3, by genotyping a subsample of the cohort in which an association between smoking and loci at the HLA class III region had been found before.
Materials and Methods
In silico tagSNP selection and assessment of HLA haplotype-dependent OR SNP alleles
All in silico analyses were based on xHLA high-density SNP genotyping data from 180 founder chromosome 6 from the CEPH collection (Miretti et al., 2005). Details on marker selection, CEPH subjects, and on their genotypings are given elsewhere (Miretti et al., 2005; de Bakker et al., 2006). A total of 1170 SNPs spanning the region between OR2B2 and MOG (Fig. 1) were initially considered and analyzed with regard to their allelic diversity (supplemental Fig. S1, available online at www.liebertpub.com). A list of all 1170 SNPs with their genomic coordinates is given in the supplemental Table S1 (available online at www.liebertpub.com). From this set of markers, we chose 110 tagSNPs capturing the haplotypic information from the whole genomic region. SNP tagging was performed using a pairwise tagging algorithm (de Bakker et al., 2006), as implemented in HAPLOVIEW v. 4 (Barrett et al., 2005), and considering a maximum intermarker distance of 650 kb and an LD coefficient (r2) threshold of ≥0.8. Loci with a minor allele frequency of less than 1%, those not conforming to Hardy-Weinberg equilibrium, or not reported by the dbSNP database (http://www.ncbi.nlm.nih.gov/projects/SNP) were excluded.
FIG. 1.
Map (not to scale) of the region encompassing the two OR clusters on chromosome 6p. Above the figure, the NCBI 36 coordinates (bp) of the chromosomal segments analyzed here are depicted. The upper plot is filled in black for OR clusters and in white for regions outside the OR clusters, while the lower part of the figure shows the OR genes/pseudogenes at their relative chromosomal locations (suppressing most of the intercluster region). Triangles indicate transcriptional orientation, and are filled in black (genes), white (pseudogenes), or in both colors (haplotype-dependent genes/pseudogenes). tel: direction to the telomere; cen: direction to the centromere.
We tested the 110 tagSNPs characterizing the OR2B2-MOG segment for association with three groups of haplotypes (A1-B8-DR3, A3-B7-DR15, and SE), the aim being to determine which tagSNPs were characteristic for each of the three haplotype groups.
Genotyping of SNP rs3749971
The genotyping of the SNP rs3749971 was performed by real-time PCR in a sample of 32 Hungarian female Caucasians (average age 46.75 years, ranging from 24 to 76 years), which was a subsample of a cohort in which a correlation had previously been found between smoking and loci of the HLA class III region (Füst et al., 2004). Based on LD, women who carried the C4A*Q0 (mono-S) genotype as well as the AGER-429C, HSPA1B-1267G, and TNF-308A alleles were considered carriers of the A1-B8-DR3 haplotype (Füst et al., 2004). Other genotypings of these subjects, details on registration of smoking habits, preparation of genomic DNA, as well as informed consent from the cohort have been provided before (Füst et al., 2004).
The PCR reactions were performed using a Stratagene realtime PCR instrument (Stratagene, Amsterdam, NL) with the following cycle conditions: 1 cycle of 94°C for 1 min and 80 cycles of 94°C for 20 s, 62°C for 20 s, 72°C for 30 s, and 78°C for 10 s. Fluorescence was measured during the 62°C (annealing) step. Twenty-microliter PCR reactions contained 100 ng of human genomic DNA; 1.5 pmol C- or T-specific reverse primers (details below); 3 pmol forward primer (rs3749971- For: AGCGAAGAGGATTGCAGATGGC); 2 μL Genetherm polymerase buffer; 0.7 mM each of dATP, dCTP, dGTP, and dTTP; 2 mM MgCl; and 2 U of GenTherm™ Taq Polymerase. Allele-specific primers were designed as molecular beacons (Jordens et al., 2000) (rs3749971-C: Fam-atacagcCTATATCTTTTCTAGGCTGTATBHQCAC and rs3749971-T: Hex-atacagcCTATATCTTTTCTAGGCTGTATBHQCAT), labeled either with FAM (6-carboxyfluorescein) or Hex (4,7,2′,4′,5′,7′-hexachloro-6-carboxyfluorescein), and quenched with Black Hole Quencher (BHQ™) attached to a T natively present within the primer binding site. Seven bases were added to the 5′ end (displayed in lower-case letters) to allow the formation of a 29-bp “hairpin” with the 10-bp complementary region to ensure fluorescence quenching of the unused primers. To assess the reproducibility of this genotyping approach, control DNA of seven individuals (including three CEPH samples) was typed 15 times independently, with 100% reproducibility.
Statistical analyses
Nucleotide diversity (π) was calculated as described by Nei (1987), using the software DNAsp (Rozas and Rozas, 1999). The two-sided Fisher’s exact test was employed for all association analyses, with a 1% level of significance. The Bonferroni correction for multiple comparisons was applied for p-values of the in silico analyses only.
Results
In silico analyses
The degree of nucleotide diversity as assessed by 1170 SNPs (elicited in the supplemental Table S1) was found to be highest between the genes OR12D3 and OR10C1 (supplemental Fig. S1), including the most polymorphic loci within this cluster (Ehlers et al., 2000). The SNP diversity outside of the OR clusters did not differ substantially from that within the clusters. SNP densities within the telomeric (0.388/kb) and the centromeric OR clusters (0.877/kb) were relatively high when compared with other genomic regions (Zhao et al., 2003).
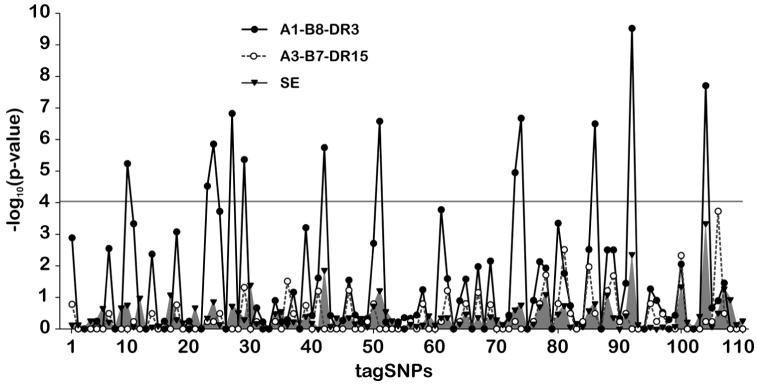
Within the panel of 180 xHLA haplotypes, 11 A1-B8-DR3, 5 A3-B7-DR15, and 59 HLA-DR SE haplotypes were found. No significant association between any of the tagSNPs and the 59 HLA-DR SE haplotypes was observed, as shown in Figure 2. A similar result was obtained with regard to the A3-B7-DR15 haplotypes, although this could be due to the low number of A3-B7-DR15 haplotypes (five) in the CEPH panel. In contrast, a significant correlation was found for 12 tagSNPs (representing 81 tagged SNPs displayed in supplemental Table S2, available online at www.liebertpub.com) when the 11 A1-B8-DR3 haplotypes were compared with the 169 non-A1-B8-DR3 haplotypes for association with the 110 tagSNPs (Fig. 2), and after setting a Bonferroni cutoff for multiple comparisons. Only one of the 81 captured SNPs is a coding, nonsynonymous SNP (rs3749971, tagged by tagSNP #51), within the gene OR12D3. In A1-B8-DR3 haplotypes, the respective allele (rs3749971T) is responsible for a Thr→Ile exchange at amino acid position 97 within the OR12D3 protein. The remaining 80 SNPs lead either to synonymous exchanges or are located in intergenic or in intronic regions. Because none of these are, to our knowledge, directly involved in any, so far known, biological process, they were not considered further.
FIG. 2.
Allelic association of 110 tagSNPs from the region comprising both HLA-linked OR clusters with A1-B8-DR3, A3-B7-DR15, and SE haplotypes. The marker rs3749971 is represented by tagSNP #51. The gray horizontal line marks the Bonferroni threshold for 110 tests with a significance level of 1%.
Since the A1-B8-DR3 haplotype was reported in another independent European cohorts to be overrepresented in smokers (Icelandic sample, Füst et al., 2004), and is also associated with various autoimmune diseases, of which some correlate with this behavioral trait, as in Graves’ disease (Hegediüs et al., 2004; Holm et al., 2005), this data demonstrate that these associations must also extend to the A1-B8-DR3-associated rs3749971T allele.
Cohort genotyping
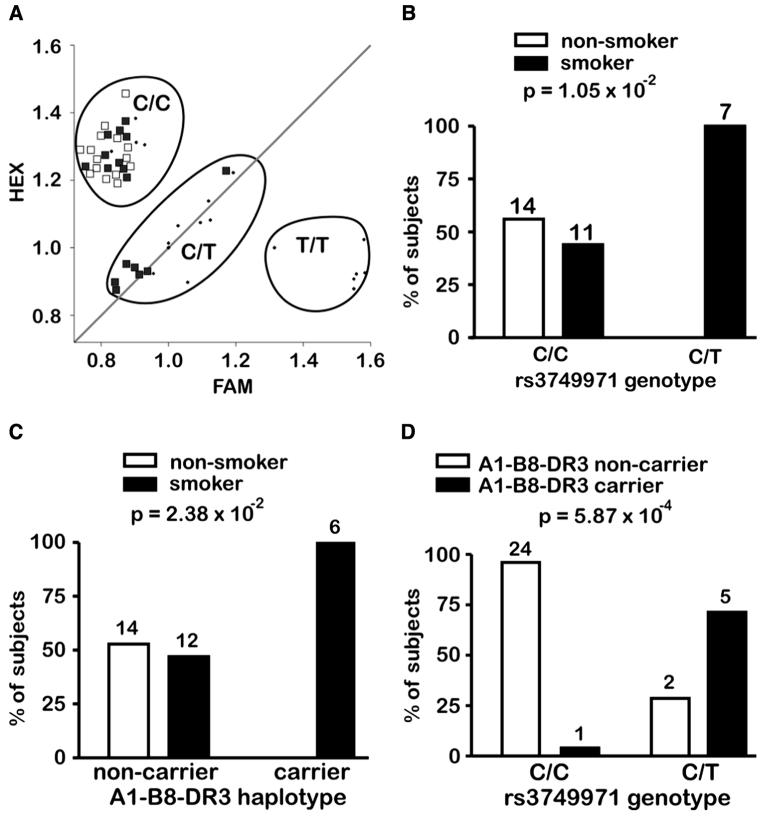
In order to validate this finding, comparing it to the previously found association, we genotyped rs3749971 in 32 female individuals from the same Hungarian cohort (Füst et al., 2004). These individuals differ in their smoking behavior and do not suffer from any autoimmune disease (Füst et al., 2004). Here we found that the correlation between smoking and rs3749971T was slightly stronger (p = 1.05×10−2, Fig. 3b) than the correlation between this trait and A1-B8-DR3 (p = 2.38×10−2, Fig. 3c). Seven and 25 subjects were rs3749971C/T and rs3749971C/C carriers, respectively. A strong association (p = 5.87×10−4) between the rs3749971T allele and the A1-B8-DR3 haplotype was observed, as five out of seven of the rs3749971T carriers but only 1/25 of the noncarriers were found to be A1-B8-DR3 positive (Fig. 3d). The group of rs3749971C/C carriers includes both smokers and nonsmokers, as shown in Figure 3a and b.
FIG. 3.
Association of smoking behavior with the rs3749971 genotype and the A1-B8-DR3 haplotype. (A) HEX and FAM are primer fluorophores marking the rs3749971 T and the C allele primers, respectively. Values indicate the normalized relative number of PCR cycles necessary to reach the genotyping threshold for each allele. HEX values around 1 (or below) are indicative for the presence of the T allele, and the same is true for FAM values, indicating the presence of the C allele. Filled squares: 17 smokers; open squares: 15 nonsmokers; dots: controls. (B) Association of smoking behavior with rs3749971 genotypes. (C) Association of smoking behavior with the carrier state of A1-B8-DR3. (D) Association of A1-B8-DR3 with rs3749971 genotypes. (B-D) The number of individuals in each category is indicated above the corresponding bars, as well as the p-values, as determined by Fisher’s exact test.
Discussion
HLA-A1-positive individuals, in contrast to those with HLA-A2 or HLA-A3, have been reported to exhibit a preference for the odor of bergamot (Milinski and Wedekind, 2001). The existence of a relationship between rs3749971T (or OR12D397Ile) and this predilection for a perfume ingredient is supported by the strong LD between HLA-A1 and rs3749971T that is described here. The fact that many tobacco brands are scented with bergamot oil components (Baker et al., 2004) provides a plausible explanation for the correlation of rs3749971T with smoking, and the identification of OR12D397Ile ligands will facilitate the design of volatiles that might be used as antidotes in individuals with a predisposition to tobacco abuse. The exchange of the hydrophilic amino acid threonine by isoleucine, a residue with a hydrophobic side chain, is expected to alter the physicochemical properties of the OR12D3 protein. However, as no X-ray crystallographic studies of ORs have been reported to date, the likely location of 97Ile within the OR12D3 protein can only be inferred from models (Man et al., 2004; Katada et al., 2005; Abaffy et al., 2007; Schmiedeberg et al., 2007) that take the structure of rhodopsin (Palczewski et al., 2000) into account. Such models locate residue 97 at the end of the first extracellular loop or at the beginning of the third transmembrane domain, close to the ligand binding site. Keller et al. (2007) have recently demonstrated that a variant of an OR gene can substantially influence sensitivity (in both intensity and pleasantness) to specific odors in humans. They showed that a mutant allele of the OR7D4 gene encoding an OR with two amino acid substitutions (residues 88 and 133) as compared to the most common allele causes functional impairment of the receptor in vitro. These substitutions also alter the perception of the smell of androstenone and androstadienone in a significant manner. Residues 88 and 133 are located within the first extracellular and the second intracellular loop. At least residue 88 is close to the beginning of the third transmembrane domain, suggesting that the region in the vicinity of residue 97 in the OR12D3 protein might indeed be involved in ligand binding. Further support for a role of amino acids close to the residue 97 in ligand binding is provided by the recently published crystal structure of the human β2 adrenergic G-protein-coupled receptor (Rasmussen et al., 2007).
Apart from social and psychological factors (Pomerleau et al., 1992; Barman et al., 2004; Lerer et al., 2006), it has been shown that the individual genetic constitution plays a role in initiating and continuing tobacco consumption (Sullivan and Kendler, 1999; Li et al., 2003). Genes within the xHLA contribute as well, as suggested by the finding of an HLA haplotype-dependent association of smoking in two independent European cohorts, with a clear-cut gender bias toward females (Füst et al., 2004). The present study confirms these results by identifying an HLA-linked OR allele that is associated with tobacco abuse. In addition, our work implies that the rs3749971T allele is involved in the smoking-induced aggravation of certain autoimmune diseases that can be observed in patients carrying A1-B8-DR3 (Hegediüs et al., 2004; Holm et al., 2005). Given the fact that the A1-B8-DR3 haplotype occurs with a frequency of 5-10% in Caucasians (Alper et al., 2006), the rs3749971 polymorphism should be suitable for large-scale screening tests, particularly among young people. Studies of the isolated OR12D3 protein will be indispensable to evaluate the consequences of the Thr97Ile exchange within this receptor on its ligand specificity.
Acknowledgments
This work was supported by the Berliner Krebsgesellschaft and by the Senate of Berlin (Ernst-von-Leyden and NaFöG fellowships to P.S.C.S.), by the Wellcome Trust (R.H., M.M. and S.B) as well as by the Monika Kutzner-Stiftung Berlin, and by the National Office for Research and Technology, Hungary.
Footnotes
Disclosure Statement
The authors declare that no competing interests exist.
References
- Abaffy T, Malhotra A, Luetje CW. The molecular basis for ligand specificity in a mouse olfactory receptor: a network of functionally important residues. J Biol Chem. 2007;282:1216–1224. doi: 10.1074/jbc.M609355200. [DOI] [PubMed] [Google Scholar]
- Alper CA, Larsen CE, Dubey DP, et al. The haplotype structure of the human major histocompatibility complex. Hum Immunol. 2006;67:73–84. doi: 10.1016/j.humimm.2005.11.006. [DOI] [PubMed] [Google Scholar]
- American Cancer Society 12th World Conference on Tobacco or Health. Tobacco Control Profiles; American Cancer Society, Atlanta. 2003. [Google Scholar]
- American Cancer Society Cancer facts and figures 2007; 2007; Atlanta: American Cancer Society; [Google Scholar]
- Baker RR, Pereira da Silva JR, Smith G. The effect of tobacco ingredients on smoke chemistry. Part I: Flavorings and additives. Food Chem Toxicol. 2004;42:S3–S37. doi: 10.1016/S0278-6915(03)00189-3. [DOI] [PubMed] [Google Scholar]
- Barman SK, Pulkkinen L, Kaprio J, Rose RJ. Inattentiveness, parental smoking and adolescent smoking initiation. Addiction. 2004;99:1049–1061. doi: 10.1111/j.1360-0443.2004.00789.x. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- de Bakker PIW, McVean G, Sabeti PC, et al. A high-resolution HLA and SNP haplotype map for disease association studies in the extended human MHC. Nat Genet. 2006;38:1166–1172. doi: 10.1038/ng1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyment DA, Ebers GC, Sadovnick AD. Genetics of multiple sclerosis. Lancet Neurol. 2004;3:104–110. doi: 10.1016/s1474-4422(03)00663-x. [DOI] [PubMed] [Google Scholar]
- Ehlers A, Beck S, Forbes SA, et al. MHC-linked olfactory receptor loci exhibit polymorphism and contribute to extended HLA-OR-haplotypes. Genome Res. 2000;10:1968–1978. doi: 10.1101/gr.10.12.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, et al. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion FMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Füst G, Arason GJ, Kramer J, et al. Genetic basis of tobacco smoking: strong association of a specific major histocompatibility complex haplotype on chromosome 6 with smoking behavior. Int Immunol. 2004;16:1507–1514. doi: 10.1093/intimm/dxh152. [DOI] [PubMed] [Google Scholar]
- Global Youth Tobacco Survey Collaborating Group Differences in worldwide tobacco use by gender: findings from the Global Youth Tobacco Survey. J Sch Health. 2003;73:207–215. doi: 10.1111/j.1746-1561.2003.tb06562.x. [DOI] [PubMed] [Google Scholar]
- Hawkes CH. Smoking is a risk factor for multiple sclerosis: a metaanalysis. Mult Scler. 2007;13:610–615. doi: 10.1177/1352458506073501. [DOI] [PubMed] [Google Scholar]
- Hegediüs L, Brix TH, Vestergaard P. Relationship between cigarette smoking and Graves’ ophthalmopathy. J Endocrinol Invest. 2004;27:265–271. doi: 10.1007/BF03345276. [DOI] [PubMed] [Google Scholar]
- Herrera BM, Cader MZ, Dyment DA, et al. Follow-up investigation of 12 proposed linkage regions in multiple sclerosis. Genes Immun. 2006;7:366–371. doi: 10.1038/sj.gene.6364308. [DOI] [PubMed] [Google Scholar]
- Holm IA, Manson JE, Michels KB, et al. Smoking and other lifestyle factors and the risk of Graves’ hyperthyroidism. Arch Intern Med. 2005;165:1606–1611. doi: 10.1001/archinte.165.14.1606. [DOI] [PubMed] [Google Scholar]
- Horton R, Wilming L, Rand V, et al. Gene map of the extended human MHC. Nat Rev Genet. 2004;5:889–899. doi: 10.1038/nrg1489. [DOI] [PubMed] [Google Scholar]
- Hunt PJ, Marshall SE, Weetman AP, et al. Histocompatibility leucocyte antigens and closely linked immunomodulatory genes in autoimmune thyroid disease. Clin Endocrinol. 2001;55:491–499. doi: 10.1046/j.1365-2265.2001.01356.x. [DOI] [PubMed] [Google Scholar]
- Jarvis MJ. Why people smoke. BMJ. 2004;328:277–279. doi: 10.1136/bmj.328.7434.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordens JZ, Lanham S, Pickett MA, et al. Amplification with molecular beacon primers and reverse line blotting for the detection and typing of human papillomaviruses. J Virol Meth. 2000;89:29–37. doi: 10.1016/s0166-0934(00)00195-6. [DOI] [PubMed] [Google Scholar]
- Katada S, Hirokawa T, Oka Y, et al. Structural basis for a broad but selective ligand spectrum of a mouse olfactory receptor: mapping the odorant-binding site. J Neurosci. 2005;25:1806–1815. doi: 10.1523/JNEUROSCI.4723-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Zhuang H, Chi Q, et al. Genetic variation in a human odorant receptor alters odour perception. Nature. 2007;449:468–472. doi: 10.1038/nature06162. [DOI] [PubMed] [Google Scholar]
- Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54:38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- Koch M, van Harten A, Uyttenboogaart M, de Keyser J. Cigarette smoking and progression in multiple sclerosis. Neurology. 2007;69:1515–1520. doi: 10.1212/01.wnl.0000277658.78381.db. [DOI] [PubMed] [Google Scholar]
- Lerer E, Kanyas K, Karni O, et al. Why do young women smoke? II. Role of traumatic life experience, psychological characteristics and serotonergic genes. Mol Psychiatry. 2006;11:771–781. doi: 10.1038/sj.mp.4001855. [DOI] [PubMed] [Google Scholar]
- Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- Linn-Rasker SP, van der Helm-van Mil AHM, van Gaalen FA, et al. Smoking is a risk factor for anti-CCP antibodies only in rheumatoid arthritis patients who carry HLA-DRB1 shared epitope alleles. Ann Rheum Dis. 2006;65:366–371. doi: 10.1136/ard.2005.041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man O, Gilad Y, Lancet D. Prediction of the odorant binding site of olfactory receptor proteins by human-mouse comparisons. Protein Sci. 2004;13:240–254. doi: 10.1110/ps.03296404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milinski M, Wedekind C. Evidence for MHC-correlated perfume preferences in humans. Behav Ecol. 2001;12:140–149. [Google Scholar]
- Miretti MM, Walsh EC, Ke X, et al. A high-resolution linkage-disequilibrium map of the human major histocompatibility complex and first generation of tag single-nucleotide polymorphisms. Am J Hum Genet. 2005;76:634–646. doi: 10.1086/429393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M. Molecular Evolutionary Genetics. Columbia University Press; New York: 1987. [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, et al. Crystal structure of rhodopsin: a G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Pomerleau OF, Flessland KA, Basson SM. Relationship of tridimensional personality questionnaire scores and smoking variables in female and male smokers. J Subst Abuse. 1992;4:143–154. doi: 10.1016/0899-3289(92)90014-o. [DOI] [PubMed] [Google Scholar]
- Rasmussen SGF, Choi H, Rosenbaum DM, et al. Crystal structure of the human β2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- Rozas J, Rozas R. DnaSP version 3: an integrated program for molecular population genetics and molecular evolution analysis. Bioinformatics. 1999;15:174–175. doi: 10.1093/bioinformatics/15.2.174. [DOI] [PubMed] [Google Scholar]
- Schmiedeberg K, Shirokova E, Weber HP, et al. Structural determinants of odorant recognition by the human olfactory receptors OR1A1 and OR1A2. J Struct Biol. 2007;159:400–412. doi: 10.1016/j.jsb.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine Tob Res. 1999;1:S51–S59. doi: 10.1080/14622299050011811. [DOI] [PubMed] [Google Scholar]
- Warren CW, Jones NR, Eriksen MP, Asma S. Global Tobacco Surveillance System (GTSS) collaborative group: patterns of global tobacco use in young people and implications for future chronic disease burden in adults. Lancet. 2006;367:749–753. doi: 10.1016/S0140-6736(06)68192-0. [DOI] [PubMed] [Google Scholar]
- Weetman AP. Graves’ disease. N Engl J Med. 2000;343:1236–1248. doi: 10.1056/NEJM200010263431707. [DOI] [PubMed] [Google Scholar]
- Younger RM, Amadou C, Bethel G, et al. Characterization of clustered MHC-linked olfactory receptor genes in human and mouse. Genome Res. 2001;11:519–530. doi: 10.1101/gr.160301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fu YX, Hewett-Emmett D, Boerwinkle E. Investigating single nucleotide polymorphism (SNP) density in the human genome and its implications for molecular evolution. Gene. 2003;312:207–213. doi: 10.1016/s0378-1119(03)00670-x. [DOI] [PubMed] [Google Scholar]
- Ziegler A, Uchańska-Ziegler B, et al. Rheumatoid arthritis etiology and HLA-linked odorant receptor gene polymorphisms: comment on the article by Klareskog. Arthritis Rheum. 2006;54:2705–2706. doi: 10.1002/art.21971. [DOI] [PubMed] [Google Scholar]