Abstract
Tasidotin, an oncolytic drug in phase II clinical trials, is a peptide analog of the antimitotic depsipeptide dolastatin 15. In tasidotin, the carboxyl-terminal ester group of dolastatin 15 has been replaced by a carboxy-terminal tert-butyl amide. As expected from studies with cemadotin, [3H]tasidotin, with the radiolabel in the second proline residue, was hydrolyzed intracellularly, with formation of N,N-dimethylvalyl-valyl-N-methylvalyl-prolyl-proline (P5), a pentapeptide also present in dolastatin 15 and cemadotin. P5 was more active as an inhibitor of tubulin polymerization and less active as a cytotoxic agent than tasidotin, cemadotin, and dolastatin 15. [3H]P5 was not the end product of tasidotin metabolism. Large amounts of [3H]proline were formed in every cell line studied, with proline ultimately becoming the major radiolabeled product. The putative second product of the hydrolysis of P5, N,N-dimethylvalyl-valyl-N-methylvalyl-proline (P4), had little activity as either an antitubulin or cytotoxic agent. In seven suspension cell lines, the cytotoxicity of tasidotin correlated with total cell uptake of the compound and was probably affected negatively by the extent of degradation of P5 to proline and, presumably, P4. The intracellular enzyme prolyl oligopeptidase probably degrades tasidotin to P5. When CCRF-CEM human leukemia cells were treated with N-benzyloxycarbonylprolylprolinal (BCPP), an inhibitor of prolyl oligopeptidase, there was a 30-fold increase in the IC50 of tasidotin and a marked increase in intracellular [3H]tasidotin. BCPP also caused a 4-fold increase in the IC50 of P5, so the enzyme probably does not convert P5 to P4. Inhibiting degradation of P5 should have led to a decrease in the IC50 obtained for P5 in the presence of BCPP.
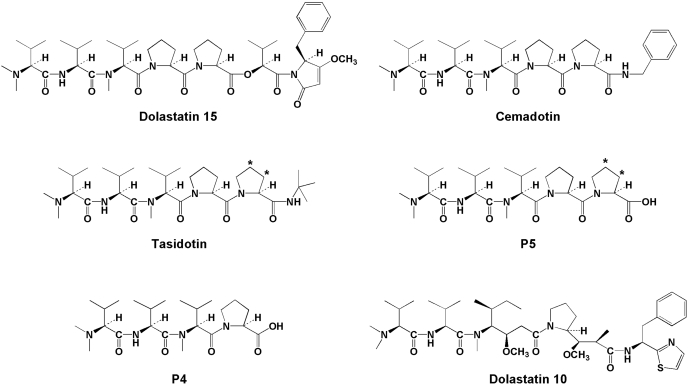
Dolastatin 15 and its analogs (structures in Fig. 1) are unusual antitubulin agents. Despite relatively feeble interactions with tubulin, the most active compounds have exceptional cytotoxicity with IC50 values often in the high picomolar or low nanomolar range (Pettit et al., 1989a, 1998; Jacobsen et al., 1991; Bai et al., 1992; Steube et al., 1992; Beckwith et al., 1993; de Aruda et al., 1995; Aherne et al., 1996; Ali et al., 1998; Haupt et al., 1998). This is true both for depsipeptides, which, like dolastatin 15, have an ester bond in the backbone chain (Pettit et al., 1998), and for analogs in which the ester bond has been replaced with an amide bond (de Aruda et al., 1995; Haupt et al., 1998). The common element in this class of agents is the amino terminal pentapeptide N,N-dimethylvalyl-valyl-N-methylvalyl-prolylproline (P5), which is esterified to a complex ester in the case of dolastatin 15 or linked via an amide bond to a benzyl group or a tert-butyl group in the cases of cemadotin and tasidotin, respectively.
Fig. 1.
Structures of dolastatins 10 and 15, tasidotin, cemadotin, and metabolites P5 and P4. Positions of tritium atoms are indicated by asterisks in tasidotin and P5.
Cemadotin as a single agent gave marginal results in past clinical trials, whereas tasidotin is presently in clinical trials. The cemadotin literature often refers to its intracellular metabolism to P5, which is described as having equivalent or greater activity with tubulin than the parent compound (de Aruda et al., 1995; Mross et al., 1996, 1998; Villalono-Calero et al., 1998; Supko et al., 2000). To our knowledge, studies documenting this transformation or the activity of cemadotin-derived P5 against tubulin were never published. However, P5 has been shown to be substantially more potent than tasidotin as an inhibitor of microtubule assembly and microtubule dynamics (Ray et al., 2007). It was also reported that P5 was more than 60-fold less cytotoxic than tasidotin against a line of MCF-7 human breast carcinoma cells that expressed a tubulin construct linked to green fluorescent protein (Ray et al., 2007).
To obtain a greater understanding of the mechanism of action of tasidotin, the compound was examined and compared with P5, N,N-dimethylvalyl-valyl-N-methylvalyl-proline (P4), cemadotin, and dolastatin 15. P5 was significantly more active than the other compounds as an inhibitor of tubulin assembly, and intracellular radiolabeled tasidotin was converted to P5. The tritium in the radiolabeled tasidotin and P5 was confined to the second proline residue (see Fig. 1), and all cell lines accumulated substantial intracellular free proline, implying further degradation of P5 to P4. Because P4 had little antitubulin activity, it seems that the dolastatin 15 class of antimitotic compounds is both activated and detoxified by cells exposed to these agents.
In this article, we present studies in which the metabolism of tasidotin in Burkitt lymphoma CA46 cells was followed in detail. We also explored whether the cytotoxicity of tasidotin in a variety of cell lines could be correlated with its intracellular metabolism.
Materials and Methods
Materials. Electrophoretically homogeneous bovine brain tubulin (Hamel and Lin, 1984), dolastatin 10 (Pettit et al., 1989b), dolastatin 15 (Pettit et al., 1991, 1994), tasidotin, P5, and P4 (Haupt et al., 1998) were prepared as described previously. [3H]P5 (2.13 Ci/mmol) and [3H]tasidotin (4.26 Ci/mmol), labeled in the second proline residue as shown in Fig. 1, were custom synthesized from P4 by ABC Laboratories (Columbia, MO). Cemadotin, as NSC 669356, was obtained from the Drug Synthesis and Chemistry Branch, National Cancer Institute (Rockville, MD). Human Burkitt lymphoma CA46 cells were obtained from the American Type Culture Collection (Manassas, VA). All other cell lines were obtained from the Screening Technologies Branch, National Cancer Institute at Frederick. The prolyl oligopeptidase inhibitor N-benzyloxycarbonylprolylprolinal (BCPP) was from BIOMOL Research Laboratories (Plymouth Meeting, PA), RPMI 1640 medium was from Mediatech (Herndon, VA), and RNase A, DNase I, and pronase were from Sigma (St. Louis, MO).
Inhibition of Tubulin Assembly. Compound effects on the assembly of purified bovine brain tubulin (Hamel and Lin, 1984) were determined as described elsewhere (Hamel, 2003). The reaction was followed turbidimetrically at 350 nm, and the extent of assembly inhibition at 20 min was determined, using various concentrations of each examined compound.
Cell Culture. Suspension and monolayer cell lines were grown in RPMI 1640 medium supplemented with 5% (except 10% for the Burkitt lymphoma CA46 cells) fetal bovine serum in a humidified 5% CO2 atmosphere at 37°C, with drugs added, as indicated, in dimethyl sulfoxide (final concentration, 0.1%). In the suspension lines, cell growth was evaluated (determination of IC50 values) by measuring cell number with a model Z1 particle counter (Beckman Coulter, Miami, FL); and in the monolayer lines, by measuring cell protein after fixation and staining with sulforhodamine B. The suspension lines were grown in 5 ml of medium in 25-cm2 tissue culture flasks (Corning Life Science, Acton, MA). The monolayer lines were grown in 96-well microtiter plates.
[3H]Tasidotin Uptake. Cells were grown in 25 ml of medium in Corning 75-cm2 culture flasks. For monolayer cells, when the cells were at near confluence, the medium was exchanged for 15 ml of fresh medium containing the indicated concentration of [3H]tasidotin, and the incubation continued for the indicated time. Monolayer cells were harvested by scraping them from the surface of the culture flask. For suspension cells, when the cells were in log phase and at approximately 106 cells/ml, the [3H]tasidotin was added at the indicated concentration, and the incubation continued for the indicated time as specified in individual experiments. Suspension cells were harvested by centrifugation. Both types of cells were washed twice with phosphate-buffered saline, pH 7.2. The cells were resuspended in 0.5 ml of water and disrupted by 10-s periods of sonication at 28 W, followed by 10-s pauses, for a total of 1 min. Protein content and radiolabel of all cell extracts were determined.
The cell extracts were heated in boiling water for 5 min and clarified by centrifugation in an Eppendorf model 5417C centrifuge (Brinkmann Instruments, Westbury, NY) for 2 min at 14,000 rpm. Additional protein was precipitated by addition of 3 parts ethanol to the supernatant, with removal of the protein by centrifugation as before. Ethanol was removed from the supernatant by vacuum centrifugation in a Vacufuge (Eppendorf North America, New York, NY). Protein content and radiolabel in the supernatants, in which negligible protein remained, and pellets were determined.
TFA to a final concentration of 0.1% was added to the supernatants, which were then subjected to HPLC on a Waters (Milford, MA) Symmetry C18 column (3.5 μm particle size, 4.6 × 150 mm), using a Hewlett Packard 1100 series HPLC system (Agilent Technologies, Santa Clara, CA) with an in-line flow detector (β-RAM model 3; IN/US, Tampa, FL). Injection volume was 0.1 ml, flow rate was 1.0 ml/min, and column temperature was 40°C. Solution A was 0.1% TFA in water, and solution B was 0.07% TFA in acetonitrile. Before sample injection, the column was equilibrated with solution A; after sample injection, the column was developed over 10 min with a linear gradient from 10% solution B to 60% solution B. The column outflow was monitored in succession with a UV monitor set at 215 nm and with the flow detector. Only the latter data are presented. Three major radiolabeled peaks were detected in varying proportions in different samples: proline, which did not bind to the column matrix; tasidotin, with an average retention time of 9.2 min; and P5, with an average retention time of 7.5 min (retention times are from the time of sample injection, and thus include the void volume of the column). Peak area of radiolabeled components was determined using Laura Lite software, obtained from IN/US.
Identification of [3H]Proline. The unbound radiolabeled peak from an extract subjected to HPLC was collected, derivatized with ninhydrin, and analyzed on a Beckman Coulter (Fullerton, CA) model 6300 amino acid analyzer. The radiolabel coeluted with derivatized proline.
Results
Peptide Effects. Table 1 presents studies comparing the activities of tasidotin and P5 with those of dolastatin 15, cemadotin, P4, and the potent antitubulin peptide dolastatin 10 (Bai et al., 1990a,b) in biochemical and cytological assays. For the biochemical study, inhibition of glutamate- and GTP-induced assembly of purified tubulin (Hamel, 2003) was examined. In the cytological studies, cytotoxicity of all the peptides was evaluated in MCF-7 human breast carcinoma cells, and most of them were evaluated in CA46 Burkitt lymphoma cells.
TABLE 1.
Peptide effects on inhibition of tubulin assembly and inhibition of cell growth
Inhibition of tubulin assembly was evaluated as described elsewhere (Hamel, 2003). In brief, 10 μM tubulin was preincubated at 30°C with varying peptide concentrations in the absence of GTP, after which GTP was added, and effects on extent of assembly after 20 min at 30°C were measured. At the IC50, assembly was reduced 50%. Inhibition of cell growth was measured either by determining increase in cell number (Burkitt lymphoma CA46 cells) or in cell protein (breast carcinoma MCF-7 cells) after a 48-h incubation at 37°C in the presence of varying concentrations of peptides. At the IC50 values, the increase in the parameter measured was reduced by 50%. Values are presented as mean ± S.D.
|
Peptide
|
IC50
|
||
|---|---|---|---|
| Tubulin Assembly | Growth of CA46 Cells | Growth of MCF-7 Cells | |
| μM | nM | ||
| Tasidotin | 10 ± 0.4 | 100 ± 5 | 150 ± 70 |
| P5 | 1.0 ± 0.2 | 3000 | 960 ± 300 |
| P4 | 23 ± 4 | 41,000 ± 6000 | |
| Dolastatin 15 | 5.6 ± 0.9 | 3 | 0.19 ± 0.06 |
| Cemadotin | 3.9 ± 0.2 | 0.44 ± 0.05 | |
| Dolastatin 10 | 0.8 ± 0.1 | 0.03 | 0.055 ± 0.04 |
As an inhibitor of tubulin assembly, tasidotin was 2- to 3-fold less active than dolastatin 15 and cemadotin, and more than 12-fold less active than dolastatin 10. P5 was a more potent inhibitor of assembly than the three compounds from which it could be derived (tasidotin, dolastatin 15, cemadotin), being almost as potent as dolastatin 10. The tetrapeptide P4, which seems to be derived from P5 intracellularly (see Results), had little ability to inhibit tubulin assembly, with an IC50 23-fold lower than that of P5. P4 was also less active than tasidotin, dolastatin 15, and cemadotin.
In the cytological assays, P5 showed substantially reduced cytotoxicity relative to tasidotin, cemadotin, and dolastatin 15. P4 was even less active. The cytotoxic activity of tasidotin was substantially less than the similar activities of dolastatin 15 and cemadotin, and these agents in turn were less potent than dolastatin 10.
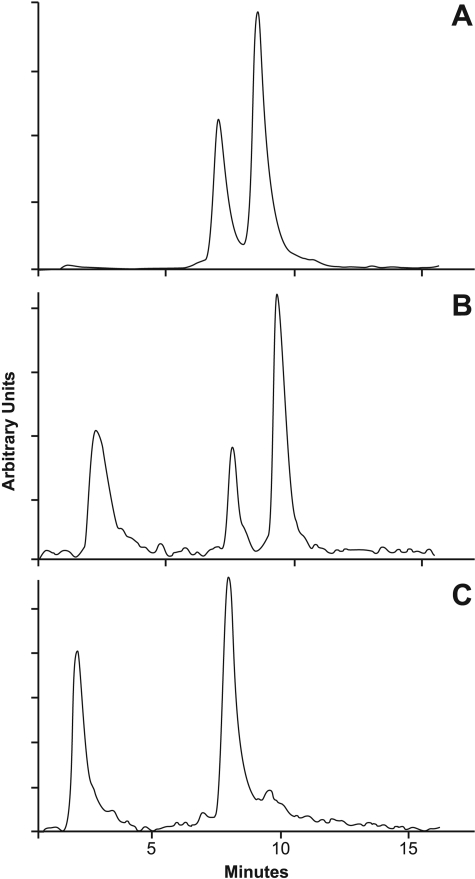
Intracellular Metabolism of [3H]Tasidotin in CA46 Cells. [3H]Tasidotin and [3H]P5 were readily resolved by HPLC (Fig. 2A). The column used to resolve the peaks was initially equilibrated with 0.1% TFA, and samples were adjusted to 0.1% TFA before application onto the column. The retention time of P5, the earlier peak, averaged 7.5 min, and that of tasidotin averaged 9.2 min.
Fig. 2.
HPLC resolution of [3H]tasidotin and [3H]P5 standards (A) and soluble intracellular radiolabel derived from [3H]tasidotin after treatment of CA46 Burkitt lymphoma cells with the compound at 0.1 μM for 5 min (B) or 96 h (C). The flow detector tracings are shown in the figure. For A, the two standards were initially run separately, and these experiments showed that the first peak is P5 (retention time from injection averaged 7.5 min) and the second, tasidotin (retention time from injection averaged 9.2 min). For B and C, cells were harvested at the indicated times and processed as described in the text.
Next, the fate of intracellular radiolabel when CA46 cells were grown in medium containing 0.1 μM [3H]tasidotin (the IC50) was examined. In these studies, cell extracts were heated in a boiling water bath and clarified by centrifugation, additional protein was precipitated with ethanol, and the final supernatants were adjusted to 0.1% TFA before chromatography, as had been done in the control study shown in Fig. 2A. After only 5 min of tasidotin exposure, intracellular radiolabel had undergone significant metabolism (Fig. 2B). Approximately 48% of the soluble intracellular radiolabel remained as tasidotin, approximately 17% had the mobility of P5, and already approximately 35% of the soluble radiolabel had apparently undergone further metabolism. The early third peak represents material that did not bind to the column matrix. This peak was identified as free [3H]proline by standard amino acid analysis chromatography. Thus, P5 probably undergoes degradation to P4, but we have no information about breakdown of P4.
A similar study performed with 0.1 μM [3H]tasidotin for 96 h (Fig. 2C) showed that ultimately very little tasidotin (approximately 6% of the radiolabel) remained in the cells. The major radiolabeled peak (approximately 56% of total radiolabel) had the mobility of P5. The proportion of radiolabel in the unbound proline peak, approximately 38%, was almost identical to what had been observed at 5 min.
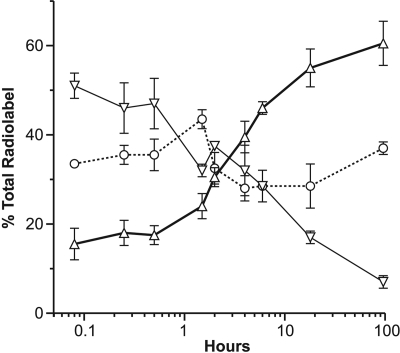
CA46 cells were then examined in detail over a 4 day time period, but 1.0 μM [3H]tasidotin was used (Fig. 3). This increased the amount of radiolabel available for analysis and minimized the possibly confounding effect of continued cell growth that occurred at the IC50. Over time, there was a steady decline in the proportion of radiolabel in the tasidotin peak and a steady increase in the amount of radiolabel in the P5 peak. The proportion of radiolabel in the proline peak varied little over time.
Fig. 3.
Metabolism of [3H]tasidotin by CA46 Burkitt lymphoma cells over time. Cells were incubated for the indicated times with 1.0 μM [3H]tasidotin, and soluble radiolabel was evaluated by HPLC and flow detector analysis. Radiolabel in the three peaks was quantitated at each time point, as indicated by the following symbols: ▿, tasidotin; ▵, P5; and ○, proline. Three independent experiments were performed, and standard deviations are indicated.
Intracellular Metabolism of [3H]Tasidotin in NCI Cell Screen Human Breast Carcinoma Cell Lines. The observation that tasidotin underwent stepwise degradation in CA46 cells, first to the tubulin-active P5, then, presumably, to the tubulin-inactive P4, raised the question of whether the potency of tasidotin in different cell lines could be related to either the rate of formation of P5 or its rate of degradation, as manifested by proline formation.
The human breast carcinoma lines in the NCI cell screen were selected for initial analysis, based on their sensitivity to cemadotin (data available at http://dtp.nci.nih.gov/). These cell lines showed similar relative sensitivities to tasidotin. The least sensitive line was NCI/ADR-RES (IC50 > 1 μM), but this line is known to be multidrug resistant, based on expression of P-glycoprotein, and dolastatin 15 is a P-glycoprotein substrate (Aherne et al., 1996). Compared with other breast carcinoma lines, relatively low amounts of tasidotin entered NCI/ADR-RES cells (data not presented), consistent with tasidotin's also being a P-glycoprotein substrate. Of the remaining lines, the greatest difference in sensitivity to tasidotin was between the more sensitive MDA-MB-435 line and the less sensitive HS 578-T line. The IC50 values in the two lines were 4 and 200 nM, respectively.
An initial comparison between extracts obtained from the two cell lines after treatment with [3H]tasidotin is presented in Fig. 4. Both lines were treated for 6 h with 0.1 μM [3H]tasidotin, and the supernatants obtained after heat- and ethanol-treatment of the cell extracts were examined by HPLC. The most prominent radiolabeled peak in the MDA-MB-435 extract (Fig. 4A) had the mobility of P5, and there was also a large proline peak. In the HS 578-T extract (Fig. 4B), the P5 peak was much smaller than the proline peak. There was little [3H]tasidotin in either extract. This initial study was consistent with P5 degradation being a significant determinant of tasidotin cytotoxicity.
Fig. 4.
Metabolism of [3H]tasidotin by MDA-MB-435 (A) and HS 578-T (B) breast carcinoma cells. Cells growing in a monolayer were incubated for 6 h with 0.1 μM [3H]tasidotin and harvested as described in the text. Soluble radiolabel was evaluated by HPLC and flow detector analysis.
Extensive experiments were performed with all the NCI cell screen breast cell lines, as well as with the melanoma lines of the cell screen. The latter lines were examined because the best clinical results thus far with tasidotin have been in patients undergoing treatment for melanoma. The goal was to determine whether there was any correlation between cell sensitivity to tasidotin and the pattern of metabolism observed. In the course of these experiments, it became clear that it was difficult to obtain reproducible data from cells growing as monolayers. The reason(s) for this are uncertain.
In the course of the monolayer studies, it was found that substantial amounts of radiolabel accumulated in the heat- and ethanol-insoluble portions of the cell extracts. It seemed likely that [3H]proline released from P5 underwent subsequent incorporation into cellular protein. Pellets were exposed to digestive enzymes to determine which ones had the capacity to solubilize radiolabel (data not presented; see next section for similar data with suspension cells). The protease pronase was most effective, releasing up to 100% of the radiolabel content from the pellet, whereas RNase was almost totally ineffective. Some radiolabel was also released by DNase. This could represent either metabolic conversion of [3H]proline into nucleotides incorporated into DNA or, more likely, considering the near total solubilization of radiolabel with pronase, release of basic proteins bound to DNA in the insoluble pellets. It is clear that a truer measure of degradation of P5 should take into account both soluble proline and proline that has been further metabolized into cellular protein. The culture medium was also examined after various incubation times, and no extracellular proline or P5 was detected.
Intracellular Metabolism of [3H]Tasidotin in NCI Cell Screen Human Leukemia Cell Lines. The inability to obtain reproducible data from cells growing in monolayer culture led to the examination of the fate of tasidotin in cultures of the leukemia lines of the NCI cell screen. These cells, as well as the CA46 Burkitt lymphoma line, yielded reproducible results.
When all cell lines were treated with 0.1 μM [3H]tasidotin, differences were observed between the cell lines in total amount of tasidotin uptake (i.e., total intracellular radiolabel) and in relative amounts of soluble proline and P5 (Fig. 5; Table 2). In all cases, at 16 h, the time point studied, minimal tasidotin was recovered from the cells. Attempts to study an earlier time point when increased amounts of tasidotin remained in the cells were unsuccessful, because the reduced amounts of radiolabel in the cells caused substantial scatter in the data.
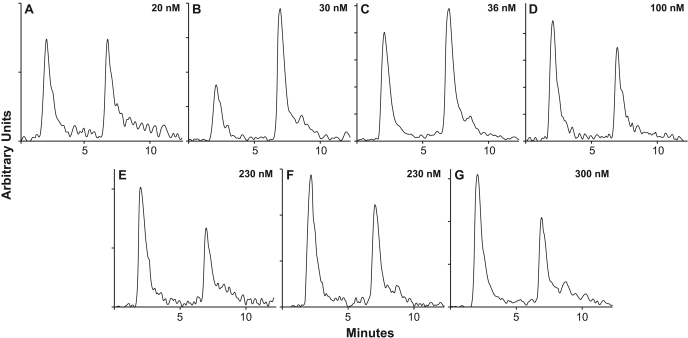
Fig. 5.
Metabolism of [3H]tasidotin by NCI cell screen leukemia cells and by Burkitt lymphoma CA46 cells. Data from representative experiments are shown. The IC50 of each cell line is shown in the upper right corner of each panel. A, SR cells. B, CCRF-CEM cells. C, HL-60 (TB) cells. D, CA46 cells. E, K-562 cells. F, MOLT-4 cells. G, RPMI-8226 cells.
TABLE 2.
Distribution of P5 and proline in suspension cell cultures
Each cell line was grown for 16 h in medium containing 0.1 μM [3H]tasidotin. Cells were harvested and extracts prepared as described under Materials and Methods. Radiolabel and protein in each extract were determined, and the extract was fractionated into heat- and ethanol-soluble supernatant and pellets, each of which was analyzed for radiolabel and protein. Based on findings with breast carcinoma cells treated with degradative enzymes (see Results), the pellet radiolabel was assumed to be derived from tasidotin-derived proline that had been incorporated into protein. The supernatant fractions were analyzed by HPLC as described under Materials and Methods, and virtually all radiolabel in all cell lines was found to be distributed between the P5 and the proline peaks. The percentages of supernatant radiolabel in the table are based on distribution between these two peaks. Figure 5 presents typical HPLC analyses obtained with each of the seven cell lines. Values are presented as mean ± S.D.
| Cell Line | IC50 | Total Tasidotin-Derived Proline (Total Tasidotin Uptake) | Total Proline in Supernatant (All Forms) | Proline in P5 in Supernatant | Free Proline in Supernatant | Proline from Pellets | Total Proline (Pellet Proline and Free Proline in Supernatant) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| nM | fmol / μg cell protein | % | % | fmol / μg cell protein | % total uptake | % | fmol / μg cell protein | fmol / μg cell protein | fmol / μg cell protein | % total uptake | |
| SR | 20 ± 1 | 20 ± 5 | 51 ± 9 | 51 ± 7 | 5.2 | 26 | 49 | 5.0 | 9.8 | 14.8 | 74 |
| CCRF-CEM | 30 ± 7 | 24 ± 9 | 52 ± 4 | 62 ± 10 | 7.7 | 32 | 48 | 11.5 | 12.5 | 16.3 | 67 |
| HL-60 (TB) | 36 ± 6 | 27 ± 3 | 54 ± 10 | 52 ± 6 | 7.6 | 28 | 48 | 7.0 | 12.4 | 19.4 | 72 |
| CA46 | 100 ± 5 | 12 ± 0.6 | 41 ± 1 | 42 ± 10 | 2.1 | 17.5 | 58 | 2.8 | 7.1 | 9.9 | 82.5 |
| K-562 | 230 ± 30 | 15.5 ± 2 | 42 ± 9 | 36 ± 6 | 2.3 | 15 | 64 | 4.2 | 9.0 | 13.2 | 85 |
| MOLT-4 | 230 ± 30 | 13 ± 3 | 46 ± 7 | 41 ± 9 | 2.5 | 19 | 59 | 3.5 | 7.0 | 10.5 | 81 |
| RPMI-8226 | 300 ± 20 | 8.4 ± 3 | 31 ± 5 | 39 ± 5 | 1.0 | 12 | 61 | 1.6 | 5.8 | 7.4 | 88 |
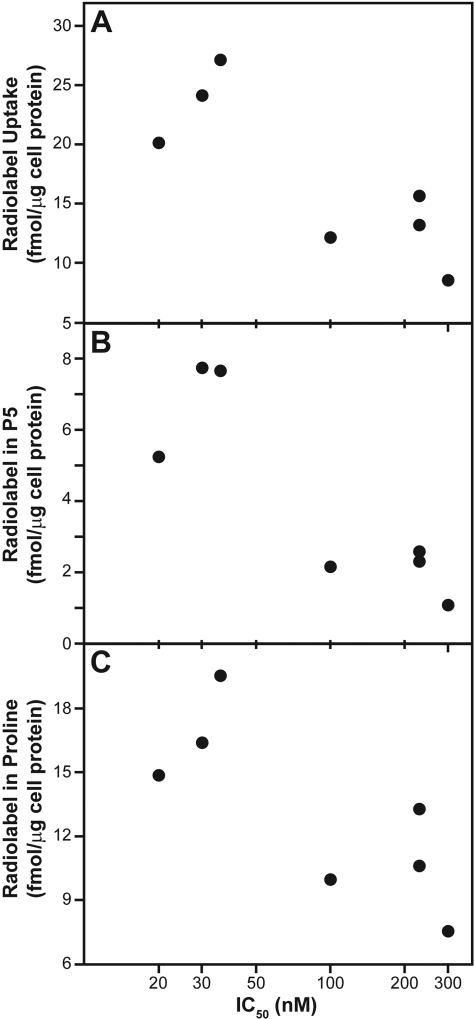
The minimal residual intracellular tasidotin prevented a conclusive determination from the Table 2 data whether it is formation or destruction of P5 that determines relative cytotoxicity of tasidotin, because the amounts of P5 and of proline are directly related to each other. Evaluation of the data showed a correlation of cytoxicity with cellular uptake of tasidotin (Fig. 6A), as well as with the relative amounts of both P5 (Fig. 6B) and proline (Fig. 6C) recovered from the cells, in that greater uptake and higher amounts of cellular P5 and radiolabeled proline were observed in the more sensitive cell lines. For proline, Fig. 6C shows the soluble proline and proline in protein combined.
Fig. 6.
Metabolism parameters of suspension cells as a function of cytotoxicity. Selected data shown in Table 2 are plotted. A, total [3H]tasidotin uptake (“total tasidotin-derived proline” column). B, soluble P5 (second value in “proline in P5 in supernatant” column). C, total proline (first value in “total proline” column).
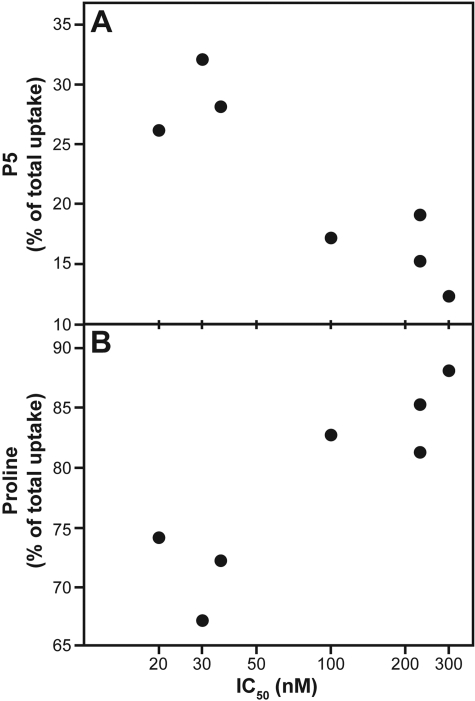
However, when the data were analyzed in terms of the percentage of total uptake as P5 (Fig. 7A) or as proline (Fig. 7B), again combining soluble proline and proline in protein, a different correlation with cytotoxicity emerged. With this modification, the correlation of higher IC50 values was with lower levels of P5 and higher levels of proline.
Fig. 7.
Metabolism parameters of suspension cells as a function of cytotoxicity. Selected data shown in Table 2 are plotted. A, soluble P5 as a percentage of total uptake (third value in “Proline in P5 in Supernatant” column). B, total proline as a percent of total uptake (second value in “total proline” column).
The examined lines can be divided into two cohorts, the three more sensitive cell lines [SR, CCRF-CEM, and HL-60 (TB)], with IC50 values of 20 to 36 nM, and the four less sensitive cell lines (CA46, K-562, MOLT-4, and RPMI-8226), with IC50 values of 100 to 300 nM. In the former, total uptake averaged 24 fmol/μg of cell protein, with 29% as P5 and 71% as proline. In the latter, total uptake averaged 12 fmol/μg of cell protein, with 16% as P5 and 84% as proline. Thus, in the less sensitive lines, despite reduced amounts of total tasidotin uptake, there was greater overall degradation of the drug. This probably indicates that the cytotoxicity of tasidotin, and presumably the dolastatin 15 class of peptides and depsipeptides as a whole, is most strongly dependent on the rate of degradation rather than the rate of formation of the active P5 pentapeptide.
Prolyl Oligopeptidase and Tasidotin Cytotoxicity. A possibility for the enzyme degrading tasidotin is a prolyl oligopeptidase, an enzyme class that cleaves to the carboxyl side of proline residues of oligopeptides (Polgár, 2002). (Presumably, an esterase performs an analogous degradation of dolastatin 15 and related depsipeptides.) The effects of BCPP, an inhibitor of this enzyme (Wilk and Orlowski, 1983), on the cytotoxicity of tasidotin and P5 were therefore explored. The enzyme degrading P5 is presently unknown. These studies were performed in CCRF-CEM leukemia cells, and combretastatin A-4, a tubulin-active agent binding at the colchicine site, and methotrexate, an inhibitor of dihydrofolate reductase, were used as controls. The 12.5 μM BCPP concentration used in these studies had negligible effects on the growth of the cells.
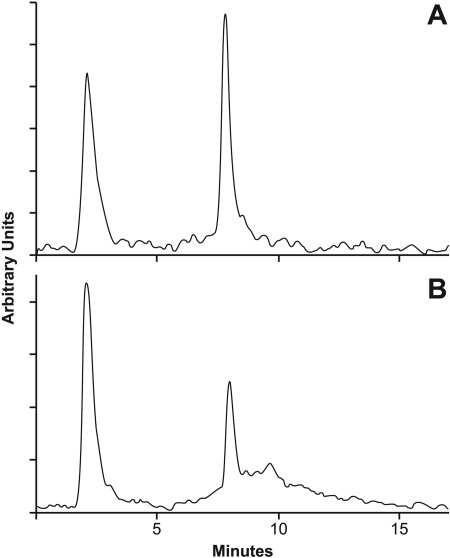
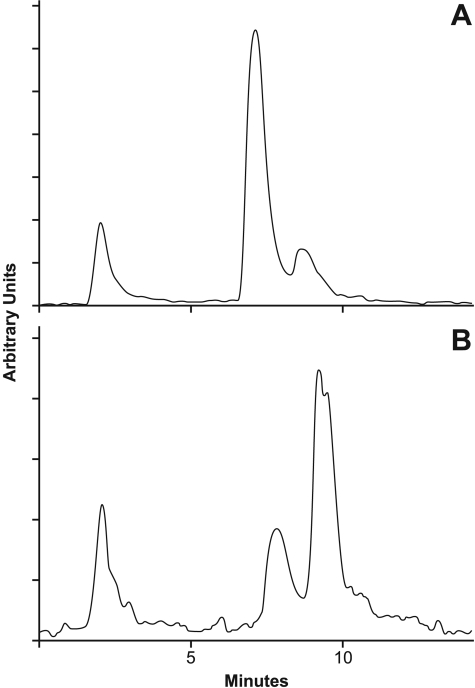
As shown in Table 3, BCPP had no effect on the inhibition of CCRF-CEM cell growth by either combretastatin A-4 or methotrexate. In contrast, the cytotoxicity of tasidotin was decreased 30-fold in the presence of BCPP, the IC50 rising from 40 nM to 1.2 μM. Consistent with this rise in the IC50, there was much more radiolabeled intracellular tasidotin in cells treated with BCPP + 20 μM [3H]tasidotin (Fig. 8B) than in those treated with [3H]tasidotin alone (Fig. 8A).
TABLE 3.
Effects of BCPP on drug IC50 values in CCRF-CEM leukemia cells
CCRF-CEM cells were cultured in various drug concentrations for 24 h, with and without 12.5 μM BCPP. Drug effects on cell growth were quantitated by measuring cell number. Two independent experiments were performed with each compound.
|
Drug
|
IC50
|
|
|---|---|---|
| − BCPP | + BCPP | |
| nM | nM (-fold difference) | |
| Tasidotin | 40 ± 10 | 1200 ± 100 (30) |
| P5 | 310 ± 9 | 1300a (4.2) |
| Combretastatin A-4 | 2.3 ± 0.3 | 1.9 ± 0.8 (0.8) |
| Methotrexate | 7.3 ± 0.8 | 8.0 ± 1 (1.1) |
The same value was obtained in both experiments.
Fig. 8.
Effect of the prolyl oligopeptidase inhibitor BCPP on metabolism of [3H]tasidotin by CCRF-CEM leukemia cells. Cells were incubated for 18 h with 20 μM [3H]tasidotin without (A) or with (B) 12.5 μM BCPP, and soluble radiolabel was evaluated by HPLC and flow detector analysis.
BCPP also unexpectedly reduced the cytotoxicity of P5 but to a much lesser extent (Table 3). Without BCPP, the IC50 of P5 was 310 nM, whereas with BCPP, the IC50 of P5 increased by approximately 4-fold, to 1.3 μM. The significance of this finding is unclear, but it does suggest that prolyl oligopeptidase is not responsible for degradation of P5 to P4. Inhibition of the enzyme degrading P5 to P4 should lead to increased, not decreased, inhibition of cell growth, because P4 is the less active peptide.
Discussion
Tasidotin is converted intracellularly to P5, as was reported (de Aruda et al., 1995; Mross et al., 1996, 1998; Supko et al., 2000; Villalono-Calero et al., 1998), but not documented in the literature, for cemadotin. As recently shown by Ray et al. (2007), P5 was more active than tasidotin as an inhibitor of tubulin assembly but less active as a cytotoxic agent. Tasidotin was also substantially less cytotoxic than cemadotin and dolastatin 15 in two cell lines, implying significant differences in uptake and/or intracellular metabolism for these different compounds.
The explanation for the limited cytotoxicity of P5, which is probably the common first intracellular metabolite of tasidotin, cemadotin, and dolastatin 15, is also probably limited cellular uptake of the pentapeptide. Although we did not specifically examine this possibility, we did find that no [3H]P5 appeared in the cell culture medium, within the experimental conditions we used, after treatment of cells with [3H]tasidotin. This suggests that the pentapeptide is unable to penetrate the cell membrane as well as the parent compounds. This may be due to the terminal carboxylate group, even though several potently cytotoxic antimitotic peptides (hemiasterlins, tubulysins, vitilevuamide) possess this moiety as part of their structures (for review, see Hamel and Covell, 2002).
Metabolism of tasidotin does not stop with formation of the potent antitubulin agent P5. At least one more degradative reaction must occur intracellularly, for all the cell lines examined produced large amounts of [3H]proline from [3H]tasidotin, particularly if heat- and ethanol-insoluble radiolabel is included. Because this insoluble radiolabel, when treated with pronase, becomes solubilized, it is likely to represent tasidotin-derived proline that was recycled by the cells. The presumptive second product of the digestion of P5, the tetrapeptide P4, had only weak activity as an antitubulin agent and negligible cytotoxic activity.
Attempts to correlate cytotoxicity of different cell lines with the intracellular metabolism of [3H]tasidotin had only limited success. This was due in part to the need for adequate intracellular radiolabel for analysis. Suspension cells were more tractable than monolayer cells for yielding reproducible data; consequently, suspension cells were used in most studies.
To obtain cells with sufficient radiolabel for reliable analysis, longer incubation times with 0.1 μM [3H]tasidotin or higher concentrations of the compound were needed. With 0.1 μM [3H]tasidotin and a 16-h incubation, in all cell lines the tasidotin was almost completely metabolized. In the seven suspension cell lines examined, there was a reasonable correlation of cytotoxicity with total tasidotin uptake. The more sensitive lines, on average, took up twice as much tasidotin as the less sensitive cell lines (24 fmol/μg cell protein versus 12 fmol/μg).
In all cell lines, radiolabel derived from [3H]tasidotin was recovered predominantly as proline and as protein containing radiolabel, presumably largely proline. The proportion of total uptake as proline ranged from 67% in the CCRF-CEM cells (IC50, 36 nM) to 88% in the least sensitive cell line examined, RPMI-8226 (IC50, 300 nM). There was a reasonable inverse correlation of cytotoxicity with the proportion of tasidotin-derived radiolabel as proline + proline in protein. These findings, combined with a greater persistence of P5 in the more sensitive cell lines, suggest that a major factor in resistance to tasidotin is the ability of a cell line to degrade P5.
However, because little tasidotin remained in cells in the 0.1 μM/16-h study, there was a similar, but direct, correlation of cytotoxicity with the proportion of tasidotin-derived radiolabel existing as P5. Because little P5 relative to tubulin is needed to poison microtubule dynamics (Ray et al., 2007), this seems to explain the greater response of the more sensitive cells to tasidotin. The window of sensitivity was rather narrow. In the more sensitive cohort (IC50 values, 20-36 nM) intracellular P5 was 5.2 to 7.7 fmol/μg cell protein, whereas in the less sensitive cohort (IC50 values, 100-300 nM) intracellular P5 was 1.0 to 2.5 fmol/μg cell protein.
Tasidotin, and presumably other depsipeptides and peptides in the dolastatin 15 class, are thus unusual antitubulin agents in that they can be both activated and deactivated by cells that they enter. Although little is known about the behavior of these compounds in specific human tissues and tumors, the ideal patient would seem to be one whose sensitive tissues, such as neutrophil progenitor cells, more rapidly degraded P5 than did the tumor being treated by tasidotin or its congeners. In addition, the findings presented here suggest that both the antitumor effect and toxicity profile of tasidotin would be enhanced if the degradation of P5 were reduced or eliminated.
Acknowledgments
We thank Suzanne Specht, National Cancer Institute at Frederick, for assistance with the amino acid analysis.
ABBREVIATIONS: P5, N,N-dimethylvalyl-valyl-N-methylvalyl-prolyl-proline; P4, N,N-dimethylvalyl-valyl-N-methylvalyl-proline; TFA, trifluoroacetic acid; BCPP, N-benzyloxycarbonylprolylprolinal; HPLC, high performance liquid chromatography; NSC 669356, cemadotin.
References
- Aherne GW, Hardcastle A, Valenti M, Bryant A, Rogers P, Pettit GR, Srirangam JK, and Kelland LR (1996) Antitumour evaluation of dolastatins 10 and 15 and their measurement in plasma by radioimmunoassay. Cancer Chemother Pharmacol 38 225-232. [DOI] [PubMed] [Google Scholar]
- Ali MA, Rosati R, Pettit GR, and Kalemkerian GP (1998) Dolastatin 15 induces apoptosis and bcl-2 phosphorylation in small cell lung cancer cell lines. Anticancer Res 18 1021-1026. [PubMed] [Google Scholar]
- Bai RL, Pettit GR, and Hamel E (1990a) Binding of dolastatin 10 to tubulin at a distinct site for peptide antimitotic agents near the exchangeable nucleotide and vinca alkaloid sites. J Biol Chem 265 17141-17149. [PubMed] [Google Scholar]
- Bai R, Pettit GR, and Hamel E (1990b) Dolastatin 10, a powerful cytostatic peptide derived from a marine animal: inhibition of tubulin polymerization mediated through the vinca alkaloid binding domain. Biochem Pharmacol 39 1941-1949. [DOI] [PubMed] [Google Scholar]
- Bai R, Friedman SJ, Pettit GR, and Hamel E (1992) Dolastatin 15, a potent antimitotic depsipeptide derived from Dolabella auricularia: interaction with tubulin and effects on cellular microtubules. Biochem Pharmacol 43 2637-2645. [DOI] [PubMed] [Google Scholar]
- Beckwith M, Urba WJ, and Longo DL (1993) Growth inhibition of human lymphoma cell lines by the marine products, dolastatins 10 and 15. J Natl Cancer Inst 85 483-488. [DOI] [PubMed] [Google Scholar]
- de Arruda M, Cocchiaro CA, Nelson CM, Grinnell CM, Janssen B, Haupt A, and Barlozzari T (1995) LU103793 (NSC D-669356): a synthetic peptide that interacts with microtubules and inhibits mitosis. Cancer Res 55 3085-3092. [PubMed] [Google Scholar]
- Hamel E (2003) Evaluation of antimitotic agents by quantitative comparisons of their effects on the polymerization of purified tubulin. Cell Biochem Biophys 38 1-21. [DOI] [PubMed] [Google Scholar]
- Hamel E and Covell DG (2002) Antimitotic peptides and depsipeptides. Curr Med Chem Anticancer Agents 2 19-53. [DOI] [PubMed] [Google Scholar]
- Hamel E and Lin CM (1984) Separation of active tubulin and microtubule-associated proteins by ultracentrifugation and isolation of a component causing the formation of microtubule bundles. Biochemistry 23 4173-4184. [DOI] [PubMed] [Google Scholar]
- Haupt A, Emling F, and Romerdahl C (1998) inventors; BASF Aktiengesellschaft, assignee. Antitumor peptides. U.S. patent 5,831,002. 1998 November 3.
- Jacobsen SE, Ruscetti FW, Longo DL, and Keller JR (1991) Antineoplastic dolastatins: potent inhibitors of hematopoietic progenitor cells. J Natl Cancer Inst 83 1672-1677. [DOI] [PubMed] [Google Scholar]
- Mross K, Herbst K, Berdel WE, Korfel A, von Broen I-M, Bankmann Y, and Hossfeld DK (1996) Phase I clinical and pharmacokinetic study of LU103793 (cemadotin hydrochloride) as an intravenous bolus injection in patients with metastatic solid tumors: a study of the AIO Phase I and APOH Group of the German Cancer Society. Onkologie 19 490-495. [Google Scholar]
- Mross K, Berdel WE, Fiebig HH, Velagapudi R, von Broen IM, and Unger C (1998) Clinical and pharmacologic phase I study of Cemadotin-HCl (LU103793), a novel antimitotic peptide, given as 24-hour infusion in patients with advanced cancer: a study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) Phase I Group and Arbeitsgruppe Pharmakologie in der Onkologie und Haematologie (APOH) Group of the German Cancer Society. Ann Oncol 9 1323-1330. [DOI] [PubMed] [Google Scholar]
- Pettit GR, Kamano Y, Dufresne C, Cerny RL, Herald CL, and Schmidt JM (1989a) Isolation and structure of the cytostatic linear depsipeptide dolastatin 15. J Org Chem 54 6005-6006. [Google Scholar]
- Pettit GR, Singh SB, Hogan F, Lloyd-Williams P, Herald DL, Burkett DD, and Clewlow PJ (1989b) The absolute configuration and synthesis of natural (-)- dolastatin 10. J Am Chem Soc 111 5463-5465. [Google Scholar]
- Pettit GR, Herald DL, Singh SB, Thornton TJ, and Mullaney JT (1991) Antineoplastic agents. 220. Synthesis of natural (-)-dolastatin 15. J Am Chem Soc 113 6692-6693. [Google Scholar]
- Pettit GR, Thornton TJ, Mullaney JT, Boyd MR, Herald DL, Singh SB, and Flahive EJ (1994) A convenient synthetic route to dolastatin 15. Tetrahedron 50 12097-12108. [Google Scholar]
- Pettit GR, Flahive EJ, Boyd MR, Bai R, Hamel E, Pettit RK, and Schmidt JM (1998) Synthesis and cancer cell growth inhibitory studies of dolastatin 15 structural modifications. Anticancer Drug Des 13 47-66. [PubMed] [Google Scholar]
- Polgár L (2002) The prolyl oligopeptidase family. Cell Mol Life Sci 59 349-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Okouneva T, Manna T, Miller HP, Schmid S, Arthaud L, Luduena R, Jordan MA, and Wilson L (2007) Mechanism of action of the microtubule-targeted antimitotic depsipeptide tasidotin (formerly ILX651) and its major metabolite tasidotin C-carboxylate. Cancer Res 67 3767-3776. [DOI] [PubMed] [Google Scholar]
- Steube KG, Grunicke D, Pietsch T, Gignac SM, Pettit GR, and Drexler HG (1992) Dolastatin 10 and dolastatin 15: effects of two natural peptides on growth and differentiation of leukemia cells. Leukemia 6 1048-1053. [PubMed] [Google Scholar]
- Supko JG, Lynch TJ, Clark JW, Fram R, Allen LF, Velagapudi R, Kufe DW, and Eder JP Jr (2000) A phase I clinical and pharmacokinetic study of the dolastatin analogue cemadotin administered as a 5-day continuous intravenous infusion. Cancer Chemother Pharmacol 46 319-328. [DOI] [PubMed] [Google Scholar]
- Villalona-Calero MA, Baker SD, Hammond L, Aylesworth C, Eckhardt SG, Kraynak M, Fram R, Fischkoff S, Velagapudi R, Toppmeyer D, et al. (1998) Phase I and pharmacokinetic study of the water-soluble dolastatin 15 analog LU103793 in patients with advanced solid malignancies. J Clin Oncol 16 2770-2779. [DOI] [PubMed] [Google Scholar]
- Wilk S and Orlowski M (1983) Inhibition of rabbit brain prolyl endopeptidase by N-benzyloxycarbonyl-prolyl-prolinal, a transition state aldehyde inhibitor. J Neurochem 41 69-75. [DOI] [PubMed] [Google Scholar]