Abstract
Study Objective:
To evaluate the efficacy and tolerability of XP13512/GSK1838262, an investigational nondopaminergic agent for the treatment of moderate-to-severe primary restless legs syndrome (RLS).
Design:
Randomized, double-blind, placebo-controlled, crossover trial.
Setting:
Nine US clinical sites.
Patients:
Thirty-eight treatment-naïve subjects with RLS (mean ± SD age 50.1 ± 13.2 years).
Interventions:
XP13512 1800 mg/day followed by placebo or placebo followed by XP13512 1800 mg/day for 14 days, with a 7-day washout between treatment periods.
Measurements and Results:
The primary endpoint was mean change from baseline International RLS Study Group rating scale (IRLS) total score on Day 14, analyzed using analysis of variance with sequence, period, and treatment as fixed effects and subjects within sequence as a random effect. XP13512 significantly reduced IRLS total score on Day 14 compared with placebo (mean ± SD: XP13512 −12.1 ± 6.5, placebo −1.9 ± 6.3; P < 0.0001). Polysomnographic data showed that XP13512 significantly improved sleep architecture on Day 14 compared with placebo (mean ± SD change from baseline sleep time [minutes]: stage 1: XP13512 −9.8 ± 23.9, placebo 0.4 ± 23.2; adjusted P < 0.0054, nominal P < 0.0001; stage 3/4 (slow-wave sleep): XP13512 22.8 ± 40.8, placebo 1.4 ± 34.3; adjusted P = 0.0092, nominal P = 0.0002). The most frequently reported adverse events were somnolence (XP13512 30.6%, placebo 2.8%) and dizziness (XP13512 27.8%, placebo 5.6%).
Conclusions:
XP13512 1800 mg/day significantly reduced RLS symptoms, improved sleep, and was generally well tolerated in subjects with moderate-to-severe primary RLS across 14 days of treatment.
Citation:
Kushida CA; Walters AS; Becker P; Thein SG; Perkins AT; Roth T; Canafax D; Barrett RW. A randomized, double-blind, placebo-controlled, crossover study of XP13512/GSK1838262 in the treatment of patients with primary restless legs syndrome. SLEEP 2009;32(2):159-168.
Keywords: Restless legs syndrome, RLS, XP13512, GSK1838262, sleep, sleep stages, polysomnography
TWO TO 3 PERCENT OF THE US POPULATION EXPERIENCE CLINICALLY RELEVANT SYMPTOMS OF PRIMARY RESTLESS LEGS SYNDROME (RLS) SEVERE enough to warrant treatment.1–3 Patients with RLS report difficulty falling asleep and exhibit abnormal sleep architecture,1,4 with a clinically significant decrease in sleep efficiency due to their symptoms. Consequently, RLS often impacts patients' daytime functioning and is a major source of morbidity and lost productivity.5
Dopaminergic agents provide important benefits for many RLS patients.5–7 However, neither ropinirole nor pramipexole has demonstrated efficacy in improving sleep architecture (eg, the time or percentage of total sleep time spent in slow wave sleep is either unchanged or reduced in these studies).6–8 Up to 30% of patients with RLS report symptoms that worsen with long-term dopaminergic treatment (augmentation).9,10 Recurrence of early-morning RLS symptoms, or rebound, may occur with short-term dopamine agonist treatment.
Early clinical studies suggested that gabapentin, approved in the United States for the treatment of postherpetic neuralgia11–13 and partial seizures,13–15 is effective in improving RLS symptoms.16–20 Gabapentin has also been shown to reduce the frequency of periodic leg movements (PLMs)21–23 and to improve sleep23 in patients with RLS. However, gabapentin is not approved for the treatment of RLS and has inherent pharmacokinetic deficiencies that may limit effectiveness. Plasma exposure to gabapentin is highly variable due to saturation of its absorption pathway in the upper intestine24 and gabapentin requires frequent dosing due to its short plasma half-life.
XP13512/GSK1838262 was developed to overcome the pharmacokinetic deficiencies of gabapentin.25 XP13512 is absorbed by high-capacity nutrient transporters throughout the gastrointestinal tract and is rapidly and extensively converted by nonspecific esterases to gabapentin. The pharmacokinetics of XP13512 provide dose-proportional gabapentin exposure. XP13512 is formulated as an extended-release tablet that allows for reduced dosing frequency.26
This study explored the efficacy and tolerability of XP13512 in subjects with moderate-to-severe primary RLS. An 1800 mg/day dose was chosen to produce maximum gabapentin levels of approximately 6–12 μg/mL in the late evening and night.26 Exploratory secondary analyses examined the effects of XP13512 on sleep quality and sleep architecture.
Methods
Design
This study (XenoPort, Inc. protocol #XP021) was a multicenter, randomized, double-blind, crossover comparison of XP13512 1800 mg/day and placebo conducted between June and December 2004 at 9 US clinical study sites. Good Clinical Practice Guidelines and the 1996 version of the Declaration of Helsinki were followed. The protocol was reviewed and approved by a central or local institutional review board, depending upon center requirements.
Inclusion and Exclusion Criteria
Men and women, aged 18 to 69 years, with a physician diagnosis of RLS based on International RLS Study Group diagnostic criteria27 and who had never received treatment for RLS were eligible for inclusion. Eligible subjects had RLS symptoms on at least 15 nights during the month prior to screening, documented RLS symptoms on at least 4 nights during the 7-day baseline period, and an International RLS Study Group rating scale (IRLS)28 total score of at least 15 at both the beginning and end of the baseline period. Enrolled subjects were otherwise healthy and free from clinically significant illness or disease. Each subject provided written informed consent prior to study participation.
Subjects experiencing daytime RLS symptoms (10:00–18:00) for at least 2 days during the week prior to baseline were excluded. Pregnancy was another exclusion criterion. Subjects were also excluded if they had a body mass index greater than 32 kg/m2, an estimated creatinine clearance less than 60 mL/minute, or a serum ferritin level less than 20 μg/mL or were currently experiencing or being treated for moderate-to-severe depression, a primary sleep disorder other than RLS, or any other serious neurologic disease or movement disorder. Dopamine agonists, levodopa/carbidopa, gabapentin, and medications used to treat sleep disorders were prohibited.
Study Conduct
Subjects were randomized in a 1:1 ratio using a computer-generated randomization schedule and numbered study drug kits to 1 of 2 treatment sequences: XP13512 1800 mg/day in Period 1 followed by placebo in Period 2 or placebo in Period 1 followed by XP13512 1800 mg/day in Period 2. There was a 7-day washout period between each 14-day treatment. XP13512 was titrated as follows: 600-mg extended-release tablets 1 hour before bedtime on Days 1 and 2; 600 mg at 17:00 and 600 mg 1 hour before bedtime on Days 3 and 4; and 600 mg at 17:00 and 1200 mg 1 hour before bedtime on Days 5 through 14. Dose reductions due to tolerability were permitted at the discretion of the investigator. Blinding was ensured by use of matching placebo and XP13512 600-mg tablets. Duplicate 5-mL blood samples for the determination of plasma gabapentin levels were obtained during each treatment period on Days -2, 4, 8, 11, and 15 approximately 10 to 12 hours after the last dose of study drug. Samples were sent to XenoPort, Inc. for blinded analysis; these data will be reported separately. Clinic visits took place at baseline (Days -2 and -1) and on Days 8 and 15 of each treatment period.
Outcome Measures
Efficacy
Primary Endpoint.
The primary efficacy endpoint was the change from baseline IRLS total score at end of treatment (Day 14). Subjects completed the IRLS to assess the previous 7 days of symptoms at baseline and on Days 8 and 15.
Secondary Endpoints.
The change from baseline IRLS total score at Day 7 and Clinical Global Impression–Improvement outcomes (1 = “very much improved”, 7 = “very much worse”) rated by investigators and subjects on Days 8 and 15 were secondary endpoints. Sleep quality, next-day functioning, number of nights with RLS symptoms, number of nights awake from RLS symptoms, and duration of time awake from RLS symptoms over the previous 7 days were also assessed on Days 8 and 15 using a 5-question exploratory sleep questionnaire designed by investigators.
Subject diaries relating to the previous 24 hours were collected on Days 8 and 15. Subjects indicated the onset and severity of RLS symptoms based on a 4-point scale (0 = “not present”, 3 = “severe”) and recorded sleep intervals and the times of study drug administration. Outcomes included duration of RLS symptoms, duration of moderate or severe symptoms, and time to RLS symptom onset.
An 8-hour overnight polysomnogram assessed wake time after persistent sleep onset (number of wake minutes after the onset of persistent sleep prior to the end of recording), wake time during sleep (number of wake minutes after the onset of persistent sleep prior to the last epoch of stage 2, 3/4, or rapid eye movement [REM] sleep [rather than end of recording]), number of awakenings, PLM frequency, number of PLM of sleep not causing arousal (PLMS), number of PLM during sleep causing awakening (PLMSW) and arousal (PLMSA), total sleep time, sleep efficiency (total sleep time/total time in bed × 100), total minutes awake, sleep architecture (percentage of sleep time spent in sleep stages 1, 2, 3/4 [slow-wave sleep], and REM and sleep latency to stages 1, 2, and REM sleep). The following indexes were calculated: PLM index (PLMI = PLM/total time in bed), PLMS index (PLMSI = PLMS/total sleep time), PLMSA index (PLMSAI = PLMSA/total sleep time), and PLMSW index (PLMSWI = PLMSW/total sleep time). Tests were performed on 2 consecutive pretreatment nights (Days -2 and -1), with the first night used as an adaptation night to document exclusionary sleep disorders. The second night provided baseline polysomnography results. The suggested immobilization test29 was administered 2 hours before the start of polysomnography at baseline (Day -2) and on Day 14 of each treatment period. During the 60-minute suggested immobilization test, subjects recorded leg discomfort every 5 minutes using a 0-to-100 visual analog scale (VAS; 0 = “no discomfort”, 100 = “extreme discomfort”).
Tolerability
Treatment-emergent adverse events (AEs) and serious AEs were recorded. AE intensity was determined by investigators as mild, moderate, or severe. A serious AE was defined as any untoward medical occurrence that was fatal or immediately life-threatening, permanently or significantly disabling, required hospitalization or prolonged hospitalization, caused a congenital anomaly or birth defect in an offspring, or was any other event that the investigator or medical monitor judged to be serious. In addition to 2 consecutive baseline assessments on Days -9 and -2; laboratory parameters were assessed on Days 4, 8, 11, and 15; vital signs on Days 8 and 15; and electrocardiogram on Day 15.
Statistical Analyses
Sample-size calculations were based on the change from baseline IRLS total scores at Day 14 in a previous randomized, double-blind, crossover study with gabapentin.23 Based on pharmacokinetic analyses, it was assumed that XP13512 1800 mg/day would be at least as effective as gabapentin 2400 mg/day.26 Assuming a standard deviation (SD) of 7, a sample size of 32 subjects was calculated to be sufficient to provide 80% power to detect a difference of 3.6 points between active treatment and placebo on the IRLS rating scale using a 2-sided t-test with a 0.05 significance level. Enrollment of 40 subjects was determined to be sufficient to ensure that at least 32 subjects (16 per sequence) were included in the modified intent-to-treat population, defined as all subjects who completed the IRLS rating scale at baseline and at least once after at least 7 days of treatment in each crossover period. All efficacy analyses were performed on the modified intent-to-treat population. The safety population included all randomized subjects who took at least 1 dose of study drug.
Changes from baseline at Days 7 and 14 were assessed for all continuous variables; scores were calculated at Days 7 and 14 for all categorical variables. Data from the 24-hour subject diary were used to calculate time to symptom onset and symptom duration. Continuous efficacy variables were analyzed using an analysis of variance model with treatment, sequence, and period as fixed effects and subject within sequence as a random effect. Least squares (LS) mean treatment difference was calculated from the model. Measurements recorded on Day -1 were used as baseline. Data are presented as pooled data for XP13512 or placebo across crossover periods. Within-treatment changes from baseline were also evaluated using a paired t-test for the primary efficacy variable of change from baseline IRLS score at Day 14. Because there were 60 secondary endpoint analyses, the Holm-Bonferroni method30 was used post hoc as a multiplicity correction to adjust P values. Nominal P values (unadjusted) are also provided for secondary endpoints. Treatment compliance was assessed by unused study tablet counts at the completion of each treatment period and review of the 24-hour RLS diary.
Results
Subject Disposition
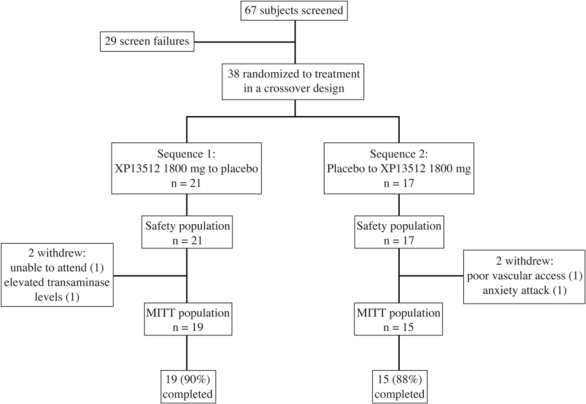
Of the 38 subjects randomly assigned at study entry, 34 (89%) completed the study (Figure 1).
Figure 1.
Subject disposition. All randomized subjects were included in the safety population. Four subjects (2 taking XP13512 and 2 taking placebo) were withdrawn from the study and excluded from the modified intent-to-treat (MITT) population.
Subject Characteristics
Overall, subjects had moderate to severe RLS; disease severity and RLS history were similar between the 2 randomly assigned groups (Table 1). At study entry, 33 subjects (86.8%) were taking medications, such as multivitamins (31.6%), atorvastatin (13.2%), ibuprofen (13.2%), tocopherol (10.5%), and calcium (10.5%). Subjects were permitted to continue these medications provided doses were stable throughout the study. The average treatment compliance was 97%. Gabapentin was not detectable in the plasma of any subject prior to the start of either treatment period.
Table 1.
Demographic and Clinical Characteristics at Baseline, Safety Population
| Demographic characteristics | XP13512 to placebo (n = 21) | Placebo to XP13512 (n = 17) | Total (N = 38) |
|---|---|---|---|
| Age, mean (SD), years | 52 (12.3) | 47.1 (14.1) | 50.1 (13.2) |
| Sex, n (%) | |||
| Male | 6 (28.6) | 10 (58.5) | 16 (42.1) |
| Female | 15 (71.4) | 7 (41.2) | 22 (57.9) |
| Racea, n (%) | |||
| White or Caucasian | 19 (90.5) | 15 (88.2) | 34 (89.5) |
| Black or African American | 1 (4.8) | 1 (5.9) | 2 (5.3) |
| Asian | 1 (4.8) | 0 (0.0) | 1 (2.6) |
| RLS history | |||
| 7-day RLS record, daysb | |||
| Mean (SD) | 6.0 (1.0) | 5.9 (1.2) | 6.0 (1.0) |
| Range | 4–7 | 4–7 | 4–7 |
| Duration of RLS symptoms, years | |||
| Mean (SD) | 13.0 (10.2) | 15.9 (18.0) | 14.3 (14.1) |
| Range | 0.6–32.1 | 0.0–57.0 | 0.0–57.0 |
| Mean baseline IRLS scorec | 20.4 | 20.4 | 20.4 |
Abbreviations: RLS refers to restless legs syndrome; IRLS, International RLS Study Group rating scale.
One subject in the XP13512 group reported race as “other.”
Number of days RLS symptoms expressed.
Score based on modified intent-to-treat (MITT) population.
Efficacy Outcomes
Change from Baseline IRLS Total Score
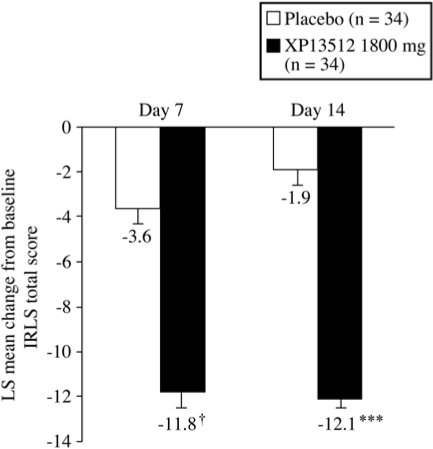
The mean change from baseline IRLS total score at the end of treatment (Day 14) was significantly greater following treatment with XP13512 compared with placebo (mean ± SD: −12.1 ± 6.5 vs −1.9 ± 6.3; P < 0.0001; Figure 2). No significant period or treatment-sequence effects were identified. XP13512-treated subjects had significantly greater reductions in IRLS total score as early as Day 7, the earliest time point evaluated, compared with placebo-treated subjects (mean ± SD change from baseline score: −11.7 ± 7.5 vs −3.7 ± 6.0; adjusted P < 0.0060, nominal P < 0.0001; Figure 2).
Figure 2.
Least squares mean ± SEM change from baseline International Restless Legs Syndrome Study Group rating scale (IRLS) total score at Day 7 (secondary endpoint) and Day 14 (primary endpoint) of treatment with XP13512 1800 mg/day (n = 34) and placebo (n = 34). ***P < 0.0001, † adjusted P < 0.0060 (nominal P < 0.0001) vs placebo, comparison across total distribution.
Investigator- and Subject-Rated Global Impression of Improvement
Significantly more subjects treated with XP13512 were rated as “much improved” or “very much improved” at Day 14 by investigators (79.5% vs 14.7%; adjusted P < 0.0060, nominal P < 0.0001) and by subjects (85.3% vs 14.7%; adjusted P < 0.0059, nominal P < 0.0001) compared with subjects treated with placebo.
Post-Sleep Questionnaire
XP13512 significantly improved scores on all post-sleep questions, except ability to function, at Day 14 compared with placebo (Table 2).
Table 2.
Summary of Responses to the Post-Sleep Questionnaire at Day 14a
| Baseline |
Day 14 |
|||||
|---|---|---|---|---|---|---|
| Sequence 1 (n = 34) | Sequence 2 (n = 34) | Placebo (n = 34) | XP13512 1800 mg (n = 34) | Adjusted P valueb | Nominal P valuec | |
| Overall quality of sleep | < 0.0058 | < 0.0001 | ||||
| Good | 1 (2.9) | 1 (2.9) | 3 (8.8) | 17 (50.0) | ||
| Reasonable | 16 (47.1) | 19 (55.9) | 18 (52.9) | 16 (47.1) | ||
| Poor | 14 (50.0) | 14 (41.2) | 13 (38.2) | 1 (2.9) | ||
| Ability to function | 0.4290 | 0.0143 | ||||
| Good | 11 (32.4) | 11 (32.4) | 14 (41.2) | 25 (73.5) | ||
| Moderate | 20 (58.8) | 20 (58.8) | 18 (52.9) | 8 (23.5) | ||
| Poor | 3 (8.8) | 3 (8.8) | 2 (5.9) | 1 (2.9) | ||
| Number of nights with RLS symptoms | < 0.0057 | < 0.0001 | ||||
| 0 | 0 (0.0) | 1 (2.9) | 1 (2.9) | 7 (20.6) | ||
| 1–2 | 0 (0.0) | 5 (14.7) | 4 (11.8) | 14 (41.2) | ||
| 3–4 | 5 (14.7) | 9 (26.5) | 12 (35.3) | 5 (14.7) | ||
| 5–6 | 15 (44.1) | 8 (23.5) | 7 (20.6) | 3 (8.8) | ||
| 7 | 14 (41.2) | 11 (32.4) | 10 (29.4) | 5 (14.7) | ||
| Number of awakenings during the night due to RLS symptoms | < 0.0056 | < 0.0001 | ||||
| 0 | 1 (2.9) | 4 (11.8) | 3 (8.8) | 16 (47.1) | ||
| 1–2 | 21 (61.8) | 20 (58.8) | 21 (61.8) | 16 (47.1) | ||
| 3–4 | 11 (32.4) | 9 (26.5) | 8 (23.5) | 2 (5.9) | ||
| ≤ 5 | 1 (2.9) | 1 (2.9) | 2 (5.9) | 0 (0.0) | ||
| Number of hours awake per night due to RLS symptoms | < 0.0055 | < 0.0001 | ||||
| 0 | 0 (0.0) | 1 (2.9) | 1 (2.9) | 8 (23.5) | ||
| < 1 | 12 (35.3) | 17 (50.0) | 12 (35.3) | 23 (67.6) | ||
| 1–< 2 | 15 (44.1) | 7 (20.6) | 14 (41.2) | 2 (5.9) | ||
| 2– < 3 | 3 (8.8) | 8 (23.5) | 5 (14.7) | 1 (2.9) | ||
| ≤ 3 | 4 (11.8) | 1 (2.9) | 2 (5.9) | 0 (0.0) | ||
Data are presented as number of subjects (%) in each category. RLS refers to restless legs syndrome.
P value for XP13512 1800 mg versus placebo, adjusted using Holm-Bonferroni methodology.
Nominal P value represents comparison of XP13512 1800 mg with placebo for all categories of response using a repeated-measures Cochran-Mantel-Haenszel test with interval scoring.
24-Hour Subject Diary
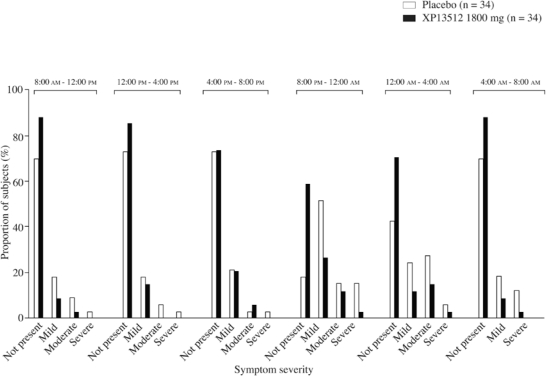
XP13512 significantly reduced the amount of time in which RLS symptoms were present over the 24-hour assessment compared with placebo (mean ± SD change from baseline: Day 7, −184.4 ± 240.7 vs −43.2 ± 287.6 minutes; adjusted P = 0.005, nominal P = 0.0001; Day 14, −205.6 ± 226.1 vs −97.9 ± 252.9 minutes; adjusted P = 0.0215, nominal P = 0.0005). At Day 14, evening and night-time symptom severities (20:00–08:00) were rated as absent or mild by 82% to 97% of XP13512-treated subjects, compared with 66% to 88% of placebo-treated subjects (Figure 3).
Figure 3.
Maximum restless legs syndrome (RLS) severity over 24 hours (6 periods of 4 hours each) on Day 14 in subjects treated with XP13512 1800 mg/day (n = 34) and placebo (n = 34).
Polysomnography
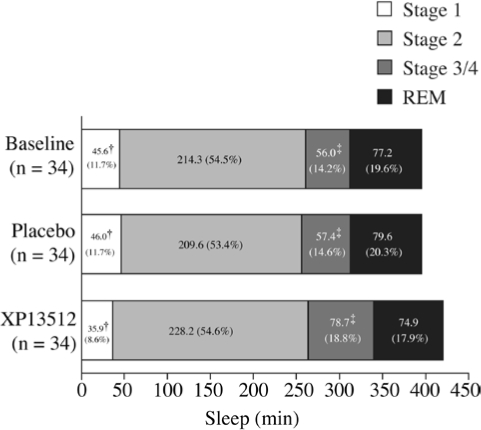
XP13512 significantly improved wake time after persistent sleep onset, wake time during sleep, and number of awakenings at Day 14 compared with placebo (Table 3). PLM parameters, including mean change from baseline PLM, PLMS, PLMSA, and PLMSW, were numerically improved with XP13512 compared with placebo, although these differences were not significant (Figure 4). XP13512 significantly shortened stage 1 sleep and extended stage 3/4 (slow-wave) sleep compared with placebo (Figure 5). REM and stage 2 sleep times were similar in the 2 treatment groups.
Table 3.
Change from Baseline Sleep and PLM Parameters Assessed by Polysomnography at Day 14
| Variable | Change from baselinea |
||||
|---|---|---|---|---|---|
| Baseline (n = 34) | Placebo (n = 34) | XP13512 (n = 34) | Adjusted P valueb | Nominal P valuec | |
| Total time in bed, min | 480.0 (0.1) | −0.1 (0.3) | −0.2 (0.8) | 1 | 0.5926 |
| Total sleep time, min | 393.2 (54.1) | −0.6 (54.8) | 24.5 (53.1) | 0.7107 | 0.0316 |
| Sleep efficiency, % | 81.9 (11.3) | −0.1 (11.4) | 5.1 (11.1) | 0.7416 | 0.0309 |
| Wake time after persistent sleep onsetd, min | 60.94 (45.6) | 6.7 (38.2) | −21.5 (50.2) | 0.0328 | 0.0009 |
| Wake time during sleepe, min | 51.8 (35.9) | 11.5 (33.8) | −14.1 (38.9) | 0.0440 | 0.0011 |
| Number of awakenings | 8.0 (3.7) | 0.4 (3.8) | −2.1 (4.0) | < 0.0053 | < 0.0001 |
| Latency to sleep stage, minf | |||||
| 1 | 13.8 (14.1) | 0.3 (20.7) | 0.3 (16.2) | 1 | 0.9687 |
| 2 | 19.6 (18.3) | −2.1 (24.7) | 2.8 (34.3) | 1 | 0.3982 |
| REM | 84.3 (50.2) | 3.4 (43.6) | 14.3 (60.4) | 1 | 0.2152 |
| Latency to persistent sleep, min | 31.2 (38.9) | −7.4 (40.5) | −4.4 (27.2) | 1 | 0.6273 |
| PLMI, no./h | 31.8 (25.3) | 0.8 (27.9) | −8.6 (23.6) | ||
| PLMSI, no./h | 22.3 (24.6) | −1.6 (23.4) | −4.9 (23.4) | ||
| PLMSAI, no./h | 9.2 (16.2) | −1.8 (13.6) | −4.9 (16.1) | ||
| PLMSWI, no./h | 0.9 (0.7) | 0.1 (0.9) | −0.3 (0.7) | ||
Abbreviations: REM refers to rapid eye movement; PLM, periodic leg movement; PLMI, PLM during time in bed index (calculated as PLM/total time in bed); PLMS, periodic leg movements of sleep not causing arousal; PLMSI, PLMS during sleep index (calculated as PLMS/total sleep time); PLMSA, PLMS that cause at least a 3-second arousal, but not an awakening; PLMSAI, PLM during sleep with arousal index (calculated as PLMSA/total sleep time); PLMSW, PLMS that cause at least one 30-second epoch of wake; PLMSWI, PLM during wakefulness index (calculated as PLMSW/total sleep time).
P value for treatment effect, adjusted using Holm-Bonferroni methodology.
Nominal P value for treatment effect from analysis of variance with treatment, period, and sequence as fixed effects, and patient within sequence as a random effect. P values for period and sequence effects were ≥ 0.1217 in the analyses of each variable.
Wake time after persistent sleep onset defined as number of wake minutes after the onset of persistent sleep prior to the end of recording.
Wake time during sleep defined as the number of wake minutes after the onset of persistent sleep prior to the last epoch of stage 2, 3/4, or REM sleep.
Latency to sleep defined as number of minutes from lights out until first epoch of stages 2, 3, 4, or REM or until the first 3 consecutive epochs of stage 1 sleep.
Latency to persistent sleep defined as time from lights out to the first epoch of 20 consecutive non wake epochs.
Figure 4.
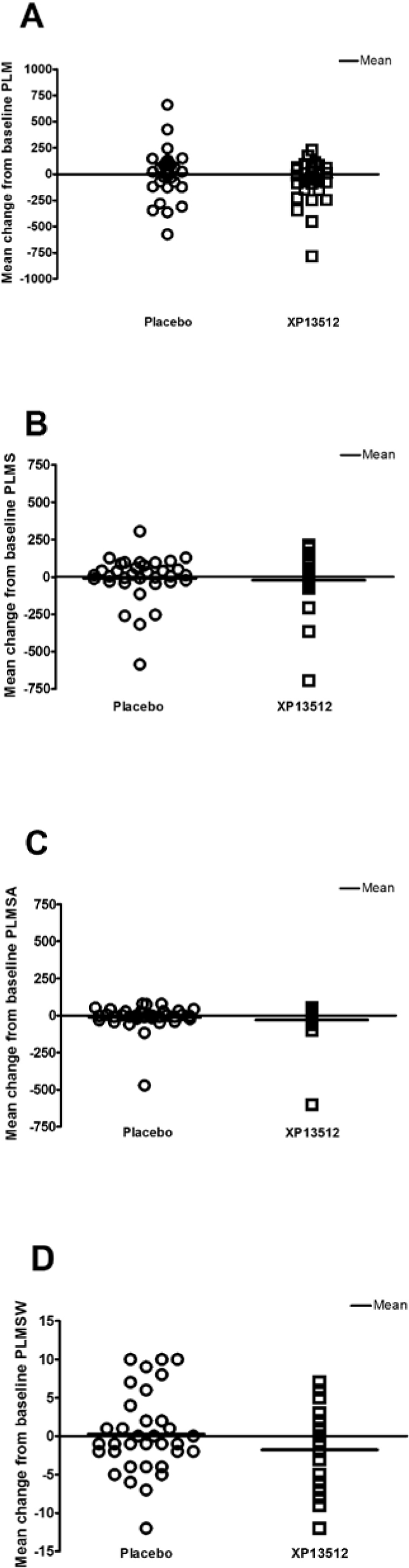
Individual and mean changes from baseline in the following parameters: periodic leg movements (PLM), periodic leg movements during sleep, not associated with an arousal (PLMS), periodic leg movements during sleep causing an arousal (PLMSA), and periodic leg movement during sleep causing an awakening (PLMSW). Scores were obtained on Day 14 in subjects treated with XP13512 1800 mg (n = 34) and placebo (n = 34).
Figure 5.
Sleep architecture in subjects treated with XP13512 1800 mg (n = 34) and placebo (n = 34) as measured by polysomnography at baseline and on Day 14. †Adjusted P < 0.0054 (nominal P < 0.0001), ‡Adjusted P = 0.0092 (nominal P = 0.0002) vs baseline; analysis of variance. REM refers to rapid eye movement.
Suggested Immobilization Test
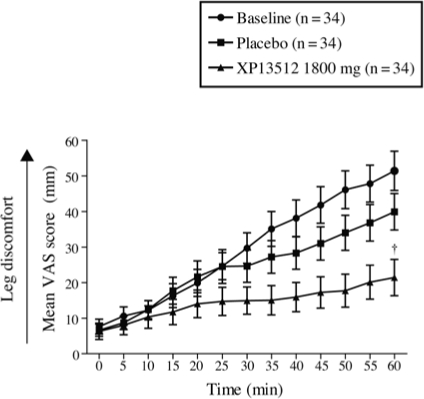
At baseline, mean VAS scores steadily increased during assessment to a maximum value at 60 minutes of 51.8 (32.1) (Figure 6). On Day 14, mean VAS scores steadily increased to a maximum value at 60 minutes of 21.8 (29.8) with XP13512 and 40.3 (29.8) with placebo (adjusted P = 0.0468; nominal P = 0.0012).
Figure 6.
Mean ± SEM leg discomfort (visual analog scale [VAS]) recorded in 5-minute intervals, 2 hours before lights out, by subjects treated with XP13512 1800 mg/day (n = 34) and placebo (n = 34) during administration of the suggested immobilization test at baseline and on Day 14. †Adjusted P = 0.0468 (nominal P = 0.0012) vs placebo, comparison across total distribution.
Tolerability
Treatment-emergent AEs were reported by 28 subjects (77.8%) receiving XP13512 and 14 (38.9%) receiving placebo (Table 4). The most commonly reported AEs were somnolence and dizziness, and all occurrences were judged to be treatment related. All but 4 AEs (1 subject with nasopharyngitis [placebo], 1 subject with insomnia [XP13512], and 2 subjects with dizziness [both XP13512]) were mild or moderate in intensity. One incident of dizziness was downgraded from severe to mild intensity following XP13512 dose reduction.
Table 4.
Most Frequently Reported Treatment-Emergent Adverse Eventsa
| Number of patients (%) |
||
|---|---|---|
| Adverse eventb | Placebo (n = 36) | XP13512 (n = 36) |
| Any | 14 (38.9) | 28 (77.8) |
| Somnolence | 1 (2.8) | 11 (30.6) |
| Dizziness | 2 (5.6) | 10 (27.8) |
| Balance disorder | 0 | 3 (8.3) |
| Dry mouth | 0 | 2 (5.6) |
| Fatigue | 0 | 2 (5.6) |
| Headache | 1 (2.8) | 2 (5.6) |
| Hypoesthesia | 0 | 2 (5.6) |
| Insomnia | 0 | 2 (5.6) |
| Nausea | 0 | 2 (5.6) |
| Nasopharyngitis | 3 (8.3) | 0 |
Reported by 2 or more subjects in any treatment group; subjects could experience more than 1 adverse event.
Adverse events reported as Medical Dictionary for Regulatory Activities (MedDRA) preferred terms.
Downward dose adjustment from XP13512 1800 mg/day to 1200 mg/day was required in 4 subjects due to dizziness (n = 2) or somnolence (n = 2) during the XP13512 period; 1 of these subjects also experienced dizziness during the placebo period. Two additional subjects did not reach the target dose of 1800 mg/day during titration in the XP13512 period, 1 because of somnolence and the other because of dizziness and fatigue. One subject required downward dose adjustment because of insomnia during the placebo period. None of these AEs led to study discontinuation.
One subject (placebo) withdrew on Day 2 due to an anxiety attack and noncardiac chest tightness that were both considered moderate in intensity and possibly related to study drug by the investigator. No serious AEs were reported. There were no clinically significant changes in vital signs, laboratory values, or electrocardiogram outcomes.
Discussion
In this double-blind crossover study of XP13512 1800 mg/day in treatment-naïve subjects with moderate to severe primary RLS, XP13512 significantly reduced RLS symptoms compared with placebo. Subjective treatment benefits based on IRLS total scores occurred as early as Day 7, the earliest time point examined, after only 2 days at the full target dose, and statistical significance was maintained until the end of treatment (Day 14). XP13512 also improved subject- and investigator-rated outcomes and subjective and objective sleep parameters.
The mean change from baseline IRLS total score after 14 days of treatment with XP13512 1800 mg/day reported here is comparable to the reduction reported in a 6-week gabapentin crossover study in patients with similar RLS disease severity.23 The treatment effect of XP13512 1800 mg, which has the potential to release 938 mg of gabapentin, is similar in magnitude to the treatment effect associated with a mean gabapentin dose of 1855 mg reported by Garcia-Borreguero et al. XP13512 doses are not directly comparable with oral gabapentin doses due to the differences in molecular weight and pharmacokinetic properties, and direct efficacy comparisons cannot be made. XP13512 is not associated with the variable and saturable absorption and relative short exposure time that is characteristic of oral gabapentin.26
To put the XP13512 findings into context with approved therapies for RLS, data from a similarly designed 4-week crossover study of ropinirole were examined.31 Patients treated with ropinirole 0.25 to 6 mg/day had reductions in IRLS scores similar to those reported in the current study, although baseline disease severity may have been slightly higher (mean IRLS score 25, compared with 20 reported here). The improvement in IRLS scores reported here is comparable to those observed in longer-term, randomized, placebo-controlled, parallel studies with ropinirole and pramipexole.32–34 However, the differing designs and the short treatment duration in the present study do not allow for a direct comparison to these dopamine agonist studies. Longer parallel-group studies are needed to examine the long-term effects of XP13512 and explore additional dosages.
The secondary exploratory outcomes reported in the present study broaden our understanding of the potential therapeutic benefits of XP13512 in RLS. Subjective improvements in sleep quality, ability to function, number of nights without RLS symptoms, awakenings, and time awake due to RLS symptoms were corroborated by objective polysomnography data. Polysomnography findings demonstrated significant differences in sleep architecture (shortened stage 1 sleep and extended stages 3/4 [slow-wave] sleep) between treatment groups. These findings are consistent with previously reported data in RLS patients and healthy adults treated with gabapentin.23,35 The improvement in slow-wave sleep observed with XP13512 differs from that observed in polysomnography studies with dopamine agonists, which have been shown to either decrease or have no effect on slow-wave sleep in RLS patients.22,36
XP13512 significantly improved mean VAS scores at 60 minutes compared with placebo on the suggested immobilization test. XP13512 improved mean VAS scores at other time points and also many polysomnography-derived secondary outcomes, although the differences were not significant compared with placebo when the post hoc Holm-Bonferroni correction was applied. The study was not powered to detect significant differences on secondary endpoints. The crossover study design was selected to allow subjects to serve as their own controls and increase the power to detect treatment effects, while reducing variability and sample size. The placebo response in patients with RLS in crossover trials, as measured on the IRLS, is relatively low and similar to that reported here: 0.4 to 2 points over 4 to 6 weeks.23,31 The interpretation of crossover studies may be confounded by treatment carryover effects. However, no significant sequence effects were observed, and gabapentin levels were not detected in any baseline measurement, including washout.
XP13512 was generally well tolerated during this study. The most commonly reported AEs were somnolence and dizziness, both of which are consistent with the known profile of oral gabapentin. The short treatment period did not allow for an assessment of long-term tolerability with XP13512. No serious AEs were reported, and the only treatment discontinuation occurred with placebo.
In summary, results from this crossover study demonstrate that XP13512 has promising efficacy and tolerability as a nondopaminergic treatment for subjects with moderate to severe primary RLS. Subjects were at the target XP13512 dose for only 2 days when improvements in IRLS score separated significantly from placebo at the earliest time point examined. These results suggest that lower doses with XP13512 should be explored.
DISCLOSURE STATEMENT
This study was funded by XenoPort, Inc., Santa Clara, CA, USA. This manuscript describes an investigational, unapproved use of XP13512/GSK1838262. Dr. Kushida has received research support through contracts with Stanford University with Boehringer Ingelheim, GlaxoSmithKline, Kyowa Pharmaceuticals, and Schwarz Pharmaceuticals.
Dr. Walters has participated in speaking engagements for Boehringer Ingelheim and GlaxoSmithKline and has consulted for Boehringer Ingelheim, GlaxoSmithKline, Jazz, Kyowa Pharmaceuticals, Novartis, Orion, Schering, UCB Pharma, and XenoPort. Dr. Becker has received research support from Arena, Boehringer Ingelheim, GlaxoSmithKline, Pfizer, Schwarz Pharmaceuticals, XenoPort; has consulted for Boehringer Ingelheim, GlaxoSmithKline, and XenoPort; and has participated in speaking engagements for Boehringer Ingelheim, GlaxoSmithKline, Sanofi-Aventis, Sepracor, and Takeda. Dr. Thein has consulted for Cephalon, Eli Lilly, INC, TransTech, UBC, and XenoPort; has participated in speaking engagements for Cephalon, Forest, Janssen, Novartis, and Sepracor; and directs operations at Pacific Research Network research center that conducts clinical trials for Abbott, Allon, Apex, Arena, Astellas, AstraZeneca, Axon, Bristol-Myers Squibb, Cephalon, Dainippon, Eisai, Elan, Eli Lilly, Epix, Forest, GlaxoSmithKline, Janssen, J&J, MAP, Martek, Merck, Mitsubishi, Myriad, Neurochem, Neurocrine, NIMH, Novartis, Ono, Organon, Ortho-McNeil, Pfizer, Rinat, Roche, Saegis, Sanofi-Aventis, Schwarz, Sepracor, Takeda, Tap, TransTech, Vanda, Vernalis, Voyager, Wyeth, and XenoPort. Dr. Perkins has received research support from XenoPort. Dr.Roth has received research support from Aventis, Cephalon, GlaxoSmithKline, Neurocrine, Pfizer, Sanofi-Aventis, Schering Plough, Sepracor, Somaxon, Syrex, Takeda, TransOral, Wyeth, XenoPort; has consulted for Abbott, Accadia, Acoglix, Actelion, Alchemers, Alza, Ancil, Arena, AstaZeneca, Aventis, BMS, Cephalon, Cypress, Dove, Elan, Eli Lilly, Evotec, Forest, GlaxoSmithKline, Hypnion, Johnson and Johnson, King, Ludbeck, McNeil, MediciNova, Merck, Neurim, Neurocrine, Neurogen, Novartis, Orexo, Organon, Orginer, Prestwick, Procter and Gamble, Pfizer, Purdue, Resteva, Roche, Sanofi, Schering Plough, Sepracor, Servier, Shire, Somaxon, Syrex, Takeda, TransOral, Vanda, Vivometrics, Wyeth, Yamanuchi, and XenoPort and has participated in speaking engagements for Sanofi and Takeda. Dr. Canafax is a former employee of XenoPort. Dr. Barrett is Chief Executive Officer and a Director of XenoPort and Director of Concert Pharmaceuticals.
ACKNOWLEDGMENTS
This report presents data generated from protocol XP021, funded by XenoPort, Inc., Santa Clara, CA. The authors acknowledge Barbara Wilson, MEd (GlaxoSmithKline, Research Triangle Park, NC) for editorial coordination and Kathy McIvor, BS (Envision Pharma, Philadelphia, PA) for editorial and writing assistance.
| Table of institutions at which the trial was performed. | |
| Investigator | Institution |
| Philip M. Becker, MD | Sleep Medicine Associates of Texas; Plano, TX |
| Aaron Ellenbogen, DO, MPH | Quest Research Institute; Southfield, MI |
| Clete A. Kushida, MD, PhD | Stanford Sleep Disorders Clinic; Stanford, CA |
| William G. Ondo, MD | Baylor School of Medicine; Houston, TX |
| A. Thomas Perkins, MD, PhD | Raleigh Neurology Associates, PA; Raleigh, NC |
| J. Steven Poceta, MD | The Scripps Research Institute, Division of Neurology; La Jolla, CA |
| Stephen G. Thein, PhD | Pacific Research Network; San Diego, CA |
| J. Catesby Ware, PhD | Sleep Disorders Center, Sentara Norfolk General Hospital; Norfolk, VA |
| Denise Sharon, PhD | Premier Medicine Sleep Center; Baton Rouge, LA |
ABBREVIATIONS
- AE,
adverse event
- ANOVA,
analysis of variance
- CGI-I,
Clinical Global Impression-Improvement
- ECG,
electrocardiogram
- IRLS,
International Restless Legs Syndrome Study Group rating scale
- IRLSSG,
-
International Restless Legs Syndrome Study Group
Latency to persistent sleep, time from lights out to the first epoch of 20 consecutive non-wake epochs
Latency to sleep, number of minutes from lights out until first epoch of NREM stages 2, 3, 4, or REM or until the first 3 consecutive epochs of stage 1 sleep
- MITT,
modified intent-to-treat
- PLMs,
periodic limb movements
- PLMI,
periodic limb movement index (calculated as PLMs/total time in bed)
- PLMS,
PLMs of sleep not causing arousal
- PLMSI,
PLMS index (calculated as PLMS/total sleep time)
- PLMSA,
PLMs of sleep that cause at least a 3-second arousal, but not an awakening
- PLMSAI,
PLMSA index (calculated as PLMSA/total sleep time)
- PLMSW,
PLMs of sleep that cause at least one 30-second epoch of wake
- PLMSWI,
PLMSW index (calculated as PLMSW/total sleep time).
- PSG,
polysomnography
- REM,
rapid eye movement
- RLS,
restless legs syndrome
- Sleep architecture,
percent sleep time spent in sleep stages 1, 2, 3/4 (slow-wave sleep)
- Sleep efficiency,
total sleep time/total time in bed × 100
- SAE,
serious adverse event
- SD,
standard deviation
- SIT,
suggested immobilization test
- VAS,
visual analog scale
- WASO,
wake time after persistent sleep onset (number of wake minutes after the onset of persistent sleep prior to the end of recording)
- WTDS,
wake time during sleep (number of wake minutes after the onset of persistent sleep prior to the last epoch of stage 2, 3/4 or REM sleep [rather than end of recording])
REFERENCES
- 1.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165:1286–92. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 2.Högl B, Kiechl S, Willeit J, et al. Restless legs syndrome: a community-based study of prevalence, severity, and risk factors. Neurology. 2005;64:1920–4. doi: 10.1212/01.WNL.0000163996.64461.A3. [DOI] [PubMed] [Google Scholar]
- 3.Nichols DA, Allen RP, Grauke JH, et al. Restless legs syndrome symptoms in primary care: a prevalence study. Arch Intern Med. 2003;163:2323–9. doi: 10.1001/archinte.163.19.2323. [DOI] [PubMed] [Google Scholar]
- 4.Saletu B, Gruber G, Saletu M, et al. Sleep laboratory studies in restless legs syndrome patients as compared with normals and acute effects of ropinirole.1. Findings on objective and subjective sleep and awakening quality. Neuropsychobiology. 2000;41:181–9. doi: 10.1159/000026658. [DOI] [PubMed] [Google Scholar]
- 5.Abetz L, Allen RP, Follet A, et al. Evaluating the quality of life of patients with restless legs syndrome. Clin Ther. 2004;26:925–35. doi: 10.1016/s0149-2918(04)90136-1. [DOI] [PubMed] [Google Scholar]
- 6.Partinen M, Hirvonen K, Jama L, et al. Efficacy and safety of pramipexole in idiopathic restless legs syndrome: a polysomnographic dose-finding study-the PRELUDE study. Sleep Med. 2006;7:407–17. doi: 10.1016/j.sleep.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Bliwise DL, Freeman A, Ingram CD, Rye DB, Chakravorty S, Watts RL. Randomized, double-blind, placebo-controlled, short-term trial of ropinirole in restless legs syndrome. Sleep Med. 2005;6:141–7. doi: 10.1016/j.sleep.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Allen RP, Becker PM, Bogan RK, et al. Ropinirole decreases periodic leg movements and improves sleep parameters in patients with restless legs syndrome. Sleep. 2004;27:907–14. doi: 10.1093/sleep/27.5.907. [DOI] [PubMed] [Google Scholar]
- 9.Winkelman JW, Johnston L. Augmentation and tolerance with long-term pramipexole treatment of restless legs syndrome (RLS) Sleep Med. 2004;5:9–14. doi: 10.1016/j.sleep.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Ondo W, Romanyshyn J, Vuong KD, Lai D. Long-term treatment of restless legs syndrome with dopamine agonists. Arch Neurol. 2004;61:1393–7. doi: 10.1001/archneur.61.9.1393. [DOI] [PubMed] [Google Scholar]
- 11.Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998;280:1837–42. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- 12.Rice AS, Maton S. Gabapentin in postherpetic neuralgia: a randomised, double blind, placebo controlled study. Pain. 2001;94:215–24. doi: 10.1016/S0304-3959(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 13.Pfizer Inc. Neurontin® Prescribing Information. [PDF] 2007 January 2007 [cited 2007 June 28]; Currently available from: http://www.pfizer.com/files/products/uspi_neurontin.pdf.
- 14.McLean MJ. Gabapentin in the management of convulsive disorders. Epilepsia. 1999;40(Suppl 6):S39–50. doi: 10.1111/j.1528-1157.1999.tb00932.x. discussion S73-4. [DOI] [PubMed] [Google Scholar]
- 15.The International Gabapentin Study Group. Anhut H, Ashman P, Feuerstein TJ, Sauermann W, Saunders M, Schmidt B. Gabapentin (Neurontin) as add-on therapy in patients with partial seizures: a double-blind, placebo-controlled study. Epilepsia. 1994;35:795–801. doi: 10.1111/j.1528-1157.1994.tb02513.x. [DOI] [PubMed] [Google Scholar]
- 16.Mellick GA, Mellick LB. Management of restless legs syndrome with gabapentin (Neurontin) Sleep. 1996;19:224–6. doi: 10.1093/sleep/19.3.224. [DOI] [PubMed] [Google Scholar]
- 17.Thorp ML, Morris CD, Bagby SP. A crossover study of gabapentin in treatment of restless legs syndrome among hemodialysis patients. Am J Kidney Dis. 2001;38:104–8. doi: 10.1053/ajkd.2001.25202. [DOI] [PubMed] [Google Scholar]
- 18.Micozkadioglu H, Ozdemir FN, Kut A, Sezer S, Saatci U, Haberal M. Gabapentin versus levodopa for the treatment of Restless Legs Syndrome in hemodialysis patients: an open-label study. Ren Fail. 2004;26:393–7. doi: 10.1081/jdi-120039823. [DOI] [PubMed] [Google Scholar]
- 19.Mellick LB, Mellick GA. Successful treatment of reflex sympathetic dystrophy with gabapentin. Am J Emerg Med. 1995;13:96. doi: 10.1016/0735-6757(95)90263-5. [DOI] [PubMed] [Google Scholar]
- 20.Adler CH. Treatment of restless legs syndrome with gabapentin. Clin Neuropharmacol. 1997;20:148–51. doi: 10.1097/00002826-199704000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Happe S, Klosch G, Saletu B, Zeitlhofer J. Treatment of idiopathic restless legs syndrome (RLS) with gabapentin. Neurology. 2001;57:1717–9. doi: 10.1212/wnl.57.9.1717. [DOI] [PubMed] [Google Scholar]
- 22.Happe S, Sauter C, Klosch G, Saletu B, Zeitlhofer J. Gabapentin versus ropinirole in the treatment of idiopathic restless legs syndrome. Neuropsychobiology. 2003;48:82–6. doi: 10.1159/000072882. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Borreguero D, Larrosa O, de la Llave Y, Verger K, Masramon X, Hernandez G. Treatment of restless legs syndrome with gabapentin: a double-blind, cross-over study. Neurology. 2002;59:1573–9. doi: 10.1212/wnl.59.10.1573. [DOI] [PubMed] [Google Scholar]
- 24.Gidal BE, Radulovic LL, Kruger S, Rutecki P, Pitterle M, Bockbrader HN. Inter- and intra-subject variability in gabapentin absorption and absolute bioavailability. Epilepsy Res. 2000;40:123–7. doi: 10.1016/s0920-1211(00)00117-0. [DOI] [PubMed] [Google Scholar]
- 25.Cundy KC, Annamalai T, Bu L, et al. XP13512 [(+/−)-1-([(alpha-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: II. Improved oral bioavailability, dose proportionality, and colonic absorption compared with gabapentin in rats and monkeys. J Pharmacol Exp Ther. 2004;311:324–33. doi: 10.1124/jpet.104.067959. [DOI] [PubMed] [Google Scholar]
- 26.Cundy KC SS, Luo W, Zou J, Moors TL, Canafax DM. Clinical pharmacokinetics of XP13512, a novel transported prodrug of gabapentin. J Clin Pharmacol. 2008 doi: 10.1177/0091270008322909. In press. [DOI] [PubMed] [Google Scholar]
- 27.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 28.Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 29.Montplaisir J, Boucher S, Nicolas A, et al. Immobilization tests and periodic leg movements in sleep for the diagnosis of restless leg syndrome. Mov Disord. 1998;13:324–9. doi: 10.1002/mds.870130220. [DOI] [PubMed] [Google Scholar]
- 30.Holm S. A simple sequentially rejective multiple test procedure. Scand J Statis. 1979;6:65–70. [Google Scholar]
- 31.Adler CH, Hauser RA, Sethi K, et al. Ropinirole for restless legs syndrome: a placebo-controlled crossover trial. Neurology. 2004;62:1405–7. doi: 10.1212/01.wnl.0000120672.94060.f1. [DOI] [PubMed] [Google Scholar]
- 32.Trenkwalder C, Garcia-Borreguero D, Montagna P, et al. Ropinirole in the treatment of restless legs syndrome: results from the TREAT RLS 1 study, a 12 week, randomised, placebo controlled study in 10 European countries. J Neurol Neurosurg Psychiatry. 2004;75:92–7. [PMC free article] [PubMed] [Google Scholar]
- 33.Walters AS, Ondo WG, Dreykluft T, Grunstein R, Lee D, Sethi K. Ropinirole is effective in the treatment of restless legs syndrome. TREAT RLS 2: a 12-week, double-blind, randomized, parallel-group, placebo-controlled study. Mov Disord. 2004;19:1414–23. doi: 10.1002/mds.20257. [DOI] [PubMed] [Google Scholar]
- 34.Winkelman JW, Sethi KD, Kushida CA, et al. Efficacy and safety of pramipexole in restless legs syndrome. Neurology. 2006;67:1034–9. doi: 10.1212/01.wnl.0000231513.23919.a1. [DOI] [PubMed] [Google Scholar]
- 35.Foldvary-Schaefer N, De Leon Sanchez I, Karafa M, Mascha E, Dinner D, Morris HH. Gabapentin increases slow-wave sleep in normal adults. Epilepsia. 2002;43:1493–7. doi: 10.1046/j.1528-1157.2002.21002.x. [DOI] [PubMed] [Google Scholar]
- 36.Saletu M, Anderer P, Saletu-Zyhlarz G, Hauer C, Saletu B. Acute placebo-controlled sleep laboratory studies and clinical follow-up with pramipexole in restless legs syndrome. Eur Arch Psychiatry Clin Neurosci. 2002;252:185–94. doi: 10.1007/s00406-002-0380-7. [DOI] [PubMed] [Google Scholar]