Abstract
Study Objectives:
Pregnant women have an increased risk of experiencing restless legs syndrome (RLS). Aim of this study was to elucidate the relationship between pregnancy-related hormonal and metabolic changes and RLS symptomatology.
Design:
Blood measurements and overnight polysomnography were performed during the third trimester of pregnancy and again 3 months after delivery. We investigated blood hormonal levels (estradiol, prolactin, progesterone, testosterone, follicle-stimulating hormone [FSH], luteinizing hormone [LH], iron, ferritin, hemoglobin) and polysomnographic sleep parameters. Subjective sleep quality and RLS symptoms were evaluated using the Pittsburgh Sleep Quality Index (PSQI) and the International RLS study group (IRLSSG) rating scale.
Setting:
Sleep laboratory.
Participants:
Ten pregnant women fulfilling the IRLSSG criteria for RLS diagnosis and 9 pregnant healthy controls underwent the protocol.
Interventions:
N/A.
Results:
Women with RLS showed higher levels of estradiol during pregnancy compared to controls (34,211 ± 6,397 pg/mL vs. 25,475 ± 7,990 pg/mL, P < 0.05). Patients also showed more periodic limb movements (PLMs) before and after delivery, particularly during sleep stage 1 and wakefulness (P < 0.05). PLMs decreased postpartum in subjects with RLS only (P < 0.05); sleep efficiency increased in women without RLS and remained unchanged in patients (P < 0.05). No significant differences were found between groups before or after delivery in plasma concentrations of prolactin, progesterone, testosterone, FSH, LH, iron, ferritin or hemoglobin.
Conclusions:
RLS in pregnant women goes along with transiently increased estradiol levels and PLM indices suggesting that estrogens play a pathophysiological role for triggering RLS symptoms during pregnancy.
Citation:
Dzaja A; Wehrle R; Lancel M; Pollmächer T. Elevated estradiol plasma levels in women with restless legs during pregnancy. SLEEP 2009;32(2):169-174.
Keywords: RLS, steroid hormones, estradiol, pregnancy, polysomnography, periodic limb movements
PREVALENCE OF THE RESTLESS LEGS SYNDROME (RLS), A COMMON NEUROLOGICAL SENSORIMOTOR DISORDER, IS FREQUENTLY REPORTED HIGHER IN women than in men.1–5 In addition, RLS significantly worsens during pregnancy and improves or even disappears after delivery.6–8 Symptoms often occur for the first time during pregnancy. Furthermore, RLS risk increases gradually with the number of pregnancies,9 and symptoms are most pronounced during the last trimester.10
In 1995, the International RLS Study Group established 4 essential criteria for RLS: (a) an urge to move the legs, accompanied by paresthesias of the legs; (b) motor restlessness with relief by movement; (c) a worsening of the symptoms at rest; and (d) worsening of symptoms in the evening or at night.11 Applying these criteria, the prevalence of RLS in the general population ranges from 5.5% to 10.6%.1–5 Most RLS patients also show repetitive, stereotyped leg movements, called periodic limb movements (PLMs) during sleep and/or wakefulness.11 Accordingly, approximately 95% of the RLS patients suffer from sleep disturbances including difficulties falling asleep, unconsolidated sleep and daytime tiredness.4,11 Most, but not all population-based studies demonstrated a higher prevalence of RLS in women than in men.1–4,12 One study of 4,310 participants reported an overall prevalence of 10.6%, increasing with age, affecting twice as many women as men.4 Particularly striking is the fact that up to 25% of pregnant women experience RLS, and symptoms usually improve or completely disappear with delivery. Manconi et al. conducted an epidemiologic study on RLS during pregnancy and the puerperium.10,13 This study revealed a prevalence rate of 26.6% among pregnant women. These observations suggest a considerable influence of pregnancy related hormonal (physiological) changes on RLS expressivity. Steroid hormones (in particular estradiol) rise during pregnancy and decline around delivery. In addition, altered sleep is common during pregnancy. Polysomnographic recordings revealed increased total sleep time during the first trimester of pregnancy, decreased stage 3 and 4 NREM sleep in each trimester, and decreased total sleep time and increased waking after sleep onset in the third trimester.14–18
The objective of the present study was to elucidate whether hormonal changes during pregnancy and postpartum are related to RLS symptoms, sleep quality, and periodic limb movements in women with and without RLS.
METHODS
Subjects
Subjects were recruited by an advertisement in a local newspaper, addressing pregnant women between 20 and 40 years of age both with and without symptoms of RLS. Subjects were paid for study participation, and screening was stopped when 10 women per each group were successfully enrolled in the study. Approval of an independent ethics committee (Bayerische Landesärztekammer) and written informed consent from all subjects were obtained. Inclusion criteria were pregnancy and age between 20 and 40 years. Patients had to satisfy the 4 standard diagnostic criteria defined by the International RLS Study Group. Twenty pregnant women, 10 suffering from RLS (mean age of onset: 22.6 ± 7.9 y), were included in the study. In the RLS group, 8 subjects reported RLS symptoms previous to the present pregnancy (preexisting RLS; mean duration of 11.2 ± 5.5 years). They all described worsening of symptoms during pregnancy, and 4 reported affected family members. One subject without RLS had to be excluded due to a stillbirth in late pregnancy. Hence, data from 10 women suffering from RLS (mean age ± SD, 31.6 ± 2.4 y) and 9 healthy controls (mean age ± SD, 32.9 ± 2.7 y) were analyzed. None of the subjects suffered from any additional disease. Of each group, 2 subjects were treated with magnesium, and one per group additionally with iron. Otherwise, all subjects were free from any medication. RLS symptomatology was comprehensively explored in a detailed clinical interview. Before inclusion, patients and controls underwent a physical examination, and a detailed medical history was documented. Exclusion criteria were any additional disease (requiring long-term medical treatment or hospitalization) within the last 10 years. Controls denied experience of RLS symptomatology in their entire lifetime.
Experimental Procedure
Around the 36th week of gestation (mean ± SD, 35.9 ± 1.9; range, 34-40 weeks) and 12 weeks postpartum, subjects underwent overnight polysomnography (PSG), 2 nights each. The first night served for adaptation purposes. Sleep data and leg movement recordings from the second night were analyzed in this study. Blood samples were taken each morning after polysomnography. Accompanying questionnaires on sleep and RLS symptoms were collected: subjects completed a daily sleep diary from about the 30th week of pregnancy until 2 weeks after delivery and again in a 4-week period 3 months following delivery. In addition, women with RLS completed the IRLSSG rating scale (IRLS) weekly during these time periods. All subjects completed the Pittsburgh Sleep Quality Index Questionnaire every 4 weeks from the 30th week of pregnancy until delivery and again 3 months after delivery. Postpartum PSG was not performed in 2 women from each group.
Blood Sampling
Blood samples were drawn between 07:30 and 08:30 each morning after the sleep recording before breakfast. Hormones were measured by electrochemiluminescence, with an Elecsys 2010 analyzer (Roche Diagnostics, Basel, Switzerland). The maximal intra- and interassay coefficients of variation at different hormone concentrations were: estradiol 5.7%, testosterone 4.6%, prolactin 4%, progesterone 2.7%, and cortisol 1.3%. Iron, ferritin, and magnesium were measured by electrochemiluminescence, with a Hitachi 912 (Roche Diagnostics, Basel, Switzerland). The maximal intra- and interassay coefficients of variation were: magnesium 1.2%, iron 1.2%, and ferritin 6%.
Sleep Recordings
Sleep recordings included electroencephalogram (EEG; C3 and C4 derivation according to the international 10/20 system, referenced to the contralateral mastoid), electrooculogram (EOG), and chin electromyogram (EMG). Polysomnographic recordings also included electrocardiogram (ECG), breathing parameters, and electromyographic (EMG) recordings of the left and the right tibialis muscle (sampling rate 250 Hz). The first night served as adaptation night; data were analyzed from the second sleep recording lasting from 23:00 to 07:00. All sleep recordings were visually scored in 30-s epochs according to standardized criteria by trained scorers blinded with respect to the group condition. The numbers of periodic leg movements of both tibialis EMG recordings were computed using an automatic scoring algorithm.19 PLM-indices for both legs were scored for each vigilance stage, as well as for time in bed (TiB).
Statistics
Statistical analysis of sleep and PLM parameters as well as of blood parameters including hormones was performed using a repeated measures analysis of variance separately for each variable, with diagnosis as between-subjects (2-level) factor and time (before or after delivery) as 2-level within-subjects factor. Post hoc tests included paired t-tests (factor time), or ANOVA (factor group). IRLS scores were compared in repeated measures ANOVA with 3-level factor time (pregnancy, 2 weeks postpartum, and 12 weeks postpartum). Correlations were performed using nonparametric Spearman correlations.
RESULTS
Table 1 shows the RLS and sleep parameters for both groups during pregnancy and postpartum, when all women (except one per group) were still lactating.
Table 1.
Questionnaire Data and Objective Sleep Parameters from Polysomnographic Recordings in Subjects With (n = 8) and Without (n = 7) RLS During the Last Trimester of Pregnancy and 3 Months Postpartum
| Pregnancy |
Postpartum |
||||||
|---|---|---|---|---|---|---|---|
| Controls | RLS | Controls | RLS | Factor RLS PF | Factor Time PF | Effect of Interaction PF | |
| RLS symptoms (IRLS score) | — | 19.78 ± 6,26 | — | 7.0 ± 6.58 | — | P < 0.001a | — |
| Sleep quality (PSQI) | 7.67 ± 3.34 | 9.14 ± 2.53 | 5.50 ± 2.12 | 4.71 ± 2.26 | NS | P11.84 < 0.01 | NS |
| PLM-Index Time in Bed (#/h) | 1.96 ± 1.32 | 42.14 ± 42.79 | 1.62 ± 1.84 | 15.44 ± 20.24 | P5.40 < 0.05 | P6.81 < 0.05 | P6.48 < 0.05 |
| PLM-Index Wake (#/h) | 5.44 ± 5.51 | 57.91 ± 51.28 | 12.60 ± 21.10 | 25.29 ± 27.93 | P4.80 < 0.05 | NS | P7.29 < 0.05 |
| PLM-Index S1 (#/h) | 1.40 ± 1.79 | 48.61 ± 36.28 | 2.96 ± 5.05 | 14.01 ± 19.40 | P8.43 < 0.05 | P11.79 < 0.005 | P14.11 < 0.005 |
| PLM-Index S2 (#/h) | 1.16 ± 1.50 | 43.86 ± 50.92 | 1.60 ± 2.51 | 18.36 ± 27.34 | NS | P4.77 < 0.05 | P5.12 < 0.05 |
| PLM-Index S3 (#/h) | 4.03 ± 8.66 | 35.61 ± 51.97 | 1.01 ± 1.76 | 6.23 ± 11.55 | NS | NS | NS |
| PLM-Index S4 (#/h) | 2.0 ± 4.78 | 26.14 ± 48.57 | 0.54 ± 0.94 | 1.29 ± 3.64 | NS | NS | NS |
| PLM-Index REM (#/h) | 0.64 ± 1.70 | 8.32 ± 10.26 | 0.0 ± 0.0 | 7.71 ± 15.07 | NS | NS | NS |
| Time In Bed (TIB, min) | 476.43 ± 8.97 | 482.00 ± 1.20 | 477.43 ± 10.82 | 480.81 ± 1.10 | NS | NS | NS |
| Total Sleep Time (TST, min) | 373.93 ± 62.17 | 394.44 ± 40.82 | 423.43 ± 33.37 | 396.31 ± 49.34 | NS | NS | NS |
| Sleep efficiency (SEI, %) | 86.20 ± 7.47 | 85.13 ± 6.52 | 93.31 ± 4.29 | 84.88 ± 8.75 | P6.18 < 0.05 | NS | NS |
| Sleep latency (S2, min) | 17.43 ± 12.73 | 17.12 ± 13.78 | 22.36 ± 16.91 | 18.19 ± 16.99 | NS | NS | NS |
| REM latency (min) | 78.10 ± 35.23 | 95.00 ± 44.55 | 67.86 ± 8.20 | 71.50 ± 37.14 | NS | P4.93 < 0.05 | NS |
| Wake (% sleep period time) | 13.04 ± 7.54 | 13.61 ± 5.76 | 6.40 ± 4.27 | 13.63 ± 8.85 | NS | NS | NS |
| S1 (% SPT) | 7.73 ± 2.73 | 6.23 ± 2.70 | 4.80 ± 1.27 | 5.49 ± 2.49 | NS | P7.89 < 0.05 | NS |
| S2 (% SPT) | 48.48 ± 6.43 | 51.53 ± 8.22 | 47.02 ± 4.55 | 46.71 ± 4.81 | NS | NS | NS |
| S3/S4 (% SPT) | 13.91 ± 4.98 | 13.33 ± 4.81 | 17.49 ± 5.25 | 15.61 ± 5.51 | NS | NS | NS |
| REM sleep (% SPT) | 15.67 ± 2.89 | 13.66 ± 3.46 | 23.40 ± 5.02 | 16.68 ± 5.61 | P6.67 < 0.05 | P12.30 < 0.005 | NS |
Mean ± SD; results from repeated measures ANOVA with factors RLS and time (pre/post partum); df (1,13) except for PSQI scores (df: 1,11);
paired T-test (n = 9), T = 6.24; NS: not significant.
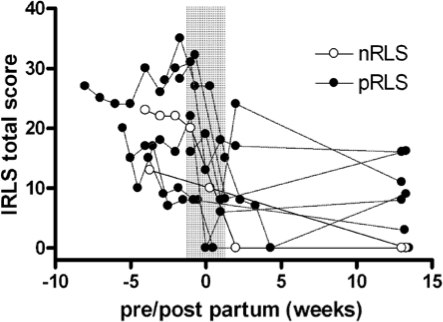
In RLS subjects the IRLS score decreased significantly during the first 2 weeks after delivery (Figure 1), and remained on a low level 12 weeks post partum (F2,16 = 36.09, P < 0.001).
Figure 1.
IRLS scores in subjects with RLS in late pregnancy and post partum, indicating a drastic decrease in subjective symptoms around delivery (nRLS-new-onset RLS; pRLS-pre-existing RLS).
Women with RLS had more periodic limb movements before (F1,13 = 6.11, P < 0.05) and after delivery (F1,13 =3.21, P < 0.1) than those without RLS (Table 1). Group effect was significant for the PLMs in wake (F1,13 = 4.80, P < 0.05) and in sleep stage 1 (F1,13 = 8.43, P < 0.05). During pregnancy, PLMs were more frequent in the RLS group throughout all sleep stages, reaching statistical significance in wake (F1,13 = 7.19, P < 0.05), sleep stage 1 (F1,13 = 11.72, P = 0.005), and sleep stage 2 (F1,13 = 4.87, P < 0.05). PLMs decreased significantly postpartum in subjects with RLS (T = 2.78, P < 0.05), while they stayed low in women without RLS.
Sleep quality increased following birth (PSQI scores, Table 1), but did not show any difference between the 2 groups. Similarly, during pregnancy, polysomnographic sleep parameters did not differ between groups (parameters are shown in Table 1). Postpartum, percentage of S1 sleep decreased, REM latency decreased, and REM sleep percentage increased in both groups, although REM sleep increase was much more pronounced in women without RLS (F1,13 =5.91, P < 0.05). Sleep efficiency increased only in women without RLS while in women suffering from RLS it remained unchanged (F1,13 = 5.35, P < 0.05).
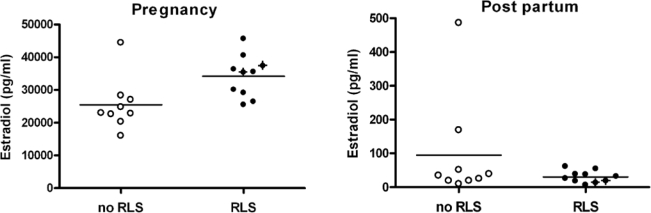
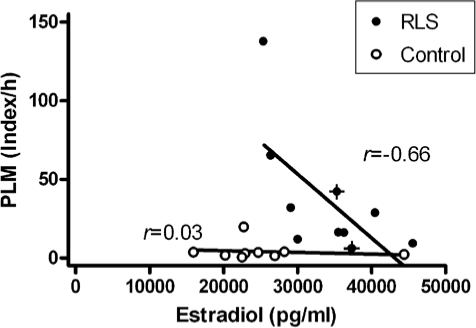
Blood parameters are given in Table 2. Estradiol levels were higher during pregnancy in both groups, but more pronounced in women with RLS during pregnancy (34,211 ± 6,397 pg/mL vs. 25,475 ± 7,990 pg/mL; F1,17 = 6.99, P < 0.05), irrespective of new-onset or preexisting RLS symptoms (see Figure 2). However, higher estradiol levels in the RLS group did not coincide with higher levels of PLM activity (Figure 3), nor did higher IRLS scores (r = −0.336, NS). PLM activity in the RLS patients even correlated negatively with estradiol levels (r = −0.66, P < 0.05), whereas no such relationship could be detected in the control group (r = 0.03, NS).
Table 2.
Hormonal and Hematologic Values in Subjects With (n = 10) and Without (n = 9) RLS During the Last Trimester of Pregnancy and 3 Months Postpartum
| Pregnancy |
Postpartum |
||||||
|---|---|---|---|---|---|---|---|
| Controls | RLS | Controls | RLS | Factor RLS PF | Factor Time PF | Effect of Interaction PF | |
| Estradiol (pg/mL) | 25,475.44 ± 7,990.48 | 34,211.0 ± 6,397.36 | 94.92 ± 154.34 | 30.73 ± 17.78 | P6.93 < 0.05 | P323.07 < 0.001 | P7.05 < 0.05 |
| Testosterone (nmol/L) | 2.56 ± 1.17 | 2.39 ± 0.95 | 1.12 ± 0.38 | 1.25 ± 0.78 | NS | P28.06 < 0.001 | NS |
| Progesterone (ng/mL) | 322.43 ± 105.57 | 302.93 ± 59.28 | 0.55 ± 0.27 | 2.0 ± 4.40 | NS | P224.63 < 0.001 | NS |
| Prolactin (ng/mL) | 211.78 ± 65.81 | 262.20 ± 55.71 | 50.76 ± 29.03 | 70.30 ± 59.50 | NS | P119.19 < 0.001 | NS |
| Cortisol (μg/L) | 391.0 ± 24.37 | 410.0 ± 74.47 | 218.0 ± 52.42 | 193.7 ± 47.80 | NS | P122.22 < 0.001 | NS |
| Iron (μg/dL) | 154.71 ± 124.96 | 62.20 ± 42.71 | 110.86 ± 28.64 | 101.4 ± 42.86 | NS | NS | NS |
| Ferritin (ng/mL) | 20.29 ± 14.30 | 13.33 ± 10.07 | 45.14 ± 29.81 | 27.67 ± 25.72 | NS | P9.34 < 0.01 | NS |
| Magnesium (mmol/L) | 0.71 ± 0.06 | 0.73 ± 0.07 | 0.78 ± 0.07 | 0.80 ± 0.08 | NS | P19.02 < 0.001 | NS |
| Hemoglobin (g/dL) | 12.44 ± 1.04 | 11.66 ± 1.04 | 13.50 ± 1.15 | 12.88 ± 1.08 | NS | P32.10 < 0.001 | NS |
Mean ± SD; results from repeated measures ANOVA with factors RLS and time (pre/post partum); df (1,17) except for progesterone and iron (df: 1,15), and ferritin (df: 1,14); NS: not significant.
Figure 2.
Significantly higher levels of estradiol are seen in RLS patients than controls during pregnancy (P < 0.05); this difference disappears after delivery (P = 0.208). New-onset RLS patients are highlighted with crosses.
Figure 3.
In RLS patients during pregnancy, higher estradiol levels decrease with higher indices of periodic leg movements; no relationship is found in control patients during pregnancy (new-onset RLS patients are highlighted with crosses).
Concentrations of prolactin, progesterone, testosterone, FSH, LH, iron, ferritin, magnesium and hemoglobin changed significantly after birth, but did not differ between the groups.
DISCUSSION
This is the first study to longitudinally investigate symptoms, subjective sleep quality, polysomnographic sleep parameters, PLM, and hormone levels in pregnant women with and without RLS. We found increased PLM indices during pregnancy and elevated estradiol levels in women with RLS compared to controls, but only minor changes in subjective sleep quality and polysomnographic sleep parameters. The most striking finding was that pregnancy-related RLS was associated with increased estradiol levels, whereas other hormonal parameters did not differentiate between groups. This effect was present in women with both preexisting and new-onset RLS, supporting previous reports of high RLS incidence in the last trimester of pregnancy when estradiol is maximally elevated.10 Hence, the present study lends empirical support to the idea that the increased prevalence of the RLS in women in general and during pregnancy in particular is related to actions of specific hormones.1–4, 6, 7, 9, 10, 13
There is strong evidence that the dopaminergic system plays a crucial pathophysiological role in RLS and PLM, supported mainly by the prominent positive effects of dopaminergic drugs20–22 and some imaging studies.23–25 Therefore, estradiol could affect RLS symptoms through this neurotransmitter system. Some studies have shown that estradiol attenuates the uptake of both endogenous and exogenous dopamine in the striatum and nucleus accumbens.26–31 Conversely, a direct antidopaminergic effect of estradiol was shown to be responsible for an increased running wheel activity in mice.32 Hence, effects of estradiol on the dopaminergic system are inconclusive and, as yet unknown factors might be causing both the elevated hormonal levels and symptoms in parallel, await further study. Reduced hemoglobin, but not elevated estradiol levels, were reported in earlier stages during pregnancy.33 Surprisingly, estradiol levels correlated negatively with PLM activity in the RLS group (Figure 3); but so far causative mechanisms are unknown. One cannot exclude the possibility that RLS symptoms induce the elevated estradiol levels, as observed in our data. However, estrogens have been clearly shown to influence nigrostriatal dopaminergic neurons,34 and estrogen effects on dopaminergic system are also targeted in Parkinson disease.35 Hence, it appears reasonable to assume effects of estradiol levels on RLS symptomatology, even if there is no consensus on the mechanisms, specific sites, or receptors where estradiol influences the synthesis, transport, or metabolism of dopamine in neurons and astrocytes.34,35 A potential explanation for the negative correlation between RLS symptoms and elevated estradiol levels is that the estrogen receptor system displays a biphasic dose-response relationship, showing the highest responses at intermediate estrogen levels.36 Of course, the small number of subjects limited our ability to draw conclusions and may have also limited our ability to assess all factors influencing RLS symptoms. Nevertheless, the effects in late pregnancy appear astonishingly stable, without indicating a difference between new-onset or preexisting RLS symptomatology. Thus, further investigations on the causative involvement of estrogens in triggering RLS and PLM are needed.
Prolactin is also strongly elevated during pregnancy and has been suggested to cause RLS symptoms13,37,38 by simultaneous decreased action of dopamine at the level of the nigrostriatal system or A11 dopaminergic neurons (localized mainly in the midbrain near the hypothalamus with projections into the cortex, the limbic system, and the spinal cord).37 The circadian rhythm of prolactin corresponds to the characteristic course of RLS symptoms over a 24-hour period, supporting this idea. However, consistent with the findings of Wetter et al.38 who did not find elevated prolactin levels in young men with and without RLS, we could not detect significant differences of prolactin levels between patients and controls during pregnancy. In both groups prolactin was elevated to a comparable extent during pregnancy and normalized after birth. Hence, so far there is no positive empirical evidence supporting a causative role of prolactin in RLS or PLMS. Apart from estradiol or prolactin, elevated levels of progesterone might be hypothesized to be involved in the generation of RLS symptoms, but were also shown to have benzodiazepine-like sleep-stabilizing effects.39 However, we did not find a significant difference of progesterone levels between women affected with RLS symptomatology and healthy controls in the present population.
Iron and iron storage indicator deficiencies often occur during pregnancy, and have been suspected to provoke RLS symptomatology.20,40,41 In agreement with previous studies, our results do not confirm a significant difference in serum iron or ferritin levels between RLS and control subjects, although both levels usually were decreased in patients. Substitution of iron or magnesium can be excluded as possible confound in the present data, as an equal and limited number of subjects was substituted in each group.
Our study is the first to longitudinally quantify symptoms, polysomnographic sleep, and PLM parameters in pregnant RLS patients and controls. The mean IRLS total scores were in the mild to moderate range during pregnancy and quite low thereafter. We showed for the first time that RLS during pregnancy is associated to increased amounts of PLM similar to idiopathic and other secondary forms of RLS, and that PLM indices decrease along with IRLS scores indices after birth.
Surprisingly, there were only minor differences between RLS patients and controls in polysomnographic parameters. Sleep efficiency and REM sleep percentage were lower in patients; but this was a reflection of steep increases after birth in controls and only slightly lower sleep efficiency in patients during pregnancy. Similarly, after birth, REM sleep latency, and stage 1 sleep decreased, and subjective sleep quality (PSQI) improved; there was no significant difference between patients and controls. One reason for the small size of these differences is that we could select only RLS patients who did not need pharmacological treatment and were able to complete polysomnographic recordings. These data strongly suggest that estrogens play an important role in RLS and PLM during pregnancy.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We thank Irene Gunst and Gabi Kohl for valuable support. This study was supported by a grant from the European Union (QLK6-CT-2000-00499).
REFERENCES
- 1.Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res. 2002;53:547–54. doi: 10.1016/s0022-3999(02)00443-9. [DOI] [PubMed] [Google Scholar]
- 2.Rothdach AJ, Trenkwalder C, Haberstock J, Keil U, Berger K. Prevalence and risk factors of RLS in an elderly population: the MEMO study. Memory and morbidity in Augsburg elderly. Neurology. 2000;54:1064–8. doi: 10.1212/wnl.54.5.1064. [DOI] [PubMed] [Google Scholar]
- 3.Tison F, Crochard A, Leger D, Bouee S, Lainey E, El Hasnaoui A. Epidemiology of restless legs syndrome in French adults: a nationwide survey: the INSTANT Study. Neurology. 2005;65:239–46. doi: 10.1212/01.wnl.0000168910.48309.4a. [DOI] [PubMed] [Google Scholar]
- 4.Berger K, Luedemann J, Trenkwalder C, John U, Kessler C. Sex and the risk of restless legs syndrome in the general population. Arch Intern Med. 2004;164:196–202. doi: 10.1001/archinte.164.2.196. [DOI] [PubMed] [Google Scholar]
- 5.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165:1286–92. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 6.Lee KA, Zaffke ME, Baratte-Beebe K. Restless legs syndrome and sleep disturbance during pregnancy: the role of folate and iron. J Womens Health Gend Based Med. 2001;10:335–41. doi: 10.1089/152460901750269652. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki K, Ohida T, Sone T, et al. The prevalence of restless legs syndrome among pregnant women in Japan and the relationship between restless legs syndrome and sleep problems. Sleep. 2003;26:673–7. doi: 10.1093/sleep/26.6.673. [DOI] [PubMed] [Google Scholar]
- 8.Sbrocca L. Ekbom’s “restless legs” syndrome or “paresthetic agitation” of the lower extremities in pregnancy. Minerva Ginecol. 1962;14:675–80. [PubMed] [Google Scholar]
- 9.McParland P, Pearce JM. Restless legs syndrome in pregnancy. Case reports. Clin Exp Obstet Gynecol. 1990;17:5–6. [PubMed] [Google Scholar]
- 10.Manconi M, Govoni V, De Vito A, et al. Restless legs syndrome and pregnancy. Neurology. 2004;63:1065–9. doi: 10.1212/01.wnl.0000138427.83574.a6. [DOI] [PubMed] [Google Scholar]
- 11.Walters AS. Toward a better definition of the restless legs syndrome. The International Restless Legs Syndrome Study Group. Mov Disord. 1995;10:634–42. doi: 10.1002/mds.870100517. [DOI] [PubMed] [Google Scholar]
- 12.Winkelman JW, Finn L, Young T. Prevalence and correlates of restless legs syndrome symptoms in the Wisconsin Sleep Cohort. Sleep Med. 2006;7:545–52. doi: 10.1016/j.sleep.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Manconi M, Govoni V, De Vito A, et al. Pregnancy as a risk factor for restless legs syndrome. Sleep Med. 2004;5:305–8. doi: 10.1016/j.sleep.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Pien GW, Schwab RJ. Sleep disorders during pregnancy. Sleep. 2004;27:1405–17. doi: 10.1093/sleep/27.7.1405. [DOI] [PubMed] [Google Scholar]
- 15.Brunner DP, Munch M, Biedermann K, Huch R, Huch A, Borbely AA. Changes in sleep and sleep electroencephalogram during pregnancy. Sleep. 1994;17:576–82. doi: 10.1093/sleep/17.7.576. [DOI] [PubMed] [Google Scholar]
- 16.Driver HS, Shapiro CM. A longitudinal study of sleep stages in young women during pregnancy and postpartum. Sleep. 1992;15:449–53. doi: 10.1093/sleep/15.5.449. [DOI] [PubMed] [Google Scholar]
- 17.Hertz G, Fast A, Feinsilver SH, Albertario CL, Schulman H, Fein AM. Sleep in normal late pregnancy. Sleep. 1992;15:246–51. doi: 10.1093/sleep/15.3.246. [DOI] [PubMed] [Google Scholar]
- 18.Lee KA, Zaffke ME, McEnany G. Parity and sleep patterns during and after pregnancy. Obstet Gynecol. 2000;95:14–8. doi: 10.1016/s0029-7844(99)00486-x. [DOI] [PubMed] [Google Scholar]
- 19.Wetter TC, Dirlich G, Streit J, Trenkwalder C, Schuld A, Pollmächer T. An automatic method for scoring leg movements in polygraphic sleep recordings and its validity in comparison to visual scoring. Sleep. 2004;27:324–8. doi: 10.1093/sleep/27.2.324. [DOI] [PubMed] [Google Scholar]
- 20.Allen R. Dopamine and iron in the pathophysiology of restless legs syndrome (RLS) Sleep Med. 2004;5:385–91. doi: 10.1016/j.sleep.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Vignatelli L, Billiard M, Clarenbach P, et al. EFNS guidelines on management of restless legs syndrome and periodic limb movement disorder in sleep. Eur J Neurol. 2006;13:1049–65. doi: 10.1111/j.1468-1331.2006.01410.x. [DOI] [PubMed] [Google Scholar]
- 22.Trenkwalder C, Hundemer HP, Lledo A, et al. Efficacy of pergolide in treatment of restless legs syndrome: the PEARLS Study. Neurology. 2004;62:1391–7. doi: 10.1212/01.wnl.0000124465.20878.84. [DOI] [PubMed] [Google Scholar]
- 23.Eisensehr I, Wetter TC, Linke R, et al. Normal IPT and IBZM SPECT in drug-naive and levodopa-treated idiopathic restless legs syndrome. Neurology. 2001;57:1307–9. doi: 10.1212/wnl.57.7.1307. [DOI] [PubMed] [Google Scholar]
- 24.Michaud M, Soucy JP, Chabli A, Lavigne G, Montplaisir J. SPECT imaging of striatal pre- and postsynaptic dopaminergic status in restless legs syndrome with periodic leg movements in sleep. J Neurol. 2002;249:164–70. doi: 10.1007/pl00007859. [DOI] [PubMed] [Google Scholar]
- 25.Turjanski N, Lees AJ, Brooks DJ. Striatal dopaminergic function in restless legs syndrome: 18F-dopa and 11C-raclopride PET studies. Neurology. 1999;52:932–7. doi: 10.1212/wnl.52.5.932. [DOI] [PubMed] [Google Scholar]
- 26.Callier S, Morissette M, Grandbois M, Di Paolo T. Stereospecific prevention by 17beta-estradiol of MPTP-induced dopamine depletion in mice. Synapse. 2000;37:245–51. doi: 10.1002/1098-2396(20000915)37:4<245::AID-SYN1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Disshorn KA, Dluzen DE. Use of in vitro superfusion to assess the dynamics of striatal dopamine clearance: influence of oestrogen. Brain Res. 1999;842:399–407. doi: 10.1016/s0006-8993(99)01863-6. [DOI] [PubMed] [Google Scholar]
- 28.Myers RE, Anderson LI, Dluzen DE. Estrogen, but not testosterone, attenuates methamphetamine-evoked dopamine output from superfused striatal tissue of female and male mice. Neuropharmacology. 2003;44:624–32. doi: 10.1016/s0028-3908(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 29.Thompson TL, Certain ME. Estrogen mediated inhibition of dopamine transport in the striatum: regulation by G alpha i/o. Eur J Pharmacol. 2005;511:121–6. doi: 10.1016/j.ejphar.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Thompson TL, Moore CC, Smith B. Estrogen priming modulates autoreceptor-mediated potentiation of dopamine uptake. Eur J Pharmacol. 2000;401:357–63. doi: 10.1016/s0014-2999(00)00432-5. [DOI] [PubMed] [Google Scholar]
- 31.Dluzen DE. Unconventional effects of estrogen uncovered. Trends Pharmacol Sci. 2005;26:485–7. doi: 10.1016/j.tips.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW. Estrogen increases locomotor activity in mice through estrogen receptor alpha: specificity for the type of activity. Endocrinology. 2003;144:230–39. doi: 10.1210/en.2002-220519. [DOI] [PubMed] [Google Scholar]
- 33.Tunc T, Karadag YS, Dogulu F, Inan LE. Predisposing factors of restless legs syndrome in pregnancy. Mov Disord. 2007;22:627–31. doi: 10.1002/mds.21291. [DOI] [PubMed] [Google Scholar]
- 34.Kipp M, Karakaya S, Pawlak J, Araujo-Wright G, Arnold S, Beyer C. Estrogen and the development and protection of nigrostriatal dopaminergic neurons: concerted action of a multitude of signals, protective molecules, and growth factors. Front Neuroendocrinol. 2006;27:376–90. doi: 10.1016/j.yfrne.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Ragonese P, D’Amelio M, Savettieri G. Implications for estrogens in Parkinson’s disease: an epidemiological approach. Ann N Y Acad Sci. 2006;1089:373–82. doi: 10.1196/annals.1386.004. [DOI] [PubMed] [Google Scholar]
- 36.Calabrese EJ, Baldwin LA. Hormesis: the dose-response revolution. Annu Rev Pharmacol Toxicol. 2003;43:175–97. doi: 10.1146/annurev.pharmtox.43.100901.140223. [DOI] [PubMed] [Google Scholar]
- 37.Trenkwalder C, Paulus W, Walters AS. The restless legs syndrome. Lancet Neurol. 2005;4:465–75. doi: 10.1016/S1474-4422(05)70139-3. [DOI] [PubMed] [Google Scholar]
- 38.Wetter TC, Collado-Seidel V, Oertel H, Uhr M, Yassouridis A, Trenkwalder C. Endocrine rhythms in patients with restless legs syndrome. J Neurol. 2002;249:146–51. doi: 10.1007/pl00007857. [DOI] [PubMed] [Google Scholar]
- 39.Lancel M, Faulhaber J, Schiffelholz T, et al. Allopregnanolone affects sleep in a benzodiazepine-like fashion. J Pharmacol Exp Ther. 1997;282:1213–8. [PubMed] [Google Scholar]
- Ghorayeb I, Bioulac B, Scribans C, Tison F. Perceived severity of restless legs syndrome across the female life cycle. Sleep Med. 2007 doi: 10.1016/j.sleep.2007.07.018. epub:doi:10.1016/j.sleep.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 41.Botez MI, Lambert B. Folate deficiency and restless-legs syndrome in pregnancy. N Engl J Med. 1977;297:670. doi: 10.1056/NEJM197709222971220. [DOI] [PubMed] [Google Scholar]