Abstract
Study Objectives:
To establish the direction and etiology of longitudinal associations between sleep problems and depression symptoms in children.
Design:
Data on twins aged 8 and 10 years were obtained. At assessments, parents completed the Child Sleep Habits Questionnaire, and twins completed the Children's Depression Inventory.
Setting:
Participants were mainly interviewed at the Institute of Psychiatry, London.
Patients or Participants:
Three hundred twin pairs initially enrolled in the study.
Interventions:
N/A.
Measurements and Results:
A genetically informative cross-lagged model examined links between sleep and depression. Sleep problems at age 8 predicted depression at age 10 (partial regression coefficient [95% confidence intervals] = 0.10 [0.01-0.18]). The converse was not found. Stability of sleep problems across time was mainly due to genes (46% of the genetic influence on sleep at 10 was due to the same genetic influence on sleep aged 8). Stability of depression was mainly due to nonshared environmental influences (19% of the nonshared environmental influence on depression at 10 was due to the same nonshared environmental influence on depression at age 8). The cross-lagged association between sleep problems at 8 and depression at 10 years was largely due to genes, although this finding was nonsignificant.
Conclusions:
This study adds to our understanding of the temporal precedence of sleep problems and depression and the risks underlying their associations. There are implications regarding the value of specifying genes linked to sleep problems and potential opportunities for informing early intervention strategies in high-risk groups at key points in the progression to developing more serious problems.
Citation:
Gregory AM; Rijsdijk FV; Lau JYF; Dahl RE; Eley TC. The direction of longitudinal associations between sleep problems and depression symptoms: a study of twins aged 8 and 10 years. SLEEP 2009;32(2):189–199.
Keywords: Depression, genetics, pediatrics, sleep, twins
SLEEP PROBLEMS AND DEPRESSION ARE INTERTWINED. FOR EXAMPLE, IN YOUTH, SLEEP PROBLEMS HAVE BEEN FOUND TO PREDICT THE SUBSEQUENT occurrence of depression symptoms and can therefore be considered risk indicators of these later difficulties1–4; and there is also some support for the converse association that depression predicts later sleep problems.5 Understanding longitudinal links between sleep problems and symptoms of depression is essential, as such information is potentially useful for informing early intervention and/or prevention strategies. Indeed, knowing why a child with sleep problems is at increased risk for developing depression can illuminate approaches for intervening to stop the progression to more serious and enduring problems. Despite the importance of understanding longitudinal associations between sleep problems and symptoms of depression, such knowledge is limited. For this reason, this study examines the order of effects of sleep problems and symptoms of depression and disentangles genetic and environmental influences on the longitudinal associations between sleep problems and depression in a sample of twins in middle-childhood.
Sleep and Depression: Longitudinal Associations
Links between sleep problems and depression have been widely established within adulthood.6–9 However, in contrast, only recently has there been interest in the longitudinal associations between sleep problems in childhood or adolescence and later depression. Regardless, there is now mounting evidence that sleep characteristics (eg, regularity and activity during sleep) and sleep problems (assessed as insomnia or symptoms of different types of sleep problems) examined early in life are risk indicators for later depression symptoms,1–4 although not all studies have found significant associations.10–12 There is less support for the converse association, that depressive symptoms in young people predict later sleep problems,1–2 although some studies have reported such links.5 Focusing research in childhood is advantageous because there is mounting evidence that a range of difficulties, including depression, typically first appear early in life.13 This suggests that, to fully understand the etiology of depression and associated problems, focusing research early in life is essential.
Sleep and Depression: Twin Studies
Many reasons have been proposed for the complex associations between sleep problems and depression. These explanations focus on abnormalities in endocrine and monoamine functioning as well as atypical circadian rhythms and interactions between circadian and sleep-wave–dependent processes.14 One area of research that has not been utilized with regard to understanding the longitudinal associations between sleep and depression is quantitative genetics. This is particularly noteworthy because the processes thought to underlie the associations between sleep and depression reflect abnormalities that are known to be influenced by genetic variation (e.g. for a discussion of genetic influences on circadian rythms see15). Twin studies allow estimations of the magnitude of genetic and environmental influences on single traits and associations between traits.16 Two relevant twin studies have examined concurrent associations between sleep problems and depression in youth. First, a study of preschool-aged children revealed that sleep problems and depression shared little genetic relatedness.17 Second, we previously reported (using data collected on this current twin sample aged 8) that the links between sleep problems and depression were largely influenced by genetic factors.18 Differences between these 2 sets of results may reflect developmental changes in the etiology of the association (eg, genes that have little effect on sleep and depression early in life starting to influence these traits later on)—or the different measures used to examine symptoms of sleep and depression. To date, twin data have not been used to examine longitudinal links between sleep problems and depression. However, researchers have examined longitudinal links within depression, with studies reporting that environmental influences making family members alike account for much of the stability in symptoms of depression within childhood19; whereas both genetic and environmental influences making family members differ appear to be important in the stability of symptoms of depression within adolescence,20 and between adolescence and young adulthood.21 In contrast with what is known about the stability of symptoms of depression, little is known about the magnitude of genetic and environmental influences with regard to the stability of sleep problems.
The absence of information about longitudinal links between sleep and depression is an important omission to our understanding of these associations. Hence we examined the longitudinal associations between these phenotypes using a genetically informative cross-lagged model that was able to estimate longitudinal associations between sleep and depression while controlling for preexisting links between these traits. Accounting for preexisting associations between sleep problems and depression is essential. If not considered, a longitudinal association between sleep problems at 8 years and depression at 10 years could simply reflect the known stability of sleep patterns and sleep problems over time1,22 and a concurrent association between sleep problems and depression at 10 years of age. Knowledge concerning the stability of individual symptoms over time and concurrent associations between traits is therefore informative when examining longitudinal associations between traits. Cross-lagged models on genetically informative data are additionally able to decompose associations into their genetic and environmental components.23 Indeed, use of a cross-lagged model of this type has provided a unique understanding of longitudinal links between various phenotypes—such as an analysis of parental negativity and antisocial behavior that found that the association was bidirectional and that genetically influenced antisocial behavior may evoke parental negativity toward children.24
Hypotheses and Research Questions
We used a genetically informative cross-lagged model to examine 2 sets of questions in a sample of twins aged 8 and 10 years. The first question concerns the longitudinal associations between sleep problems and depression. Based on previous research, we hypothesized that sleep difficulties predict symptoms of depression to a greater extent than depression symptoms predict later sleep problems. We also expected stability of sleep problems and depression symptoms over time (which would partly account for the longitudinal associations between the phenotypes). The second issue concerns the magnitude of genetic and environmental influences on these associations. Given the paucity of research examining longitudinal associations between sleep problems and depression in young twins, a specific pattern of genetic and environmental influences on the longitudinal associations was not hypothesized, and this remains an exploratory question. However, based on previous research, we did expect the stability of depressive symptoms over time to be due to both genetic and environmental influences.
METHOD
Participants
Participants are members of the Emotions, Cognitions, Heredity and Outcome (ECHO) study, which consists of 300 twin pairs selected from a larger ongoing study of twins born in England and Wales during 1994–1996, known as the Twins Early Development Study (TEDS).25,26 At Wave 1 of data collection, 300 eight-year-old twin pairs (mean = 8 years 6 months; range = 8 years 2 months to 8 years 11 months) were selected from TEDS. Of these 300 pairs, 247 scored highly on parent-reported anxiety at age 7 years, and 53 control twin pairs were selected to ensure full coverage of the full range of scores on test measures.18 Anxiety was the focus when selecting participants, as the current data were collected as part of a study investigating anxiety and links with other problems (eg. depression and sleep problems). The design maximizes power and ensures inclusion of children with high levels of emotional symptoms. However, to generalize results to an unselected population, a weight is included in all analyses (see below). Because data from 11 families were considered unusable at Wave 1 due to autistic spectrum disorders, severe receptive language impairments, and persistent attention problems in at least 1 of the twins, only 289 families were contacted at Wave 2, approximately 2 years later. Of these, 250 (87%) agreed to participate (mean age = 10 years 2 month; range 9 years 7 months to 10 years 10 months). Data collection for ECHO mainly took place at the Institute of Psychiatry (London, United Kingdom), with a small number of families visited in their homes. The ECHO study received ethical approval from the Maudsley Hospital Ethics Committee (London), and informed consent from parents was obtained by mail before data collection. The ECHO participants (43% boys) were mainly white (87%) and were from higher-than-average socioeconomic-status families.18
Procedures and Instruments
Zygosity
A parent-rated instrument was used to assign twin zygosity. This resulted in unambiguously identifying 95% of the twin pairs as monozygotic (MZ) or dizygotic (DZ). For the remaining 5%, DNA was collected from cheek swabs, and zygosity was assigned using highly polymorphic markers that yield an accuracy of 99.9%.27 The total ECHO sample comprises 100 MZ twin pairs, 199 DZ twin pairs, and 1 pair of twins for which zygosity is unclear and who refused to provide DNA; their data are therefore excluded from genetic analyses.
Anxiety-selection Variable
Anxiety was examined in the TEDS sample when the participants were aged 7 years using the parent-report Anxiety Related Behaviours Questionnaire (ARBQ).28 This is a 21-item scale reflecting commonly assessed anxiety-related behaviors in young children, including general distress (negative mood), separation anxiety, shyness/ inhibition, and fears. Each item was rated on a 3-point scale (0 = never; 1 = sometimes; 2 = often) and was taken from existing, reliable, and valid measures.29–33 The internal consistency (α) of this measure was 0.81. Twins with at least 1 member scoring in the top 15% of this measure were selected as high-anxiety pairs in the ECHO study, whereas control pairs both scored below this cutoff. The entire sample was analyzed as 1 group, and a weight was included to account for this selection process.
Child Sleep Habits Questionnaire
Parents reported on their 8-year-old children's sleep problems using the abbreviated version of the Child Sleep Habits Questionnaire (CSHQ).34 This version of the CSHQ consists of 33 items, including “child struggles at bedtime (cries, refuses to stay in bed, etc)” each rated on a 3-point scale (1 = rarely; 3 = usually). The CSHQ taps into 8 aspects of commonly reported childhood sleep problems: bedtime resistance, sleep-onset delay, sleep duration, sleep anxiety, night waking, parasomnias, sleep-disordered breathing, and daytime sleepiness. Parents reported on the most recent typical week. Where necessary, items (eg, “child goes to bed at the same time at night”) were recoded, so a higher score on this scale indicates greater sleep problems. The psychometric properties of this measure have been examined in both community and clinical populations of children aged 4 to 10 years.34 Results show adequate internal consistency in both populations (α = 0.68 for the community sample; α = 0.78 for the clinical sample) and acceptable test-retest reliability. Total scores were able to consistently differentiate the community and clinical sample. For the purposes of this study, we used a composite measure of sleep problems, as in our prior analyses.18 The reasons for this were 2-fold. First, the internal consistency of the total sleep problems score was good (0.78 at both 8 and 10 years), suggesting that items on the CSHQ tap into the same general construct. We therefore liken our analyses to those focusing upon general measures of “internalizing” or “externalizing” problems in other twin studies of developmental psychopathology.35,36 A second (and related point) concerned the distribution of the CSHQ subscales, some of which need to be considered as ordinal rather than continuous. Multivariate genetic analysis of ordinal variables involves multidimensional integration of the assumed multivariate normal distribution, which is computationally intensive when the number of dimensions are high and requires relatively large samples to produce reliable estimates.
Children's Depression Inventory
Depressive symptoms were examined by the Children's Depression Inventory (CDI),37 a 27-item self-report questionnaire that examines affective, cognitive, and behavioral signs of depression. For each item, the child must select 1 of 3 statements that best reflects how he or she has been feeling for the past few weeks. For example, the child can choose between the statements “I have fun in many things,” “I have fun in some things,” and “nothing is fun at all.” Each statement is coded 0 (least depressive) to 2 (most depressive), and a sum score of all the responses is made. The discriminant validity of the CDI has been examined in clinical samples of children (6-16 years), with results suggesting that, although the measure may lack sensitivity as a diagnostic tool, it is appropriate as a symptom inventory (as assessed in terms of accuracy of prediction of children with diagnoses of depression).38,39 One item concerning thoughts about suicide was removed from the questionnaire for ethical reasons (it was deemed inappropriate for 8-year-old children). Furthermore, as with our previous report,18 to avoid the potential problems of overlapping items, 2 items pertaining to trouble sleeping and tiredness were removed from the CDI. The internal consistency (α) of the remaining items was 0.82 at 8 years and 0.80 at 10 years.
Statistics
Weight
A weight that controlled for bias associated with ascertainment across waves (oversampling children with high anxiety scores in ECHO) was constructed for analyses. This used the ratio of the selection probability of high-symptom families to that of nonsymptom control families. Effectively, weighting involves assigning lower weights to individuals from overrepresented categories and higher weights to individuals from underrepresented categories in the sample relative to the population distribution.
Twin Modeling: General
As would be expected for data on developmental psychopathology, the data were slightly positively skewed. However, the skew was considered acceptable (ranging from 0.98 for depression at 8 years to 1.4 for sleep problems at 10 years), so the data were not transformed because of skew. However, as is standard in twin modeling, variables were age and sex regressed prior to modeling. Descriptive statistics and genetic models were run in MX,40 which is one of the most popular statistical programs designed to deal with genetically sensitive data. This software controls for nonindependence of data from family members and can incorporate sampling weights into descriptive and model-fitting analyses.
Twin studies compare within-pair similarity (examined in terms of correlations and covariances) for groups of MZ twins, who are genetically identical, and DZ twins who share, on average, half of their segregating genes.16 It is assumed that environmental influences are equally similar for MZ and DZ twins—so any increased similarity between MZ, as compared with DZ, twins must be explained by genetic factors. In addition to estimating additive genetic influences (A) on single traits and associations between traits, twin data can be used to disentangle shared environmental (C), and nonshared environmental (E) influences. Shared environmental influences make individuals within a family similar to one another and can therefore be estimated by calculating the similarity between identical twins that is not due to genetic influences. Nonshared environmental influences act to make family members different, and estimates also incorporate error. Nonshared environmental influence can therefore be estimated by examining the association between MZ twin pairs (if there is not a perfect correlation between MZ twins, we assume that nonshared environmental influence and/or measurement error are playing a role).
Cross-lagged Model: General
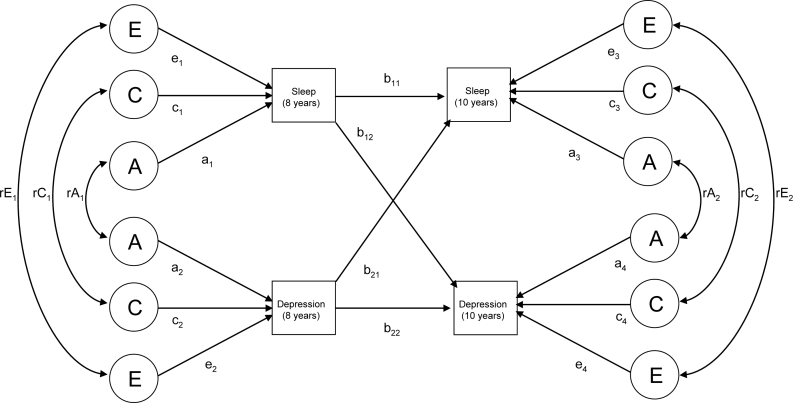
A cross-lagged model (see Figure 1a) was used to examine the concurrent and longitudinal associations between sleep problems and depression. Cross-lagged models have been previously used to examine longitudinal associations between different variables (parenting and externalizing problems) in twin samples.23,24 A cross-lagged model was used here to obtain information about (1) the magnitude of the total genetic and environmental influences on individual traits (univariate estimates); (2) the magnitude of genetic and environmental influences on the cooccurrence of sleep problems and depression within single time points (bivariate estimates); (3) the longitudinal associations between sleep and depression (the cross-lagged association); and (4) the genetic and environmental influences on univariate traits at 10 years as a function of those that also influenced sleep problems and depression at 8 years and those that were “new” at 10 years (decomposition of year-10 variables).
Figure 1a.
A path diagram to represent the cross-lagged model.
Cross-lagged Model: Univariate Estimates
The magnitude of genetic and shared and nonshared environmental influences on variance of sleep problems and depression at 8 years are calculated using path-tracing rules. For example, sleep problems at 8 years is influenced by genetic (a1 2), shared environmental (c1 2), and nonshared environmental (e1 2) pathways. When calculating the variance of traits at 10 years, influences on year-8 variables are also considered (please see section below, “Cross-lagged models: decomposition of sleep problems and depression at 10 years,” for further information).
Cross-lagged Model: Bivariate Estimates
The cross-lagged model was also used to estimate genetic and shared and nonshared environmental influences on the associations between sleep problems and depression at both 8 years and then again at 10 years. At 8 years, the bivariate association between sleep and depression was calculated as [a1 × rA1 × a2] + [c1 × rC1 × c2] + [e1 × rE1 × e2] = [the phenotypic correlation between sleep problems and depression at 8 years]. The rA1, rC1, and rE1 pathways estimate the extent to which factors influencing sleep problems are the same as those influencing depression within time points. At age 10 years, the bivariate association between sleep and depression was calculated by summing all of the legitimate paths between the 2 phenotypes. For example, the genetic influence was calculated by [b11 × a1 × rA1 × a2 × b22] + [b11 × genetic variance on sleep problems at 8 × b12] + [b21 × a2 × rA1× a1 × b12] + [b21 × genetic variance on depression at 8 × b22] + [a3 × rA2 × a4].
Cross-lagged Model: Cross-lagged Associations
The across-age associations are represented in Figure 1a as partial regression coefficients (paths b11, b22, b12, b21). The value of each pathway is independent of the other associations being addressed. For example, the value of pathway b12 is the contribution of sleep at 8 years to depression at 10 years (after accounting for the effects of depression at 8 years on depression at 10 years and the association between sleep and depression at 8 years).
Cross-lagged Model: Decomposition of Sleep Problems and Depression at 10 Years
From the estimated values of the different pathways of the model, genetic and environmental influences on sleep problems assessed at 10 years can be decomposed into (1) cross-age stability effects (genetic and environmental effects influencing sleep problems at 8 years; the genetic path is calculated by b11 2 × a1 2); (2) cross-lagged effects (genetic and environmental effects influencing depression at 8 years; the genetic path is calculated by b21 2 × a2 2); (3) common effects from age 8 (genetic and environmental influences common to sleep problems and depression at 8 years; the genetic path is calculated as 2[b11 × a1 × rA1 × a2 × b21]); and (4) specific effects from age 10 (the unique contributions to sleep problems at 10 years; the genetic path is calculated by a3 2). Genetic and environmental influences on depression at 10 years were decomposed into the same 4 main components. As with other complex analyses using data from the ECHO sample,41,42 sex differences were not examined due to the small sample size. Of note, preliminary analyses did not reveal any mean differences between boys and girls for any of the key variables reported here (ie, sleep at 8, depression at 8, sleep at 10, depression at 10).
Model-fit is derived by computing the difference between log-likelihood (−2LL) statistics of the raw data generated from the model and that from a saturated model, which estimates the maximum number of parameters to describe variances, covariances, and means of the variables. This model-fit index is distributed as χ2, and a lower value suggests a better fit.
RESULTS
Descriptive Statistics and Phenotypic Correlations
Raw means and standard deviations for the sleep problems and symptoms of depression at 8 and 10 years are presented in Table 1. This table also shows the phenotypic correlations between sleep problems and depression symptoms at 8 and 10 years. The magnitude of the association between sleep problems and depression symptoms was small and similar at both 8 and 10 years (r = 0.20, for information about interpreting the magnitude of correlations, see Cohen43). There was stability over time for both sleep problems (r = 0.69) and depression symptoms (r = 0.49). The across-trait and time correlations were small but significant for both sleep at 8 years and depression symptoms at 10 years (r = 0.20) and for depression symptoms at 8 and sleep at 10 years (r = 0.18). These correlations are noteworthy in that the magnitude of the across-trait across-time correlations is almost identical to the across-trait within-time correlations.
Table 1.
| Variables | Twin pairs, no. | Mean (SD) | Phenotypic correlations (95% confidence interval) |
|||
|---|---|---|---|---|---|---|
| Aged 8 |
Aged 10 |
|||||
| Sleep problems | Depression | Sleep problems | Depression | |||
| Aged 8 | ||||||
| Sleep problems | 279 | 44.05 (6.63) | 1 | |||
| Depression | 288 | 9.06 (6.36) | 0.20 (0.12–0.28) | 1 | ||
| Aged 10 | ||||||
| Sleep problems | 241 | 42.88 (6.08) | 0.69 (0.64–0.74) | 0.18 (0.09–0.26) | 1 | |
| Depression | 250 | 7.41 (5.31) | 0.20 (0.11–0.29) | 0.49 (0.42–0.55) | 0.20 (0.10–0.29) | 1 |
Note. Means and SDs are on raw (nonregressed) data to be maximally relevant to the reader. Scores on the sleep-problems scales used here can range from 33 to 99. Scores on the depression scales used here can range from 0 to 54. Correlations are on age- and sex-regressed variables. Estimates are derived from weighted analyses to account for the selected nature of the sample.
As assessed by Child Sleep Habits Questionnaire.
As assessed by the Children's Depression Inventory.
Twin Correlations
The twin correlations are presented in Table 2. The univariate (within-trait, within-time) twin correlations are presented along the diagonal axis. For sleep problems at both 8 and 10 years, the magnitude of the MZ twin correlation is much greater than the DZ twin correlation, suggesting strong genetic influence. For example, at 8 years of age, the MZ twin correlation is r = 0.73, whereas the DZ twin correlation is r = 0.40. For depression symptoms, the discrepancy between the MZ and DZ correlations is more modest (at 8 years MZ = 0.26, DZ = 0.22), suggesting weaker genetic influence but some shared environmental effects. The MZ correlations for depression symptoms are much lower than those for sleep problems, suggesting greater nonshared environmental influence on depression symptoms, as compared with sleep problems.
Table 2.
Multivariate Twin Correlations (with 95% Confidence Intervals) for Sleep Problems and Depression at Ages 8 and 10
| Twin number | Twin 1 |
||||
|---|---|---|---|---|---|
| MZ twins | Variable | Sleep at 8 | Depression at 8 | Sleep at 10 | Depression at 10 |
| Twin 2 | Sleep at 8 | 0.73 (0.63–0.80) | |||
| Depression at 8 | 0.25 (0.14–0.35) | 0.26 (0.07–0.43) | |||
| Sleep at 10 | 0.59 (0.51–0.66) | 0.23 (0.12–0.33) | 0.80 (0.72–0.85) | ||
| Depression at 10 | 0.23 (0.12–0.33) | 0.36 (0.23–0.47) | 0.17 (0.07–0.28) | 0.47 (0.33–0.59) | |
| DZ twins | Variable | Sleep at 8 | Depression at 8 | Sleep at 10 | Depression at 10 |
| Twin 2 | Sleep at 8 | 0.40 (0.27–0.51) | |||
| Depression at 8 | 0.14 (0.04–0.24) | 0.22 (0.09–0.35) | |||
| Sleep at 10 | 0.32 (0.20–0.43) | 0.12 (0.01–0.23) | 0.37 (0.21–0.50) | ||
| Depression at 10 | 0.21 (0.10–0.32) | 0.23 (0.12–0.34) | 0.18 (0.06–0.29) | 0.38 (0.23–0.51) | |
Note. All correlations come from age and sex regressed data. Estimates are derived from weighted analyses to account for the selected nature of the sample.
The within-time associations between sleep problems and depression symptoms are higher for MZ than DZ twins at 8 years (MZ = 0.25, DZ = 0.14), suggesting genetic influence on this association. At age 10 years, the MZ/ DZ correlations are more similar (MZ = 0.17, DZ = 0.18), suggesting that environmental factors are likely to play a larger role in explaining this association.
The within-trait across-time correlations (ie, sleep at age 8 and at age 10) are higher for MZ than DZ twins for both sleep problems (MZ = 0.59, DZ = 0.32) and depression symptoms (MZ = 0.36, DZ = 0.23), suggesting that genes influence the stability of symptoms over time.
Finally, MZ and DZ differences in the across-trait, across-time correlations suggest genetic influence on the association between depression symptoms at 8 and sleep at 10 (MZ = 0.23, DZ = 0.12) but less so on the association between sleep problems at 8 and depression symptoms at 10 (MZ = 0.23 and DZ = 0.21).
Cross-lagged Model: General
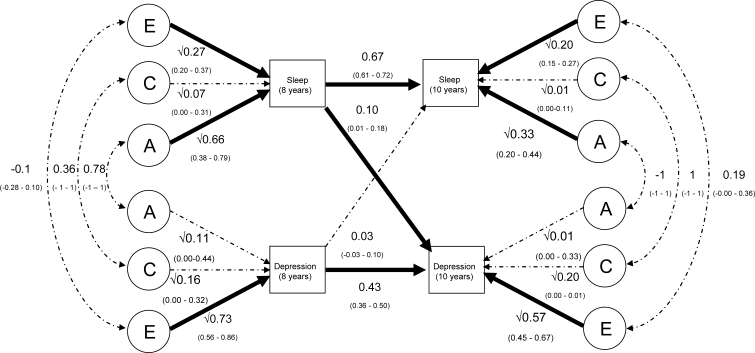
The estimated values of the paths in the cross-lagged model are presented in Figure 1b. This model fits the data significantly less well than does the saturated model (Δχ2 = 396.62, Δdf = 62, P < 0.001). Poor fits are commonly found using multivariate genetic models in general and the cross-lagged model in particular.23,24 This is due to the data not meeting the assumptions of multivariate normality and equal variances between MZ and DZ twins.
Figure 1b.
A path diagram presenting results from the cross-lagged model of the associations between sleep and depression at 8 and 10 years of age. Note. Significant pathways are in bold font. The association between variables are calculated by summing the values of legitimate paths between the variables. For example, the correlation between sleep problems and depression at 10 years (r = 0.20) is due to A = 0.04; C = 0.07; E = 0.09. The genetic path is calculated as [0.67 × √0.66 × 0.78 × √0.11 × 0.43] + [0.67 × 0.66 × 0.10] + [0.03 × √0.11 × 0.78× √0.66 × 0.10] + [0.03 × 0.11 × 0.43] + [√0.33 × −1 × √0.01]).
Cross-lagged Model: Univariate Estimates
From the cross-lagged model, it is possible to estimate total genetic and environmental influences on each variable using path-tracing rules. Genetic and shared and nonshared environmental influences on sleep problems and depression and their association at age 8 years have been reported previously.18 As reported previously, sleep problems at 8 were mainly influenced by genetic factors (estimated here at 66%) with smaller environmental influences (shared = 7%; nonshared = 27%). At age 10, a similar pattern of results emerged (see Table 3). Specifically, genetic factors accounted for 63% of the variability in sleep problems at 10 years (calculated by summing genetic influence shared with sleep at 8 [0.292], those shared with depression at 8 [0.000], those due to the association between sleep and depression at 8 [0.009], and new effects at 10 [0.332]), with smaller shared (4%) and nonshared (32%) environmental influences. Genetic influences on depression symptoms at 8 years were small (11%), with modest shared (16%) and large nonshared (73%) environmental influences (see Figure 1b). A similar pattern of results was reported at 10 years (see Table 3).
Table 3.
Genetic and Shared and Nonshared Environmental Influences (with 95% Confidence Intervals) on Sleep Problems and Depression at Age 10
| Genetic influences | Shared environmental influences | Nonshared environmental influences | |
|---|---|---|---|
| Sleep problems at 10 years | 0.633(0.47 – 0.73) | 0.043 (0.00 – 0.18) | 0.324(0.26 – 0.40) |
| ACE on sleep problems at 10 years due to | |||
| Sleep problems at 8 years (cross age-stability effects) | 0.292(0.17 – 0.39)[46%] | 0.032 (0.00 – 0.14)[74%] | 0.121(0.09 – 0.17)[37%] |
| Depression at 8 years (cross-lagged effects) | 0.000 (0.00 – 0.00)[0%] | 0.000 (0.00 – 0.00)[0%] | 0.001 (0.00 – 0.01)[0%] |
| Common effects at 8 years | 0.009 (−0.01 – 0.04)[3%] | 0.002 (0.00 – 0.02)[5%] | −0.002 (−0.01 – 0.00)[−1%] |
| Specific effects at 10 years | 0.332(0.20 – 0.43)[52%] | 0.009 (0.00 – 0.11)[21%] | 0.204(0.15 – 0.27)[63%] |
| Depression at 10 years | 0.057(0.00 – 0.39) | 0.238(0.00 – 0.35) | 0.706(0.58 – 0.81) |
| ACE on depression at 10 years due to | |||
| Depression at 8 years (cross-age stability effects) | 0.020 (0.00 – 0.09)[35%] | 0.031 (0.00 – 0.07)[13%] | 0.136(0.09 – 0.19)[19%] |
| Sleep problems at 8 years (cross-lagged effects) | 0.006 (0.00 – 0.02)[11%] | 0.001 (0.00 – 0.01)[0%] | 0.003 (0.00 – 0.01)[0%] |
| Common effects at 8 years | 0.017 (0.00 – 0.05)[30%] | 0.003 (−0.01– 0.02)[1%] | −0.004 (−0.01 – 0.00)[−1%] |
| Specific effects at 10 years | 0.013 (0.00 – 0.33)[23%] | 0.203 (0.00 – 0.31)[85%] | 0.571(0.45 – 0.67)[81%] |
Note: Percentages of total influences due to different influences are provided in brackets. Total genetic and shared and nonshared environmental contributions to the 10-year traits are presented in italics. These genetic and shared and nonshared environmental contributions are then broken down into (1) those uniquely contributed by influences on sleep problems at 8 years, (2) those uniquely contributed by influences on depression at 8 years, (3) those resulting from the preexisting association between sleep problems and depression at 8 years (common effects at 8 years), and (4) those specific to the age-10-year traits (specific effects at 10 years). For each difficulty, percentages within each column should add up to 100%, although not all do because of rounding. Significant pathways are in bold font.
Cross-lagged Model: Bivariate Estimates
The bivariate association between sleep problems and depression at 8 years (r = 0.20) is due to correlations between genetic influences (calculated by √0.66 × 0.78 × √0.11 = 0.21), shared environmental influences (0.04) and nonshared environmental influences (−0.04). The association between sleep problems and depression at 10 years was calculated by summing the values of all of the legitimate pathways between these 2 variables (the phenotypic correlation of 0.20 was due to A = 0.04; C = 0.07; E = 0.09; see footnote of Figure 1b for further information).
Cross-lagged Model: Cross-lagged Associations
Figure 1b presents the partial regression coefficients for the longitudinal associations between sleep and depression and within each measure over time. The values of the pathways control for associations between sleep problems and depression at 8 years of age. As would be expected, sleep problems at 8 predict sleep problems at 10 and depression symptoms at 8 predict depression symptoms at 10. The cross-lagged paths also suggest that sleep problems at 8 years significantly predict depression at 10 years—but the converse association (whereby depression at 8 predicts sleep at 10) is not significant. The influence of each pathway on variances at 10 years can be obtained by squaring the partial regression coefficients, demonstrating that the variance of sleep problems at 10 is influenced by sleep problems at 8 (44% [calculated by 0.6672]) and depression at 8 (0%). The variance of depression symptoms at 10 can be explained by depression at 8 (19%) and sleep problems at 8 (1%).
Cross-lagged Model: Decomposition of Sleep Problems and Depression at 10 Years
From the cross-lagged model, it is possible to calculate the proportions of the genetic, shared environmental, and nonshared environmental influences on the year-10 variables that are shared with the year-8 variables and those that represent “new” influences (age-specific variance) at 10 years of age. For example, from Table 3, it is possible to see that 46% of the genetic influences on sleep problems at 10 are due to genetic influences on sleep problems at 8 (genetic influence shared with sleep problems at 8 years/ total genetic influence on sleep problems at 10 years = 0.292 / 0.633). Most of the remaining 54% of the genetic influence on sleep problems at 10 is due to specific effects to the 10-year assessment (these account for 52% of the genetic influences on sleep problems at 10 years). The small and nonsignificant shared environmental influence on sleep problems at 10 was mainly due to cross-age stability effects. The nonshared environmental influence on sleep problems at 10 is largely due to nonshared environmental influences on sleep problems at 8 (37%, calculated as 0.121/0.324) and specific effects at 10 (63%).
Variance in depression at 10 was mainly explained by shared and nonshared environmental influences, although there was a small nonsignificant genetic influence. The nonsignificant genetic influence could be explained by genetic influences on depression at 8 years (35%), sleep problems at 8 years (11%), common effects at 8 years (30%), and specific effects at 10 years (23%). The shared environmental influence was mainly due to depression at 8 years (13%) and specific influence at 10 years (85%). Nonshared environmental influences on symptoms of depression at 10 years came mainly from depression at 8 years (19%) and specific effects at 10 years (81%).
DISCUSSION
The results of this study sit well with the hypotheses proposed in the introduction. First, sleep problems at 8 years predicted symptoms of depression at 10 years, whereas depression symptoms did not predict later sleep problems. This highlights a possible unidirectional association between the 2 phenotypes when considering sleep problems and depression as single categories of difficulties (although it is important to note that other studies have found that depression symptoms also predict later sleep problems). Although further research is needed to explore associations between specific types of sleep problems and depression, these results are consistent with the possibility that early treatment of these sleep problems might be protective against depression. Second, there was stability of both sleep problems and depression from ages 8 to 10 years, which partially accounted for the longitudinal association between phenotypes. Third, genetic and, to a lesser extent, nonshared environmental influences were most important in explaining the stability of sleep problems over the 2-year gap, whereas nonshared environment was the most robust influence on stability of depression symptoms. Finally, there was a complex pattern of influences on the longitudinal association between sleep problems and depression. These influences were nonsignificant—suggesting that there was not power to distinguish sources of influence on the small cross-lagged association between sleep and later depression. Nonetheless, genetic factors appeared to be most important in explaining this link, and, of the small and nonsignificant genetic influence on depression at 10 years, 11% was due to genetic influences on sleep problems at 8 years and 30% was due to genetic influences on the association between sleep problems and depression at 8 years. It would be unwise to place great importance on these latter findings given the nonsignificant pattern of results. Although not central to the hypotheses and aims of this report, this study also highlighted a similar magnitude of genetic and environmental influences at 8 years and 10 years on sleep problems (at both time points, genetic influences were most significant) and also on depression (at both time points nonshared environmental influences were most significant). As reported previously,18 the concurrent associations between sleep problems and depression at 8 was mainly due to genes. However, at 10 years, nonshared environmental influence on the concurrent association between the phenotypes was most substantial, with smaller shared and genetic influences, possibly reflecting an increase in nonshared environmental experiences as children grow older.
Limitations
Despite strengths of this study including the use of twin pairs assessed longitudinally to thoroughly explore associations between sleep problems and depression, certain limitations need to be acknowledged. First, the sample size was small, which means that there was not sufficient power to decompose all of the longitudinal associations between sleep problems and depression into genetic and environmental influences. This suggests that examining the issues outlined here in a larger twin sample would be fruitful. Nonetheless, there was power to highlight other important findings (e.g., the unidirectional link between sleep problems and depression assessed by different raters and interesting patterns of genetic and environmental influences on the stability of these problems over time).
A second set of limitations concerns the measures used to examine sleep problems and depression in children. We employed one of the most widely used and well-validated measures of sleep problems in children (the CSHQ), and we focused on the total sleep problems score. However, it is important to note that, despite phenotypic overlap between sleep problems of different types,44 it is possible that certain sleep problems predict later depression to a greater extent than others45 and that the directionality of the association between sleep disturbances differs depending on the conceptualization of sleep problems (eg, it is possible that sleep disordered breathing predicts depression and not vice versa; whereas there could be a bidirectional association between sleep-anxiety and depression). Furthermore, despite genetic overlap between sleep problems of different types,46,47 there are also likely to be etiologic differences between different sleep problems. Despite this limitation, it is important to note that, even had we focused upon a more homogeneous measure of sleep (such as sleeplessness), this may have comprised etiologically distinct subcomponents. Our data were not appropriate for considering these issues because our small sample meant that we needed to analyze our data as continuous variables in our multivariate model and the subscales of the CSHQ were not all appropriate for this purpose. It therefore remains possible that, had we examined different types of sleep problems separately, we would have seen patterns of results different than those presented here. This issue needs to be explored in a larger sample. A further issue concerning the use of the CSHQ to examine sleep problems is that it is a parent-report measure and parents may not be accurate reporters of their children's sleep difficulties. Indeed, differences are reported when we compare parent and child reports in terms of both frequency of difficulties and genetic and environmental influences on difficulties.44,48 Furthermore, whereas links between sleep and depression have been highlighted when subjectively assessing the former,49 there is less evidence for an association when using objective measures of sleep in this age group.50–52 The use of symptom-count measures is common in large samples. However, before the clinical implications of this research can be established, there is a need to examine whether different processes are at play with regard to extreme difficulties, as compared with general difficulties in the full range. A much larger sample than that employed in this study is necessary to address this issue.
A third set of limitations is relevant to all studies employing twins to estimate genetic and environmental influences on measured traits. Various assumptions are made in twin studies, and these have been challenged. This is an ongoing debate, but twin researchers typically argue that, although twin studies are not perfect, they provide one of the very best techniques available to estimate genetic and environmental influences on traits, and, so long as estimates from genetic models are interpreted generally, they are useful.53
Implications
Despite limitations, the results reported here have implications for theory, research, and practice. With regard to theory, it is noteworthy that our results demonstrated that sleep problems predict later depression but that the converse association was not found. This pattern is consistent with other findings emerging in the literature.1,2 If this specific direction of effects is confirmed, this has implications for (1) understanding the natural course of these associations and (2) early-intervention strategies. From the perspective of clinical and social policy goals, what is most needed is information that can inform early-intervention strategies in targeted high-risk samples. Thus, if it can be established that early disruptions in sleep (including a genetic disposition for sleep difficulties early in life) represent a pathway to developing depression, early treatment to improve sleep could represent a window of opportunity for prevention. Clearly there is a need for more-specific models and theories designed to account for the associations between sleep and depression—especially with a developmental framework for understanding key times in development that create both vulnerabilities and opportunities for intervention.
The pattern of genetic and environmental influences on the phenotypes also has important implications for theory. Furthermore, the finding that environmental influences are key in explaining childhood depression needs to be reflected in models of the development of this difficulty (for a discussion of risk factors for child psychopathology, see Goodyer54). Although this finding is consistent with previous research suggesting that the heritability of depression in children is low and that this increases into adolescence,19,55 it is important to note that the nonshared environmental influence reported here was particularly large and was therefore unexpected—although this may partly be explained by measurement error (which is estimated as nonshared environmental influence).
There are important research implications from both the phenotypic and genetic results. The pattern of results presented here is consistent (although not demonstrative) of the suggestion that sleep problems cause later depression. There could be great value in future studies designed in ways that examine the possibility of a causal association more directly56 and potential mechanisms linking sleep regulation and affect regulation in the developmental progression and maintenance of depressive disorders in youth. As for the genetic findings reported here, the patterns of results support mounting evidence to suggest that genetic factors are important in explaining a range of sleep difficulties. Furthermore, this study is novel in suggesting that genetic factors are key in explaining the stability of sleep difficulties over time. These findings emphasize the relative dearth of knowledge concerning the specific genes involved in common sleep difficulties57 and indicate that further research in this area is likely to prove fruitful. Although further research is needed, our results tentatively suggest the possibility that some of the genes influencing early sleep problems may also influence the development of depression.
As mentioned earlier, there are also potential clinical implications of this study regarding the value of early assessment and treatment of childhood sleep problems, particularly in samples at high risk for developing depression. Further research is needed to establish whether treating sleep disturbances (and, if so, which particular sleep disturbances) can reduce the likelihood of the subsequent emergence of symptoms of depression. Although sleep problems at 8 years accounted for just a small proportion of the variance in symptoms of depression at 10 years, it is becoming increasingly apparent that most common difficulties suffered by children are best explained by multiple influences of small effect size. We argue that childhood sleep problems need to be given serious consideration despite having just a small influence on later depression because sleep problems (assessed in various ways) have negative consequences on mood, attention, and social and academic function,58–60 as well as being associated with difficulties later in life.10,61,62 Finally, in comparison with other risk indicators of later problems, sleep problems are more easily broached and readily discussed with families, often without the negative stigma that can unfortunately be associated with discussion of mental health problems in children, thus creating added opportunities for both research and clinical settings.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We would like to thank all participants from the ECHO study. We are also grateful to Jeanette Augustin, Philippa Carter, David Clark, Georgina Hosang, Orla Jordan, Helen Mathews, Fiona Mcleod, Peter McGuffin, Maria Napolitano, Robert Plomin, Jasmine Singh, and Lucy Stirling.
Financial support: The ECHO study was supported by a Career Development Award from the UK MRC to the last author. Alice Gregory is supported by a Leverhulme Research Fellowship.
REFERENCES
- 1.Gregory AM, O'Connor TG. Sleep problems in childhood: a longitudinal study of developmental change and association with behavioral problems. J Am Acad Child Adolesc Psychiatry. 2002;41:964–71. doi: 10.1097/00004583-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatr Res. 2006;40:700–8. doi: 10.1016/j.jpsychires.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 3.Ong SH, Wickramaratne P, Tang M, Weissman MM. Early childhood sleep and eating problems as predictors of adolescent and adult mood and anxiety disorders. J Affect Disord. 2006;96:1–8. doi: 10.1016/j.jad.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 4.Roberts RE, Roberts CR, Chen IG. Impact of insomnia on future functioning of adolescents. J Psychosom Res. 2002;53:561–9. doi: 10.1016/s0022-3999(02)00446-4. [DOI] [PubMed] [Google Scholar]
- 5.Patten CA, Choi WS, Gillin JC, Pierce JP. Depressive symptoms and cigarette smoking predict development and persistence of sleep problems in US adolescents. Pediatrics. 2000;106:art–e23. doi: 10.1542/peds.106.2.e23. [DOI] [PubMed] [Google Scholar]
- 6.Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–8. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 7.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders—an opportunity for prevention. JAMA. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 8.Livingston G, Blizard B, Mann A. Does sleep disturbance predict depression in elderly people—a study in inner London. Br J Gen Prac. 1993;43:445–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Riemann D, Voderholzer U. Primary insomnia: a risk factor to develop depression? J Affect Disord. 2003;76:255–9. doi: 10.1016/s0165-0327(02)00072-1. [DOI] [PubMed] [Google Scholar]
- 10.Gregory AM, Caspi A, Eley TC, Moffitt TE, O'Connor TG, Poulton R. Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. J Abnorm Child. 2005;33:157–63. doi: 10.1007/s10802-005-1824-0. [DOI] [PubMed] [Google Scholar]
- 11.Johnson EO, Chilcoat HD, Breslau N. Trouble sleeping and anxiety/depression in childhood. Psychiatry Res. 2000;94:93–102. doi: 10.1016/s0165-1781(00)00145-1. [DOI] [PubMed] [Google Scholar]
- 12.Stoleru S, Nottelmann ED, Belmont B, Ronsaville D. Sleep problems in children of affectively ill mothers. J Child Psychol Psychiatry. 1997;38:831–41. doi: 10.1111/j.1469-7610.1997.tb01601.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, Poulton R. Prior juvenile diagnoses in adults with mental disorder - Developmental follow-back of a prospective-longitudinal cohort. Arch Gen Psychiatry. 2003;60:709–17. doi: 10.1001/archpsyc.60.7.709. [DOI] [PubMed] [Google Scholar]
- 14.Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin psychiatry. 2005;66:1254–69. doi: 10.4088/jcp.v66n1008. [DOI] [PubMed] [Google Scholar]
- 15.Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Ann Rev Physiol. 2001;63:647–76. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- 16.Plomin R, DeFries JC, McClearn GE, McGuffin P. 4th ed. New York, NY: Worth Publishers; 2001. Behavioral Genetics. [Google Scholar]
- 17.Van den Oord EJCG, Boomsma DI, Verhulst FC. A study of genetic and environmental effects on the co-occurrence of problem behaviors in three-year-old twins. J Abnorm Psychol. 2000;109:360–72. doi: 10.1037/0021-843X.109.3.360. [DOI] [PubMed] [Google Scholar]
- 18.Gregory AM, Rijsdijk F, Dahl RE, McGuffin P, Eley TC. Associations between sleep problems, anxiety and depression in twins at 8 years of age. Pediatrics. 2006;118:1124–32. doi: 10.1542/peds.2005-3118. [DOI] [PubMed] [Google Scholar]
- 19.Scourfield J, Rice F, Thapar A, Harold GT, Martin N, McGuffin P. Depressive symptoms in children and adolescents: changing aetiological influences with development. J Child Psychol Psychiatry. 2003;44:968–76. doi: 10.1111/1469-7610.00181. [DOI] [PubMed] [Google Scholar]
- 20.O'Connor TG, Neiderhiser JM, Reiss D, Hetherington EM, Plomin R. Genetic contributions to continuity, change, and co-occurrence of antisocial and depressive symptoms in adolescence. J Child Psychol Psychiat. 1998;39:323–36. [PubMed] [Google Scholar]
- 21.Lau JYF, Eley TC. Changes in genetic and environmental influences on depressive symptoms across adolescence and young adulthood. Br J Psychiatry. 2006;189:422–7. doi: 10.1192/bjp.bp.105.018721. [DOI] [PubMed] [Google Scholar]
- 22.Thorleifsdottir B, Bjornsson JK, Benediktsdottir B, Gislason T, Kristbjarnarson H. Sleep and sleep habits from childhood to young adulthood over a 10-year period. J Psychosom Res. 2002;53:529–37. doi: 10.1016/s0022-3999(02)00444-0. [DOI] [PubMed] [Google Scholar]
- 23.Burt SA, McGue M, Krueger RF, Iacono WG. How are parent-child conflict and childhood externalizing symptoms related over time? Results, from a genetically informative cross-lagged study. Dev Psychopathol. 2005;17:145–65. doi: 10.1017/S095457940505008X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsson H, Viding E, Rijsdijk FV, Plomin R. Relationships between parental negativity and childhood antisocial behavior over time: a bidirectional effects model in a longitudinal genetically informative design. J Abnorm Child Psychol. 2008;36:633–645. doi: 10.1007/s10802-007-9151-2. [DOI] [PubMed] [Google Scholar]
- 25.Oliver BR, Plomin R. Twins' Early Development Study (TEDS): a multivariate, longitudinal genetic investigation of language, cognition and behavior problems from childhood through adolescence. Twin Res Human Genetics. 2007;10:96–105. doi: 10.1375/twin.10.1.96. [DOI] [PubMed] [Google Scholar]
- 26.Trouton A, Spinath FM, Plomin R. Twins Early Development Study (TEDS): A multivariate, longitudinal genetic investigation of language, cognition and behavior problems in childhood. Twin Res. 2002;5:444–8. doi: 10.1375/136905202320906255. [DOI] [PubMed] [Google Scholar]
- 27.Price TS, Freeman B, Craig IW, Petrill SA, Ebersole L, Plomin R. Infant zygosity can be assigned by parental report questionnaire data. Twin Res. 2000;3:129–33. doi: 10.1375/136905200320565391. [DOI] [PubMed] [Google Scholar]
- 28.Eley TC, Bolton D, O'Connor TG, Perrin S, Smith P, Plomin R. A twin study of anxiety-related behaviours in pre-school children. J Child Psychol Psychiatry. 2003;44:945–60. doi: 10.1111/1469-7610.00179. [DOI] [PubMed] [Google Scholar]
- 29.Achenbach TM. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. Manual for the Child Behaviour Checklist and 1991 profile. [Google Scholar]
- 30.Behar L, Stringfield S. A behavior rating scale for the preschool child. Dev Psychol. 1974;33:3–66. [Google Scholar]
- 31.Berg CZ, Whitaker A, Davies M, Flament MF, Rapoport JL. The survey form of the Leyton Obsessional Inventory-Child Version: Norms from an epidemiological study. J Am Acad Child Adolesc Psychiatry. 1988;27:759–63. doi: 10.1097/00004583-198811000-00017. [DOI] [PubMed] [Google Scholar]
- 32.Elander J, Rutter M. Use and development of the Rutter parents' and teachers' scales. Int J Methods Psychiatric Res. 1996;6:63–78. [Google Scholar]
- 33.Goodman R, Scott S. Comparing the strengths and difficulties questionnaire and the Child Behavior Checklist: Is small beautiful? J Abnorm Child. 1999;27:17–24. doi: 10.1023/a:1022658222914. [DOI] [PubMed] [Google Scholar]
- 34.Owens JA, Spirito A, McGuinn M. The Children's Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–51. [PubMed] [Google Scholar]
- 35.Jaffee SR, Moffitt TE, Caspi A, Taylor A, Arseneault L. Influence of adult domestic violence on children's internalizing and externalizing problems: an environmentally informative twin study. J Am Acad Child Adolesc Psychiatry. 2002;41:1095–103. doi: 10.1097/00004583-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 36.van der Valk JC, Verhulst FC, Stroet TM, Boomsma DI. Quantitative genetic analysis of internalising and externalising problems in a large sample of 3-year-old twins. Twin Res. 1998;1:25–33. doi: 10.1375/136905298320566456. [DOI] [PubMed] [Google Scholar]
- 37.Kovacs M. The Children's Depression Inventory (CDI) Psychopharmacol Bull. 1985;21:995–8. [PubMed] [Google Scholar]
- 38.Fundudis T, Berney TP, Kolvin I, et al. Reliability and validity of two self-rating scales in the assessment of childhood depression. Br J Psychiatry. 1991;159:36–40. [PubMed] [Google Scholar]
- 39.Hodges K. Depression and anxiety in children: A comparison of self-report questionnaires to clinical interview. Psychol Assess: J Consult Clin Psychol. 1990;2:376–81. [Google Scholar]
- 40.Neale MC. 4th ed. Richmond, VA: Department of Psychiatry; 1997. Mx: Statistical Modeling. [Google Scholar]
- 41.Eley TC, Gregory AM, Clark DM, Ehlers A. Feeling anxious: a twin study of panic/somatic symptoms, anxiety sensitivity and heart-beat perception in children. J Child Psychol Psychiatry. 2007;48:1184–91. doi: 10.1111/j.1469-7610.2007.01838.x. [DOI] [PubMed] [Google Scholar]
- 42.Gregory AM, Rijsdijk FV, Lau JYF, Napolitano M, McGuffin P, Eley TC. Genetic and environmental influences on interpersonal cognitions and associations with depressive symptoms in 8-year-old twins. J Abnorm Psychol. 2007;116:762–75. doi: 10.1037/0021-843X.116.4.762. [DOI] [PubMed] [Google Scholar]
- 43.Cohen J. 2nd ed. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1988. Statistical Power Analysis for the Behavioral Sciences. [Google Scholar]
- 44.Gregory AM, Rijsdijk FV, Eley TC. A twin-study of sleep difficulties in school-aged children. Child Dev. 2006;77:1668–79. doi: 10.1111/j.1467-8624.2006.00966.x. [DOI] [PubMed] [Google Scholar]
- 45.Gregory AM, Van den Ende J, Willis TA, Verhulst FC. Parent-reported sleep problems during development predicts self-reported anxiety/ depression, attention problems and aggression later in life. Arch Ped Adolesc Med. 2008;162:330–5. doi: 10.1001/archpedi.162.4.330. [DOI] [PubMed] [Google Scholar]
- 46.Gregory AM. A genetic decomposition of the association between parasomnias and dyssomnias in 8 year old twins. Arch Ped Adolesc Med. 2008;162:299–304. doi: 10.1001/archpedi.162.4.299. [DOI] [PubMed] [Google Scholar]
- 47.Hublin C, Kaprio J, Partinen M, Koskenvuo M. Parasomnias: co-occurrence and genetics. Psychiatr Genet. 2001;11:65–70. doi: 10.1097/00041444-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Owens JA, Spirito A, McGuinn M, Nobile C. Sleep habits and sleep disturbance in elementary school-aged children. J Dev Behav Pediatr. 2000;21:27–36. doi: 10.1097/00004703-200002000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Bertocci MA, Dahl RE, Williamson DE, et al. Subjective sleep complaints in pediatric depression: A controlled study and comparison with EEG measures of sleep and waking. J Am Acad Child Adolesc Psychiatry. 2005;44:1158–66. doi: 10.1097/01.chi.0000179057.54419.17. [DOI] [PubMed] [Google Scholar]
- 50.Dahl RE, Puig-Antich J, Ryan ND, et al. EEG sleep in adolescents with major depression—the role of suicidality and inpatient status. J Affect Disord. 1990;19:63–75. doi: 10.1016/0165-0327(90)90010-6. [DOI] [PubMed] [Google Scholar]
- 51.Forbes EE, Bertocci MA, Gregory AM, et al. Objective sleep in pediatric anxiety disorders and major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2008;47:148–55. doi: 10.1097/chi.0b013e31815cd9bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Puig-Antich J, Goetz R, Hanlon C, et al. Sleep architecture and REM-sleep measures in prepubertal children with major depression—a controlled study. Arch Gen Psychiatry. 1982;39:932–9. doi: 10.1001/archpsyc.1982.04290080046007. [DOI] [PubMed] [Google Scholar]
- 53.Martin N, Boomsma DI, Machin G. A twin-pronged attack on complex traits. Nat Genet. 1997;17:387–92. [Google Scholar]
- 54.Goodyer IM. Family relationships, life events and childhood psychopathology. J Child Psychol Psychiatry. 1990;31:161–92. doi: 10.1111/j.1469-7610.1990.tb02277.x. [DOI] [PubMed] [Google Scholar]
- 55.Silberg J, Pickles A, Rutter M, et al. The influence of genetic factors and life stress on depression among adolesent girls. Arch Gen Psychiatry. 1999;56:225–32. doi: 10.1001/archpsyc.56.3.225. [DOI] [PubMed] [Google Scholar]
- 56.Hill AB. Environment and disease—association or causation. Proc Royal Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 57.Dauvilliers Y, Maret S, Tafti M. Genetics of normal and pathological sleep in humans. Sleep Med Rev. 2005;9:91–100. doi: 10.1016/j.smrv.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 58.Alfano CA, Ginsburg GS, Kingery JN. Sleep-related problems among children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46:224–32. doi: 10.1097/01.chi.0000242233.06011.8e. [DOI] [PubMed] [Google Scholar]
- 59.Goodnight JA, Bates JE, Staples AD, Pettit GS, Dodge KA. Temperamental resistance to control increases the association between sleep problems and externalizing behavior development. J Fam Psychol. 2007;21:39–48. doi: 10.1037/0893-3200.21.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sadeh A, Gruber R, Raviv A. Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child Dev. 2002;73:405–17. doi: 10.1111/1467-8624.00414. [DOI] [PubMed] [Google Scholar]
- 61.Thunstrom M. Severe sleep problems in infancy associated with subsequent development of attention-deficit/hyperactivity disorder at 5.5 years of age. Acta Paediatrica. 2002;91:584–92. doi: 10.1080/080352502753711731. [DOI] [PubMed] [Google Scholar]
- 62.Wong MM, Brower KJ, Fitzgerald HE, Zucker RA. Sleep problems in early childhood and early onset of alcohol and other drug use in adolescence. Alcoholism: Clin – Exp Res. 2004;28:578–87. doi: 10.1097/01.alc.0000121651.75952.39. [DOI] [PubMed] [Google Scholar]