Abstract
Objectives:
Stimulant medications appear effective at restoring simple alertness and psychomotor vigilance in sleep deprived individuals, but it is not clear whether these medications are effective at restoring higher order complex cognitive capacities such as planning, sequencing, and decision making.
Design:
After 44 hours awake, participants received a double-blind dose of one of 3 stimulant medications or placebo. After 45–50 hours awake, participants were tested on computerized versions of the 5-Ring Tower of Hanoi (TOH), the Tower of London (TOL), and the Wisconsin Card Sorting Test (WCST).
Setting:
In-residence sleep-laboratory facility at the Walter Reed Army Institute of Research.
Participants:
Fifty-four healthy adults (29 men, 25 women), ranging in age from 18 to 36 years.
Interventions:
Participants were randomly assigned to 1 of 3 stimulant medication groups, including caffeine, 600 mg (n = 12), modafinil, 400 mg (n = 12), dextroamphetamine, 20 mg (n = 16), or placebo (n = 14).
Measurements and Results:
At the doses tested, modafinil and dextroamphetamine groups completed the TOL task in significantly fewer moves than the placebo group, and the modafinil group demonstrated greater deliberation before making moves. In contrast, subjects receiving caffeine completed the TOH in fewer moves than all 3 of the other groups, although speed of completion was not influenced by the stimulants. Finally, the modafinil group outperformed all other groups on indices of perseverative responding and perseverative errors from the WCST.
Conclusions:
Although comparisons across tasks cannot be made due to the different times of administration, within-task comparisons suggest that, at the doses tested here, each stimulant may produce differential advantages depending on the cognitive demands of the task.
Citation:
Killgore WDS; Kahn-Greene ET; Grugle NL; Killgore DB; Balkin TJ. Sustaining Executive Functions During Sleep Deprivation: A Comparison of Caffeine, Dextroamphetamine, and Modafinil. SLEEP 2009;32(2):205–216.
Keywords: Sleep deprivation, stimulants, caffeine, modafinil, dextroamphetamine, executive function, prefrontal cortex, problem solving, tower, Wisconsin Card Sorting Test
ADEQUATE SLEEP IS ESSENTIAL FOR OPTIMAL COGNITIVE PERFORMANCE. WITHOUT SUFFICIENT SLEEP, MENTAL PROCESSES BECOME SLOWED, INSTABLE, and prone to errors.1 Sleep loss makes it difficult to sustain alertness, attention, reaction time, and psychomotor vigilance.1,2 Sleep deprivation also adversely affects mood and emotional processes,3–5 which may indirectly influence many aspects of higher order cognition such as judgment and decision-making.6 Although contradictory findings have been reported,7 sleep deprivation has generally been shown to adversely affect a number of complex cognitive processes that are subsumed under the construct of executive functions.8–11
Executive functions include a broad spectrum of complex higher-order cognitive abilities mediated to a large extent by regions of the prefrontal cortex and associated multimodal regions of the posterior cortex.12 Executive functions include the cognitive abilities necessary to plan and coordinate actions, to monitor and adjust behavior as necessary, and to focus attention and suppress distractions.11 Total sleep deprivation has been shown to reduce many of these functions, including the ability to think divergently,10,13 to switch flexibly among semantic categories,14 to avoid stereotypical responses and maintain adherence to cognitive rules,15 and to monitor and correct errors during ongoing behavior.16 Recent studies have suggested that sleep deprivation also impairs a number of higher-order capacities that involve emotional and behavioral control, including the ability to inhibit inappropriate responses,17 the ability to make decisions that lead to advantageous outcomes,18,19 the propensity to engage in risky behaviors,20,21 and the willingness to make utilitarian decisions that violate personal moral beliefs.22
From a neurobiological perspective, executive functions are believed to be mediated predominantly by regions of the prefrontal cortex,23 particularly the mid-dorsolateral, mid-ventrolateral, and dorsal anterior cingulate gyrus,24 although these functions also rely on a broadly distributed system of cortical networks that potentially includes posterior parietal cortex and temporal regions among others.12 Interestingly, sleep deprivation is associated with significant declines in the glucose metabolism of several prefrontal and other cortical regions that are commonly associated with complex executive functions and cognitive control abilities, including dorsolateral, ventrolateral, medial prefrontal cortices, and dorsal anterior cingulate gyrus.25,26 These absolute decreases in prefrontal glucose metabolism are often associated with greater compensatory responses within adjacent prefrontal regions or even spatially distal heteromodal regions such as the parietal cortex during cognitive challenge tasks when measured using functional magnetic resonance imaging.27,28 Molecular imaging techniques have recently shown significant up-regulation of A1 adenosine receptors in the brain following 24 hours of sleep deprivation, most prominently within the orbitofrontal region of the prefrontal cortex.29 Together, these findings suggest that sleep deprivation alters normal cerebral functioning, particularly—though not exclusively—within the prefrontal cortex, and that these changes may account for some of the deficits in higher order cognitive abilities that are observed during prolonged wakefulness.
Stimulant medications are often used to sustain performance during periods of sleepiness or prolonged wakefulness. Commonly used stimulants include caffeine, dextroamphetamine, and modafinil. These stimulants each have different mechanisms of action and varying side effect profiles.30 Moderate doses of caffeine (200 mg) have been found to improve cognitive function, including visual vigilance, learning, and memory, in sleep-deprived individuals.31 Similarly, dextroamphetamine and modafinil effectively sustain alertness and simple cognitive performance.32,33 Recent head-to-head comparison of these 3 stimulants during 85 hours of sleep deprivation suggests that, at appropriate doses, all 3 are equally effective at temporarily reversing deficits in psychomotor vigilance within the first 2 to 4 hours after administration, but that dextroamphetamine and modafinil have longer durations of action than caffeine.2 Preliminary results from Wesensten and colleagues (2005) suggested that, while these stimulants were all effective at sustaining alertness and psychomotor vigilance, they each showed evidence of selective enhancement of different aspects of executive functioning. That study found no effects of stimulants on executive functions such as verbal fluency, Stroop Color-Word Interference, or the Wisconsin Card Sorting Test after 3 nights of sleep deprivation, but did suggest a possible advantage for caffeine and modafinil on a cognitive estimation task.2 Recent data suggest that these 3 stimulants do have differential effects on complex cognitive-affective processes such as the ability to identify and appreciate subtle humor in jokes and cartoons when sleep deprived.34 Further, impairments in emotional decision-making during sleep deprivation appear to be unaffected by stimulants such as caffeine.19 Because the term “executive function” encompasses a broad range of highly complex multi-modal cognitive capacities, each involving prefrontal (and other) cortical networks with unique as well as shared neural systems, it is conceivable that the various stimulant medications may differentially enhance some executive function systems while leaving other systems relatively unaffected.
Only one published study has directly compared the effects of caffeine, dextroamphetamine, and modafinil on tasks of executive functioning in sleep deprived individuals.2 In that study, however, probe tasks of executive function were not the primary objective of the resarch and were not administered until 4.5 to 10.5 hours after drug administration, leading to the possibility that the effectiveness of these stimulants on such tasks may have been underestimated. Furthermore, critical aspects of executive functioning, namely strategic planning and sequencing abilities, were not assessed. Therefore, in the present double-blind placebo controlled study, we directly compared the effects of these 3 stimulants on the specific aspects of executive function and working memory measured by the Tower of London (i.e., planning and visuospatial working memory), Tower of Hanoi (planning, strategy, sequencing, inhibition of pre-potent responses), and the Wisconsin Card Sorting Test (abstract concept formation, mental set shifting), in individuals deprived of sleep for 2 consecutive nights. It was hypothesized that performance on these measures of executive function would be more impaired in the placebo group relative to the 3 stimulant groups. Furthermore, if these 3 stimulants improve executive function by enhancing simple alertness and vigilance, then all 3 should have similar effectiveness in reversing the executive function deficits produced by sleep loss. If, however, the mechanism of action of each stimulant acts differentially on the specific prefrontal and other heteromodal associative systems required to complete each task, then within a particular task, some stimulants might selectively enhance task performance more than other stimulants.
METHODS
Participants
A total of 54 healthy volunteers (29 males and 25 females) took part in a larger study to examine the effects of stimulant medications on cognitive functioning during sleep deprivation. While the present findings are new and have not been published previously, other data from this larger study have been reported elsewhere.20,34–38 Participants included 42 civilians and 12 military personnel. Age ranged from 18 to 36 (M = 23.5, SD = 4.0) and educational level ranged from 11 to 18 years of schooling (M = 14.5, SD = 1.9). Performance IQ, as assessed by the Wechsler Abbreviated Scale of Intelligence (WASI; The Psychological Corporation; San Antonio, Texas) ranged from 85 to 126 (M = 104.6, SD = 11.0). All subjects were in good health as determined by a physical examination prior to entry. Exclusionary criteria included tobacco use in the previous 3 years, a history of alcohol or drug abuse, mental or psychiatric illness, sleep problems, pregnancy, or significant medical/health issues that could have placed the subject at risk while participating in the study. Monetary compensation was provided to the participants upon the completion of the study (or prorated for early withdrawal). The study was approved by the Walter Reed Army Institute of Research Human Use Review Committee and the U.S. Army Human Subjects Research Review Board.
Volunteers were randomly assigned to one of 4 conditions: placebo (n = 14), caffeine 600 mg (n = 12), dextroamphetamine 20 mg (n = 16), or modafinil 400 mg (n = 12). Drug group status was maintained in a double-blind until completion of the study. Due to some computer failures, data for the WCST were available for only 49 of the 54 participants (i.e., placebo [n = 9], caffeine 600 mg [n = 12], dextroamphetamine 20 mg [n = 16], or modafinil 400 mg [n = 12]. The choice of stimulants and dosages for this study was made to allow direct comparison with previous studies from our laboratory that suggest that the current dosages of these three medications produce comparable levels of psychomotor vigilance performance during sleep deprivation.2,39
Assessment Tasks
Psychomotor Vigilance Test (PVT)
To assess simple reaction time/psychomotor speed, a modified 5-minute version of the PVT was administered on a palm-held computer every 2 hours throughout the duration of the study. This version has been shown to provide valid and reliable estimates of psychomotor vigilance during sleep deprivation.40 During this task, participants monitored a screen and pressed a response button on the hand held unit each time a “bulls-eye” target appeared. The interstimulus interval was varied pseudorandomly between presentations to minimize anticipation of the stimulus. Average reaction time from all trials was scored for each administration. Because comprehensive PVT data encompassing the entire study are reported elsewhere, we simply report drug group differences here in order to provide a context in which to interpret the executive function results.
Tower of London (TOL)
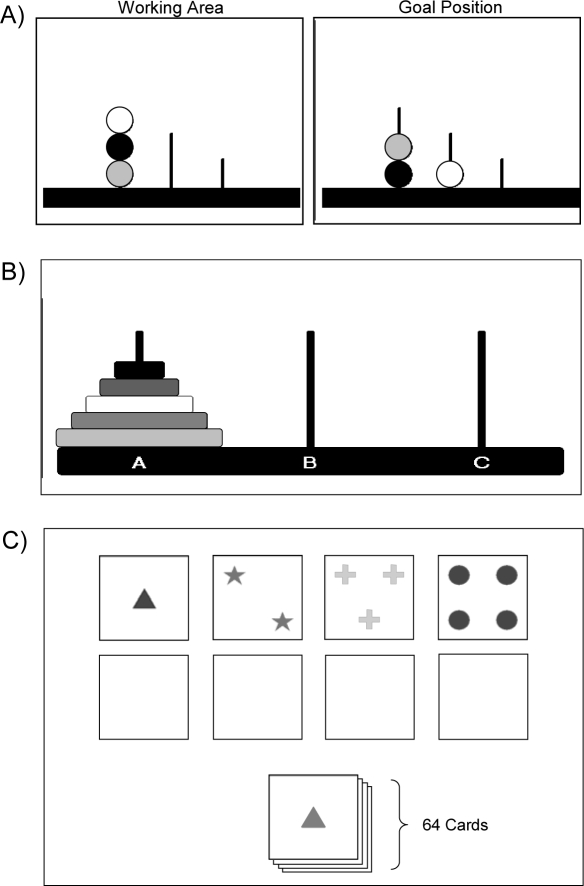
Participants completed a 10-trial computerized version of the TOL (Colorado Assessment Tests, http://www.catstests.com). Each trial presented volunteers with a series of 3 “pegs” of differing lengths, each with 3 different colored “beads” arranged in randomly appearing configurations upon the pegs (Figure 1). The beads were moved on the screen by pointing the cursor with the computer mouse. Clicking on a bead selected it and allowed it to be moved to another location with the mouse. A second click released the bead to stay in its designated location. The examinee was required to rearrange the beads so that the final configuration matched a pre-specified goal pattern. Movement of the beads was constrained by the rule that only one bead could be moved at a time, and additional beads were not permitted to be placed on a peg that was already full. Completion of one trial immediately initiated the next trial. Initial trials were simple and could be completed in as few as 3 moves, whereas later trials were more complex and required a minimum of ≥ 7 bead placements to complete perfectly. The goal was to complete the task in as few moves and as quickly as possible. Dependent variables from this task included the number of bead placements or “moves” required to match the goal arrangement, and the average latency between the last bead placement and the pick-up of the next bead—an index of planning time and cognitive processing.
Figure 1.
Three executive function tasks were used. (A) The Tower of London (TOL): moving only one bead at a time, subjects must rearrange the 3 colored beads within the Working Area so that they match the arrangement shown in the Goal Position. (B) The Tower of Hanoi (TOH): by moving only one colored ring at a time, subjects must rearrange the stack of 5 rings so that they are positioned in the same order on peg C. (C) The Wisconsin Card Sorting Test-64 card computerized version (WCST): Subjects are given a stack of 64 cards and must match each card to one of 4 “key” cards at the top of the screen. Unbeknownst to the subject, the sorting principle changes after 10 consecutive correct responses. Cards may be sorted according to color, shape, or number of symbols on the card.
Tower of Hanoi (TOH)
Participants completed the 5-ring version of the TOH on computer (Colorado Assessment Tests, http://www.catstests.com). On the screen, participants were presented with 3 “pegs” holding a stack of 5 “rings” of graduated sizes and different colors (Figure 1). The rings were moved on the screen by using the computer mouse to place the cursor on the desired ring and clicking on that ring to select it. Once selected, the ring could be moved to another peg using the mouse. A second click released the ring to stay in its designated location. The goal was to move the stack of rings from the leftmost peg, one at a time, and replicate the stack on the rightmost peg. Placement of the rings was constrained by the rule that a larger ring could never be placed on top of a smaller ring. If accomplished without any errors, the 5-ring puzzle can be completed in no less than 31 moves (participants were not made aware of this, however). Participants were instructed to complete this puzzle in the fewest number of moves and as quickly as possible. Upon completion of the first trial, participants were instructed to complete it again as quickly as possible. Four trials of the same 5-ring puzzle were completed in one session, with each trial separated by a rest interval of 10 seconds. Dependent measures included the number of ring placements or “moves” required to complete the task, and the average latency between the last ring placement and the pick-up of the next ring—a measure of planning time and cognitive processing.
Wisconsin Card Sorting Task (WCST)
The 64-card computer-based version of the WCST (Psychological Assessment Resources, Lutz, FL) was used to assess the ability to form abstract concepts, learn from feedback, and shift mental set. When taking this test, participants were seated in front of a computer screen and were presented with a virtual deck of cards positioned at the bottom of the computer screen and 4 key cards located side-by-side across the upper half of the screen (Figure 1). The key cards each displayed a different design: (1) one red triangle, (2) two green stars, (3) three yellow crosses, and (4) four blue circles. The card deck contained 64 cards of varying shapes (stars, triangles, crosses, circles), colors (yellow, blue, red, green), and numbers of shapes (one, two, three, four). Participants were told to match each card from the deck to one of the 4 key cards by using the computer mouse to click on the appropriate option. Once an option was selected, the computer moved the card to a position just below the associated key card. After each trial, participants received combined auditory and visual feedback indicating whether they had made a correct or incorrect choice. Without warning, the sorting principle changed (i.e., from color to shape to number) after the examinee had completed 10 consecutive correct matches. The total number of errors, perseverative responses (responses that follow a previously reinforced principle that is no longer correct), perseverative errors (i.e., errors that follow a previously reinforced principle that is no longer correct), conceptual level responses (consecutive runs of 3 correct answers), failures to maintain set (i.e., when an examinee switches to an incorrect sorting principle after 5 consecutive correct responses), and number of categories completed were calculated according to the test manual.
Procedure
Participants arrived at the laboratory on Wednesday evening of the acclimation day (Day 0) at 18:00. During the acclimation period, the participants were administered the WASI to obtain a baseline level of intellectual functioning and were then given training in basic study procedures. Prior to the sleep deprivation phase of the study, volunteers received one controlled night of sleep in the sleep laboratory. Enforced bedtime began at 23:00, as volunteers were given 8 hours of time in bed in private darkened rooms. Thursday morning (Day 1) participants were awakened at 07:00 and remained awake until 20:00 Saturday evening (Day 3), for a total of 61 hours of continuous sleep deprivation. Volunteers completed the PVT every 2 hours while awake. When not taking scheduled cognitive tests, participants were allowed to eat, read, play games, and watch television; however, no food was permitted for 3 hours prior to and 4 hours after the administration of the test article. Between 02:50–03:00 Saturday morning (Day 3; 44 hours sleep deprivation), the participants received one of 3 stimulant medications (caffeine 600 mg, modafinil 400 mg, or dextroamphetamine 20 mg) or a placebo administered in 4 identically prepared capsules. All test substances were administered double-blind so that neither the volunteers nor the investigators were aware of the conditions until the completion of the final run of the study. The executive function tests were administered between 04:00 and 07:40 that morning. The WCST was administered from 04:00 (1 hour after drug administration; 45 hours awake) to 04:15. The TOH administration began at 06:30 (3.5 hours after drug administration; 47.5 hours awake) and ended at 06:50, and the TOL began at 07:30 (4.5 hours after drug administration; 48.5 hours awake) and ended at 07:40. Participants were allowed to sleep at 20:00 Saturday evening (Day 3) until 08:00 Sunday morning (Day 4). The TOH and TOL were re-administered at 12:40 and 13:30 respectively to provide a post-recovery sleep comparison. Participants were released from the laboratory at 18:00 Sunday evening.
Data Analysis
The “executive function testing period” began at 04:00 and ended at approximately 07:40 Days 3 and 4. During this period, 2 PVT and SSS assessments were administered (at 04:20 and 06:20). The PVT for the 2 sessions most proximal to the administration of the executive function tasks (i.e., 04:20 and 06:20) were each converted into a measure of speed (1/RT*1000), then normalized as a percentage of the mean score at rested baseline (i.e., the first 8 administrations on the baseline day from 08:20–22:20) to obtain an estimate of alertness and vigilance during the executive function testing period. Higher scores indicate better PVT speed performance relative to baseline. The PVT and SSS scores from the 2 sessions were subjected to repeated measures analyses of variance (ANOVA) with drug condition as the between-groups variable and time of testing as the repeated measure. Drug group effects and interactions were evaluated via Bonferroni post hoc comparisons with α = 0.05. Executive function tasks (i.e., TOL, TOH, WCST) were each subjected to a one-way ANCOVA to evaluate drug group effects. Because performance on these tasks is known to be influenced by intelligence and formal education, we included these variables as well as study week (i.e., drift in the sampling population over the course of the investigation) as covariates in the analyses. Tukey post hoc comparisons, with α = 0.05, were used to evaluate pair-wise drug group effects. Because the TOL, TOH, and WCST do not share a common normative base or relative scaling, it is psychometrically inappropriate to compare directly across tasks, not to mention the potential confound produced by administering the tasks at different points in the circadian phase. Therefore, analyses were restricted to comparisons between stimulant conditions separately for each task.
RESULTS
Psychomotor Vigilance
Repeated measures ANOVA showed a main effect of time of testing, F(1,49) = 5.05, P = 0.029, a significant drug effect, F(3,49) = 12.77, P < 0.0001, and drug by time of testing interaction, F(3,49) = 4.45, P = 0.008. As evident in Figure 2, Bonferroni post hoc comparisons showed that this interaction was produced by a significant (P ≤ 0.001) worsening of the PVT speed performance (as a % of baseline) for the placebo group, whereas none of the 3 stimulant groups showed significant changes from the beginning to the end of the executive function testing block.
Figure 2.
Measures of (A) objective alertness (Palm PVT) and (B) subjective sleepiness (Stanford Sleepiness Scale) were taken at 2 points during the executive functioning test period. Whereas all 3 stimulant conditions showed sustained vigilance performance and subjective alertness during the testing block, the placebo group showed significant worsening of vigilance speed and increased ratings of sleepiness. *P ≤ 0.001 Bonferroni corrected difference between testing sessions and between drug groups.
Subjective Sleepiness
Repeated measures ANOVA showed no main effect of time of testing on SSS scores, F(1,50) = 1.23, P = 0.274, but did reveal a significant effect of drug condition, F(3,50) = 9.97, P < 0.0001, and a significant drug by time of testing interaction, F(3,50) = 4.44, P = 0.008. Figure 2 shows that the 3 drug groups remained stable in their sleepiness scores from the beginning of the executive function block to the end, whereas Bonferroni post hoc comparisons showed that the placebo group showed increased subjective sleepiness over the same period (P ≤ 0.001).
Tower of London
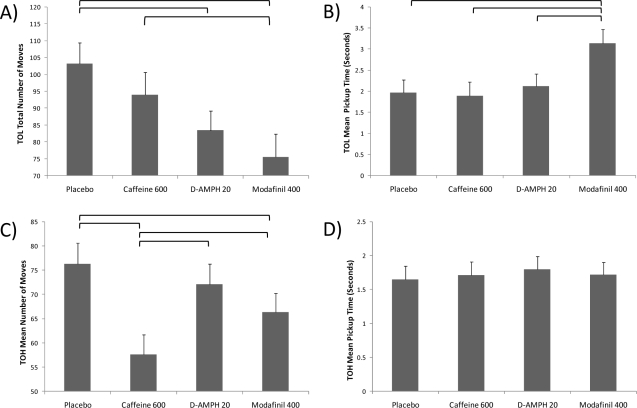
For the TOL, there was a significant main effect of drug group on the total number of moves taken across the 10 trials, F(3,47) = 3.56, P = 0.021. Tukey post hoc comparisons revealed that the modafinil group solved the problems with significantly (P < 0.01) fewer moves than the placebo group and the caffeine group, but did not differ significantly from the dextroamphetamine group (Figure 3). The dextroamphetamine group also performed the task in significantly fewer moves than the placebo group (P < 0.01). There was also a significant main effect of drug group on the average time taken to pick up each new bead during the task, F(3,47) = 3.12, P = 0.035. Tukey post hoc tests showed that the average amount of time between bead placement and pick up of the next bead—a putative measure of cognitive processing—was actually slowest for the modafinil group relative to the other 2 drug groups or placebo (P < 0.01). In contrast, the average total time for each trial of the TOL (i.e., pickup and placement of all beads) did not differ across drug groups, F(3,47) = 1.85, P = 0.152. Similarly, the total time to complete the entire TOL test did not differ across the four groups, F(3,47) = 0.46, P = 0.715. When tested on the same TOL tasks following a full night of recovery sleep, no drug group differences were apparent for any of these variables.
Figure 3.
Four drug conditions (i.e., placebo, caffeine 600 mg, dextroamphetamine 20 mg, and modafinil 400 mg) were compared on the Tower of London (TOL) and Tower of Hanoi (TOH). (A) Stimulants had a significant effect on the total number of moves taken to complete the 10 TOL trials (P < 0.05). Brackets show significant drug group differences after Tukey post hoc correction (P < 0.05), with modafinil and dextroamphetamine outperforming placebo. (B) Modafinil was associated with significantly longer mean pickup time than the other 3 groups after Tukey correction for multiple comparisons (P < 0.05). (C) Stimulants also had a significant effect on the mean number of moves taken to solve the 5 ring puzzle across the 4 repeated administrations (P < 0.05). Brackets showing Tukey paired comparisons show that the caffeine group outperformed the other 3 groups (P < 0.05). (D) There were no significant differences in mean TOH ring pickup time among the 4 conditions.
Tower of Hanoi
For the TOH, 4 attempts were given to solve the same 5-ring version of the puzzle. The total number of moves and pick-up times were averaged across the 4 trials for each subject. There was a significant main effect of drug group on the average number of moves to solve the puzzle across the 4 trials, F(3,38) = 3.20, P = 0.034 (see Figure 2). Tukey post hoc comparisons showed that the caffeine group solved the TOH 5-ring task with significantly (P < 0.01) fewer moves than either the placebo, dextroamphetamine, or modafinil groups (Figure 3). The modafinil group also performed the task in significantly fewer moves than the placebo group (P < 0.01). Interestingly, there was no significant difference among the groups with regard to the average pick-up time for each ring, F(3,38) = 0.93, P = 0.963, suggesting that these effects were not due simply to faster processing or motor speed. When the TOH was re-administered following a full night of recovery sleep, no drug group differences were apparent for total moves or for average pick-up times.
Wisconsin Card Sorting Test-64
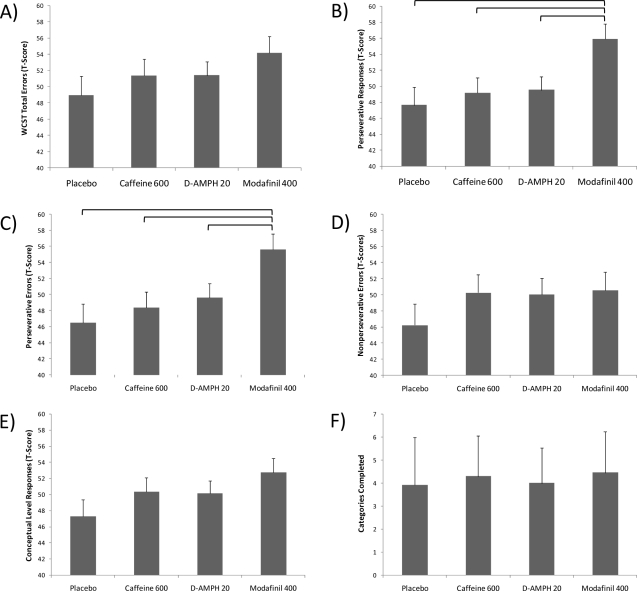
Figure 4 shows the group performances on the 64-item computerized version of the WCST. Most performances were transformed into normalized T-scores to correct for age and gender. These scores have a mean of 50 and a standard deviation of 10. Consequently, higher T-scores reflect better performance relative to the normative comparison group. As evident in Figure 4, there was no significant effect of drug group on the number of errors made on the test, F(3,42) = 0.93, P = 0.434. There was, however, a significant main effect of drug group for the number of perseverative responses (norm adjusted T-scores), F(3,42) = 3.35, P = 0.028, and the number of perseverative errors (norm adjusted T-scores), F(3,42) = 3.53, P = 0.023. Tukey post hoc comparisons showed that the modafinil group demonstrated significantly better performance (i.e., fewer perseverative responses and perseverative errors) than the placebo, caffeine, or dextroamphetamine groups (Figure 4). In contrast, there was no significant main effect of drug group on other measures of WCST performance, including non-perseverative errors, F(3,42) = 0.63, P = 0.598, conceptual level responses, F(3,42) = 1.27, P = 0.298, number of categories completed, F(3,42) = 10.01, P = 0.398, failure to maintain set, F(3,42) = 0.46, P = 0.711, or learning to learn, F(3,39) = 0.51, P = 0.676.
Figure 4.
Four drug conditions (placebo, caffeine 600 mg, dextroamphetamine 20 mg, and modafinil 400 mg) were compared on the various indices from the Wisconsin Card Sorting Test-64 card computerized version (WCST). Although there was no difference among the stimulants with regard to (A) the Total number of Errors (standardized T-score for age and gender), there was a significant effect of drug group for (B) Perseverative Responses (standardized T-Score for age and gender), with modafinil showing the highest T-score (i.e., fewest Perseverative Responses) relative to the other groups after Tukey correction for multiple comparisons (P < 0.05). (C) Modafinil also outperformed (Tukey post hoc, P < 0.05) the other 3 groups with regard to Perseverative Errors (standardized T-Score for age and gender). In contrast, there were no drug group effects for (D) Non-Perseverative Errors (T-scores), (E) Conceptual Level Responses (T-scores), or (F) total number of Categories Completed.
Inter-Task Correlations
Finally, it was also of interest to examine the strength of relationships among the various executive function tasks during the sleep deprivation and post-recovery phases. As evident in Table 1, during sleep deprivation, the mean number of moves required to complete the TOL was moderately positively correlated with the number of moves required to complete the TOH and the number of errors on the WCST and inversely related to the number of WCST categories completed. Speed of TOL pickup time, number of moves to complete the TOH, and mean pickup time for the TOH were unrelated to any other variables. WCST variables were generally correlated with one another but not with other variables. Interestingly, after a night of recovery sleep, the correlation between the mean number of moves to complete the TOH and TOL showed a reversal of their relationship from the sleep deprived state, with better performance on one task associated with worse performance on the other.
Table 1.
Correlations Among Executive Function Tasks During Sleep Deprivation Phase (Below Diagonal) and Post-Recovery Phase (Above Diagonal)
| TOL No. Moves | TOL Pickup Time | TOH No. Moves | TOH Pickup Time | WCST No. Errors | WCST No. PE | WCST No. Cat | |
|---|---|---|---|---|---|---|---|
| TOL No. Moves | – | −0.47** | −0.54** | −0.10 | |||
| TOL Pickup Time | −0.19 | – | 0.07 | 0.11 | |||
| TOH No. Moves | 0.38* | −0.03 | – | −0.27 | |||
| TOH Pickup Time | −0.02 | 0.02 | −0.07 | – | |||
| WCST No. Errors | 0.40** | −0.03 | 0.19 | 0.16 | – | ||
| WCST No. PE | −0.21 | 0.16 | 0.07 | −0.07 | −0.61 | – | |
| WCST No. Cat | −0.39** | 0.12 | −0.15 | 0.13 | −0.87** | 0.52** | – |
Note.
P < 0.05,
P < 0.01.
TOL = Tower of London; TOH = Tower of Hanoi; WCST = Wisconsin Card Sorting Test; PE = Perseverative Errors; Cat = Categories Completed
DISCUSSION
Although equally effective at restoring psychomotor vigilance speed and subjective sleepiness after 2 nights of continuous sleep deprivation, the 3 stimulant medications (at the dosages tested here) showed different patterns of effectiveness for sustaining performance on three tasks designed to measure separate but overlapping components of executive functioning. Specifically, modafinil (400 mg) and dextroamphetamine (20 mg) were effective at improving/sustaining sleep deprived performance on the TOL relative to placebo, whereas caffeine (600 mg) was not. On the other hand, the presently tested dose of caffeine was significantly more effective than either of the other 2 stimulants or placebo at sustaining sleep deprived TOH performance. Finally, the current dose of modafinil was more effective than both of the other stimulants and placebo at reducing perseverative responses and perseverative errors on the WCST during sleep deprivation. These differential findings underscore the heterogeneity of the concept of executive functions41 and further suggest that the 3 stimulant medications at the dosages employed in the present study are not identical in their effects on some aspects of executive functions in individuals undergoing continuous sleep deprivation.
Although all are considered to be tasks of executive function, the TOL, TOH, and WCST each measure distinct as well as broadly overlapping cognitive processes. Studies using functional neuroimaging techniques suggest that many tasks defined as measuring “executive functions” commonly activate a network of prefrontal brain regions including the mid-dorsolateral, mid-ventrolateral, and dorsal anterior cingulate gyrus.24 The TOL is generally considered to be a measure of planning ability42 that draws heavily—though not exclusively—upon the resources of the prefrontal cortex.43 This task activates the dorsolateral and rostrolateral prefrontal cortex as part of a larger network that may include premotor cortex, anterior cingulate gyrus, basal ganglia, and parietal regions.44,45 In addition to executive functions such as planning and sequencing, the TOL places strong cognitive processing demands on visuospatial working memory, as the examinee must initially plan out the solution to the problem in advance and maintain that solution in working memory while completing the necessary sequence of motor movements.46–48 However, this task appears to correlate most strongly with measures of fluid intelligence and problem solving, and once these capacities have been accounted for, other functions such as inhibitory capacity and working memory appear to contribute very little additional information to the prediction of TOL scores.48 Using a non-computerized version of the TOL task, Horne13 found that sleep deprived subjects took significantly longer to plan each solution than well-rested control subjects, but they did not differ from controls with regard to total time to solve the problem once they began moving the beads. This suggests that sleep deprivation primarily affects the planning and mental manipulation stage of processing for this task rather than the motivation or motor sequencing once a plan is in mind.
In the present study, 400 mg of modafinil significantly enhanced the ability of sleep deprived volunteers to complete the TOL solutions more efficiently (i.e., with fewer moves) than other participants that received placebo, but also more effectively than those that received equally alerting doses of caffeine. Although there were no effects of the drugs on total solution time, volunteers in the modafinil group did take significantly longer on average between each bead pick-up than did volunteers in all 3 of the other groups, suggesting that the more efficient solutions made by the modafinil group may have been partly a function of greater forethought and planning in the process of solving the puzzles.46 In other words, relative to the other conditions, the modafinil group appeared to be less impulsive and more deliberate in their approach to the task, thus ensuring that their solutions were closer to optimal on each move. Such findings are consistent with previous neuropsychological findings showing reduced impulsiveness and slowing of responses with modafinil,49 and recent functional neuroimaging evidence that suggests that modafinil may enhance prefrontal and executive network activity during sleep deprivation in healthy subjects50 and in patients with prefrontal pathology such as schizophrenia,51 particularly within dorsolateral regions. Dextroamphetamine (20 mg) was also more effective than placebo at sustaining the efficiency of TOL performance, but did not differ significantly from the other 2 stimulants.
The findings presented here suggest that relative to placebo, sleep deprived administration of the presently tested dose of modafinil was associated with more efficient and deliberative TOL solutions. Because there are currently no normative data available for this version of the computerized TOL in rested volunteers it is also plausible that sleep deprivation did not impair performance on this task and that the findings simply reflect enhanced cognitive performance above normal baseline, as has been shown in previous research with rested subjects.49 This seems unlikely, however, in light of the prior findings showing that sleep deprivation does impair performance on other versions of this task.13
In contrast to the findings for the TOL, sleep deprived performance on the TOH was significantly enhanced/sustained specifically by the administration of 600 mg of caffeine. In fact, the caffeine group not only outperformed the placebo group, but also showed significantly better performance than both the modafinil and dextroamphetamine groups on this task, despite essentially equivalent levels of subjective and objective alertness during the same testing period. Despite the similarities in form, the TOL and TOH appear to require somewhat different cognitive processes.52 In fact, these 2 tasks show only moderate correlations with one another, and the percentage of the variance that is not shared between them ranges from 84% to 93% across studies.47,48 Similarly, in the sleep deprivation condition of the present study, the strongest correlations between the TOL and TOH only accounted for 16% of the variance, suggesting that the greatest proportion of variability was accounted for by other factors.
The modest correlations between these 2 tasks may be due to the unique cognitive demands and problem solving strategies engendered by each. Relative to our version of the TOL, which only included puzzles that could be solved in ≤ 7 moves, our TOH task relied on strategic implementation of a repetitive multi-stage solution to the puzzle. Because the 5-ring TOH cannot be solved in fewer than 31 moves, a limit far beyond normal capacity of human working memory, the examinee must engage in a “recursive” process that involves accomplishing a number of sub-goals, which ultimately leads to the long term goal of a correct solution. In addition to the implementation of this recursive strategy, successful completion of the puzzle also requires the examinee to inhibit the natural inclination to move each ring to its final destination prematurely,48 and instead place the rings in counterintuitive locations. These counterintuitive moves lead to preliminary subgoals that must first be achieved before the final puzzle can be solved. This sets up what Goel & Grafman53 describe as a “goal-subgoal conflict” where the examinee must move progressively toward a larger goal by breaking the larger task down into a series of intermediate solutions involving iterations of counterintuitive sequences. Using functional neuroimaging, frontopolar regions (i.e., Brodmann's Area 10) have been shown to be critical for the ability to complete multiple subgoal tasks while keeping an overarching goal in mind.54 This same anterior brain region appears to be activated when an individual evaluates internally generated information during reasoning and problem solving.55 A recent functional neuroimaging study showed that caffeine significantly increased activity within a similar region of prefrontal cortex including bilateral medial frontopolar and anterior cingulate cortex during a working memory task.56 When considered in this context, the present findings tentatively suggest that caffeine may be particularly effective at sustaining frontopolar cortical systems that are important for the ability to mentally divide problems into subgoals while simultaneously evaluating progress against a larger goal.
The TOH and TOL share many similarities in their layout and administration and it would make sense that they would draw upon similar cognitive abilities and neural systems. In fact, evidence from a number of neuroimaging studies suggests that the 2 tasks do share common patterns of activation, particularly within the right dorsolateral prefrontal cortex and bilateral parietal and premotor regions.57,58 Functional neuroimaging studies have not directly compared these 2 tasks in the same participants, however, so it is not currently possible to determine the overlap of neuroanatomical regions involved in solving the puzzles. In the present study, participants always completed the tasks in the same order (i.e., TOH one hour before TOL) and there is the possibility that experience with the first task may have led to negative transfer, thereby affecting the learning and performance of the second task. Because we were interested in comparisons among drug groups within the same task and all groups completed the tasks in the identical order, this is unlikely to be a major confound because the interference effects should have been distributed equally for all groups. Furthermore, even if one or more of the drug conditions differentially affected the negative transfer of learning relative to the other groups, such data are important, because one aspect of executive function is the ability to ignore irrelevant distractions and resolve conflicting interference.11 The present findings also showed that the 2 tasks were moderately positively correlated with one another during sleep deprivation (i.e., subjects that solved the TOH in fewer moves also solved the TOL with fewer moves as well). This suggests that there is some similarity in the cognitive processes involved. Interestingly, this pattern was reversed following a night of recovery sleep, in that subjects that solved the TOH most efficiently tended to require many more moves to solve the TOL problems. The causes of this shift are uncertain, but underscore the fact that despite their inherent similarities, there are also significant differences in the processes required to solve these 2 puzzles. These findings also suggest that there may be some negative transfer, particularly after a night of recovery sleep, wherein the establishment of an effective strategy for one task may actually interfere with the ability to solve a similar appearing task that requires a different strategy.
There are also alternative explanations for the modest correlations between the TOL and TOH, however. It should be noted that in many studies, including the present investigation, a large number of executive function tasks are typically given as part of an extensive battery of tasks, and it is conceivable that time-on-task and fatigue-related effects may diminish the strength of the inter-task correlations. Nevertheless, despite the similarity in appearance between the TOL and TOH, these data suggest that these 2 tasks actually measure unique as well as shared aspects of planning and sequencing ability, and further suggest that caffeine may be particularly effective at sustaining the specific types of inhibitory and strategic problem solving required by the TOH relative to modafinil or dextroamphetamine. In contrast, the fluid intelligence and spatial working memory aspects of the TOL appear to be more effectively sustained by modafinil and dextroamphetamine.
The third executive function task used in this study was the WCST, which has long been considered to be the classical test of executive functions. Although many of the standard indices of the WCST showed no group differences, there was a significant effect of drug group with regard to perseverative responses and perseverative errors. Both of these indices provide information about how effectively an examinee is able to use ongoing feedback to recognize that the conditions of the task have changed and make appropriate shifts in behavior away from the formerly reinforced strategy and toward a new and successful strategy for solving the problem. Perseveration of thought and behavior is considered to be one of the hallmarks of dysfunction within the prefrontal cortex59 and perseverative errors have been particularly associated with lesions to the left prefrontal cortex60 and prefrontal white matter.61 Greater perseveration has also been found to occur as a result of sleep deprivation.15 Presently, sleep deprived participants in the modafinil (400 mg) group showed significantly less perseveration on incorrect strategies than did volunteers in the other 3 groups. This suggests that, at the dosages tested here, the 3 stimulants may have differential effects on the executive processes required by the WCST. Relative to clinical normative data, however, none of the groups in the present study performed outside of the normal range on the WCST, suggesting that the group differences reported here represent subclinical levels of severity.
The current findings differ somewhat from our previous study,2 which found no effects of equivalent doses of the same three stimulants on WCST performance. The divergent findings are likely due primarily to differences in the duration of sleep deprivation, the plasma concentration of the stimulants at testing, time of day of administration of the WCST, and baseline levels of cognitive ability. In our previous study, participants completed the WCST after 71.5 hours of sleep deprivation, whereas the test was administered after only 45 hours of sleep deprivation in the present study. The greater duration of sleep deprivation in the Wesensten et al. (2005) study may have, in and of itself, been enough to obscure any stimulant effects. In addition, the WCST was administered 6.5 hours after drug administration in the previous study, whereas the present WCST was given within one hour after drug administration. Consequently, the current testing occurred much closer to the peak plasma concentrations of all of the stimulants, which may account for the measureable differences observed among the stimulants. Furthermore, the time of WCST administration also differed between the 2 studies. Whereas the Wesensten et al. study administered the WCST at 06:35, during the early phase of the circadian upswing, the current administration of the test occurred at 04:00, proximal to the circadian trough of core body temperature. Finally, the previous study did not include a rested baseline test of global intellectual function, whereas the present study used an estimate of full scale intelligence as a statistical covariate to account for individual differences in cognitive ability among drug groups. Thus differences in findings across our 2 studies may be accounted for by these methodological variations.
From the present data, it is impossible to identify with certainty the specific executive systems that may be affected by each of the stimulants because of the complexity of the WCST and the multiple systems that it recruits. The WCST is sensitive to lesions to the dorsolateral prefrontal cortex,62 but its specificity for prefrontal damage has been questioned in recent years.63 Electrophysiological and functional neuroimaging studies suggest that while successful WCST performance does recruit prefrontal cortex,64 it also appears to recruit a number of non-frontal cortical regions such as the parietal cortex, basal ganglia, and cerebellum.63,65 A recent fMRI study demonstrated that the various cognitive components of the WCST involve several different cerebral networks, including systems for sustaining simple working memory (i.e., right ventrolateral prefrontal cortex), mental manipulation and complex working memory (i.e., right dorsolateral prefrontal cortex), maintaining visual representations in working memory (i.e., parietal cortex and retrosplenium), error detection (i.e., rostral anterior cingulate cortex and temporoparietal junction), and allocation of attention during increases in working memory load (caudal anterior cingulate and right dorsolateral prefrontal cortex), and shifting of mental set (i.e., cerebellum).66 While it appears that 400 mg of modafinil was effective at reducing perseverations on the WCST during sleep deprivation, it remains uncertain which of these neural systems may be most affected by modafinil and can account for the effects seen here.
In summary, these findings suggest that, at the doses used here, all 3 stimulants appear similarly effective at sustaining subjective and objective alertness, but each showed differential effectiveness for sustaining specific aspects of executive functioning and working memory. At the dosages used here, modafinil, and to a lesser extent, dextroamphetamine, both appeared to be effective at sustaining performance on tasks requiring visuospatial working memory and problem solving, while modafinil alone was effective at suppressing perseverative responses on a complex reasoning task. The current dose of caffeine, on the other hand, was most effective for a task requiring inhibitory behavior and the ability to focus on achieving multistep subgoals to achieve a larger goal. While it is not clear why there would be a differential effect on the various aspects of executive function, it is likely that the three stimulants each activate specific networks specialized for different cognitive processes. Because we only tested one dosage level for each stimulant, it is not possible to adequately address issues of equipotentiality of the specific dosages and stimulants for sustaining executive functioning. It is likely that at other dosage levels, further variability in the effects of each stimulant might have been observed. Future studies may consider using functional neuroimaging techniques to directly compare the relative activation produced by each of these stimulants at varying dosages during controlled executive function tasks such as those used here. Because the effects of these stimulants are often task specific, it will be important to compare drug effects within and across tasks.
When considering these findings, a few limitations should be kept in mind. First, it is critical to highlight the fact that the 3 tasks were administered at different time points over a 3.5-hour block beginning near the circadian trough and continuing through its subsequent upswing. Consequently, the circadian drive for sleep would be presumed to have been greatest for earlier tasks relative to those administered later, and may have differentially affected performance on the 3 tasks. This confound makes it inappropriate to compare directly across tasks. However, the goal of the present study was not to compare performance across tasks (which would have been inappropriate due to their different scaling and lack of common normative base), but rather was to compare across drug groups within each task. In other words, for each given executive function task, this study was designed to compare the effects of the 3 stimulants on that task. To that end, a second limitation is that caffeine, modafinil, and dextroamphetamine have notably different half-lives. The half-life of caffeine is 5 to 6 hours, the shortest of the 3 stimulants.67,68 In contrast, the half-life of modafinil is about 10-12 hours (product monograph, Provigil), and the half-life of dextroamphetamine is approximately 12 hours (product monograph, Dexedrine). Since the tasks were administered between 1 and 4.5 hours after the drug administration, it is possible that some of the observed differences across the 3 tasks may have been due to the different plasma concentrations at the time of testing. This is unlikely, however, as all tasks were administered well within their respective half-lives, and all 3 stimulants were significantly more effective than placebo but not significantly different from one another with regard to PVT performance and SSS scores across this same time period. Thirdly, this study used a single administration of each drug, so it is not possible to determine any potential effects on executive functioning with the sustained use of the stimulants. Future studies should examine the effects of repeated or sustained administration of stimulants on executive functioning because this may be more representative of real-life patterns of stimulant use. It will also be important to examine whether the effects of stimulants on executive function are similar in situations involving chronic sleep restriction, a common problem in many occupational settings, as opposed to continuous sleep deprivation as in the present study.
The tasks used here only represent a small sample of the range of cognitive processes subsumed under the construct of “executive functions.” Future research should examine the effects of sleep loss and stimulants on other dimensions of executive functioning, such as the ability to update, rehearse, and manipulate information in working memory, a capacity that is well measured by the classic “n-back” tasks.69 Such tasks draw heavily upon the resources of the prefrontal cortex, particularly the dorsolateral and anterior cingulate regions.69,70 An additional limitation is that the current study lacked rested-baseline comparison data for the executive function tasks. Unfortunately, the very nature of these specific executive function tasks precluded this possibility. The assessment of executive function by the 3 tasks used here was predicated on the novelty of the task. For this reason, the executive function tasks were not repeatable, and we were, therefore, constrained to a between groups comparison among the 4 drug conditions at a particular point in time. It is, therefore, impossible to rule out individual differences in baseline cognitive ability that may have contributed to the results. This is unlikely, however, as we did statistically covary for baseline level of intellectual functioning in the analyses using an abbreviated intelligence test given when the participants were rested. Regardless, future studies should consider using executive function tasks that may be repeated within a short time span. Finally, the sample sizes were also relatively small, which may have limited the power to detect significant differences between groups, potentially leading to type II errors. Since some of the stimulant effects may be more subtle, it will be important for future studies to utilize larger sample sizes.
With these limitations in mind, these findings suggest that while caffeine, dextroamphetamine, and modafinil all appear effective at sustaining alertness, they each show different degrees of effectiveness at restoring/sustaining executive functions depending on the particular task in question. Such findings raise the possibility that optimal sustainment of executive functioning during periods of sleep loss may require an understanding of how these stimulants may operate in combination. Until such research is available, the present findings underscore the importance of obtaining sufficient sleep in order to perform adequately on high-level problem solving tasks. While stimulants may generally sustain alertness and vigilance during periods of inadequate sleep, they do not necessarily improve all aspects of cognition equally.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Killgore works part-time as a contract researcher for the US Army. Dr. Balkin owns a private consulting business. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This material has been reviewed by the Walter Reed Army Institute of Research and there is no objection to its presentation and/or publication. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Army, the Department of Defense, the U.S. Government, or any of the institutions with which the authors are affiliated.
REFERENCES
- 1.Doran SM, Van Dongen HP, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Archives Italiennes de Biologie (Pisa) 2001;139:253–67. [PubMed] [Google Scholar]
- 2.Wesensten NJ, Killgore WDS, Balkin TJ. Performance and alertness effects of caffeine, dextroamphetamine, and modafinil during sleep deprivation. J Sleep Res. 2005;14:255–66. doi: 10.1111/j.1365-2869.2005.00468.x. [DOI] [PubMed] [Google Scholar]
- 3.Kahn-Greene ET, Lipizzi EL, Conrad AK, Kamimori GH, Killgore WDS. Sleep deprivation adversely affects interpersonal responses to frustration. Pers Individ Dif. 2006;41:1433–43. [Google Scholar]
- 4.Kahn-Greene ET, Killgore DB, Kamimori GH, Balkin TJ, Killgore WDS. The effects of sleep deprivation on symptoms of psychopathology in healthy adults. Sleep Med. 2007;8:215–21. doi: 10.1016/j.sleep.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Selvi Y, Gulec M, Agargun MY, Besiroglu L. Mood changes after sleep deprivation in morningness-eveningness chronotypes in healthy individuals. J Sleep Res. 2007;16:241–4. doi: 10.1111/j.1365-2869.2007.00596.x. [DOI] [PubMed] [Google Scholar]
- 6.Damasio AR. Descartes' error: Emotion, reason and the human brain. New York: Grosset/Putnam; 1994. [Google Scholar]
- 7.Binks PG, Waters WF, Hurry M. Short-term total sleep deprivations does not selectively impair higher cortical functioning. Sleep. 1999;22:328–34. doi: 10.1093/sleep/22.3.328. [DOI] [PubMed] [Google Scholar]
- 8.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–29. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 9.Nilsson JP, Soderstrom M, Karlsson AU, et al. Less effective executive functioning after one night's sleep deprivation. J Sleep Res. 2005;14:1–6. doi: 10.1111/j.1365-2869.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- 10.Harrison Y, Horne JA. The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl. 2000;6:236–49. doi: 10.1037//1076-898x.6.3.236. [DOI] [PubMed] [Google Scholar]
- 11.Jones K, Harrison Y. Frontal lobe function, sleep loss and fragmented sleep. Sleep Med Rev. 2001;5:463–75. doi: 10.1053/smrv.2001.0203. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter PA, Just MA, Reichle ED. Working memory and executive function: evidence from neuroimaging. Curr Opin Neurobiol. 2000;10:195–9. doi: 10.1016/s0959-4388(00)00074-x. [DOI] [PubMed] [Google Scholar]
- 13.Horne JA. Sleep loss and «divergent» thinking ability. Sleep. 1988;11:528–36. doi: 10.1093/sleep/11.6.528. [DOI] [PubMed] [Google Scholar]
- 14.Harrison Y, Horne JA. Sleep deprivation affects speech. Sleep. 1997;20:871–7. doi: 10.1093/sleep/20.10.871. [DOI] [PubMed] [Google Scholar]
- 15.Gottselig JM, Adam M, Retey JV, Khatami R, Achermann P, Landolt HP. Random number generation during sleep deprivation: effects of caffeine on response maintenance and stereotypy. J Sleep Res. 2006;15:31–40. doi: 10.1111/j.1365-2869.2006.00497.x. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh S, Cheng IC, Tsai LL. Immediate error correction process following sleep deprivation. J Sleep Res. 2007;16:137–47. doi: 10.1111/j.1365-2869.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- 17.Drummond SP, Paulus MP, Tapert SF. Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. J Sleep Res. 2006;15:261–5. doi: 10.1111/j.1365-2869.2006.00535.x. [DOI] [PubMed] [Google Scholar]
- 18.Killgore WDS, Balkin TJ, Wesensten NJ. Impaired decision-making following 49 hours of sleep deprivation. J Sleep Res. 2006;15:7–13. doi: 10.1111/j.1365-2869.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- 19.Killgore WDS, Lipizzi EL, Kamimori GH, Balkin TJ. Caffeine effects on risky decision-making after 75 hours of sleep deprivation. Aviat Space Environ Med. 2007;78:957–62. doi: 10.3357/asem.2106.2007. [DOI] [PubMed] [Google Scholar]
- 20.Killgore WDS. Effects of sleep deprivation and morningness-eveningness traits on risk-taking. Psychol Rep. 2007;100:613–26. doi: 10.2466/pr0.100.2.613-626. [DOI] [PubMed] [Google Scholar]
- 21.McKenna BS, Dicjinson DL, Orff HJ, Drummond SP. The effects of one night of sleep deprivation on known-risk and ambiguous-risk decisions. J Sleep Res. 2007;16:245–52. doi: 10.1111/j.1365-2869.2007.00591.x. [DOI] [PubMed] [Google Scholar]
- 22.Killgore WDS, Killgore DB, Day LM, Li C, Kamimori GH, Balkin TJ. The effects of 53 hours of sleep deprivation on moral judgment. Sleep. 2007;30:345–52. doi: 10.1093/sleep/30.3.345. [DOI] [PubMed] [Google Scholar]
- 23.Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York: Oxford; 2002. [Google Scholar]
- 24.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–83. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 25.Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 26.Wu JC, Gillin JC, Buchsbaum MS, et al. Frontal lobe metabolic decreases with sleep deprivation not totally reversed by recovery sleep. Neuropsychopharmacology. 2006;31:2783–92. doi: 10.1038/sj.npp.1301166. [DOI] [PubMed] [Google Scholar]
- 27.Drummond SP, Meloy MJ, Yanagi MA, Orff HJ, Brown GG. Compensatory recruitment after sleep deprivation and the relationship with performance. Psychiatry Res. 2005;140:211–23. doi: 10.1016/j.pscychresns.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Stricker JL, Brown GG, Wetherell LA, Drummond SP. The impact of sleep deprivation and task difficulty on networks of fMRI brain response. J Int Neuropsychol Soc. 2006;12:591–7. doi: 10.1017/S1355617706060851. [DOI] [PubMed] [Google Scholar]
- 29.Elmenhorst D, Meyer PT, Winz OH, et al. Sleep deprivation increases A1 adenosine receptor binding in the human brain: a positron emission tomography study. J Neurosci. 2007;27:2410–5. doi: 10.1523/JNEUROSCI.5066-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caldwell JA, Caldwell JL. Fatigue in military aviation: an overview of US military-approved pharmacological countermeasures. Aviat Space Environ Med. 2005;76:C39–51. [PubMed] [Google Scholar]
- 31.Lieberman HR, Tharion WJ, Shukitt-Hale B, Speckman KL, Tulley R. Effects of caffeine, sleep loss, and stress on cognitive performance and mood during U.S. Navy SEAL training. Psychopharmacology (Berl.) 2002;164:250–61. doi: 10.1007/s00213-002-1217-9. [DOI] [PubMed] [Google Scholar]
- 32.Caldwell JA, Smythe NK, Leduc PA, Caldwell JL. Efficacy of Dexedrine for maintaining aviator performance during 64 hours of sustained wakefulness: a simulator study. Aviat Space Environ Med. 2000;71:7–18. [PubMed] [Google Scholar]
- 33.Caldwell JAJ, Caldwell JL, Smythe NKI, Hall KK. A double-blind, placebo-controlled investigation of the efficacy of modafinil for sustaining the alertness and performance of aviators: A helicopter simulator study. Psychopharmacology (Berl.) 2000;150:272–82. doi: 10.1007/s002130000450. [DOI] [PubMed] [Google Scholar]
- 34.Killgore WDS, McBride SA, Killgore DB, Balkin TJ. The effects of caffeine, dextroamphetamine, and modafinil on humor appreciation during sleep deprivation. Sleep. 2006;29:841–7. doi: 10.1093/sleep/29.6.841. [DOI] [PubMed] [Google Scholar]
- 35.Huck NO, McBride SA, Kendall AP, Grugle NL, Killgore WD. The effects of modafinil, caffeine, and dextroamphetamine on judgments of simple versus complex emotional expressions following sleep deprivation. Int J Neurosci. 2008;118:487–502. doi: 10.1080/00207450601125907. [DOI] [PubMed] [Google Scholar]
- 36.Killgore WDS, Killgore DB. Morningness-eveningness correlates with verbal ability in women but not men. Percept Mot Skills. 2007;104:335–8. doi: 10.2466/pms.104.1.335-338. [DOI] [PubMed] [Google Scholar]
- 37.Killgore WDS, McBride SA. Odor identification accuracy declines following 24 h of sleep deprivation. J Sleep Res. 2006;15:111–6. doi: 10.1111/j.1365-2869.2006.00502.x. [DOI] [PubMed] [Google Scholar]
- 38.Killgore WD, Rupp TL, Grugle NL, Reichardt RM, Lipizzi EL, Balkin TJ. Effects of dextroamphetamine, caffeine and modafinil on psychomotor vigilance test performance after 44 h of continuous wakefulness. J Sleep Res. 2008:309–21. doi: 10.1111/j.1365-2869.2008.00654.x. [DOI] [PubMed] [Google Scholar]
- 39.Wesensten NJ, Belenky G, Kautz MA, Thorne DR, Reichardt RM, Balkin TJ. Maintaining alertness and performance during sleep deprivation: modafinil versus caffeine. Psychopharmacology (Berl.) 2002;159:238–47. doi: 10.1007/s002130100916. [DOI] [PubMed] [Google Scholar]
- 40.Thorne DR, Johnson DE, Redmond DP, Sing HC, Belenky G, Shapiro JM. The Walter Reed palm-held psychomotor vigilance test. Behav Res Methods. 2005;37:111–8. doi: 10.3758/bf03206404. [DOI] [PubMed] [Google Scholar]
- 41.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex «Frontal Lobe» tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 42.Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- 43.Baker SC, Rogers RD, Owen AM, et al. Neural systems engaged by planning: a PET study of the Tower of London task. Neuropsychologia. 1996;34:515–26. doi: 10.1016/0028-3932(95)00133-6. [DOI] [PubMed] [Google Scholar]
- 44.Boghi A, Rasetti R, Avidano F, et al. The effect of gender on planning: An fMRI study using the Tower of London task. Neuroimage. 2006;33:999–1010. doi: 10.1016/j.neuroimage.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 45.Dagher A, Owen AM, Boecker H, Brooks DJ. Mapping the network for planning: a correlational PET activation study with the Tower of London task. Brain. 1999;122(Pt 10):1973–87. doi: 10.1093/brain/122.10.1973. [DOI] [PubMed] [Google Scholar]
- 46.Asato MR, Sweeney JA, Luna B. Cognitive processes in the development of TOL performance. Neuropsychologia. 2006;44:2259–69. doi: 10.1016/j.neuropsychologia.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Welsh MC, Satterlee-Cartmell T, Stine M. Towers of Hanoi and London: contribution of working memory and inhibition to performance. Brain Cogn. 1999;41:231–42. doi: 10.1006/brcg.1999.1123. [DOI] [PubMed] [Google Scholar]
- 48.Zook NA, Davalos DB, Delosh EL, Davis HP. Working memory, inhibition, and fluid intelligence as predictors of performance on Tower of Hanoi and London tasks. Brain Cogn. 2004;56:286–92. doi: 10.1016/j.bandc.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 49.Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, Sahakian BJ. Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology (Berl.) 2003;165:260–9. doi: 10.1007/s00213-002-1250-8. [DOI] [PubMed] [Google Scholar]
- 50.Thomas RJ, Kwong K. Modafinil activates cortical and subcortical sites in the sleep-deprived state. Sleep. 2006;29:1471–81. doi: 10.1093/sleep/29.11.1471. [DOI] [PubMed] [Google Scholar]
- 51.Hunter MD, Ganesan V, Wilkinson ID, Spence SA. Impact of modafinil on prefrontal executive function in schizophrenia. Am J Psychiatry. 2006;163:2184–6. doi: 10.1176/appi.ajp.163.12.2184. [DOI] [PubMed] [Google Scholar]
- 52.Welsh MC, Revilla V, Strongin D, Kepler M. Towers of Hanoi and London: is the nonshared variance due to differences in task administration? Percept Mot Skills. 2000;90:562–72. doi: 10.2466/pms.2000.90.2.562. [DOI] [PubMed] [Google Scholar]
- 53.Goel V, Grafman J. Are the frontal lobes implicated in «planning» functions? Interpreting data from the Tower of Hanoi. Neuropsychologia. 1995;33:623–42. doi: 10.1016/0028-3932(95)90866-p. [DOI] [PubMed] [Google Scholar]
- 54.Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–51. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- 55.Christoff K, Gabrieli J. The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28:168–86. [Google Scholar]
- 56.Koppelstaetter F, Poeppel TD, Siedentopf CM, et al. Does caffeine modulate verbal working memory processes? An fMRI study. Neuroimage. 2008;39:492–9. doi: 10.1016/j.neuroimage.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 57.Fincham JM, Carter CS, van Veen V, Stenger VA, Anderson JR. Neural mechanisms of planning: a computational analysis using event-related fMRI. Proc Natl Acad Sci U S A. 2002;99:3346–51. doi: 10.1073/pnas.052703399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schall U, Johnston P, Lagopoulos J, et al. Functional brain maps of Tower of London performance: a positron emission tomography and functional magnetic resonance imaging study. Neuroimage. 2003;20:1154–61. doi: 10.1016/S1053-8119(03)00338-0. [DOI] [PubMed] [Google Scholar]
- 59.Sullivan EV, Mathalon DH, Zipursky RB, Kersteen-Tucker Z, Knight RT, Pfefferbaum A. Factors of the Wisconsin Card Sorting Test as measures of frontal-lobe function in schizophrenia and in chronic alcoholism. Psychiatry Res. 1993;46:175–99. doi: 10.1016/0165-1781(93)90019-d. [DOI] [PubMed] [Google Scholar]
- 60.Goldstein B, Obrzut JE, John C, Ledakis G, Armstrong CL. The impact of frontal and non-frontal brain tumor lesions on Wisconsin Card Sorting Test performance. Brain Cogn. 2004;54:110–6. doi: 10.1016/S0278-2626(03)00269-0. [DOI] [PubMed] [Google Scholar]
- 61.Arnett PA, Rao SM, Bernardin L, Grafman J, Yetkin FZ, Lobeck L. Relationship between frontal lobe lesions and Wisconsin Card Sorting Test performance in patients with multiple sclerosis. Neurology. 1994;44:420–5. doi: 10.1212/wnl.44.3_part_1.420. [DOI] [PubMed] [Google Scholar]
- 62.Barcelo F, Knight RT. Both random and perseverative errors underlie WCST deficits in prefrontal patients. Neuropsychologia. 2002;40:349–56. doi: 10.1016/s0028-3932(01)00110-5. [DOI] [PubMed] [Google Scholar]
- 63.Barcelo F, Santome-Calleja A. [A critical review of the specificity of the Wisconsin card sorting test for the assessment of prefrontal function] Rev Neurol. 2000;30:855–64. [PubMed] [Google Scholar]
- 64.Berman KF, Ostrem JL, Randolph C, et al. Physiological activation of a cortical network during performance of the Wisconsin Card Sorting Test: a positron emission tomography study. Neuropsychologia. 1995;33:1027–46. doi: 10.1016/0028-3932(95)00035-2. [DOI] [PubMed] [Google Scholar]
- 65.Nagahama Y, Fukuyama H, Yamauchi H, et al. Cerebral activation during performance of a card sorting test. Brain. 1996;119(Pt 5):1667–75. doi: 10.1093/brain/119.5.1667. [DOI] [PubMed] [Google Scholar]
- 66.Lie CH, Specht K, Marshall JC, Fink GR. Using fMRI to decompose the neural processes underlying the Wisconsin Card Sorting Test. Neuroimage. 2006;30:1038–49. doi: 10.1016/j.neuroimage.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 67.Whitsett TL, Manion CV, Christensen HD. Cardiovascular effects of coffee and caffeine. Am J Cardiol. 1984;53:918–22. doi: 10.1016/0002-9149(84)90525-3. [DOI] [PubMed] [Google Scholar]
- 68.Patwardhan RV, Desmond PV, Johnson RF, Schenker S. Impaired elimination of caffeine by oral contraceptive steroids. J Lab Clin Med. 1980;95:603–8. [PubMed] [Google Scholar]
- 69.Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–61. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- 70.D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]