Abstract
Study Objectives:
Obstructive Sleep Apnea (OSA) is associated with a poor prognosis in patients with coronary artery disease. We hypothesized that abnormalities of coronary blood flow (CBF) associated with obstructive apneas may predispose patients to ischemia. We aimed to determine CBF during respiratory events in patients with OSA.
Setting:
University Hospital.
Patients:
Ten subjects undergoing elective percutaneous coronary intervention
Design:
We measured CBF and myocardial work (rate-pressure product [RPP]) in a non-culprit coronary artery in patients sleeping in the cardiac catheterization laboratory. Hemodynamic responses were matched to spontaneously occurring respiratory events.
Measurements and Results:
Events comprised a mixture of obstructive apneas, central apneas and hypopneas. RPP increased at the termination of each type of respiratory event. Following the rise in RPP, there was a delay, identified with breakpoint analysis, before CBF began to increase (P < 0.001) that differed in duration with event type: 8 sec for obstructive apnea, 5 sec for central apnea, and 4 sec for hypopnea. The delay in CBF with obstructive apnea was associated with an increase in coronary vascular resistance of 16% ± 4% (P < 0.05). Stepwise multilinear regression analysis showed the increase in CBF was predicted by the rise in RPP (R = 0.52, P < 0.001) and presence of arousal from sleep (R = 0.30, P < 0.05), but not the degree of O2 desaturation.
Conclusion:
Following obstructive apneas there is a transient uncoupling of CBF from myocardial work and an increase in CVR. This disturbed flow-metabolic coupling may lead to nocturnal myocardial ischemia in patients with both OSA and coronary artery disease.
Citation:
Hamilton GS; Meredith IT; Walker AM; Solin P. Obstructive sleep apnea leads to transient uncoupling of coronary blood flow and myocardial work in humans. SLEEP 2009;32(2):263-270.
Keywords: Sleep apnea, blood flow, coronary circulation
OBSTRUCTIVE SLEEP APNEA (OSA) IS A COMMON CONDITION AFFECTING 24% OF MEN AND 9% OF WOMEN AGED 30–60 YEARS1 AND HAS BEEN independently associated with a variety of cardiovascular diseases.2 In patients with coronary artery disease, the presence of OSA adversely affects prognosis and is an independent predictor of cardiovascular mortality.3,4 Furthermore, sudden cardiac death in patients with OSA is 2.57 times as likely to occur between midnight and 06:00 as in a control population.5 Patients with both OSA and coronary artery disease also have a higher rate of nocturnal ischemic events than those with OSA alone.6
The mechanisms underlying these associations are incompletely understood, but the termination of apnea may represent a time of elevated risk. Peled et al.6 have demonstrated that nocturnal ischemic events predominantly occur during the rebreathing phase following the obstructive apnea, not during the apnea itself. This post-apneic phase is the time of increased heart rate and blood pressure and thus greatest myocardial energy demand.7 As this is also the time of maximal oxygen desaturation, myocardial oxygen transport may be impaired should there be inadequate compensatory CBF changes.
Limited data exist on the effects of OSA on coronary blood flow (CBF). Animal studies show CBF increases with OSA, but the relevance of hypoxia or other factors in driving CBF changes is uncertain.8,9 Notably, no study has explored in detail the relationship of CBF with myocardial work during OSA, and no human data exist on the subject. Accordingly, we undertook a study examining CBF in an angiographically smooth appearing coronary artery during spontaneously occurring episodes of sleep apnea, in patients undergoing elective coronary angioplasty to another vessel.
METHODS
Study Population
Ten patients (8 males, 2 females), with stable but symptomatic ischemic heart disease, were studied following elective coronary angioplasty and stenting. Patients were recruited based on the suitability of their coronary anatomy and willingness to sleep in the cardiac catheterization laboratory, not on the presence of known pre-existing OSA. Clinical characteristics are shown in Table 1. Subjects had uncomplicated single or double vessel coronary artery disease requiring percutaneous coronary angioplasty. Angiographically smooth or mildly irregular non-culprit coronary arteries were selected for flow determinations.
Table 1.
Clinical Characteristics of Study Subjects
| Patients (n = 10) | |
|---|---|
| Age (y) | 60.3 ± 9.1 |
| Sex, M/F | 8/2 |
| BMI | 29.8 ± 4.4 |
| Ex-smoker | 7 (70%) |
| Diabetes mellitus | 3 (30%) |
| Hypertension | 5 (50%) |
| Hyperlipidemia | 10 (100%) |
| Regular snoring | 7 (70%) |
| Epworth Sleepiness Scale score | 9.1 ± 6.2 |
| Midazolam dose (mg) | 3.9 ± 1.7 |
| Overnight AHI (events/h) | 20.8 ± 19.3 |
Data are mean ± SD or number (%) of patients with the factor. AHI indicates apnea-hypopnea index on subsequent overnight polysomnography, not during the percutaneous coronary intervention.
The left anterior descending artery was studied in 4 patients; the circumflex artery in 4; the left main coronary artery in 1; and the right coronary artery in 1. The vessel subtended viable myocardium, as defined by no Q waves on ECG and no hypokinesis, akinesis, or dyskinesis on left ventriculography. Exclusion criteria included unstable angina, significant left ventricular impairment or valvular disease, left main stem or triple vessel disease coronary disease. All patients were offered an overnight polysomnogram and follow-up with a sleep physician at the completion of the study.
Study Protocol
Written informed consent was obtained from all patients prior to study entry, and the study protocol was approved by the Human Research Ethics Committee at Monash Medical Centre. Patients were admitted on the morning of their coronary procedure and all usual medications were administered as directed by the treating doctor. Prior to angioplasty the patients were intrumented for standard polysomnography, with the exception of thoracic respiratory bands, which were omitted so as not to interfere with fluoroscopic screening during angioplasty. Sleep study data were acquired in the cardiac catheterization laboratory using a portable system (Compumedics Siesta, Abbotsford, Victoria, Australia) with the following signals measured: electroencephalogram (C3/A2, C4/A1, O1/A2, O2/A1), electro-oculogram (bilateral), electrocardiogram, submental electromyogram, oxyhemoglobin saturation (finger pulse oximetry), abdominal excursion (piezoelectric band), nasal pressure for airflow estimation, and oronasal thermistor.
Following successful and uncomplicated coronary angioplasty, a Doppler coronary blood flow velocity wire (Cardiometrics FloWire, California, USA) was positioned in an adjacent segment of smooth or mildly irregular conduit coronary artery to obtain a stable coronary flow signal. Sedation with intravenous midazolam was allowed to help facilitate sleep. The patient was then left to sleep for a 30-min period, during which there was continuous acquisition of polysomnographic, respiratory, and hemodynamic data (coronary blood flow velocity, central aortic blood pressure, and heart rate). After the 30-min period, the patient was awakened, and the study was terminated.
Data Analysis
Data were analyzed on 2 software systems – Profusion PSG (Compumedics, Abbotsford, Victoria, Australia) for sleep and respiratory data, and Chart (AD Instruments, Sydney, Australia) for hemodynamic data. A visual record of the hemodynamic data was continuously sent to Profusion PSG to ensure precise time-matching of sleep, respiratory, and hemodynamic data.
The stage of sleep was determined using standard criteria.10 Respiratory events were scored according to recently published guidelines.11 Coronary flow velocity was measured continuously. To calculate coronary blood flow (CBF), coronary diameter was measured by quantitative coronary angiography using standard techniques.12,13 CBF was calculated from the product of average peak velocity (APV) and vessel cross-sectional area.13,14 Myocardial work was calculated using rate-pressure product (RPP), the product of mean aortic blood pressure and heart rate.15 Coronary vascular resistance (CVR) was calculated from mean arterial blood pressure (MABP)/CBF; and CBF corrected for myocardial work was determined by the ratio CBF:RPP.13 All beat-to-beat hemodynamic data were averaged over 1 sec, then matched to the corresponding sleep stage and to all respiratory events. Hemodynamic changes (%) related to a respiratory event were calculated from a baseline value, represented by an average over a 10-sec period, starting 20 sec prior to the end of each event. Data for each of the 3 types of respiratory event (obstructive apnea, central apnea, or hypopnea) were pooled and averaged for each patient; individual patient data were pooled to create a mean value for each type of respiratory event.
Statistical Analysis
Demographic data are expressed as mean ± SD. Changes from baseline in CBF and RPP at each time point within a respiratory event are expressed as mean ± SEM. Change in CBF and RPP over the period from 15 sec prior to and 15 sec after the end of the respiratory event were compared using 2-way repeated analysis of variance. Breakpoints identifying the time when CBF and RPP began to increase were determined using an analytical process based on polynomial regression analysis and analysis of variance.16,17 Values for CBF and RPP versus time were each fitted with 2 regression lines that intersected at a test point that was progressively moved across the range of data, summing the residual sums of squares at each point, with the breakpoint defined as the time-point at which the residual sums of squares reached a minimum. Breakpoints therefore identified the time when CBF and RPP began to increase. The breakpoint, slope, and identity of each regression line for CBF and RPP versus time were then compared using the method described by Glantz.18 Changes from baseline in CVR were compared using one-way repeated measures ANOVA for each of obstructive apnea, central apnea, and hypopnea. The Holm-Sidak test was used post hoc to identify the origin of any differences found using ANOVA.
Forward stepwise multiple linear regressions were used to determine the predictors of maximal CBF response, the maximal CVR, and the maximal CBF change corrected for RPP during respiratory events. All statistical tests (other than those described by Glantz18) were performed using SigmaStat 3.0 (Systat Software, CA, USA), and statistical significance was accepted at P < 0.05.
RESULTS
Ten patients (8M, 2F) aged 60.3 ± 9.1 years and with a BMI of 29.8 ± 4.4 kg/m2 were studied. The characteristics of these patients are summarized in Table 1. Sleep was fragmented with many sleep-wake transitions. There were 94 epochs of stage 1 sleep; 169 epochs of stage 2 sleep; and 7 epochs of stage 3/4 sleep. There was no REM sleep. One patient had good quality sleep with stable respiration throughout, while the other 9 patients had unstable breathing patterns with frequent respiratory events (Table 2). Overall there were large numbers of obstructive apneas (OA, n = 45), central apneas (CA, n = 70), and hypopneas (HP, n = 62). The characteristics of these respiratory events are summarized in Table 2. CA exhibited a pattern of cyclical periodic breathing, commonly seen in fragmented sleep and drowsy wakefulness, but not the pattern of Cheyne-Stokes respiration. Following the study, 8 patients agreed to have an overnight sleep study. Total apnea-hypopnea index (AHI) ranged from 0–60.2/h, with a mean of 20.8/h. All those with obstructive apneas during the CBF study had evidence of OSA on overnight sleep study (except one patient who declined to have the overnight study) with a mean AHI of 28.4/h.
Table 2.
Characteristics of Respiratory Events
| OA | CA | HP | |
|---|---|---|---|
| No. of events | 45 | 70 | 62 |
| No. of patients with event | 5 | 5 | 6 |
| SpO2 min (%) | 92.9 ± 3.4 | 91.2 ± 3.6 | 92.1 ± 3.2 |
| O2 desaturation (%) | 3.3 ± 2.5 | 2.5 ± 2.6 | 2.2 ± 1.5 |
| Events with arousal (%) | 71 | 50 | 53 |
OA = Obstructive Apnea, CA = Central Apnea, HP = Hypopnea. For SpO2 min and O2 desaturation (%), values are represented as mean ± standard deviation.
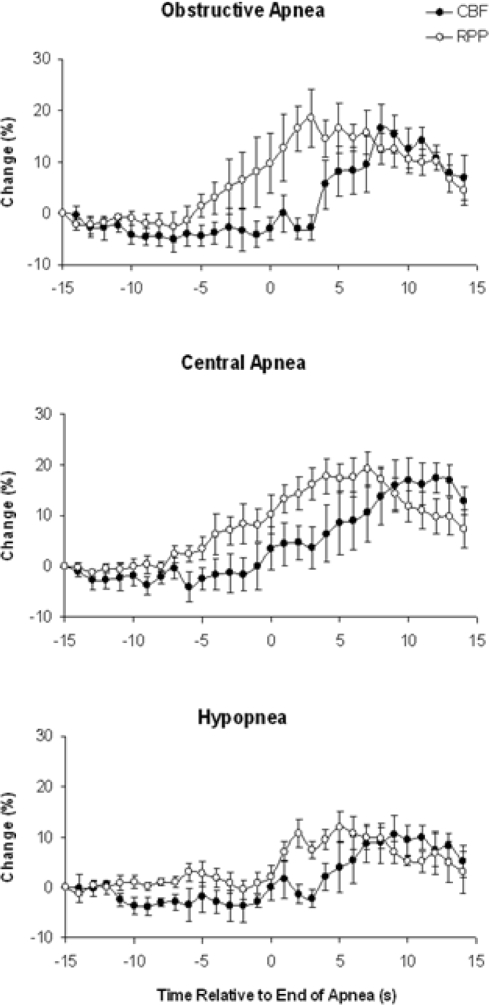
CBF and RPP before and after termination of each type of respiratory event are shown in Figure 1. CBF and RPP increased significantly (P < 0.05) following OA (17% ± 5% and 19% ± 6% respectively), CA (17% ± 3% and 19% ± 3%), and HP (10% ± 4% and 12% ± 3%). Examples of recordings from a single patient are demonstrated in Figure 4.
Figure 1.
Change in CBF and RPP from 15 sec before to 15 sec after the end of OA, CA, and HP. Data represent mean ± SEM for pooled results of 5 patients with OA, 5 patients with CA, and 6 patients with HP. Note the dissociation between CBF and RPP with obstructive apnea.
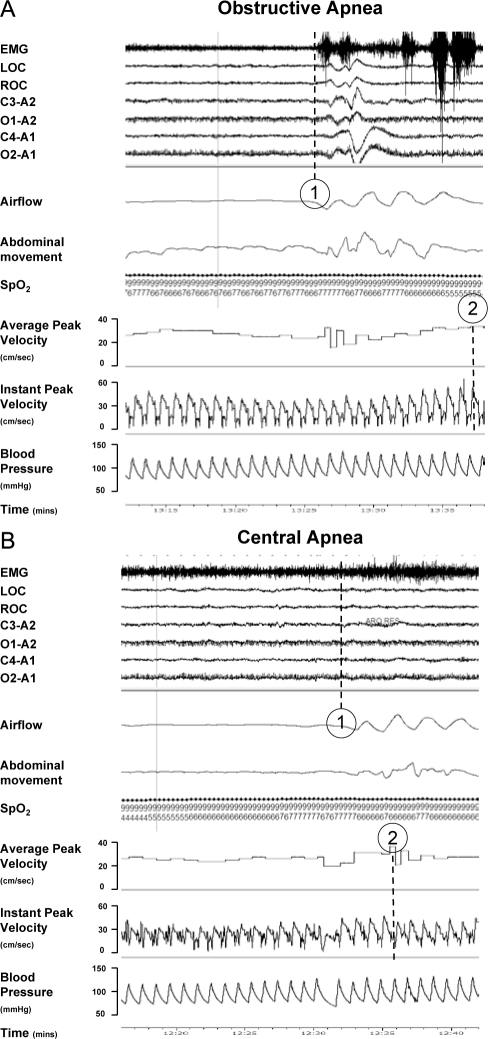
Figure 4.
Illustrative clinical recordings from a single patient for (A) obstructive apnea and (B) central apnea. The average and instant peak velocity represents the coronary blood flow. Following obstructive apnea (A) note the delay (~ 10 sec) between the termination of the obstructive apnea event (1) and the peak coronary blood flow response (2). Note the lesser delay (~ 4 sec) after termination of central apnea (B).
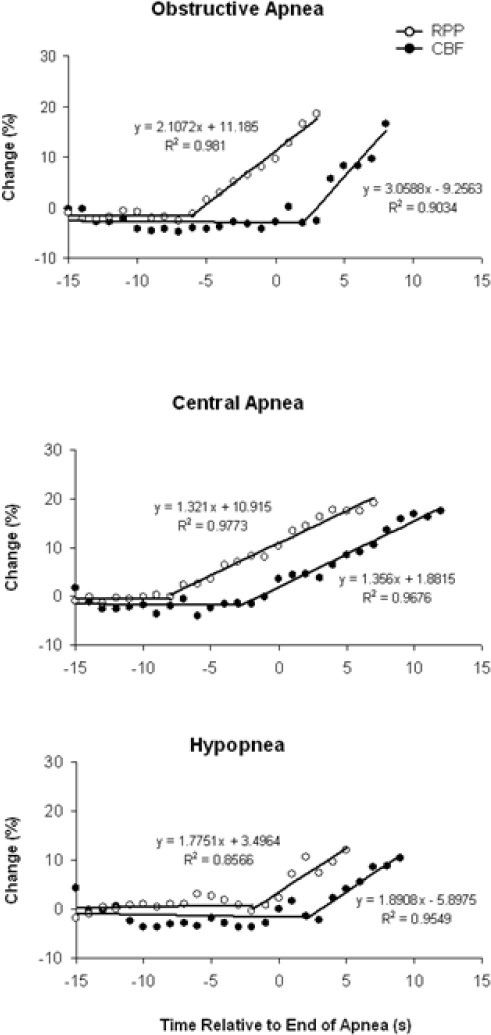
Bilinear regression curves demonstrating the time course of CBF and RPP are shown in Figure 2. With OA, the increase in CBF followed the increase in RPP with a delay of 8 sec, and then increased more steeply than the RPP rise, signified by significant differences (P < 0.001) of intercept, slope, and coincidence of regression lines. In comparison, the increase in CBF for CA and HP followed the increase in RPP with a delay of 5 sec and 4 sec, respectively, and then increased at the same rate as the RPP rise (P < 0.001 for difference of intercept and coincidence; P = NS for difference of slope).
Figure 2.
Bilinear regression curves demonstrating the relationship between CBF and RPP. Data represent mean values for pooled results of 5 patients with OA, 5 patients with CA, and 6 patients with HP. For OA, the increase in CBF follows the increase in RPP with a delay of 8 sec, then the slope of rise is more steep (P < 0.001 for difference of intercept, slope, and coincidence). For CA and HP, the increase in CBF follows the increase in RPP with a delay of 5 and 4 sec, respectively, then increases at the same rate. (P < 0.001 for difference in intercept and coincidence. P = NS for difference of slope.)
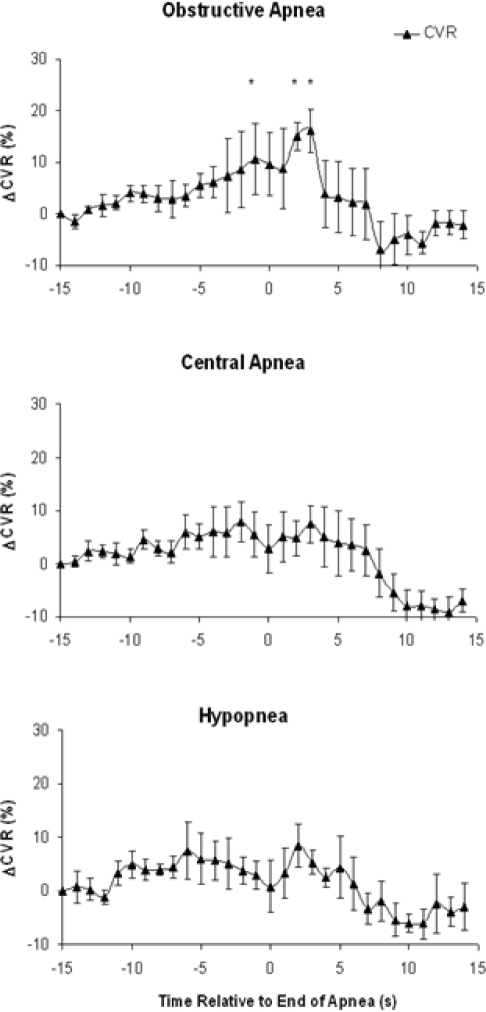
CVR before and after each type of respiratory event is shown in Figure 3. With OA, CVR increased shortly after the termination of the apnea and peaked at 16% ± 4% above baseline (P < 0.005), 3 sec after the resumption of ventilation, before declining again. CVR did not change for either CA or HP over this time period (Fig. 3). Multivariate predictors of the maximal CVR increase following OA were assessed using a forward stepwise regression analysis. There was no effect of the presence of arousal, hypoxia associated with the event, or sleep stage; the only independent predictor was β-blocker use (R = 0.45, R2 = 0.20, F = 10.9, P < 0.01).
Figure 3.
Change in CVR from 15 sec before to 15 sec after the end of OA, CA, and HP. Data represents mean ± SEM for pooled results of 5 patients with OA, 5 patients with CA, and 6 patients with HP. With OA, CVR increases post apnea, peaking 16% ± 4% above baseline 3 sec following the end of the apnea (* P < 0.05). There is no significant increase in CVR with CAs or HPs.
The maximal CBF response seen after the termination of OA was predicted by a linear regression model: R = 0.60, F = 11.9, P < 0.001. Independent predictors of maximal CBF were the maximal RPP change (R = 0.52, F =18.4, P < 0.001), and also the presence of arousal from sleep with the respiratory event (R = 0.30, F = 5.95, P < 0.05). The magnitude of CBF increase was not predicted by the degree of oxygen desaturation, minimal SpO2, sleep stage, or use of β-blockers. In addition, the univariate regression of minimal SpO2 with CBF revealed no evidence of a relationship.
CBF corrected for RPP decreased shortly after the termination of each respiratory event. The maximal CBF/RPP decline was predicted by a linear regression model: R = 0.53, F = 35.2, P < 0.001. Independent predictors of the nadir in CBF/RPP were the type of respiratory event (OA < CA < HP, R = 0.51, F = 61.9, P < 0.001) and the minimal SpO2 reached (R = −0.15, F = 5.8, P < 0.05). The degree of O2 desaturation, presence of arousal, sleep stage, and β-blocker use did not predict the magnitude of decline in CBF/RPP.
DISCUSSION
This is the first study to quantify the effect of sleep apnea on CBF in humans. We found that CBF increases following the termination of a respiratory event, with the pattern of CBF change depending on the nature of the breathing disorder. With OA, CBF becomes uncoupled from myocardial work (RPP), reflected by a significant delay of 8 sec before CBF follows the increase in RPP. Once CBF begins to increase, the slope of response is steeper than that of RPP, compensating for the initial delay. Both features of the response to OA contrast with CA and HP, where the rise in CBF follows that of RPP with lesser delays of 5 and 4 sec, respectively, and where the slopes of CBF and RPP change are the same.
We quantified the extent of CBF and RPP mismatch by calculating a CBF/RPP ratio. Stepwise regression analysis showed that the fall in CBF corrected for RPP (CBF/RPP) was strongly influenced by the type of respiratory event, with OA predicting a greater fall in CBF/RPP than CA and HP. The exaggerated deficit in flow seen in OA was explained by an increase in CVR of 16% ± 4%, 3 sec following the termination of OA, whereas there was no change in CVR following CA or HP. Stepwise regression analysis revealed that RPP and cortical arousal influenced the magnitude of CBF increase following OA, but the degree of oxygen desaturation was not an independent predictor of the magnitude of CBF increase or the fall in CBF/RPP.
The myocardium has limited anaerobic capacity, and as a consequence, myocardial oxygen consumption is tightly coupled to oxygen delivery—increases in myocardial energy demand (myocardial work) are closely matched by increases in CBF. Following from this, an impaired CBF response to increased myocardial work leads to a proportional reduction in coronary venous oxygen tension, that is, myocardial hypoxia.19 The major determinants of myocardial work are heart rate and systemic blood pressure, and the multiplication product of the two (rate-pressure product, RPP) is an excellent and accurate correlate of myocardial oxygen consumption.15,20 Hence CBF and RPP should maintain a close and proportional relationship as myocardial work changes under physiological stimulation. Both obstructive and central sleep apneas are associated with acute increases in heart rate and blood pressure, and decreases in arterial oxygen saturation.7 These changes are maximal during the period of hyperpnea that follows apnea resolution.7,21 At this time of resumption of ventilation, coronary arterial oxygen content is at a minimum.22 Consequently, a rise in CBF following the apnea is vital to maintain adequate myocardial tissue oxygenation in the face of increased myocardial energy demand and arterial hypoxia. Underscoring the importance of this compensation, there is clinical evidence suggesting an imbalance between myocardial oxygen supply and demand can occur at the time of apnea resolution. Nocturnal ischemia or angina in patients with OSA and coronary artery disease is common and predominantly occurs in this early post-apneic period.6 Our study provides a potential explanation for this clinical finding, as we have demonstrated a delayed and impaired CBF response to the increase in myocardial work that occurs with OA.
The differing coronary hemodynamic effects we observed between OA, CA, and HP can be explained by considering their specific respiratory and cardiovascular characteristics. Periodic central apneas are driven by an unstable ventilatory control system and are not associated with excessive swings in intrathoracic pressure.23 However, CA does generate oscillations in heart rate and blood pressure with peaks occurring after the resumption of ventilation, even in the absence of hypoxia, CO2 retention, or arousal from sleep,24 so that CBF would be anticipated to rise to meet the exaggerated metabolic demand. Our data confirm this expectation, as both RPP and CBF increased in response to CA, peaking 7 to 12 sec after the end of the apnea. There was only a short delay (5 sec) before the CBF increased in response to the rising myocardial energy demand, and then CBF increased proportionally to the increase in RPP. Additionally, there was no rise in CVR during this initial delay, and CVR fell after the end of the apnea, at the time when CBF had significantly increased.
In contrast, obstructive apneas are associated with large, negative swings in intrathoracic pressure, as respiratory effort continues against an obstructed oropharynx.25 Cardiac output is impaired during the apnea (due to a combination of reduced preload and increased afterload),26 then surges once the obstruction is relieved and the heart rate increases, contributing to the increase in blood pressure seen following the event. Arousal from sleep is common with obstructive apneas and exacerbates the increase in heart rate and blood pressure through activation of the sympathetic nervous system.27 Our data show that while the RPP increases as expected following obstructive apneas, there is a transient period when the CBF response is inadequate, in two important respects. First, toward the end of the apnea and in the immediate post apneic period, CBF does not rise in concert with RPP, as would normally be required to meet increasing myocardial metabolic needs. Rather, there is a delay of 8 sec before the CBF begins to rise following the RPP increase. Moreover, during this delay, the CBF corrected for RPP is falling, signifying a worsening of flow-metabolic demand coupling. Stepwise regression analysis showed that the magnitude of this fall is greater for OA than for CA or HP, independent of other contributing factors. This mismatch between flow-metabolic demand coupling with OA is likely to be clinically relevant in the setting of underlying coronary artery disease, as it has recently been shown that obstructive apneas can acutely change the physiological significance of an intermediate grade coronary stenosis.28 In this case report of a patient with obstructive sleep apnea occurring during coronary angiography, fractional flow reserve measurements varied from above to below the ischemic threshold. The presence of OSA therefore converted a functionally benign coronary lesion to a clinically significant one. Furthermore, repeated obstructive events throughout the night (as in severe obstructive sleep apnea) may lead to cumulative adverse ischemic effects—exacerbating the effects of a single event of short duration.
We identified a rise in CVR as the basis for the impaired CBF response in obstructive events. With OA, CVR rose throughout the period of delayed CBF response, peaking at 16% above baseline. Immediately following this peak, CVR began to fall and CBF subsequently increased. By contrast, there were no CVR increases associated with CA or HP events.
The exact basis for this imbalance between CBF and myocardial work and rise in CVR is uncertain, but we postulate it is due to an imbalance of factors affecting coronary resistance vessels, including α-adrenoreceptor mediated vasoconstriction, β-adrenoreceptor mediated vasodilation, hypoxia-mediated vasodilation, and local production of endothelial derived vasodilators, such as nitric oxide (NO) and prostaglandins. In chronically instrumented pigs, airway obstruction with arousal from rapid eye movement sleep is associated with a 24% increase in CVR following the termination of the apnea.9 Vasoconstriction was mediated by the sympathetic nervous system, acting via α receptors. Normally, an increase in sympathetic activity leads to a net reduction in coronary vascular resistance and an increase in CBF. This occurs through activation of dilating β-adrenoreceptors,29 but also via metabolic vasodilation, with activation of other local endothelial mediators.30 An increase in cardiac sympathetic activity at the end of an apnea may tip the balance away from β-adrenoreceptor mediated vasodilation, towards α-adrenoreceptor vasoconstriction, particularly in subjects with associated endothelial dysfunction and impaired production of NO. In keeping with the likelihood of sympathetically induced α-adrenoreceptor mediated vasoconstriction in our study subjects, β-blocker use was the only independent predictor of a CVR increase during OA. In further support of our hypothesis, Jones et al have demonstrated that α-adrenoreceptor activation is an important determinant of coronary vascular resistance in the setting of abnormal endothelial dependent relaxation.31 Although our studies utilized angiographically smooth or minimally irregular vessel, all patients had established coronary atherosclerosis and thus were likely to have had a degree of coronary endothelial dysfunction. Thus, similar CBF and CVR responses may not be seen in patients without coronary artery disease, but this remains to be determined. The potential interaction of endothelial dysfunction and the adrenergic system on CBF during OSA, as suggested by our results, are intriguing and warrant further dedicated study.
The hemodynamic responses seen with hypopneas were less dramatic than those seen for either OA or CA. The magnitudes of rise in CBF and RPP were less, with CBF peaking only 10% ± 4% above baseline, and there was no increase in CVR in the early post-hypopneic period. Furthermore, the delay from the beginning of the RPP to CBF rise was only 4 sec, with no evidence that the slope of the CBF response exceeded that of the RPP increase. The lesser impact on the coronary circulation of HP is in keeping with how these events differ physiologically from OA and CA. Intrathoracic pressure swings and the resultant hemodynamic stress would be expected to be of lower magnitude than that seen with obstructive apneas, and accordingly, the impact on CBF and its regulation would also be predicted to be less, as our data show.
Although arterial oxygen desaturation with respiratory events contributes to myocardial hypoxia, the exact role it plays in the coronary hemodynamic response to apnea remains controversial. Under baseline conditions, hypoxia leads to coronary vasodilation, whereas hyperoxia vasoconstricts.32 The system is more complex in the setting of hypoxia with associated upper airway obstruction, as other physiological factors (both mechanical and neurohumeral) affect CBF to a significant degree. In particular, there are conflicting data as to whether the increase in blood pressure and CBF seen with obstructive apnea is independent from, or driven by, the accompanying hypoxia.8,21,33,34 In our study, neither the degree of oxygen desaturation nor the minimum SpO2 level were significant predictors of the maximal CBF rise. Although hypoxia has the potential to increase CBF via local vasodilation and activation of the sympathetic nervous system in sleep apnea, our analysis suggests that any such effects of hypoxia on CBF are overwhelmed by the autonomic response to apnea termination, by lung reinflation, or by cortical arousal. In keeping with this suggestion, each of these factors has been associated with increases in heart rate and blood pressure at the end of both obstructive and central apneas.21,24,35 A caveat of this is that the degree of O2 desaturation seen in our study was mild, so we can not exclude the possibility that arterial hypoxia becomes a significant mediator of the rise in CBF at more extreme levels of oxygen desaturation, as has been suggested by animal studies.8,36
There are a number of limitations to our study. Patient numbers were small, as the measurement technique is invasive, and limited numbers of patients are prepared or able to sleep in the cardiac catheterization laboratory. With these limitations, it was not possible to select patients based on any underlying sleep apnea, nor study a control group without breathing disturbances. It was also therefore not possible to control for potential confounding factors between patients with different types of breathing disturbances. As the total number of patients was small (n = 10), the bilinear regression curves outlined in the breakpoint analysis of Figure 2 have low statistical power, and we can not exclude the possibility that the findings arose from individual patient characteristics rather than type of respiratory event. On the other hand, clearly significant predictors were identified despite the small subject numbers, increasing confidence in the physiological factors that emerged from the analysis. Finally, our results are entirely consistent with those obtained in animal studies, and the findings of our study—the first to specifically assess CBF in human sleep apnea—provide important direction for further study of CBF during human OSA.
We measured beat-to-beat changes in coronary blood velocity and assumed constant cross-sectional vessel area at the site of the flow wire when calculating CBF. Coronary vessel diameter variations due to flow mediated dilatation may have introduced error into our calculations of CBF; nevertheless, we feel this is unlikely to affect our comparison of respiratory event types—the same limitation would apply to all types of respiratory events, as the extent of flow changes were similar in each. In addition, given that the perturbations in coronary blood velocity were modest, any diameter change was likely to have been relatively small. The strength of this technique, however, is that transient alterations in CBF (such as those occurring with sleep apnea) can be accurately tracked.14 Other techniques for measuring CBF, such as positron emission tomography,37 are not able to assess CBF changes over such a short time period.
All patients in our study received sedation during their coronary catheterization, so we can not exclude that CBF responses to apnea may be different in spontaneous sleep. Midazolam has been shown to decrease the responsiveness of the sympathetic nervous system when used for sedation in humans.38 It also has been shown to decrease baseline coronary blood flow (by 24%), but as it also decreases myocardial oxygen consumption to the same degree (26%), it is unlikely to alter flow-metabolic coupling in the myocardium. This raises uncertainty regarding how much of its effect is due to deepening sleep itself, rather than the pharmacological effects of the medication on the heart.38 Importantly, there was no evidence of ischemia nor change in CVR with midazolam administration per se. In any case, our study focused on the respective changes in CBF and RPP occurring with individual respiratory events, not the absolute baseline values. As midazolam decreases sympathetic responsiveness, the use of this medication in our study may have attenuated the degree of vasoconstriction occurring with apnea termination, thus strengthening the significance of our findings.
Our new data offer potential explanations for the mechanisms behind the increased risk of nocturnal ischemia, arrhythmias, and, potentially, sudden cardiac death in patients with both OSA and coronary artery disease. Following obstructive apneas we have demonstrated a mismatch between CBF and myocardial energy demand in angiographically normal coronary vessels due to a transient rise in CVR. The short duration of flow/work mismatch may not have clinical sequelae in patients without coronary stenosis, but may well become important in those with either intermediate severity or hemodynamically significant lesions. In this setting, the dissociation between CBF and myocardial work is likely to become accentuated and may lead to myocardial ischemia. In those with more severe OSA, significant arterial oxygen desaturation resulting from the apneas is likely to worsen myocardial oxygen supply, increasing the risk of ischemia.
In summary, this novel study provides the first CBF measurements in human OSA and suggests potential mechanisms of adverse cardiovascular outcomes in patients with OSA that warrant further investigation. Because OSA can be effectively treated, the possibility that OSA is present should be considered in patients with coronary artery disease, particularly those who complain of nocturnal ischemia.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We are grateful to Kathryn Stevens, B.AppSc, who provided technical assistance with the sleep studies, and the staff of the coronary catheterization laboratory at Monash Medical Centre.
Site of Study: Monash Institute of Medical Research, Monash University, Clayton, Victoria, Australia
Funding source: National Health and Medical Research Council of Australia
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 3.Peker Y, Hedner J, Kraiczi H, Loth S. Respiratory disturbance index: an independent predictor of mortality in coronary artery disease. Am J Respir Crit Care Med. 2000;162:81–6. doi: 10.1164/ajrccm.162.1.9905035. [DOI] [PubMed] [Google Scholar]
- 4.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 5.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–14. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 6.Peled N, Abinader EG, Pillar G, Sharif D, Lavie P. Nocturnal ischemic events in patients with obstructive sleep apnea syndrome and ischemic heart disease: effects of continuous positive air pressure treatment. J Am Coll Cardiol. 1999;34:1744–9. doi: 10.1016/s0735-1097(99)00407-6. [DOI] [PubMed] [Google Scholar]
- 7.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L, Sica AL, Greenberg H, Scharf SM. Role of hypoxemia and hypercapnia in acute cardiovascular response to periodic apneas in sedated pigs. Respir Physiol. 1998;111:257–69. doi: 10.1016/s0034-5687(98)00007-3. [DOI] [PubMed] [Google Scholar]
- 9.Pinto JM, Garpestad E, Weiss JW, Bergau DM, Kirby DA. Hemodynamic changes associated with obstructive sleep apnea followed by arousal in a porcine model. J Appl Physiol. 1993;75:1439–43. doi: 10.1152/jappl.1993.75.4.1439. [DOI] [PubMed] [Google Scholar]
- 10.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington, DC: Government Printing Office; 1968. [DOI] [PubMed] [Google Scholar]
- 11.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 12.Duffy SJ, Castle SF, Harper RW, Meredith IT. Contribution of vasodilator prostanoids and nitric oxide to resting flow, metabolic vasodilation, and flow-mediated dilation in human coronary circulation. Circulation. 1999;100:1951–7. doi: 10.1161/01.cir.100.19.1951. [DOI] [PubMed] [Google Scholar]
- 13.Farouque HM, Worthley SG, Meredith IT, Skyrme-Jones RA, Zhang MJ. Effect of ATP-sensitive potassium channel inhibition on resting coronary vascular responses in humans. Circ Res. 2002;90:231–6. doi: 10.1161/hh0202.103713. [DOI] [PubMed] [Google Scholar]
- 14.Doucette JW, Corl PD, Payne HM, et al. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation. 1992;85:1899–911. doi: 10.1161/01.cir.85.5.1899. [DOI] [PubMed] [Google Scholar]
- 15.Kitamura K, Jorgensen CR, Gobel FL, Taylor HL, Wang Y. Hemodynamic correlates of myocardial oxygen consumption during upright exercise. J Appl Physiol. 1972;32:516–22. doi: 10.1152/jappl.1972.32.4.516. [DOI] [PubMed] [Google Scholar]
- 16.Grant DA, Franzini C, Wild J, Eede KJ, Walker AM. Autoregulation of the cerebral circulation during sleep in newborn lambs. J Physiol. 2005;564:923–30. doi: 10.1113/jphysiol.2005.083352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orr GW, Green HJ, Hughson RL, Bennett GW. A computer linear regression model to determine ventilatory anaerobic threshold. J Appl Physiol. 1982;52:1349–52. doi: 10.1152/jappl.1982.52.5.1349. [DOI] [PubMed] [Google Scholar]
- 18.Glantz S. Primer of biostatistics. 5th ed. San Francisco: McGraw-Hill; 2002. [Google Scholar]
- 19.Tune JD, Gorman MW, Feigl EO. Matching coronary blood flow to myocardial oxygen consumption. J Appl Physiol. 2004;97:404–15. doi: 10.1152/japplphysiol.01345.2003. [DOI] [PubMed] [Google Scholar]
- 20.Baller D, Bretschneider HJ, Hellige G. Validity of myocardial oxygen consumption parameters. Clin Cardiol. 1979;2:317–27. doi: 10.1002/clc.4960020502. [DOI] [PubMed] [Google Scholar]
- 21.Ringler J, Basner RC, Shannon R, et al. Hypoxemia alone does not explain blood pressure elevations after obstructive apneas. J Appl Physiol. 1990;69:2143–8. doi: 10.1152/jappl.1990.69.6.2143. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Sica AL, Scharf SM. Mechanisms of acute cardiovascular response to periodic apneas in sedated pigs. J Appl Physiol. 1999;86:1236–46. doi: 10.1152/jappl.1999.86.4.1236. [DOI] [PubMed] [Google Scholar]
- 23.Bradley TD, Floras JS. Sleep apnea and heart failure: Part II: central sleep apnea. Circulation. 2003;107:1822–6. doi: 10.1161/01.CIR.0000061758.05044.64. [DOI] [PubMed] [Google Scholar]
- 24.Lorenzi-Filho G, Dajani HR, Leung RST, Floras JS, Bradley TD. Entrainment of blood pressure and heart rate oscillations by periodic breathing. Am J Respir Crit Care Med. 1999;159:1147–54. doi: 10.1164/ajrccm.159.4.9806081. [DOI] [PubMed] [Google Scholar]
- 25.Bradley TD, Floras JS. Sleep apnea and heart failure: Part I: obstructive sleep apnea. Circulation. 2003;107:1671–8. doi: 10.1161/01.CIR.0000061757.12581.15. [DOI] [PubMed] [Google Scholar]
- 26.Tolle FA, Judy WV, Yu PL, Markand ON. Reduced stroke volume related to pleural pressure in obstructive sleep apnea. J Appl Physiol. 1983;55:1718–24. doi: 10.1152/jappl.1983.55.6.1718. [DOI] [PubMed] [Google Scholar]
- 27.O'Donnell CP, Ayuse T, King ED, Schwartz AR, Smith PL, Robotham JL. Airway obstruction during sleep increases blood pressure without arousal. J Appl Physiol. 1996;80:773–81. doi: 10.1152/jappl.1996.80.3.773. [DOI] [PubMed] [Google Scholar]
- 28.Ciaramita JP, Parham WA, Herrmann S, Khoukaz S, Kern MJ. The influence of obstructive sleep apnea on FFR measurements for coronary lesion assessment. J Interv Cardiol. 2004;17:331–7. doi: 10.1111/j.1540-8183.2004.04025.x. [DOI] [PubMed] [Google Scholar]
- 29.Kirby DA, Pinto JM, Weiss JW, Garpestad E, Zinkovska S. Effects of beta adrenergic receptor blockade on hemodynamic changes associated with obstructive sleep apnea. Physiol Behav. 1995;58:919–23. doi: 10.1016/0031-9384(95)00150-h. [DOI] [PubMed] [Google Scholar]
- 30.Zeiher AM, Drexler H, Wollschlager H, Just H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991;83:391–401. doi: 10.1161/01.cir.83.2.391. [DOI] [PubMed] [Google Scholar]
- 31.Pepperell JCT, Maskell NA, Jones DR, et al. A randomized controlled trial of adaptive ventilation for Cheyne-Stokes breathing in heart failure. Am J Respir Crit Care Med. 2003;168:1109–14. doi: 10.1164/rccm.200212-1476OC. [DOI] [PubMed] [Google Scholar]
- 32.McNulty PH, King N, Scott S, et al. Effects of supplemental oxygen administration on coronary blood flow in patients undergoing cardiac catheterization. Am J Physiol Heart Circ Physiol. 2005;288:H1057–62. doi: 10.1152/ajpheart.00625.2004. [DOI] [PubMed] [Google Scholar]
- 33.Schneider H, Schaub CD, Chen CA, et al. Neural and local effects of hypoxia on cardiovascular responses to obstructive apnea. J Appl Physiol. 2000;88:1093–102. doi: 10.1152/jappl.2000.88.3.1093. [DOI] [PubMed] [Google Scholar]
- 34.Okabe S, Hida W, Kikuchi Y, et al. Role of hypoxia on increased blood pressure in patients with obstructive sleep apnoea. Thorax. 1995;50:28–34. doi: 10.1136/thx.50.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trinder J, Merson R, Rosenberg JI, Fitzgerald F, Kleiman J, Douglas Bradley T. Pathophysiological interactions of ventilation, arousals, and blood pressure oscillations during Cheyne-Stokes respiration in patients with heart failure. Am J Respir Crit Care Med. 2000;162:808–13. doi: 10.1164/ajrccm.162.3.9806080. [DOI] [PubMed] [Google Scholar]
- 36.Bao G, Randhawa PM, Fletcher EC. Acute blood pressure elevation during repetitive hypocapnic and eucapnic hypoxia in rats. J Appl Physiol. 1997;82:1071–8. doi: 10.1152/jappl.1997.82.4.1071. [DOI] [PubMed] [Google Scholar]
- 37.Di Carli MF, Tobes MC, Mangner T, et al. Effects of cardiac sympathetic innervation on coronary blood flow. N Engl J Med. 1997;336:1208–15. doi: 10.1056/NEJM199704243361703. [DOI] [PubMed] [Google Scholar]
- 38.Marty J, Gauzit R, Lefevre P, et al. Effects of diazepam and midazolam on baroreflex control of heart rate and on sympathetic activity in humans. Anesth Analg. 1986;65:113–9. [PubMed] [Google Scholar]