Abstract
Study Objective:
To investigate the incidence of overactive bladder (OAB) and urgency incontinence (UI) in men with obstructive sleep apnea syndrome (OSAS).
Design:
Prospective questionnaire study
Setting:
Saarland University Hospital
Patients:
All male patients who underwent full-night in-laboratory polysomnography between November 2006 and April 2007.
Interventions:
Overactive bladder symptom score (OABSS) and International Consultation on Incontinence Questionnaire, Short-Form (ICIQ-SF).
Measurements and Results:
OSAS severity was assessed according to the apnea-hypopnea-index (AHI). Return rate of questionnaires was 100% (n = 100). Patients with upper airway resistance syndrome (UARS) served as controls. Evaluation of OABSS revealed that patients with moderate and severe OSAS presented with a significantly higher incidence of symptoms of OAB than patients with mild OSAS and UARS (P < 0.05). Further, the ICIQ-SF revealed a higher occurrence of UI in patients with severe OSAS than in those with mild OSAS and UARS (P < 0.05).
Conclusions:
Increasing severity of OSAS appears to be associated with an increasing occurrence of overactive bladder and urgency incontinence in men. This relationship may have clinical implications for the treatment of affected patients.
Citation:
Kemmer H; Mathes AM; Dilk O; Gröschel A; Grass C; Stöckle M. Obstructive Sleep Apnea Syndrome Is Associated with Overactive Bladder and Urgency Incontinence in Men. SLEEP 2009;32(2):271-275.
Keywords: Obstructive sleep apnea syndrome, overactive bladder, urgency incontinence, upper airway resistance syndrome
IN RECENT STUDIES, 4% TO 5 % OF MIDDLE AGED MEN ARE SUPPOSED TO SUFFER FROM OBSTRUCTIVE SLEEP APNEA SYNDROME (OSAS).1 OSAS IS FREQUENTLY associated with clinical complications such as hypertension, ischemic heart disease and increased risk of stroke.2–4 Several studies have also shown a relationship between OSAS and urologic symptoms like erectile dysfunction5 and nocturia.6 The symptom of nocturia has been defined by the International Continence Society as “the complaint that the individual has to wake up at night one or more times to void”7 and can be caused by a variety of diseases,8 like myocardial insufficiency, benign prostate hyperplasia and overactive bladder (OAB). The present theory about the mechanism resulting in increased nocturia in patients with OSAS describes nocturnal polyuria to be caused by elevated nocturnal atrial natriuretic peptide (ANP) excretion.6 This is explained by the generation of negative pressure in the chest caused by partial or full obstruction of the airway and sustained ventilatory effort, which causes the heart to receive a false signal of volume overload. The hormonal response to this signal is an increase in ANP secretion. Improvements of nocturia in OSAS patients have been described with nasal CPAP treatment.9
In contrast to nocturia, the overactive bladder (OAB) syndrome affects patients particularly at daytime. OAB is defined as a symptom syndrome of urinary urgency, with or without urgency incontinence, usually with urinary frequency and nocturia, in the absence of infections or other obvious pathologic features.10 The key symptom of OAB is urgency, the sudden, compelling desire to void that is difficult to defer.11 Several reasons11 are thought to account for the development of OAB: morphologic changes of the detrusor (e.g., patchy denervation of detrusor muscle bundles), neurologic changes (e.g., ischemic nerve damage), age-related causes of urinary dysfunction, and metabolic causes (e.g., disturbed serotonin metabolism). The prevalence of OAB in Europe is reported to be 4.6% to 15.0 % in men and 14.0% to 40.0% in women.12
In the past years, we observed a high number of patients with diagnosed and treated manifestations of OSAS, complaining not only about nocturia, but also specifically about daytime symptoms of urinary urgency and frequency. Further diagnostic procedures in our department revealed a frequent occurrence of OAB in men suffering from OSAS. To confirm these subjective suggestions from our observations, this study was initiated to investigate the occurrence of OAB and urgency incontinence in patients with mild, moderate, and severe OSAS. Patients with upper airway resistance syndrome served as a reference group.
METHODS
Patients
After obtaining informed consent for this study, all male patients with symptoms of sleep disorders who underwent full-night polysomnography as a diagnostic procedure for clinical purposes between November 2006 and April 2007 were screened for comorbidities and previous surgery. Patients with neurologic disorders (e.g., multiple sclerosis, disk herniation, stroke, or diabetes) or previous urogenital operations (e.g., radical prostatectomy, transurethral resection) were excluded (n = 47). The remaining patients (n = 100) were asked to answer our standardized questionnaires for the purpose of this study.
Polysomnography
All patients underwent full-night polysomnography for at least one night. The patients went to bed between 22:00 and 23:00; all patients were awakened at 07:00. Central and occipital EEG leads, bilateral electrooculograms, and submental electromyograms were used to monitor sleep. An anterior tibialis electromyogram was recorded to detect leg movements. A bipolar ECG was simultaneously recorded for cardiac monitoring. Blood oxygen levels were determined by finger pulse oximetry. Respiratory airflow was monitored using a nasal cannula and an oral thermistor. Respiratory effort was monitored using rib and abdominal piezoelectric strain gauges. Body position was monitored using a sleep position sensor. All signals were acquired digitally (Alice IV, Respironics, USA). Daytime sleepiness was assessed by a comprehensive history of a board-certified sleep specialist with the aid of the Epworth Sleepiness Scale.13 Apnea was defined as a complete cessation of airflow ≥ 10 seconds. Hypopnea was defined as a flow reduction in combination with an oxygen desaturation ≥ 4% or an arousal in the EEG. The severity of OSAS was defined by the Apnea Hypopnea Index (AHI; calculated by dividing the number of apneas and hypopneas by the hours of sleep), where a frequency of 5–15 events/h was considered mild (n = 33), 15–30 as moderate (n = 24) and > 30 events/h as severe (n = 14). Upper airway resistance syndrome (UARS) was diagnosed in case of excessive daytime sleepiness, with arousal index > 20 events/h and AHI < 5 events/h.14
Questionnaires
All patients were asked to answer the overactive bladder symptom score (OABSS),15 the International Consultation on Incontinence Questionnaire, Short-Form (ICIQ-SF),16 and the International Prostate Symptoms Score (IPSS).17
The OABSS sums the score of 4 symptoms (daytime frequency, nighttime frequency, urgency and urgency incontinence) and was been developed and validated in 2006. OAB was defined as ≥ 4 points on the OABSS. Consequently, the incidence of OAB reflects the percentage of patients with a score ≥ 4 on the OABSS. The severity of OAB was measured by the sum of points in the OABSS for each patient, including those with < 4 points.
The results of the ICIQ-SF were calculated as the sum of scores obtained from the frequency of urinary incontinence episodes (from 0 or “never,” increasing by 1 unit up to 5 or “always”), perceived quantity of urine losses (0 or “’no loss,” increasing by 2 units up to 6 or “a large amount”) and quality of life (QoL) (from 0 or “no interference” of urinary incontinence with life, increasing by 1 unit up to 10 or “maximum interference”). The possible range of the score sum is between 0–21 points.
The IPSS is a frequently used standardized 8 question screening tool (7 symptom questions and 1 quality of life question) to evaluate syptoms of benign prostate hyperplasia. The possible range of the score sum is between 0–35. A score sum between 0 and 7 describes a patient suffering from mild symptoms of bening prostate hyperplasia. A patient with a score of 8 to 19 points suffers from moderate disease, and a patient with 20 to 35 points has severe prostate hyperplasia.
Statistical Analysis
Statistical analyses were performed using the software SigmaPlot 9.0 (Systat Software Inc., Erkraht, Germany). Data were analysed using a Kruskal-Wallis analysis of variance (ANOVA) on ranks, followed by a Dunn's test for post hoc multiple comparison. These data are given as median ± 95th percentiles. Frequencies of occurrence were analyzed using a χ2-test. These data are given as the percentage of all patients in one defined group. To adjust for potential confounders, a multiple linear regression analysis was performed, using the software BiAS 8.3.8 (epsilon, Frankfurt, Germany). For all analyses, a P-value of < 0.05 was considered statistically significant.
RESULTS
In total, 147 patients were screened for being suitable for this study; 47 patients were excluded because of neurological disorders or previous urogential operations. Consequentially, 100 male patients were included; the return rate of questionnaires was 100% (n = 100). All patients with incomplete questionnaires or without evidence of OSAS or UARS in the polysomnogram were excluded (n = 15). Of the remaining 85 patients, 14 patients with UARS were classified as reference group. Therefore, 71 patients were classified in the different OSAS groups according to the above mentioned criteria. Table 1 shows the biometric patient data and the proportion of the different groups.
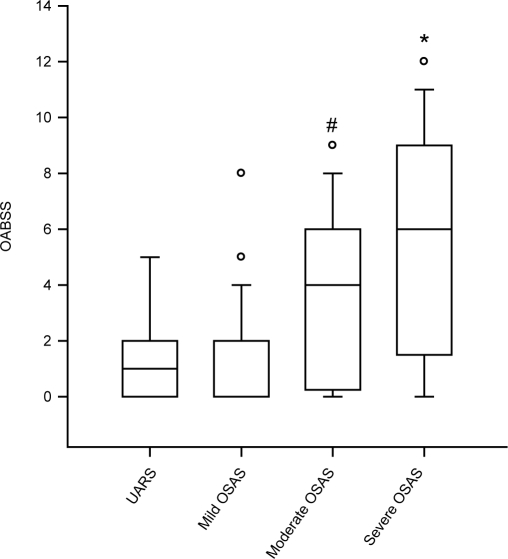
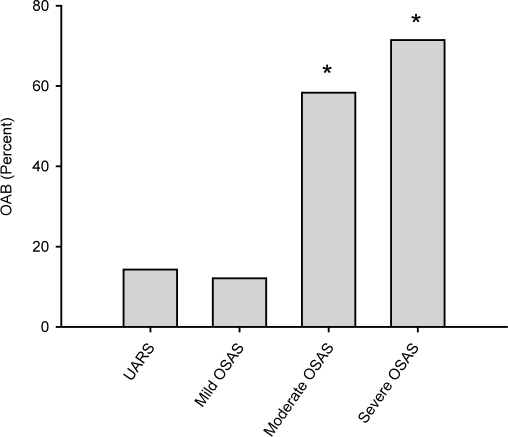
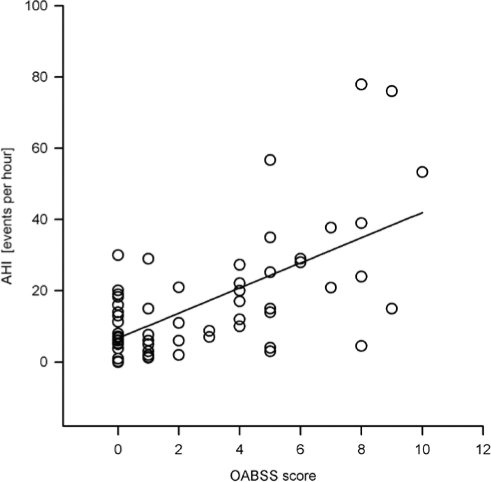
Analysis of the OABSS questionnaire revealed a significant difference of the sum score between groups (P < 0.001) (Figure 1). OABSS mean and median was significantly higher in both the severe OSAS group and the moderate OSAS group, compared with mild OSAS patients and UARS patients. Consequentially, the incidence of OAB was found to be significantly different between groups (P < 0.001) (Figure 2). Incidence of OAB was significantly higher in patients with severe and moderate OSAS, compared with mild OSAS and UARS. A linear regression analysis of the AHI and OABSS scores is given in Figure 3. The overall prevalence of OAB in patients with OSAS was 39% (n = 28). Power of the performed tests for OABSS, OAB and linear regression with α = 0.05 is 1.000.
Figure 1.
Severity of overactive bladder in different stages of obstructive sleep apnea syndrome. Overactive bladder symptom score (OABSS) in patients with upper airway resistance syndrome (UARS), mild, moderate, and severe obstructive sleep apnea syndrome (OSAS). With higher stages of OSAS, the severity of OAB as measured in the OABSS is increasing significantly. An asterisk (*) indicates p < 0.05 vs. UARS and mild OSAS; a pound sign (#) indicates P < 0.05 vs. mild OSAS. Data are median ± 95th percentiles; circles indicate outliners.
Figure 2.
Occurrence of overactive bladder in patients with different stages of obstructive sleep apnea syndrome. Percentage of patients with overactive bladder (OAB), defined as a score of 4 and above in the overactive bladder symptom score evaluation, in patients with upper airway resistance syndrome (UARS), mild, moderate, and severe obstructive sleep apnea syndrome (OSAS). Note that in patients with severe or moderate OSAS, the percentage of patients with OAB is significantly increased. An asterisk (*) indicates P < 0.05 vs. UARS and mild OSAS.
Figure 3.
Linear regression analysis of the AHI and the OABSS score. Linear regression analysis of the apnea-hypopnea-index (AHI) with the overactive bladder symptom score (OABSS) reveals a correlation between raw data of the AHI with OABSS scores (R = 0.740).
Subdomain analysis of the OABSS revealed that daytime symptoms were noted more frequently in the severe OSAS groups (daytime score 1.07 ± 0.73), compared to the UARS (daytime score 0.28 ± 0.46), mild (daytime score 0.36 ± 0.54) and moderate (daytime score 0.66 ± 0.56) OSAS groups; this difference was statistically significant with respect to severe OSAS vs. UARS (P < 0.001) and mild OSAS (P < 0.001). Power of performed test with α = 0.05 is 0.931.
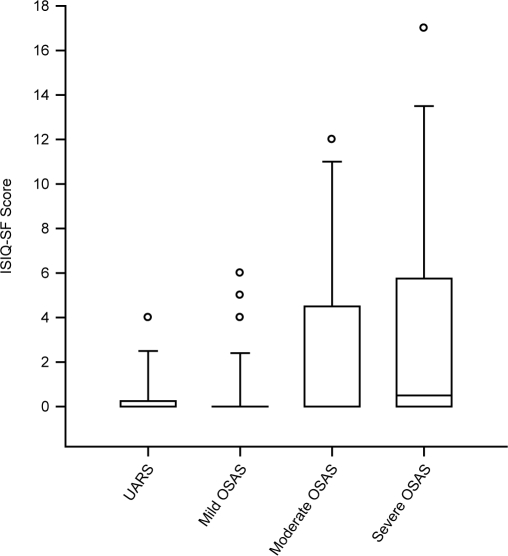
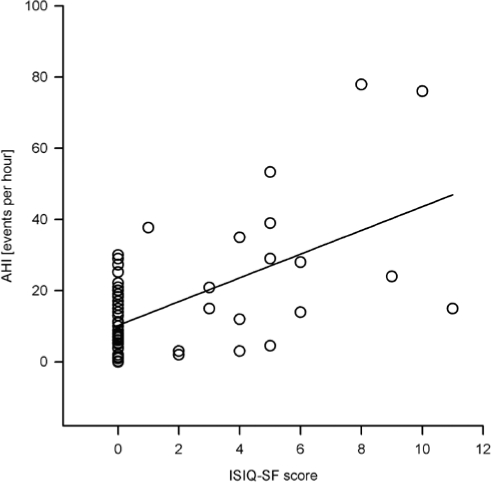
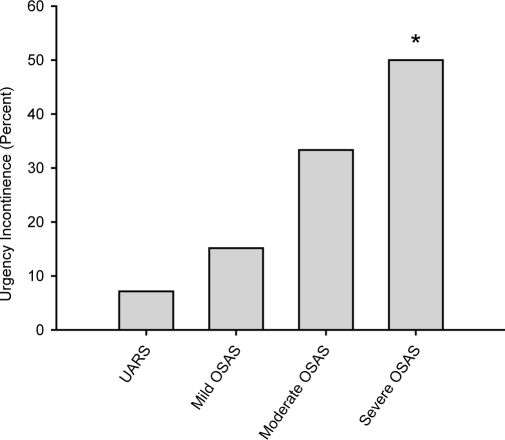
Analysis of the ICIQ-SF revealed a significant difference between groups (P = 0.012) (Figure 4). The mean total sum score was higher in men with severe and moderate OSAS, compared with mild OSAS and UARS; however, in a post hoc multiple comparison test, this difference was not statistically significant between specific groups. A linear regression analysis of the AHI and ICIQ-SF scores is given in Figure 5. Power of the performed tests for ICIQ-SF and linear regression with α = 0.05 is 1.000. Subgroup analysis of the ICIQ-SF with respect to the section of urgency incontinence revealed a significantly higher incidence in patients with severe OSAS, compared to mild OSAS (P = 0.019) and UARS (P = 0.011) (Figure 6). The overall prevalence of UI in patients with OAB was 50 % (n = 14).
Figure 4.
Incontinence Questionnaire Scores in different stages of obstructive sleep apnea syndrome. International Consultation on Incontinence Questionnaire, Short- Form (ISIQ-SF) score in patients with upper airway resistance syndrome (UARS), and mild, moderate, and severe obstructive sleep apnea syndrome (OSAS). Sum scores were significantly different between groups (P = 0.012). Data are median ± 95th percentiles; circles indicate outliners.
Figure 5.
Linear regression analysis of the AHI and the ISIQ-SF score. Linear regression analysis of the apnea-hypopnea-index (AHI) with the International Consultation on Incontinence Questionnaire, Short- Form (ISIQ-SF) score reveals a moderate correlation between raw data of the AHI with ICIQ-SF scores (R = 0.582).
Figure 6.
Occurrence of urgency incontinence in different stages of obstructive sleep apnea syndrome. Subgroup analysis of the International Consultation on Incontinence Questionnaire, Short- Form (ISIQ-SF) regarding urgency incontinence in patients with upper airway resistance syndrome (UARS), mild, moderate, and severe obstructive sleep apnea syndrome (OSAS). In patients with severe OSAS, symptoms of urgency incontinence were higher than in the reference group UARS or mild OSAS. An asterisk (*) indicates P < 0.05 vs. UARS and mild OSAS
A statistically significant difference was noted between groups with respect to IPSS (P = 0.004), but not regarding age (P = 0.251) or body mass index (BMI) (0.073). Nevertheless, age and BMI were included in the following multiple regression analysis, as they are recognized risk factors for OSAS. To predict for different OSAS groups, the independent variables age, BMI, IPSS, and OABSS were evaluated in a multiple linear regression analysis (full model unadjusted multiple R = 0.55; P < 0.001). The partial regression coefficient was significant for OABSS (β = 0.129; P = 0.001), but not for the variables age (β = 0.002; P = 0.70), BMI (β = 0.024; P = 0.098), or IPSS (β = 0.017; P = 0.392).
DISCUSSION
This study shows that moderate and severe OSAS is associated with OAB and urgency incontinence in men. Our data demonstrate that patients with moderate and severe OSAS score significantly higher in the OABSS compared with patients with mild OSAS and UARS. Daytime symptoms of urinary urgency are seen more frequently in patients with severe OSAS, compared to mild OSAS and UARS. Further, severe OSAS seems to be associated with urgency incontinence, as evaluated in the ISIQ-SF.
According to our data, the overall occurrence of OAB in patients with OSAS was found to be 39% and was therefore considerably higher as in the average male European population. Based on the OABSS, OAB was more frequently diagnosed in patients with higher stages of OSAS (moderate and severe OSAS). On the other hand, we are able to demonstrate an occurrence of OAB in patients with UARS (14%) and mild OSAS (12%) that appears to be similar to the average prevalence of OAB in European men. Thus, it may be assumed that patients with moderate and severe OSAS are more likely to present with symptoms of OAB compared with the average population.
OSAS has already been demonstrated to cause erectile dysfunction18,19 and nocturia.9 The symptom of nocturia describes the nocturnal need to void. In contrast, patients with OAB complain of urgency and frequency or even urgency incontinence particularly during daytime.11 Subgroup analysis of the OABSS reveals that patients with severe OSAS complain significantly more often about daytime symptoms of urinary urgency, compared to mild OSAS and UARS. Nevertheless, the OABSS contains questions about nighttime frequency, as this could also be among the symptoms of OAB. Therefore, our findings may not solely be attributable to OAB. To analyse this objection, further urodynamic evaluations may be needed.
Fanfulla et al.5 suggested that the mechanism for the association between OSAS and erectile dysfunction may be mediated by hypoxia-induced occult nerve dysfunction. Similar to the mechanism in erectile dysfunction, overactive bladder induced by nerve destruction has been reported e.g. in cases of diabetes20 or stroke.21 Thus, it is tempting to speculate that the mechanism of OSAS induced OAB may be related to hypoxia as well. Furthermore, symptoms of OSAS as nocturia and erectile dysfunction are reported to improve with nasal CPAP treatment.9,22 Therefore, assumptions can be made that positive airway pressure therapy may reduce OABSS scores in affected patients as well.
Our data for the ICIQ-SF show that patients with higher stages of OSAS present with increasing severity of incontinence. These results correspond well with our data on OAB. Based on subgroup analysis, UI occurred significantly more often in patients with severe OSAS. Although the sum scores may appear to be low, compared to the range of the questionnaire, this is not an unusual finding in patients with incontinence.23 The quality of life index within the questionnaire generates a high impact and may be low even in patients with severe urinary incontinence.23
This study is limited to the extent that some potential confounders, like diuretics intake and the relevance of nocturia, could not be completely eliminated. Further urodynamic studies will be needed to underline our results, as the evaluation of urinary symptoms was based on subjective criteria within the questionnaires.
The results of this investigation may have clinical implications for the management of affected patients. OAB and even more urgency incontinence are reported to have severe implications on social interactions, sleep, mental and sexual health, and overall health-related quality of life of affected patients.24 Therefore, all OSAS patients should consequently be screened for overactive bladder symptoms and urgency incontinence. To evaluate the treatment options in patients with OSAS and OAB or urgency incontinence, and to analyze the possibility of CPAP treatment as optional treatment, further studies will be needed.
CONCLUSIONS
This study shows that obstructive sleep apnea syndrome is associated with overactive bladder and urgency incontinence. These findings have clinical implications, as untreated OAB and UI may cause a relevant attenuation in quality of life in affected patients. Therefore, OAB should be investigated in all patients with higher stages of OSAS.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We would like to thank PD Dr. Gräber and Dr. Ong from the Department of Statistics, Saarland University Hospital, for their kind help with data evaluation and interpretation.
Institution where the work was performed:
Saarland University Hospital Department of Urology and Pediatric Urology D-66421 Homburg (Saar), Germany
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Caples SM, Garcia-Touchard A, Somers VK. Sleep-disordered breathing and cardiovascular risk. Sleep. 2007;30:291–303. doi: 10.1093/sleep/30.3.291. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–14. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 4.Bassetti C, Aldrich MS. Sleep apnea in acute cerebrovascular diseases: final report on 128 patients. Sleep. 1999;22:217–23. doi: 10.1093/sleep/22.2.217. [DOI] [PubMed] [Google Scholar]
- 5.Fanfulla F, Malaguti S, Montaga T, et al. Erectile dysfunction in men with obstructive sleep apnea: an early sign of nerve involvement. Sleep. 2000;23:775–781. [PubMed] [Google Scholar]
- 6.Umlauf MG, Chasens ER, Greevy RA, Arnold J, Burgio KL, Pillion DJ. Obstructive sleep apnea, nocturia and polyuria in older adults. Sleep. 2004;27:139–44. doi: 10.1093/sleep/27.1.139. [DOI] [PubMed] [Google Scholar]
- 7.Van Kerrebroeck P, Abrams P, Chaikin D, et al. The standardization of terminology in nocturia: report from the standardization subcommittee of the International Continence Society. BJU Int. 2002;90:11–15. doi: 10.1046/j.1464-410x.90.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 8.Walmsley K, Staskin DR. Nocturia: when is it not related to overactive bladder? Curr Urol Rep. 2003;4:441–5. doi: 10.1007/s11934-003-0024-0. [DOI] [PubMed] [Google Scholar]
- 9.Margel D, Shochat T, Getzler O, Livne PM, Pillar G. Continuous positive airway pressure reduces nocturia in patients with obstructive sleep apnea. Urology. 2006;67:974–7. doi: 10.1016/j.urology.2005.11.054. [DOI] [PubMed] [Google Scholar]
- 10.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology. 2003;61:37–49. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 11.Chu FM, Dmochowski R. Pathophysiology of overactive bladder. Am J Med. 2006;119:3–8. doi: 10.1016/j.amjmed.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Wein AJ, Rovner ES. Definition and epidemiology of overactive bladder. Urology. 2002;60:7–12. doi: 10.1016/s0090-4295(02)01784-3. [DOI] [PubMed] [Google Scholar]
- 13.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 14.Stoohs RA, Knaack L, Blum HC, Janicki J, Hohenhorst W. Differences in clinical features of upper airway resistance syndrome, primary snoring, and obstructive sleep apnea/ hypopnea syndrome. Sleep Med. 2008;9:121–8. doi: 10.1016/j.sleep.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Homma Y, Yoshiada M, Seki N, et al. Symptom assessment tool for overactive bladder syndrome–overactive bladder symptom score. Urol. 2006;68:318–23. doi: 10.1016/j.urology.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 16.Avery K, Donovan J, Abrams P. Validation of a new questionnaire for incontinence: the international consultation on incontinence questionnaire (ICI-Q) Neurourol Urodyn. 2001;20:86. [Google Scholar]
- 17.Barry MJ, Fowler FJ, Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–57. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 18.Margel D, Cohen M, Livne PM, Pillar G. Severe, but not mild, obstructive sleep apnea syndrome is associated with erectile dysfunction. Urology. 2004;63:545–9. doi: 10.1016/j.urology.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Hirshkowitz M, Karacan I, Arcasoy MO, Acik G, Narter EM, Williams RL. Prevalence of sleep apnea in men with erectile dysfunction. Urology. 1990;36:232–4. doi: 10.1016/0090-4295(90)80262-l. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi C, Sakakibara R, Uchiyama T, et al. Overactive bladder in diabetes: A peripheral or central mechanism? Neurourol Urodyn. 2007;26:807–13. doi: 10.1002/nau.20404. [DOI] [PubMed] [Google Scholar]
- 21.Andersson KE. Mechanisms of disease: central nervous system involvement in overactive bladder syndrome. Nat Clin Pract Urol. 2004;1:103–8. doi: 10.1038/ncpuro0021. [DOI] [PubMed] [Google Scholar]
- 22.Goncalves MA, Guilleminault C, Ramos E, Palha A, Paiva T. Erectile dysfunction, obstructive sleep apnea syndrome and nasal CPAP treatment. Sleep Med. 2005;6:333–9. doi: 10.1016/j.sleep.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Scheer I, Andrews V, Thakar R, Sultan AH. Urinary incontinence after obstetric anal sphincter injuries (OASIS)-is there a relationship? Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:179–83. doi: 10.1007/s00192-007-0431-8. [DOI] [PubMed] [Google Scholar]
- 24.Epstein LB, Goldberg RP. The overactive bladder and quality of life. Int J Fertil Womens Med. 2005;50:30–6. [PubMed] [Google Scholar]