Abstract
Essential oils that contain large concentrations of germacrene D are typically accompanied by cadinane sesquiterpenoids. The acid-catalyzed cyclization of germacrene D to give cadinane and selinane sesquiterpenes has been computationally investigated using both density functional (B3LYP/6-31G*) and post Hartree-Fock (MP2/6-31G* *) ab initio methods. The calculated energies are in general agreement with experimentally observed product distributions, both from acid-catalyzed cyclizations as well as distribution of the compounds in essential oils.
Keywords: germacrene D, cadinene, muurolene, amorphene, selinene, density functional theory, ab initio molecular orbital theory
1. Introduction
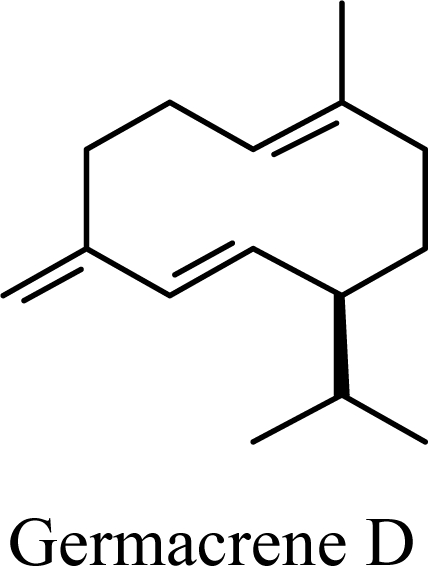
It is generally observed that essential oils containing large concentrations of the sesquiterpene germacrene D are typically accompanied by cadinane and muurolane sesquiterpenoids [1–6] and germacrene D has been suggested to serve as biogenetic precursor to a number of different sesquiterpenoid skeletons [7,8]. Bülow and König have demonstrated that germacrene D readily undergoes acid-catalyzed cyclization to give cadinane, muurolane, and amorphane sesquiterpenes [8]. In addition, there is concern that skeletal rearrangements may occur during the hydrodistillation of plant materials to obtain essential oils [9–12]. In this work, an ab initio investigation using density functional theory (B3LYP/6-31G*) and molecular orbital theory (MP2/6-31G**) of the acid-catalyzed cyclization of germacrene D has been carried out in order to test the hypothesis that the relative ratios of cadinane and muurolane sesquiterpenoids found in essential oil compositions as well as experimental cyclization of germacrene D reflect the energetic differences of the sesquiterpenoids and their carbocation precursors.
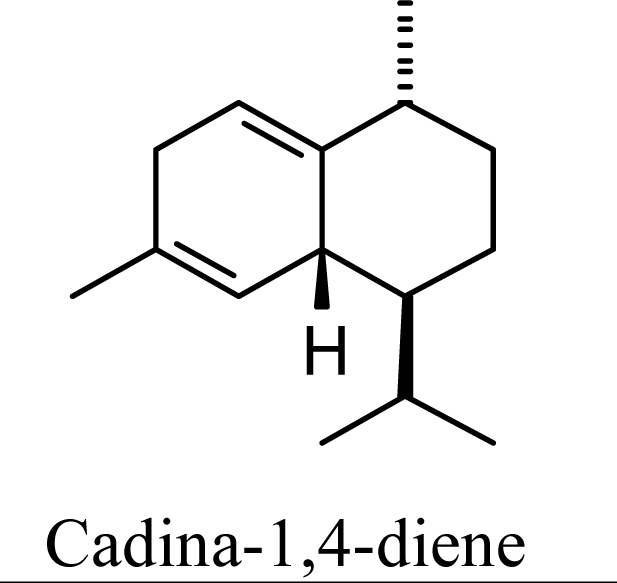
The essential oil compositions of Beilschmiedia [1], Cedrela [2],Croton [3], Eugenia [4], Ocotea [5], and Piper [6] species generally show large concentrations of germacrene D accompanied by smaller concentrations of γ-muurolene, α-muurolene, γ-cadinene, δ-cadinene, cadina-1,4-diene, and α-cadinene. Overall, the ratios of these materials from the essential oils above are: germacrene D (81.5%), γ-muurolene (1.2%), α-muurolene (1.1%), γ-cadinene (3.2%), δ-cadinene (12.2%), cadina-1,4-diene (0.8%), and α-cadinene (0.4%).
2. Results and Discussion
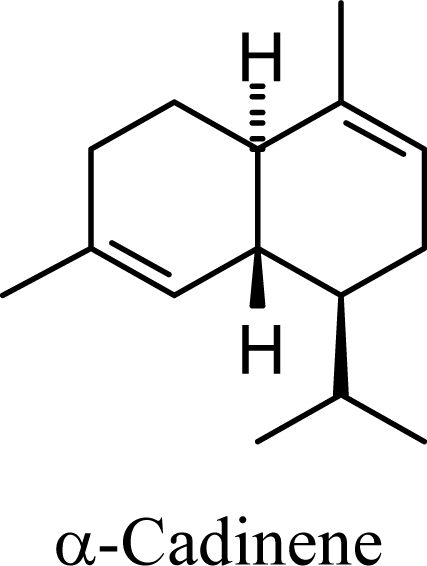
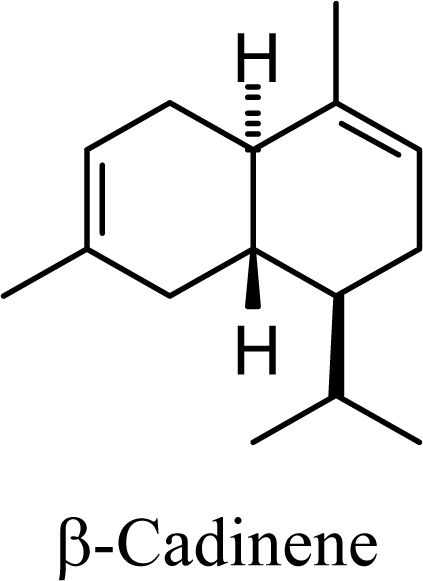
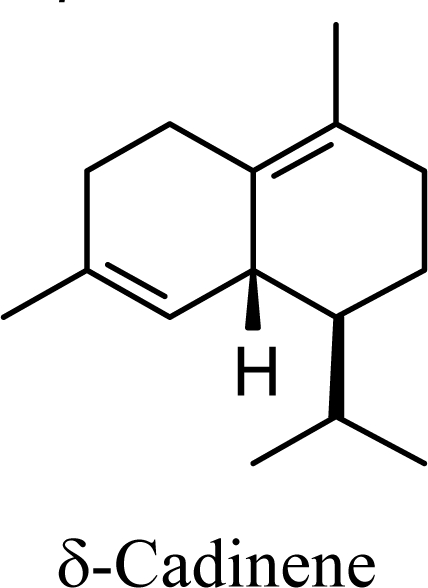
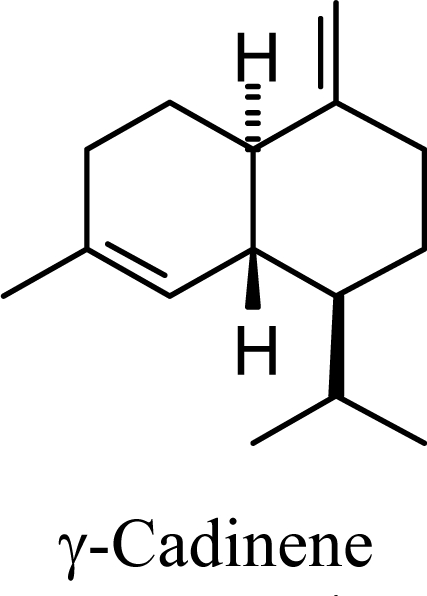
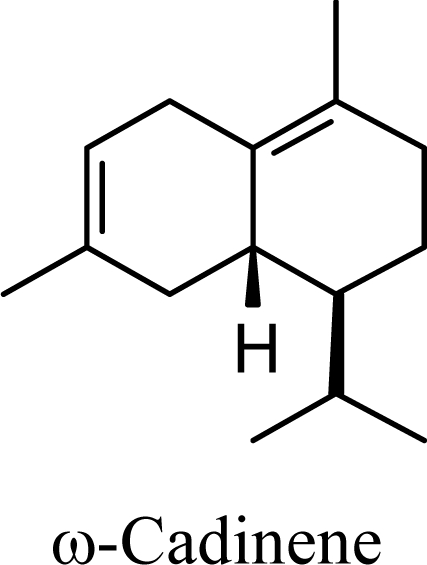
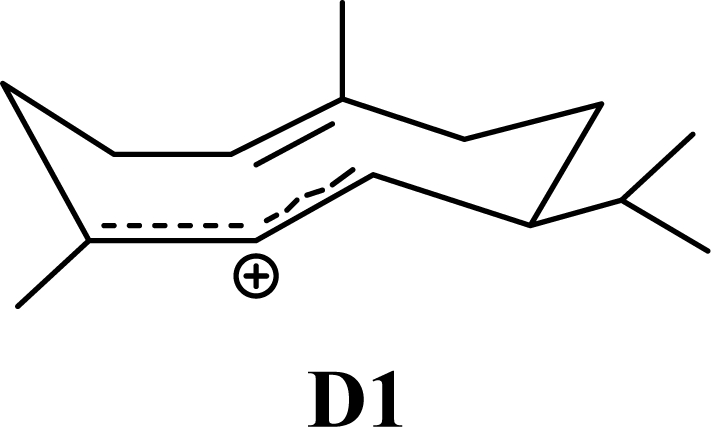
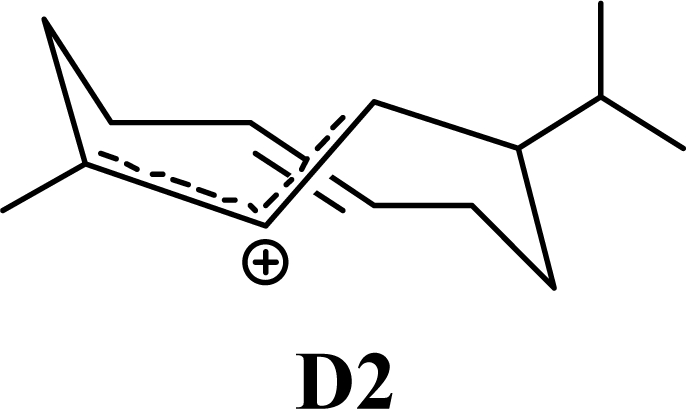
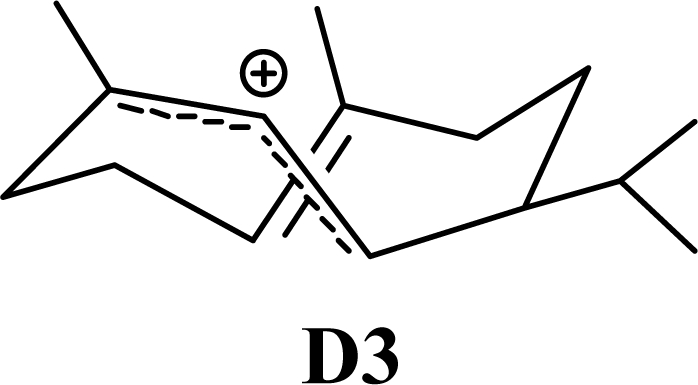
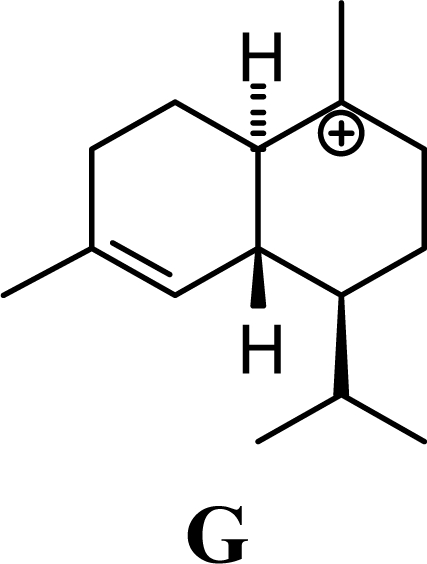
The DFT (B3LYP/6-31G*) and post-HF (MP2/6-31G* *) relative enthalpies (H(0K)) and free energies (G°, from the DFT calculations, or G, based on HF 6-31G** calculated entropies at 25°C) for germacrene D and the sesquiterpene hydrocarbons are summarized in Table 1. The relative energies of the carbocation intermediates are listed in Table 2. The boat-chair-chair conformation of the germacrenyl carbocation (D1) that leads to the cadinenyl cation (G) is lower in energy than either of the conformations that lead to muurolenyl (D2 → H) or amorphenyl (D3 → J) carbocations by 0.65 and 1.25 kcal/mol, respectively. Cadinenyl carbocation G is slightly higher in energy than muurolenyl carbocation H (0.75 kcal/mol). The cadinenyl carbocation manifold leads, by loss of a proton, directly to α-cadinene, δ-cadinene, and γ-cadinene, with δ-cadinene the lowest energy, followed by α-cadinene (2.90 kcal/mol higher) and γ-cadinene (4.96 kcal/mol). Bülow and König [8] had found that acid-catalyzed cyclizations of germacrene D generally give a preponderance of δ-cadinene, followed by γ-cadinene, and lesser amounts of α-cadinene. The abundant δ-cadinene is consistent with the ab initio calculations, but the calculated energies of α-cadinene and γ-cadinene are not in agreement with the experimental results, and would predict α-cadinene to be more abundant than γ-cadinene. An analysis of a number of essential oils from many different families, e.g., Apiaceae [13], Asteraceae [14-19], Cistaceae [20], Clusiaceae [21–25], Cupressaceae [26–29], Euphorbiaceae [3], Heteropyxidaceae [30,31], Lamiaceae [32–37], Lauraceae [1,5,38–40], Meliaceae [2], Myrtaceae [4,41], Pinaceae [42], and Piperaceae [6], reveals that δ-cadinene is also the most abundant cadinane sesquiterpene found. In addition, γ-cadinene is more abundant in these essential oils than α-cadinene. α-Cadinene has been shown to undergo acid-catalyzed rearrangement to give β-cadinene [43], which in turn, has been found to isomerize to ω-cadinene [44-46], in agreement with the calculated energies; β-cadinene and ω-cadinene are lower in energy than α-cadinene by 1.88 kcal/mole and 2.90 kcal/mol, respectively. Bülow and König [8] reported that ω-cadinene can be formed from δ-cadinene in a 1.4:1 ratio, consistent with the nearly equal calculated energies.
Table 1.
Ab Initio Enthalpies and Free Energies for Sesquiterpene Hydrocarbons.
| Compound | Relative Energies (kcal/mol) | Compound | Relative Energies (kcal/mol) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| B3LYP/6-31G* | MP2/6-31G** | B3LYP/6-31G* | MP2/6-31G** | ||||||
| H(0K) | G° | H(0K) | G | H(0K) | G° | H(0K) | G | ||
 |
33.86 | 33.18 | 41.96 | 41.05 | 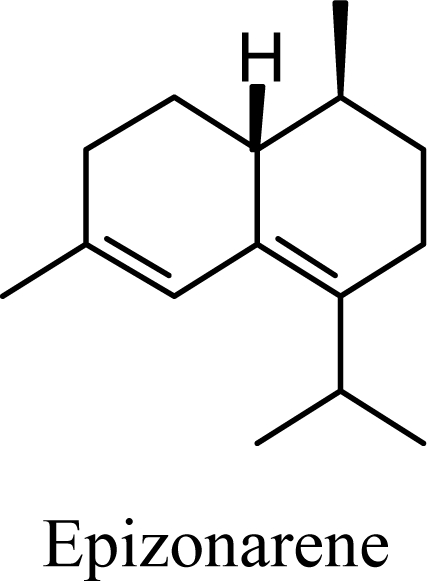 |
2.09 | 1.84 | 3.74 | 3.51 |
 |
8.62 | 8.86 | 10.21 | 10.17 | 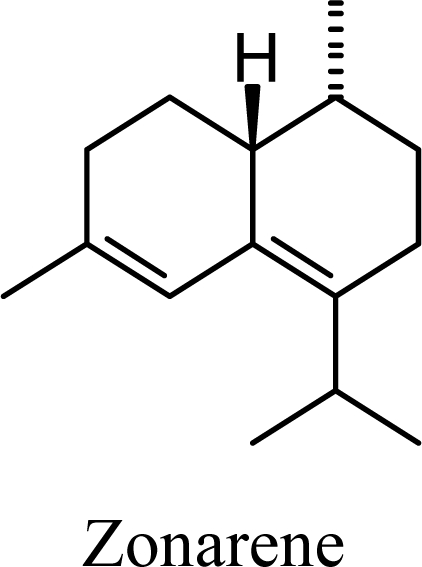 |
2.49 | 2.57 | 4.13 | 3.96 |
 |
6.74 | 7.17 | 8.06 | 8.05 | 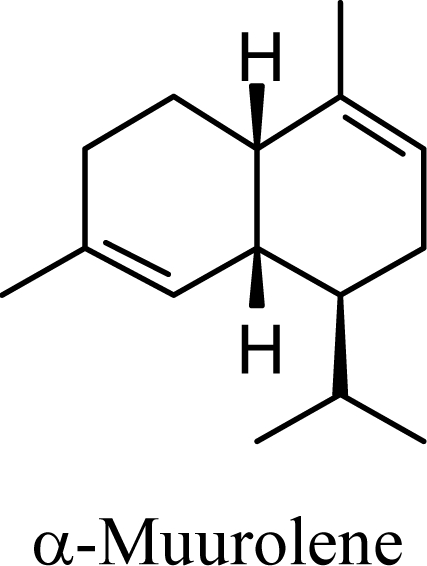 |
6.32 | 6.28 | 7.86 | 7.78 |
 |
5.72 | 5.58 | 7.92 | 7.69 | 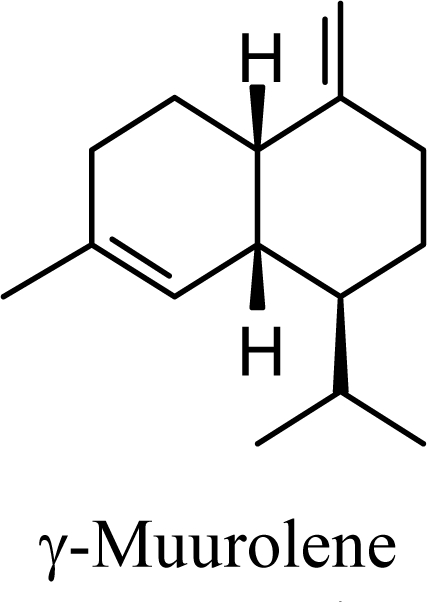 |
10.53 | 10.78 | 11.05 | 11.13 |
 |
10.68 | 11.00 | 11.79 | 11.91 | 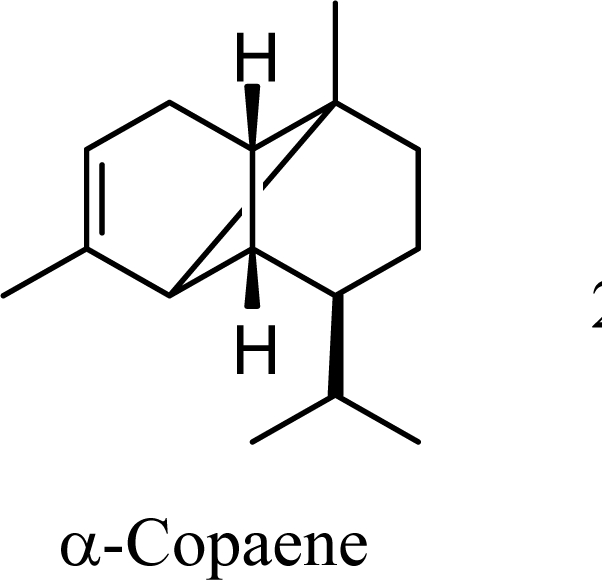 |
20.45 | 21.49 | 13.42 | 14.33 |
 |
5.72 | 5.37 | 8.64 | 8.27 | 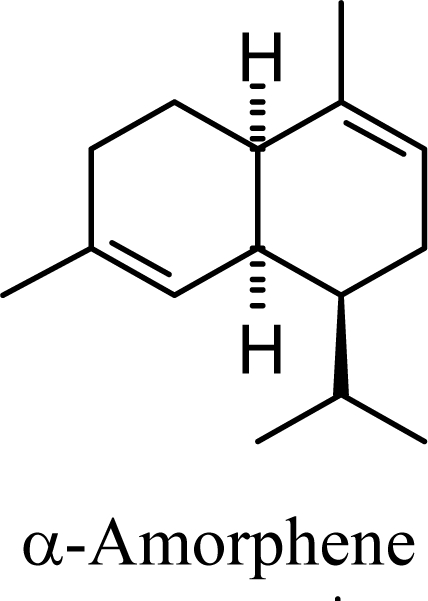 |
12.67 | 12.43 | 13.56 | 13.39 |
 |
5.58 | 5.90 | 7.52 | 7.48 | 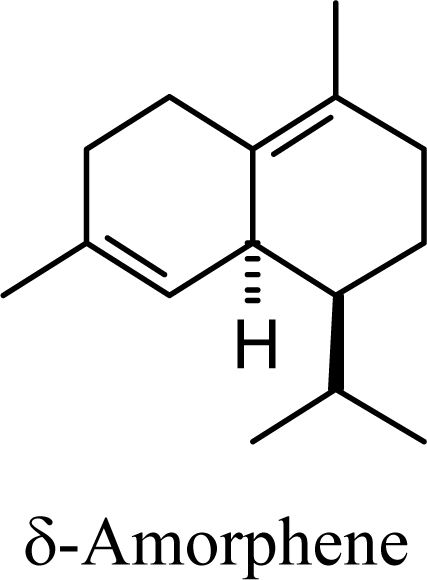 |
8.04 | 8.08 | 9.44 | 9.34 |
 |
20.02 | 20.03 | 20.61 | 20.73 | 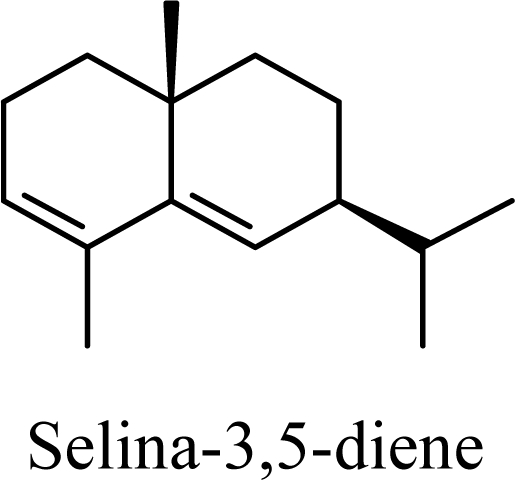 |
2.79 | 3.34 | 2.04 | 2.32 |
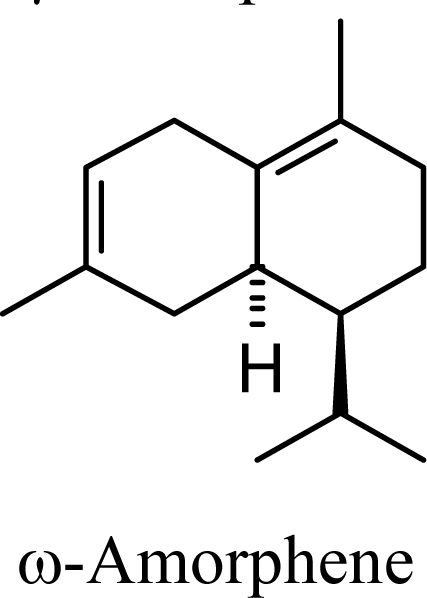 |
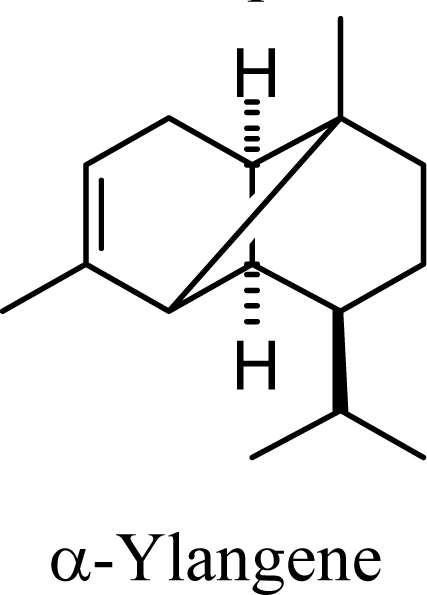
6.41 | 6.46 | 8.19 | 8.06 | 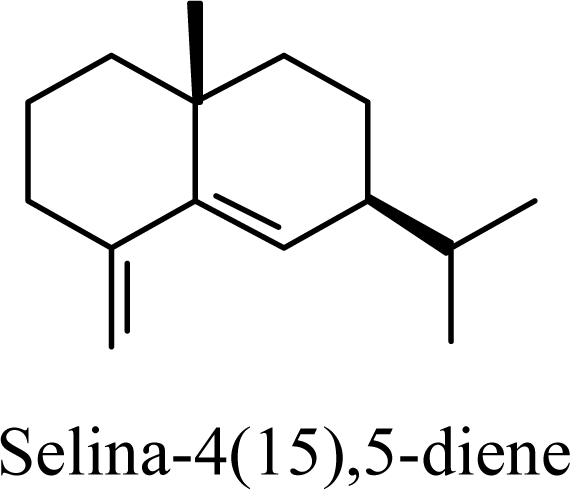 |
8.84 | 9.46 | 7.13 | 7.45 |
 |
20.55 | 21.82 | 13.17 | 14.08 | 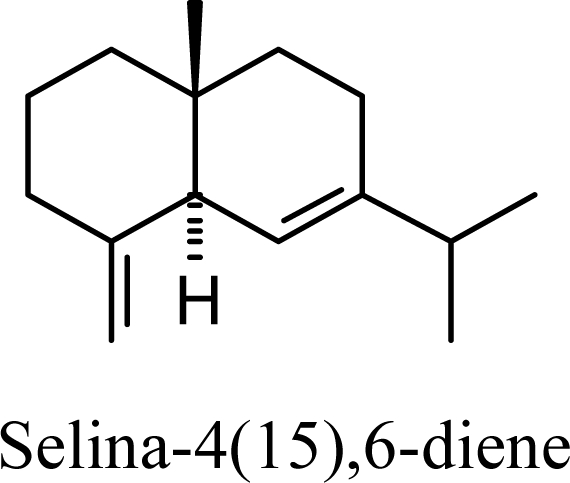 |
16.91 | 17.23 | 15.71 | 15.98 |
 |
0 | 0 | 0 | 0 | 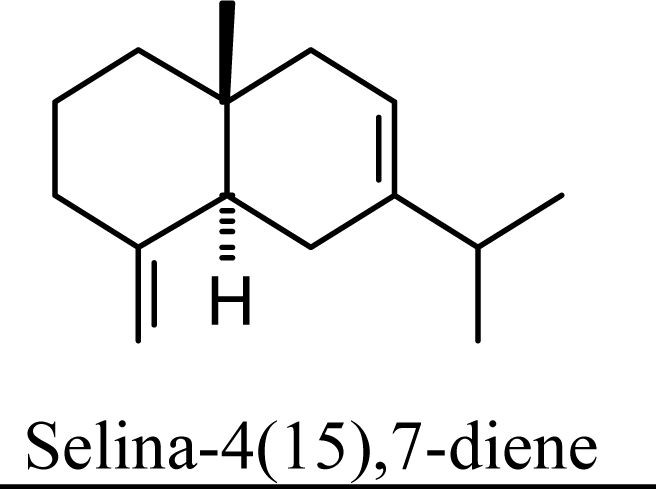 |
14.61 | 14.90 | 13.39 | 13.71 |
Table 2.
Ab Initio Enthalpies and Free Energies for Sesquiterpenyl Carbocations.
| Carbocation | Relative Energies (kcal/mol) | Carbocation | Relative Energies (kcal/mol) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| B3LYP/6-31G* | MP2/6-31G** | B3LYP/6-31G* | MP2/6-31G** | ||||||
| H(0K) | G° | H(0K) | G | H(0K) | G° | H(0K) | G | ||
 |
4.35 | 4.29 | 7.40 | 6.81 | 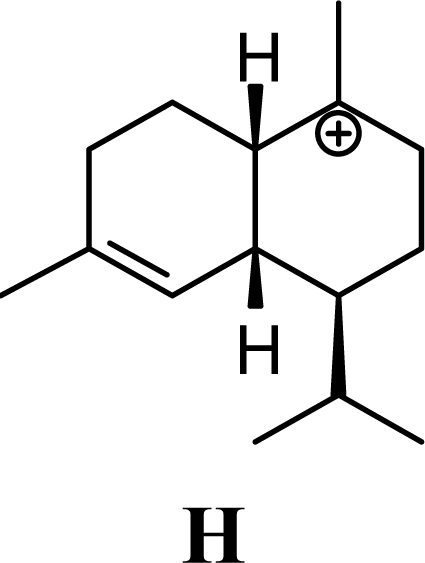 |
1.68 | 1.40 | 1.55 | 1.51 |
 |
5.00 | 4.52 | 7.21 | 6.81 | 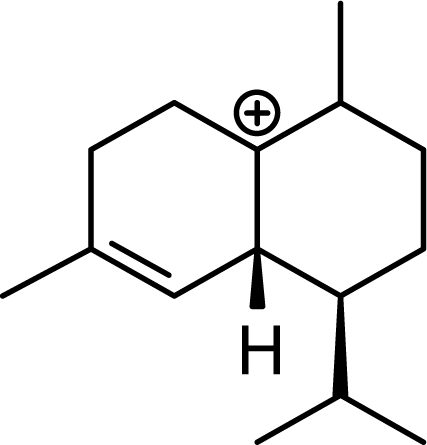 |
0 | 0 | 0 | 0 |
 |
5.60 | 5.77 | 7.75 | 7.19 | 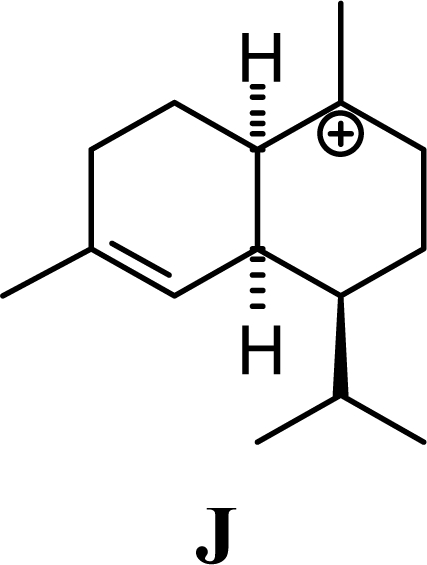 |
8.15 | 8.42 | 8.19 | 8.42 |
 |
15.19 | 15.45 | 11.94 | 11.58 | 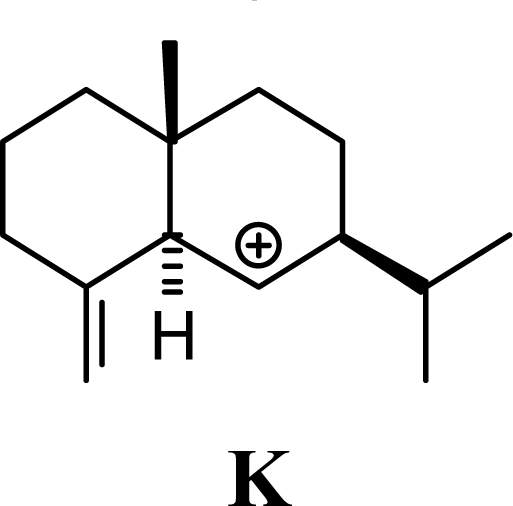 |
26.33 | 26.08 | 22.31 | 22.95 |
 |
2.43 | 1.92 | 1.94 | 1.92 | 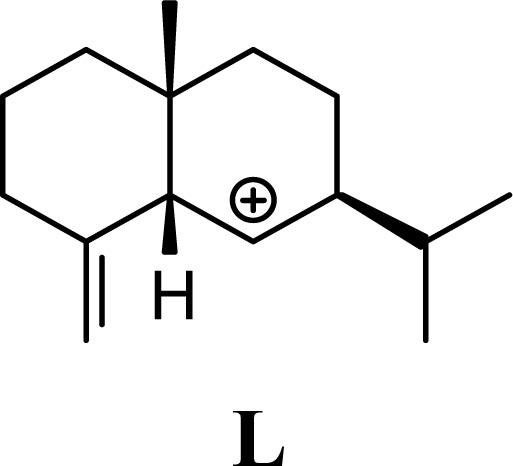 |
23.05 | 23.23 | 18.91 | 19.06 |
The muurolenyl carbocation, H, leads directly to α-muurolene, γ-muurolene, and α-copaene. Of these, α-muurolene is lowest in energy, followed by γ-muurolene (4.21 kcal/mol higher) and α-copaene (14.13 kcal/mol higher). In the acid-catalyzed cyclizations, γ-muurolene generally predominates over α-muurolene, but the ratio depends on the acidic conditions used [8]. Notably, γ-muurolene also predominates over α-muurolene in essential oils, but we have found α-muurolene to generally predominate over γ-muurolene in the Lauraceae [1,5] while γ-muurolene predominates in the Piperaceae [6]. Of the essential oils examined in this study, δ-cadinene predominates (49% of the cadinane sesquiterpenes), followed by γ-muurolene and γ-cadinene (17% and 15%, respectively), then α-muurolene (9%), α-cadinene (3%) and cadina-1,4-diene (3%); very similar to the average percentages observed in the acid-catalyzed cyclizations of germacrene D (δ-cadinene, 42%; γ-muurolene, 17%; γ-cadinene, 19%; α-muurolene, 11%; α-cadinene, 8%; and cadina-1,4-diene, 3%). This would suggest that the relative distributions of cadinane sesquiterpenes observed, both from acid-catalyzed cyclization and present in essential oils represent equilibrium concentrations and depend on the energies of the respective compounds, and the only real inconsistency is the calculated energy of α-cadinene.
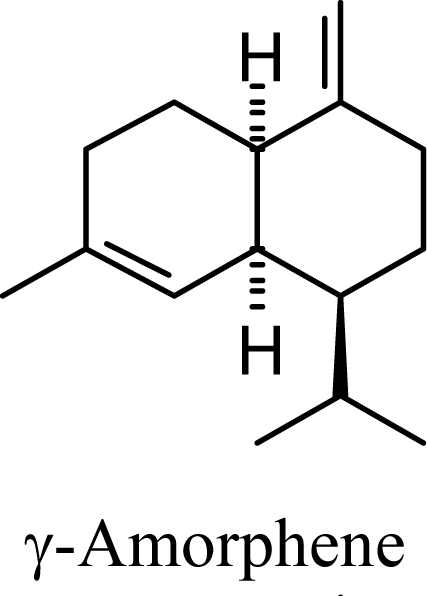
Amorphenes are generally present only in small amounts, if at all, in essential oils. Bülow and König [8], however, did find relatively high concentrations of δ-amorphene, α-amorphene, and γ-amorphene, in the acid-catalyzed cyclizations of germacrene D; up to 6% of each, depending on the conditions. The smaller amounts of amorphenes, compared to cadinenes and muurolenes reflects the relatively high energy of the amorphenyl carbocation compared to the cadinenyl and muurolenyl carbocations; about 6 kcal/mol higher. Reaction of germacrene D with strong Lewis acids (AlCl3 or ZnCl2) produces large amounts of epizonarene (as much as 80%) and zonarene (up to 25%). These two compounds are calculated to be the thermodynamically most stable of the cadinane sesquiterpenes in this investigation, with epizonarene slightly lower in energy (about 0.4 kcal/mol) than zonarene. Although these are the most stable of the cadinane sesquiterpenes, these materials are generally found only in small quantities in plant essential oils. Zonarene is, however, the major hydrocarbon component of the brown seaweed Dictyopteris zonarioides [47], and epizonarene is somewhat abundant in Cedrela fissilis leaf oil (2.5%) [48], the hexane extract of Tanacetum longifolium aerial parts (14.7%) [49], and the essential oil of Teucrium leucocladum (4.5%) [50].
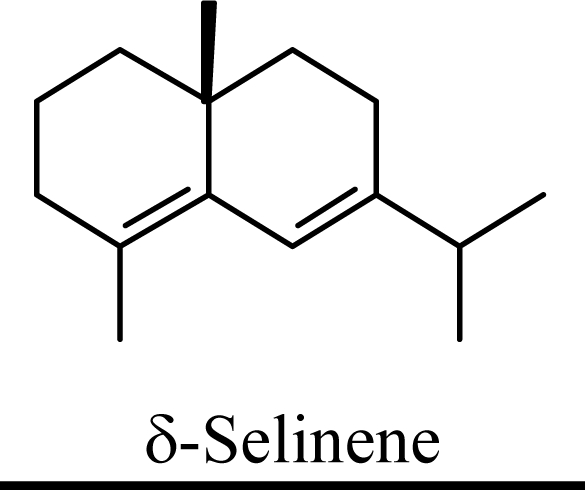
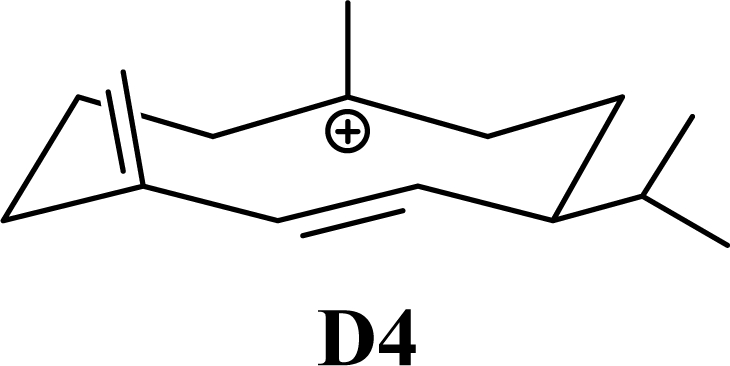
Protonation of C(1) of germacrene D leads to the selinane sesquiterpenes by way of carbocation D4 with ring closure to give selinyl carbocations K and L. The calculated energies of these carbocation intermediates are much higher than those leading to the cadinane sesquiterpenes. δ-Selinene, however, is lower in energy than any of the cadinenes, muurolenes, or amorphenes, and this is consistent with the experimental observation; longer reaction times lead to increasing concentrations of δ-selinene at the expense of the cadinanes [8].
3. Computational Methods
All calculations were carried out using SPARTAN ′06 for Windows [51]. The hybrid B3LYP functional [52,53] and the 6–31G* basis set [54] were used for the optimization of all stationary points in the gas phase. Single-point Hartree-Fock ab initio energies were calculated using the DFT geometries (above) at the 6–31G* * [54] level, followed by a correlation energy calculation using the second-order Møller-Plesset model (MP2) [54]. Frequency calculations were used to characterize stationary points as minima. All enthalpies reported are zero-point (ZPE) corrected with unscaled frequencies, but with no thermal corrections; they are, therefore, H(0K). Entropies were calculated using the linear harmonic oscillator approximation.
References and Notes
- 1.Setzer WN, Haber WA. Leaf essential oil composition of five species of Beilschmiedia from Monteverde, Costa Rica. Nat Prod Commun. 2007;2:79–83. [Google Scholar]
- 2.Eason HM, Setzer WN. Bark essential oil composition of Cedrela tonduzii C. DC. (Meliaceae) from Monteverde, Costa Rica. Rec Nat Prod. 2007;1:24–27. [Google Scholar]
- 3.Setzer WN. Chemical compositions of the bark essential oils of Croton monteverdensis and Croton niveus from Monteverde, Costa Rica. Nat Prod Commun. 2006;1:567–572. [Google Scholar]
- 4.Cole RA, Haber WA, Setzer WN. Chemical composition of essential oils of seven species of Eugenia from Monteverde, Costa Rica. Biochem Syst Ecol. 2007;35:877–886. [Google Scholar]
- 5.Takaku S, Haber WA, Setzer WN. Leaf essential oil composition of 10 species of Ocotea (Lauraceae) from Monteverde, Costa Rica. Biochem Syst Ecol. 2007;35:525–532. [Google Scholar]
- 6.Setzer WN, Park G, Agius BR, Stokes SL, Walker TM, Haber WA. Chemical compositions and biological activities of leaf essential oils of twelve species of Piper from Monteverde, Costa Rica. Molecules. 2008;13 (submitted) [Google Scholar]
- 7.Yoshihara K, Ohta Y, Sakai T, Hirose Y. Germacrene D, a key intermediate of cadinene group compounds and bourbonenes. Tetrahedron Lett. 1969:2263–2264. [Google Scholar]
- 8.Bülow N, König WA. The role of germacrene D as a precursor in sesquiterpene biosynthesis: investigations of acid catalyzed, photochemically and thermally induced rearrangements. Phytochemistry. 2000;55:141–168. doi: 10.1016/s0031-9422(00)00266-1. [DOI] [PubMed] [Google Scholar]
- 9.Bartley JP, Foley P. Supercritical fluid extraction of Australian-grown ginger (Zingiber officinale) J Sci Food Agric. 1994;66:365–371. [Google Scholar]
- 10.Asfaw N, Storesund HJ, Skattebøl L, Aasen AJ. Coexistence of chrysanthenone, filifolone, and (Z)-isogeranic acid in hydrodistillates. Artefacts! Phytochemistry. 2001;58:489–492. doi: 10.1016/s0031-9422(01)00254-0. [DOI] [PubMed] [Google Scholar]
- 11.Babu KGD, Kaul VK. Variation in essential oil composition of rose-scented geranium (Pelargonium sp.) distilled by different distillation techniques. Flavour Fragr J. 2005;20:222–231. [Google Scholar]
- 12.Teixeira S, Mendes A, Alves A, Santos L. Simultaneous distillation-extraction of high-value volatile compounds from Cistus ladanifer L. Anal Chim Acta. 2007;584:439–446. doi: 10.1016/j.aca.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 13.Palá-Paúl J, Pérez-Alonso MJ, Velasco-Negueruela A, Vadaré J, Villa AM, Sanz J, Brophy JJ. Essential oil composition of the different parts of Eryngium bourgatii Gouan from Spain. J Chromatogr A. 2005;1074:235–239. doi: 10.1016/j.chroma.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 14.Roussis V, Tsoukatou M, Petrakis PV, Chinou I, Skoula M, Harborne JB. Volatile constituents of four Helichrysum species growing in Greece. Biochem Syst Ecol. 2000;28:163–175. [Google Scholar]
- 15.Juteau F, Masotti V, Bessière JM, Viano J. Compositional characteristics of the essential oil of Artemisia campestris var glutinosa. Biochem Syst Ecol. 2002;30:1065–1070. [Google Scholar]
- 16.Maia JGS, Zoghbi MdGB, Andrade EHA, da Silva MH, Luz AIR, da Silva JD. Essential oils composition of Eupatorium species growing wild in the Amazon. Biochem Syst Ecol. 2002;30:1071–1077. [Google Scholar]
- 17.Chericoni S, Flamini G, Campeol E, Cioni PL, Morelli I. GC-MS analyses of the essential oil from the aerial parts of Artemisia verlotiorum: variability during the year. Biochem Syst Ecol. 2004;32:423–429. [Google Scholar]
- 18.Gauvin A, Smadja J. Essential oil composition of four Psiadia species from Reunion Island: A chemotaxonomic study. Biochem Syst Ecol. 2005;33:705–714. [Google Scholar]
- 19.Saroglou V, Dorizas N, Kypriotakis Z, Skaltsa H. Analysis of the essential oil composition of eight Anthemis species from Greece. J Chromatogr A. 2006;1104:313–322. doi: 10.1016/j.chroma.2005.11.087. [DOI] [PubMed] [Google Scholar]
- 20.Demetzos C, Angelopoulou D, Perdetzoglou D. A comparative study of the essential oils of Cistus salviifolius in several populations of Crete (Greece) Biochem Syst Ecol. 2002;30:651–665. doi: 10.1016/s0305-1978(00)00071-5. [DOI] [PubMed] [Google Scholar]
- 21.Nogueira PCdL, Bittrich V, Shepherd GJ, Lopes AV, Marsaioli AJ. The ecological and taxonomic importance of flower volatiles of Clusia species (Guttiferae) Phytochemistry. 2001;56:443–452. doi: 10.1016/s0031-9422(00)00213-2. [DOI] [PubMed] [Google Scholar]
- 22.Schwob I, Bessière JM, Viano J. Composition of the essential oils of Hypericum perforatum L. from southeastern France. C R Biol. 2002;325:781–785. doi: 10.1016/s1631-0691(02)01489-0. [DOI] [PubMed] [Google Scholar]
- 23.Schwob I, Bessiere JM, Masotti V, Viano J. Changes in essential oil composition in Saint John's wort (Hypericum perforatum L.) aerial parts during its phenological cycle. Biochem Syst Ecol. 2004;32:735–745. [Google Scholar]
- 24.Petrakis PV, Couladis M, Roussis V. A method for detecting the biosystematic significance of the essential oil composition: The case of five Hellenic Hypericum L. species. Biochem Syst Ecol. 2005;33:873–898. [Google Scholar]
- 25.Saroglou V, Marin PD, Rancic A, Veljic M, Skaltsa H. Composition and antimicrobial activity of the essential oil of six Hypericum species from Serbia. Biochem Syst Ecol. 2007;35:146–152. [Google Scholar]
- 26.Adams RP. Systematics of the one seeded Juniperus of the eastern hemisphere based on leaf essential oils and random amplified polymorphic DNAs (RAPDs) Biochem Syst Ecol. 2000;28:529–543. doi: 10.1016/s0305-1978(99)00096-4. [DOI] [PubMed] [Google Scholar]
- 27.Salido S, Altarejos J, Nogueras M, Sánchez A, Pannecouque C, Witvrouw M, De Clercq E. Chemical studies of essential oils of Juniperus oxycedrus ssp. badia. J Ethnopharmacol. 2002;81:129–134. doi: 10.1016/s0378-8741(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 28.Cavaleiro C, Salgueiro LR, da Cunha AP, Figueiredo AC, Barroso JG, Bighelli A, Casanova J. Composition and variability of the essential oils of the leaves and berries from Juniperus navicularis. Biochem Syst Ecol. 2003;31:193–201. [Google Scholar]
- 29.Vichi S, Riu-Aumatell M, Mora-Pons M, Guadayol JM, Buxaderas S, López-Tamames E. HS-SPME coupled to GC/MS for quality control of Juniperus communis L. berries used for gin aromatization. Food Chem. 2007;105:1748–1754. [Google Scholar]
- 30.Sibanda S, Chigwada G, Poole M, Gwebu ET, Noletto JA, Schmidt JM, Rea AI, Setzer WN. Composition and bioactivity of the leaf essential oil of Heteropyxis dehniae from Zimbabwe. J Ethnopharmacol. 2004;92:107–111. doi: 10.1016/j.jep.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Van Vuuren SF, Viljoen AM, Özek T, Demirci B, Başer KHC. Seasonal and geographic variation of Heteropyxis natalensis essential oil and the effect thereof on the antimicrobial activity. S Afr J Bot. 2007;73:441–448. [Google Scholar]
- 32.Pereira SI, Santos PAG, Barroso JG, Figueiredo AC, Pedro LG, Salgueiro LR, Deans SG, Scheffer JJC. Chemical polymorphism of the essential oils from populations of Thymus caespititius grown on the island S. Jorge (Azores) Phytochemistry. 2000;55:241–246. doi: 10.1016/s0031-9422(00)00278-8. [DOI] [PubMed] [Google Scholar]
- 33.Skaltsa HD, Mavrommati A, Constantinidis T. A chemotaxonomic investigation of volatile constituents in Stachys subsect. Swainsonianeae (Labiatae) Phytochemistry. 2001;57:235–244. doi: 10.1016/s0031-9422(01)00003-6. [DOI] [PubMed] [Google Scholar]
- 34.Veličković DT, Randjelović NV, Ristić MS, Šmelcerović AA, Veličkoviú AS. Chemical composition and antimicrobial action of the ethanol extracts of Salvia pratensis L., Salvia glutinosa L. and Salvia aethiopis L. J Serb Chem Soc. 2002;67:639–646. [Google Scholar]
- 35.Couladis M, Chinou IB, Tzakou O, Loukis A. Composition and antimicrobial activity of the essential oil of Ballota pseudodictamnus L. Bentham. Phytother Res. 2002;16:723–726. doi: 10.1002/ptr.1043. [DOI] [PubMed] [Google Scholar]
- 36.Ložiene K, Vaièiuniene J, Venskutonis PR. Chemical composition of the essential oil of different varieties of thyme (Thymus pulegioides) growing wild in Lithuania. Biochem Syst Ecol. 2003;31:249–259. [Google Scholar]
- 37.Skaltsa HD, Demetzos C, Lazari D, Sokovic M. Essential oil analysis and antimicrobial activity of eight Stachys species from Greece. Phytochemistry. 2003;64:743–752. doi: 10.1016/s0031-9422(03)00386-8. [DOI] [PubMed] [Google Scholar]
- 38.Telascrea M, de Araújo CC, Marques MOM, Facanali R, de Moraes PLR, Cavalheiro AJ. Essential oil from the leaves of Cryptocarya mandioccana Meisner (Lauraceae): Composition and intraspecific chemical variability. Biochem Syst Ecol. 2007;35:222–232. [Google Scholar]
- 39.Setzer WN, Stokes SL, Penton AF, Takaku S, Haber WA, Hansell E, Caffrey CR, McKerrow JH. Cruzain inhibitory activity of leaf essential oils of Neotropical Lauraceae and essential oil components. Nat Prod Commun. 2007;2:1203–1210. [Google Scholar]
- 40.Agius BR, Setzer MC, Stokes SL, Walker TM, Haber WA, Setzer WN. Composition and bioactivity of essential oils of Lauraceae from Monteverde, Costa Rica. Int J Essent Oil Ther. 2008 (in press) [Google Scholar]
- 41.Cole RA, Haber WA, Lawton RO, Setzer WN. Leaf essential oil composition of three species of Myrcianthes from Monteverde, Costa Rica. Chem Biodiv. 2008 doi: 10.1002/cbdv.200890120. (in press) [DOI] [PubMed] [Google Scholar]
- 42.Nicolić B, Ristić M, Bojoviæ S, Marin PD. Variability of the needle essential oils of Pinus heldreichii from different populations in Montenegro and Serbia. Chem Biodiv. 2007;4:905–916. doi: 10.1002/cbdv.200790079. [DOI] [PubMed] [Google Scholar]
- 43.Fringuelli F, Pizzo F, Taticchi A, Ferreira VF, Michelotti EL, Porter B, Wenkert E. Diels-Alder reactions of cycloalkenones. 4. Short syntheses of some cadinenes. J Org Chem. 1985;50:890–891. [Google Scholar]
- 44.Vlahov R, Holub M, Herout V. On terpenes. CLXXXIV. Sesquiterpenic hydrocarbons from the essential oil of Mentha piperita of Bulgarian origin. Coll Czech Chem Comm. 1967;32:822–829. [Google Scholar]
- 45.Connell DW, Hildebrand RP, Sutherland MD. Terpenoid chemistry XIV: the significance of the term “δ-cadinene”. Tetrahedron Lett. 1968:519–523. [Google Scholar]
- 46.Nagasampagi BA, Yankov L, Dev S. Sesquiterpenoids from the wood of Cedrela toona Roxb – partial synthesis of T-muurolol, T-cadinol and cubenol, structures of δ-cadinene and δ-cadinol. Tetrahedron Lett. 1968:1913–1918. [Google Scholar]
- 47.Fenical W, Sims JJ, Wing RM, Radlick PC. Zonarene, a sesquiterpene from the brown seaweed Dictyopteris zonarioides. Phytochemistry. 1972;11:1161–1163. [Google Scholar]
- 48.Maia BHLNS, de Paula JR, Sant'Ana J, da Silva MFdGF, Fernandes JB, Vieira PC, Costa MdSS, Ohashi OS, Silva JNM. Essential oils of Toona and Cedrela species (Meliaceae): Taxonomic and ecological implications. J Braz Chem Soc. 2000;11:629–639. [Google Scholar]
- 49.Mahmood U, Kaul VK, Singh B. Sesquiterpene and long chain ester from Tanacetum longifolium. Phytochemistry. 2002;61:913–917. doi: 10.1016/s0031-9422(02)00396-5. [DOI] [PubMed] [Google Scholar]
- 50.El-Shazly AM, Hussein KT. Chemical analysis and biological activities of the essential oil of Teucrium leucocladum Boiss. (Lamiaceae) Biochem Syst Ecol. 2004;32:665–674. [Google Scholar]
- 51.SPARTAN ′06 for Windows. Wavefunction, Inc; Irvine, California: 2006. [Google Scholar]
- 52.Becke AD. Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys. 1993;98:5648–5652. [Google Scholar]
- 53.Lee C, Yang W, Parr RG. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 54.Hehre WJ, Radom L, Schleyer PvR. Ab initio Molecular Orbital Theory. Wiley; New York: 1986. [Google Scholar]


