Abstract
A dihydroxotin(IV) porphyrin functionalized single-walled carbon nanotubes (SWNTs) nanohybrid is obtained. Solubility of the nanohybrid in organic solvents is determined by UV-Vis-NIR absorption spectroscopy. Electron absorption and fluorescence spectra investigations demonstrate that efficient electron transfer occurs within the nanohybrid at the photoexcited state and the charge-separated state of the nanohybrid is observed by transient absorption spectrum. The results illustrate that this soluble electron donor–acceptor nanohybrid might be a good candidate as a light harvesting material in molecular photoelectronic devices.
Keywords: Carbon nanotubes, nanohybrid material, porphyrin, light harvesting, Fluorescence
1. Introduction
The novel and unique electronic properties exhibited by SWNTs make them excellent candidates for applications in the area of optoelectronics and photovoltaic devices [1]. It is generally acknowledged that many of these potential applications require the chemical modification of carbon nanotubes with specific functionalities via covalent or noncovalent methods. The presence of extended, delocalized π-electron systems makes this carbon material very useful for managing charge transfer when carbon nanotubes are combined with electron donors, such as porphyrin [2] and phthalocyanine [3]. These arrangements might be used to construct photovoltaic cells [4]. Although much progress has been made, it is widely recognized that the poor solubility of the SWNTs-based nanocomposites has hindered their chemical manipulation and practical applications greatly [4,5]. Thus, it is essential to realize their potential applications in nanotechnology by solubilizing functional carbon nanotube composites to increase their dispersibility in a particular medium.
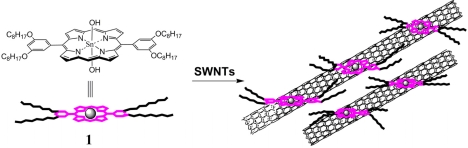
Porphyrins are ideal building blocks for the design of novel artificial photosynthetic supramolecular systems. In particular, the metalloporphyrins have been of great interest because metal–ligand interactions could provide a convenient method for constructing functional supramolecular arrays [6]. Consequently, the dihydroxotin(IV) porphyrin (Sn(OH)2DPP, 1) is employed to functionalize the SWNTs to yield a nanoscale hybrid (SWNT–SnDPP) (Scheme 1) and the attached Sn(OH)2DPP makes the SWNTs dispersible in organic solvents. The solubility of the SWNT–SnDPP nanohybrid in solvents is determined by UV-Vis-NIR absorption spectroscopy. Further spectroscopic investigations demonstrate that electron transfer occurs between the porphyrins and SWNTs. The transient absorption spectrum confirms that the photoexcited process generates charge-separated states within the nanohybrid and that the SWNTs serve as the electron acceptor component in this donor–acceptor nanohybrid. The electron transfer and the light harvesting features of the SWNT-SnDPP nanohybrid suggest that this novel soluble nanohybrid presents a potential candidate for nanoscale photoelectronic and photovoltaic devices.
Scheme 1.
Schematic view of the Sn(IV) porphyrin (1) functionalized SWNTs.
2. Results and Discussion
2.1. Characterization of the nanohybrid
The transmission electron micrograph (TEM) shown in Figure 1 demonstrates that the surfaces of SWNTs samples before functionalization with Sn(OH)2DPP are highly smooth. After functionalization, nanotube bundles exfoliate to thin bundles and black nanoparticals are observed to be coated on the surfaces of nanotubes (Figure 1b), which are similar to the functionalized SWNTs materials previously reported in the literature [7,8]. This demonstrates that the tin porphyrins are immobilized on the sidewalls of SWNTs. The porphyrins are attached on the nanotubes' surface forming nano-sized clusters with diameters ranging from several to tens of nanometers which presumably consist of aggregated porphyrin molecules [7–10]. Energy-dispersive X-ray Spectroscopic (EDS, Figure 1c) analysis of the SWNT–SnDPP sample confirms a spatial localization of the expected tin on the functionalized tubes with ∼ 5 wt% Sn in SWNT–SnDPP.
Figure 1.
TEM images of purified SWNTs dispersed in DMF via sonication for 30 minutes (a), SWNT–SnDPP dispersed in THF via sonication for 1 minute (b) and EDS analysis of SWNT–SnDPP (c). Measurements performed at room temperature.
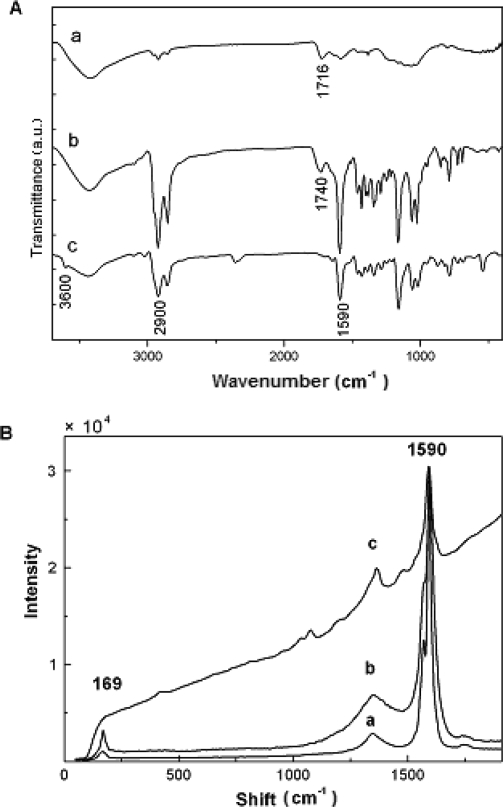
The strong signal at 1740 cm−1 in the FT-IR spectrum of SWNT–SnDPP nanohybrid (Figure 2A–b) is assigned to the carbonyl stretch of the ester, which is consistent with other ester functionalized SWNTs [7]. Furthermore, the OH signal of the free tin porphyrin 1 at 3600 cm−1 (Figure 2A–c) disappears after the hybrid formation. These results indicate that the tin porphyrin is covalently linked to the carboxylic SWNTs through the formation of ester bonds, as reported previously [7,8,11,12]. In Figure 2A–b, the alkyl chain C–H vibration at 2900 cm−1 can also be seen, together with signals at 1580–1600 cm−1 (benzenoid ring stretch), 1100–1200 cm−1 (pyrrole vibration), all of which are consistent with the presence of porphyrin.
Figure 2.
(A) FT-IR spectra of: SWNTs (a), SWNT–SnDPP (b) and Sn(OH)2DPP (c). All IR samples are prepared as pellets by using spectroscopic grade KBr at room temperature. (B) Raman spectra of SWNTs (a) SWNT–SnDPP after TGA treatment in nitrogen, 5 °C/min (b) and SWNT–SnDPP (c). Raman spectra are measured at room temperature with the 514.5 nm line of an Ar ion laser as the excitation source.
As in other well dispersed functionalized carbon nanotubes, the Raman spectrum of the SWNT– SnDPP sample (Figure 2B–c) is overwhelmed by the luminescence background [4,7]. The luminescence interference is eliminated after thermal defunctionalization of the SWNT–SnDPP sample (Figure 2B–b). When the porphyrin substituents on the nanotube surfaces are removed after thermal treatment, the spectrum shows the characteristic Raman peaks of SWNTs at ∼ 169 cm−1 (redial breathing mode), ∼ 1300 cm−1 (disorder mode) and ∼ 1590 cm−1 (tangential mode) (Figure 2B–b), which are similar to those of purified SWNTs (Figure 2B–a).
2.2. Estimation of the solubility of the nanohybrid
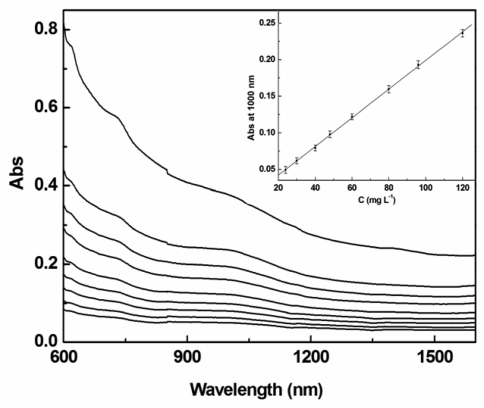
Many studies and applications of SWNTs are greatly hindered by processing and manipulation difficulties owing to their poor dispersibility in common organic solvents. Therefore, solubilization of SWNTs by using covalent and noncovalent methods has been developed to overcome these difficulties [4,5,13]. Here we employ tin porphyrin to functionalize and disperse SWNTs. The backbone of the tin porphyrin we use is modified with long alkyl chains and adopts stable six-coordinate structures with two trans OH axial ligands on Sn(IV). The structural design of this tin porphyrin is based on three considerations: 1) The OH axial ligands of the tin porphyrins can be displaced easily by carboxylate and phenoxide [11,12]. There are large numbers of COOH, OH groups on both the sidewalls and the ends of the nanotubes after acid treatment of SWNTs [14], which could react with the axial OH of the tin porphyrin; 2) Long alkyl chains will increase solubility of SWNTs in organic solvents and stabilize SWNTs dispersion; 3) 119Sn NMR can be used to further explore the interaction between SWNTs and the functional groups, which is difficult in most other cases for SWNT materials. Indeed, after immobilization by tin porphyrins, the functionalized SWNTs can be well dispersed in organic solvents such as tetrahydrofuran (THF), chloroform and toluene, and as such can form a stable suspension. Solution phase UV-Vis-NIR spectroscopy has been reported to demonstrate a linear relationship between the absorbance and the concentration of SWNTs in solvents, which has been used to determine the solubility of SWNTs [15]. The UV-Vis-NIR spectra of samples with different concentrations in THF at room temperature are shown in Figure 3. The observed spectra are dependent on the concentration in a linear fashion (Figure 3 inset), following Lambert-beer's law. The solubility of SWNT-SnDPP in typical organic solvents is given in Table 1. The values are the average of triplicate measurements.
Figure 3.
Absorption spectra of SWNT–SnDPP nanohybrid in room temperature THF at different concentrations (from top to bottom: saturated solution, 120, 96, 80, 60, 48, 40, 30 and 24 mg L−1). Inset shows the linear relationship of the absorption at 1000 nm versus concentration; slope = 0.002, R = 0.9997.
Table 1.
Room temperature solubility of SWNT-SnDPP nanohybrid. a
| Solvent | Solubility (mg/L) b |
|---|---|
| 1,2-Dichlorobenzene | 440 ± 4.6 |
| Chloroform | 358 ± 4.9 |
| Toluene | 237 ± 4.4 |
| Tetrahydrofuran | 209 ± 3.2 |
| Hexane | ——c |
| Ethanol | ——c |
| Methanol | ——c |
The sonicator bath water temperature rises to ca. 35 °C over the course of 0.5 hour.
Measurement is carried out independently three times (n = 3), and the values are the average of three measurements.
Solubility in these solvents is < 1 mg L–1 (sonication for over 2 hours).
2.3. NMR investigations
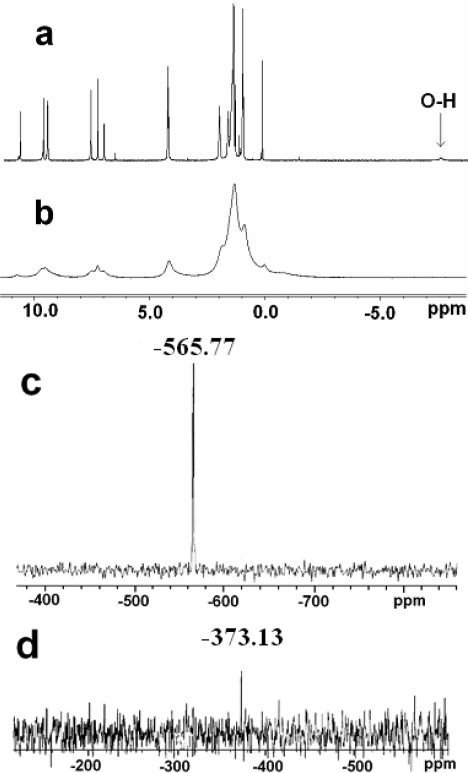
The solubilization of carbon nanotubes provides opportunities for solution NMR studies of the nanotubes, which is difficult in many cases for SWNTs. 1H NMR and 119Sn NMR spectra of SWNT– SnDPP and Sn(OH)2DPP are shown in Figure 4. The 1H NMR signal at high field (−7.85 ppm, Figure 4a) is attributed to the proton of the OH group bound to Sn(IV), which disappears in the spectrum of SWNT–SnDPP (Figure 4b). This result indicates that OH should be replaced by the carboxylate group on the SWNTs [11,12]. All other 1H NMR peaks of the hybrid appear at similar positions as those for free Sn(OH)2DPP, but all the peaks are significantly broadened. We argue that this broadening is caused for the following reasons: 1) Strong interaction between porphyrin and SWNTs; 2) The loss of symmetry which comes from the restriction of the degrees of conformational freedom in solution; 3) The presence of large diamagnetic SWNTs ring currents (vide infra) [4,5,13,16–18].
Figure 4.
NMR (CDCl3, 300 MHz, room temperature): 1H NMR spectra: (a) Sn(OH)2DPP, (b) SWNT–SnDPP; 119Sn NMR spectra: (c) Sn(OH)2DPP, (d) SWNT–SnDPP.
The presence of 119Sn in this nanohybrid and its good solubility enable observation of the 119Sn NMR signal. The spectrum of Sn(OH)2DPP shows a 119Sn peak at −565.77 ppm (Figure 4c), while the 119Sn peak of the SWNT–SnDPP appears at −373.13 ppm (Figure 4d). The significant downfield shift demonstrates that the immobilization of porphyrin onto the sidewalls of SWNTs has great influence on the chemical environment of the tin atom. For the nanohybrid, the down-field in particular indicates that tin porphyrins are experiencing a strong magnetic field as they are positioned over a region of the nanotubes in which there exist strong extended diamagnetic ring current circulations around the nanotube axis [16–18].
2.4. Absorption spectra investigations
The room temperature UV-Vis-NIR absorption spectrum of SWNT–SnDPP in THF shows a Soret band at ∼ 420 nm and characteristic broad bands of van Hove singularities at ∼ 1000 and ∼ 1800 nm, corresponding separately to porphyrin and transitions associated with the S22 and S11 bands of SWNTs (Figure 5). Compared with the absorption of Sn(OH)2DPP, the spectrum of SWNT–SnDPP shows a modest red shift and broadening of the Soret band. The observed red-shift and broadening features indicate that tin porphyrins covalently linked to SWNTs cause some alteration of the electronic state of porphyrin in the ground state, which is similar to other reported porphyrin meso-phenyl ester linked nanotubes [7,8].
Figure 5.
UV-Vis-NIR absorption spectra of Sn(OH)2DPP (dotted line) and SWNT–SnDPP (solid line) in THF at room temperature (Cporphyrin = 1.1×10−5 mol L−1, 25 °C). The inset shows the enlarged Soret-band region.
2.5. Fluorescence and transient absorption spectra investigations
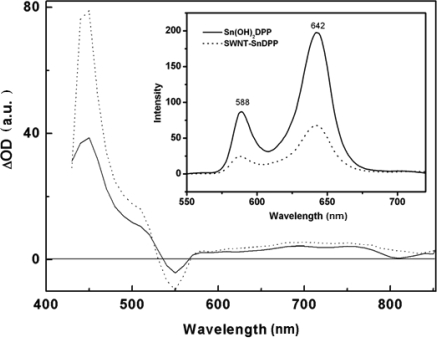
In order to probe excited-state interactions of tin porphyrin and SWNTs, the fluorescence emission spectra of SWNT–SnDPP and Sn(OH)2DPP are compared (Figure 6 inset). Upon excitation of the tin porphyrin moiety at its Soret band, the solution of SWNT-SnDPP exhibits 66% quenching of fluorescence at 588 nm and 642 nm. The efficient fluorescence quenching indicates that the excited tin porphyrin strongly interacts with SWNTs. The quenching which comes from the electron transfer between photoexcited electron-donor porphyrin and electron-acceptor SWNTs will be discussed hereinafter.
Figure 6.
Differential absorption spectrum obtained upon nanosecond flash photolysis (532 nm) of SWNT–SnDPP (dotted line) and Sn(OH)2DPP (solid line) in argon saturated THF at room temperature with a 50 ns time delay. Inset shows the fluorescence spectra of Sn(OH)2DPP and SWNT–SnDPP in THF (Cporphyrin = 1.1×10−5 mol L−1) at room temperature.
Transient absorption measurements are performed to get more information about the porphyrin– SWNTs interactions and possible deactivation mechanism of the excited state of this nanohybrid. Pumping light into the Sn(OH)2DPP ground state with short 532 nm laser pulses leads to the observation of the long-lived and molecular-oxygen-sensitive triplet spectra (Figure 6 solid line), besides bleaching in the Soret-and Q-band region. The characteristic triplet maximum appears at around 690 and 750 nm, whilst the SWNT–SnDPP nanohybrid gives a different transient absorption spectrum which is much broader and extends to above 800 nm in the near infrared range (Figure 6 dotted line). These features are indicative of the presence of porphyrin based oxidized products, i.e. the π-radical cation [2]. At increasing times after excitation, diminishing of these features indicates the return of the charge-separated state to the ground state. However, a reliable kinetic analysis of the decay dynamics is prevented by the superimposing features of the residue triplet excited state. All the studies above reflect a rapid excited state quenching of tin porphyrin due to the electron-transfer process from tin porphyrin to SWNTs, where tin porphyrin acts as a light harvesting antenna.
3. Experimental Section
3.1. Instruments and Measurements
The separation of SWNT from the impurities is performed with a centrifuge (Eppendorf 5810R, Germany), and filtration is performed through nylon membrane (0.22/0.1μm, 47 mm; Whatman Internantional Ltd., Maidstone, UK). Water bath sonication is performed with a KQ-400KDE (400 W, 40 kHz, Kunshan Sonicator Instrument Co. Inc.) sonicator. Transmission electron microscope (TEM) images are obtained on a FEI TECNAI-20 instrument (Fei, Tokyo, Japan) equipped with energydispersive X-ray spectroscopy (EDS) capabilities. Sample preparation involves sonicating the materials in THF for 1 minute and dropping the resulting suspension onto carbon-coated copper grids. NMR spectra are recorded on a BRUKER (300 MHz) spectrometer (Bruker GmbH, Bremen. Germany). FTIR spectra are obtained with a BRUKER TENSOR 27 instrument. All IR samples are prepared as pellets using spectroscopic grade KBr. Raman spectra are measured by a Reinishaw in Via Raman Microscope at room temperature with the 514.5 nm line of an Ar ion laser as the excitation source. UV-Vis-NIR spectra are obtained with a JASCO V-570 spectrometer (JASCO International Co., Ltd, Tokyo, Japan) using a quartz cell with a path length of 10 mm. Fluorescence spectra are obtained with a VARIAN Cary Eclips spectrometer (Varian Inc. Palo Alto, CA, USA). Nanosecond laser flash photolysis experiments are performed on a LP 920 pump-probe spectroscopic setup (Edinburgh Instruments Ltd, Livingston, UK). The solutions are purged with nitrogen for 20 minutes before each measurement and all measurements are performed at room temperature. The excitation source is the unfocused third harmonic (532 nm, 7 ns fwhm) output of a Nd:YAG laser (Continuum surelite II); the probe light source is a pulse-xenon lamp. The signals are detected with a LP900 from Edinburgh analytical instruments and recorded on a Tektronix TDS 3012B oscilloscope and computer. All the experiments are performed at least three times independently and at room temperature.
3.2. Materials
Trans-dihydroxo[5,15-bis(3,5-dioctyloxylphenyl)porphyrin] tin(IV) complex (Sn(OH)2DPP, 1) is prepared according to Crossley et al [19]. The pristine SWNTs are obtained by the arcing method [20] and purified using previously reported methods [14]. This treatment generates surface functionalities, particularly carboxylic acids at nanotube ends and sidewall defect sites. Hexane, chloroform and o-dichlorobenzene (ODCB) are distilled from calcium hydride before use. THF and toluene are dried and distilled from sodium. All other chemicals (AR) obtained from commercial sources are used without further purification.
3.3. Preparation of Tin porphyrin functionalized SWNTs nanohybrid (SWNT–SnDPP)
As shown in Scheme 1, the purified SWNTs sample (50 mg) is added to a solution of Sn(OH)2DPP in THF (10 mL, 10 mg mL−1), and the mixture is sonicated for 12 hours and kept below 40 °C. After the removal of THF on a rotary evaporator, the solid mixture is extracted with hexane coupled with repeated ultrasonication and centrifuging (4000 rpm, 10 minutes). The solid is collected by filtration and washes with CH2Cl2 to remove free Sn(OH)2DPP. Then, THF (60 mL) is added to the solid sample to dissolve the Sn(OH)2DPP–attached SWNTs and sonicated for 1 minute. After centrifuging (11000 rpm) for 10 minutes, the supernatant soluble SWNT–SnDPP samples are combined, follow by a complete evaporation of the solvent, and subsequently the residue is dried at 60 °C for 8 hours under vacuum to finally obtain tin porphyrin functionalized nanotubes (58 mg).
3.4. Determination of solubility
Typically, the tetrahydrofuran solution of SWNT–SnDPP is prepared as follows: the sample of SWNT-SnDPP nanohybrid (3.0 mg) is dissolved in THF (25 ml) completely to give a homogeneous black-red solution with a final concentration of 120 mg L−1. The solution is separated into several portions and diluted to different concentrations (96, 80, 60, 48, 40, 30 and 24 mg L−1) and their absorption spectra are measured immediately. The absorption spectra are dependent on the solution concentration in a linear fashion, following Lambert-Beer's law. Thus, a standard curve of absorbance versus concentration at certain wavelength (1000 nm) can be obtained. A saturated solution is then diluted to comply with Lambert-Beer's law and from standard curve, the concentration may be determined. Subsequently, the solubility of SWNT–SnDPP in the solvent could be calculated. The values of solubility are the average of triplicate independent measurements.
4. Conclusions
A soluble SWNTs-based donor-acceptor nanohybrid is obtained by covalently functionalized SWNTs with dihydroxotin(IV) porphyrins and this nanohybrid is studied with TEM, FTIR, Raman, NMR and UV-Vis-NIR spectra. The steady fluorescence and transient absorption measurements of the nanohybrid demonstrate electron transfer properties and the tin porphyrin may play a role as a light harvesting antenna to give electron transfer. Furthermore, our results show that strategic modification of nanohybrids may be used to increase the dispersibility of such hybrids in various solvents. Both the efficient electron-transfer and good dispersibility of this novel soluble nanohybrid suggest that it may be a good candidate for potential applications in optoelectronic devices and artificial photosynthetic systems.
Acknowledgements
We gratefully acknowledge the financial support from the “973” Program (#2006CB932900 and 2006CB932700) from MST and The Natural Science Foundation of China (#20572048, 20421202 and 20644004)
References
- 1.Dresselhaus MS, Dresselhaus G, Avouris P, editors. Carbon Nanotubes: Synthesis, Structure, Properties and Applications. Springer Verlag; Berlin, Germany: 2001. [Google Scholar]
- 2.Rahman GMA, Guldi DM, Campideli S, Prato M. Electronically interacting single wall carbon nanotube–porphyrin nanohybrids. J Mater Chem. 2006;16:62–65. [Google Scholar]
- 3.Xu H, Chen H, Shi M, Bai R, Wang M. A novel donor–acceptor heterojunction from singlewalled carbon nanotubes functionalized by erbium bisphthalocyanine. Mater Chem Phys. 2005;94:342–346. [Google Scholar]
- 4.Sun YP, Fu K, Lin Y, Huang W. Functionalized carbon nanotubes: properties and applications. Acc Chem Res. 2002;35:1096–1104. doi: 10.1021/ar010160v. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Harmon MA, Hu H, Chen Y, Rao AM, Eklund PC, Haddon RC. Solution properties of single-walled carbon nanotubes. Science. 1998;282:95–98. doi: 10.1126/science.282.5386.95. [DOI] [PubMed] [Google Scholar]
- 6.Sanders JKM, Bampos N, Clyde-Watson Z, Darling SL, Hawley JC, Kim HJ, Mak CC, Webb SJ. In: The Porphyrin Handbook. Kadish KM, Smith KM, Guilard R, editors. Academic Press; London: 2000. [Google Scholar]
- 7.Li H, Martin RB, Harruff BA, Carino RA, Allard LF, Sun YP. Single-walled carbon nanotubes tethered with porphyrins: synthesis and photophysical properties. Adv Mater. 2004;16:896–900. [Google Scholar]
- 8.Baskaran D, Mays JW, Zhang XP, Bratcher MS. Carbon nanotubes with covalently linked porphyrin antennae: photoinduced electron transfer. J Am Chem Soc. 2005;127:6916–6917. doi: 10.1021/ja0508222. [DOI] [PubMed] [Google Scholar]
- 9.Guldi DM, Taieb H, Rahman GMA, Tagmatarchis N, Prato M. Novel photoactive singlewalled carbon nanotubeporphyrin polymer wraps: efficient and long-lived intracomlex charge separation. Adv Mater. 2005;17:871–875. [Google Scholar]
- 10.Murakami H, Nomura T, Nakashima N. Noncovalent porphyrin-functionalized single-walled carbon nanotubes in solution and the formation of porphyrinnanotube nanocomposites. Chem Phys Lett. 2003;378:481–485. [Google Scholar]
- 11.Arnold DP, Blok J. The coordination chemistry of tin porphyrin complexes. Coor Chem Rev. 2004;248:299–319. [Google Scholar]
- 12.Hawley JC, Bampos N, Abraham RJ, Sanders JKM. Carboxylate and carboxylic acid recognition by tin(IV) porphyrins. Chem Commun. 1998:661–662. [Google Scholar]
- 13.Sun YP, Huang W, Lin Y, Fu K, Kitaygorodskiy A, Riddle LA, Yu YJ, Carroll DL. Soluble dendron-functionalized carbon nanotubes: preparation, characterization, and properties. Chem Mater. 2001;13:2864–2869. [Google Scholar]
- 14.Liu J, Rinzler AG, Dai H, Hafner JH, Bradley RK, Boul PJ, Lu A, Iverson T, Shelimov K, Huffman CB, Rodriguez-Macias F, Shon Y, Lee TR, Colbert DT, Smalley RE. Fullerene Pipes. Science. 1998;280:1253–1256. doi: 10.1126/science.280.5367.1253. [DOI] [PubMed] [Google Scholar]
- 15.Bahr JL, Mickelson ET, Bronikowski MJ, Smalley RE, Tour JM. Dissolution of small diameter single-wall carbon nanotubes in organic solvents? Chem Commun. 2001:193–194. [Google Scholar]
- 16.Haddon RC. Magnetism of the carbon allotropes. Nature. 1995;378:249–255. [Google Scholar]
- 17.Ramirez AP, Haddon RC, Zhou O, Fleming RM, Zhang J, McClure SM, Smalley RE. Magnetic susceptibility of molecular carbon: nanotubes and fullerite. Science. 1994;265:84–86. doi: 10.1126/science.265.5168.84. [DOI] [PubMed] [Google Scholar]
- 18.Hemraj-Benny T, Wong SS. Silylation of single-walled carbon nanotubes. Chem Mater. 2006;18:4827–4839. [Google Scholar]
- 19.Crossley MJ, Thordarson P, Wu RAS. Efficient formation of lipophilic dihydroxotin(IV) porphyrins and bisporphyrins. J Chem Soc Perkin Trans 1. 2001:2294–2302. [Google Scholar]
- 20.Lv X, Du F, Ma Y, Wu Q, Chen Y. Synthesis of high quality single-walled carbon nanotubes at large scale by electric arc using metal compounds. Carbon. 2005;43:2020–2022. [Google Scholar]