Abstract
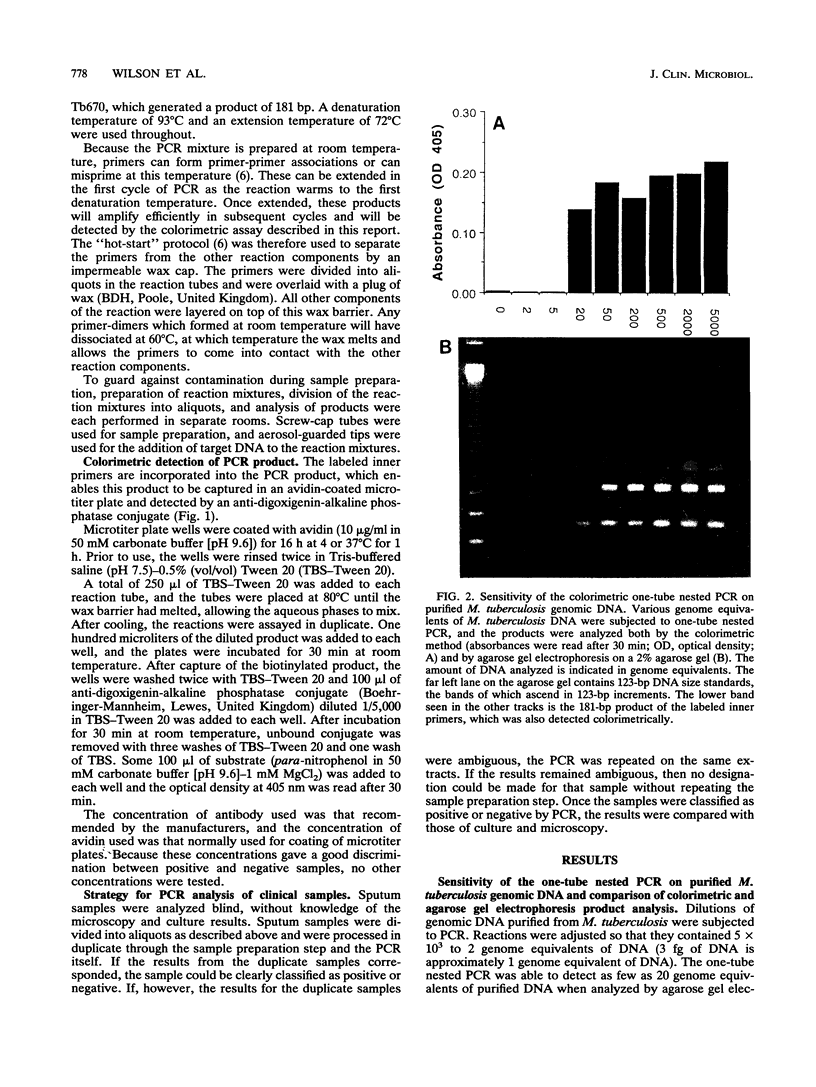
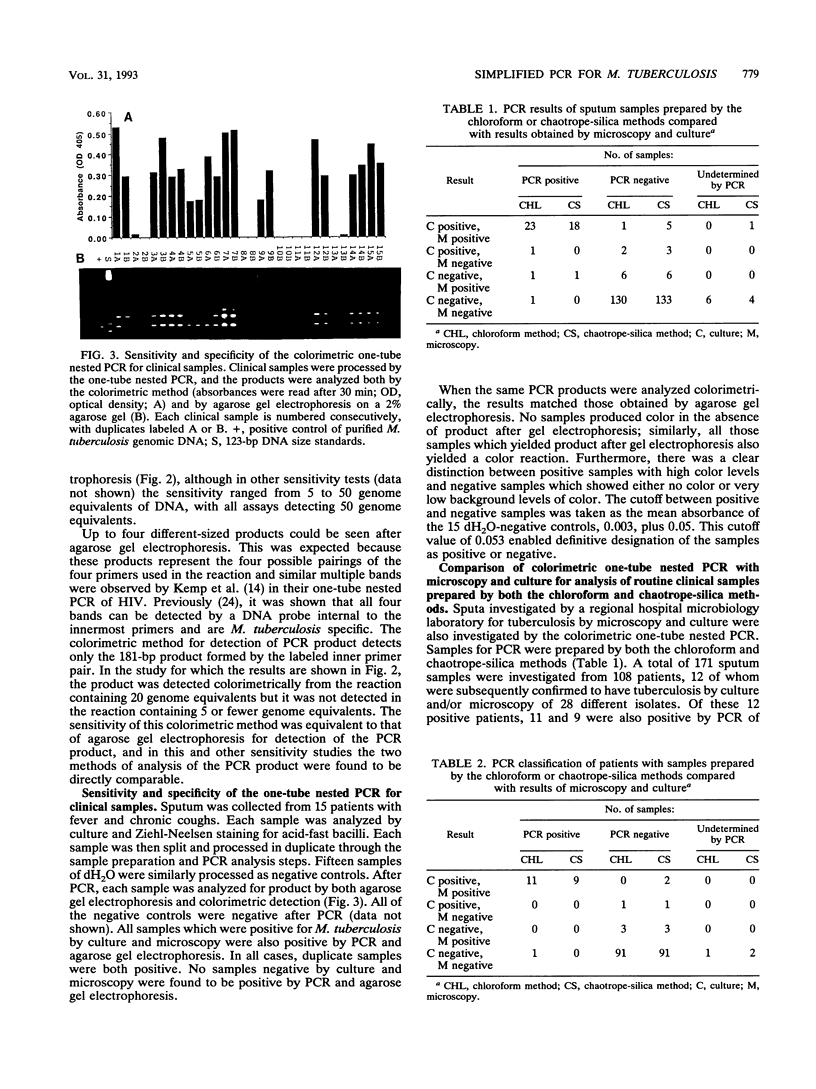
The complexity, expense, and susceptibility to contamination of the polymerase chain reaction (PCR) are all issues which need to be overcome if PCR is to be used outside of research laboratories. We addressed these problems with respect to the diagnosis of tuberculosis. First, we simplified the procedure for extracting Mycobacterium tuberculosis DNA from sputum samples. Two methods of sample preparation were compared: the chaotrope-silica method and a novel, more simple chloroform method. Second, we developed a colorimetric method for product detection. This method was as sensitive and specific as agarose gel electrophoresis for detection of PCR product. By using a one-tube nested protocol, 5 to 50 genome equivalents of M. tuberculosis DNA were detected. The simplified colorimetric PCR was compared with microscopy and culture for detection of M. tuberculosis in clinical specimens of sputum. A total of 171 sputum samples were investigated from 108 patients, 12 of whom were subsequently found to have tuberculosis by culture and/or microscopy. PCR of samples prepared by the chaotrope-silica method had a sensitivity of 75% and a specificity of 100% whereas PCR of samples prepared by the chloroform method had a sensitivity of 92% and a specificity of 99% when compared with the sensitivities and specificities of the combined classical microbiological methods for the diagnosis of tuberculosis. The simplified colorimetric PCR in combination with the chloroform sample preparation method was at least as sensitive as microscopy but had a greater specificity because samples with atypical mycobacteria were not detected by PCR. The sensitivity of the method for detection of smear-negative and extrapulmonary tuberculosis remains to be investigated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boom R., Sol C. J., Salimans M. M., Jansen C. L., Wertheim-van Dillen P. M., van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990 Mar;28(3):495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson-Noel A., Aznar C., Chureau C., Nguyen S., Pierre C., Bartoli M., Bonete R., Pialoux G., Gicquel B., Garrigue G. Diagnosis of tuberculosis by DNA amplification in clinical practice evaluation. Lancet. 1991 Aug 10;338(8763):364–366. doi: 10.1016/0140-6736(91)90492-8. [DOI] [PubMed] [Google Scholar]
- Brisson-Noël A., Gicquel B., Lecossier D., Lévy-Frébault V., Nassif X., Hance A. J. Rapid diagnosis of tuberculosis by amplification of mycobacterial DNA in clinical samples. Lancet. 1989 Nov 4;2(8671):1069–1071. doi: 10.1016/s0140-6736(89)91082-9. [DOI] [PubMed] [Google Scholar]
- Brook M. G., Bannister B. A. Childhood tuberculosis: clinical aspects. CDR (Lond Engl Rev) 1991 Nov 8;1(12):R134–R136. [PubMed] [Google Scholar]
- Chou Q., Russell M., Birch D. E., Raymond J., Bloch W. Prevention of pre-PCR mis-priming and primer dimerization improves low-copy-number amplifications. Nucleic Acids Res. 1992 Apr 11;20(7):1717–1723. doi: 10.1093/nar/20.7.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Portillo P., Murillo L. A., Patarroyo M. E. Amplification of a species-specific DNA fragment of Mycobacterium tuberculosis and its possible use in diagnosis. J Clin Microbiol. 1991 Oct;29(10):2163–2168. doi: 10.1128/jcm.29.10.2163-2168.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach K. D., Cave M. D., Bates J. H., Crawford J. T. Polymerase chain reaction amplification of a repetitive DNA sequence specific for Mycobacterium tuberculosis. J Infect Dis. 1990 May;161(5):977–981. doi: 10.1093/infdis/161.5.977. [DOI] [PubMed] [Google Scholar]
- Eisenach K. D., Sifford M. D., Cave M. D., Bates J. H., Crawford J. T. Detection of Mycobacterium tuberculosis in sputum samples using a polymerase chain reaction. Am Rev Respir Dis. 1991 Nov;144(5):1160–1163. doi: 10.1164/ajrccm/144.5.1160. [DOI] [PubMed] [Google Scholar]
- Festenstein F., Grange J. M. Tuberculosis and the acquired immune deficiency syndrome. J Appl Bacteriol. 1991 Jul;71(1):19–30. [PubMed] [Google Scholar]
- Godfrey-Faussett P., Wilkins E. G., Khoo S., Stoker N. Tuberculous pericarditis confirmed by DNA amplification. Lancet. 1991 Jan 19;337(8734):176–177. doi: 10.1016/0140-6736(91)90840-l. [DOI] [PubMed] [Google Scholar]
- Hermans P. W., van Soolingen D., Dale J. W., Schuitema A. R., McAdam R. A., Catty D., van Embden J. D. Insertion element IS986 from Mycobacterium tuberculosis: a useful tool for diagnosis and epidemiology of tuberculosis. J Clin Microbiol. 1990 Sep;28(9):2051–2058. doi: 10.1128/jcm.28.9.2051-2058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D. J., Churchill M. J., Smith D. B., Biggs B. A., Foote S. J., Peterson M. G., Samaras N., Deacon N. J., Doherty R. Simplified colorimetric analysis of polymerase chain reactions: detection of HIV sequences in AIDS patients. Gene. 1990 Oct 15;94(2):223–228. doi: 10.1016/0378-1119(90)90391-4. [DOI] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Lundeberg J., Wahlberg J., Holmberg M., Pettersson U., Uhlén M. Rapid colorimetric detection of in vitro amplified DNA sequences. DNA Cell Biol. 1990 May;9(4):287–292. doi: 10.1089/dna.1990.9.287. [DOI] [PubMed] [Google Scholar]
- Manjunath N., Shankar P., Rajan L., Bhargava A., Saluja S., Shriniwas Evaluation of a polymerase chain reaction for the diagnosis of tuberculosis. Tubercle. 1991 Mar;72(1):21–27. doi: 10.1016/0041-3879(91)90020-s. [DOI] [PubMed] [Google Scholar]
- Nisar M., Davies P. D. Tuberculosis--on the increase? Respir Med. 1991 May;85(3):175–176. doi: 10.1016/s0954-6111(06)80074-0. [DOI] [PubMed] [Google Scholar]
- Pierre C., Lecossier D., Boussougant Y., Bocart D., Joly V., Yeni P., Hance A. J. Use of a reamplification protocol improves sensitivity of detection of Mycobacterium tuberculosis in clinical samples by amplification of DNA. J Clin Microbiol. 1991 Apr;29(4):712–717. doi: 10.1128/jcm.29.4.712-717.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues L. C., Smith P. G. Tuberculosis in developing countries and methods for its control. Trans R Soc Trop Med Hyg. 1990 Sep-Oct;84(5):739–744. doi: 10.1016/0035-9203(90)90172-b. [DOI] [PubMed] [Google Scholar]
- Sudre P., ten Dam G., Kochi A. Tuberculosis: a global overview of the situation today. Bull World Health Organ. 1992;70(2):149–159. [PMC free article] [PubMed] [Google Scholar]
- Thierry D., Brisson-Noël A., Vincent-Lévy-Frébault V., Nguyen S., Guesdon J. L., Gicquel B. Characterization of a Mycobacterium tuberculosis insertion sequence, IS6110, and its application in diagnosis. J Clin Microbiol. 1990 Dec;28(12):2668–2673. doi: 10.1128/jcm.28.12.2668-2673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]