Abstract
Additional specific rates of solvolysis have been determined, mainly in fluoroalcohol containing solvents, for benzenesulfonyl chloride (1) and p-nitrobenzene-sulfonyl chloride (2). For trans-β-styrenesulfonyl chloride (3), a study has been carried out in 43 pure and binary solvents, covering a wide range of hyroxylic solvent systems. For the specific rates of solvolyses of each of the three substrates, a good correlation was obtained over the full range of solvents when the extended Grunwald-Winstein equation was applied. The sensitivities to changes in solvent nucleophilicity and solvent ionizing power are similar to values determined earlier and an SN2 process is proposed. For 3, kinetic solvent isotope effects of 1.46 for kH2O/kD2O and 1.76 for kMeOH/kMeOD were determined. These are also compared to literature values for other sulfonyl chlorides.
Keywords: Solvolysis, sulfonyl transfer, benzenesulfonyl chloride, para-nitrobenzenesulfonyl chloride, trans-β-styrenesulfonyl chloride, Grunwald-Winstein equation, Linear Free Energy Relationships, kinetic solvent isotope effect, general base catalysis
1. Introduction
The syntheses of biologically active sulfonamides usually involve nucleophilic attack of an amine on a sulfonyl chloride, such as benzenesulfonyl chloride (1) or p-nitrobenzenesulfonyl chloride (2). Several thousand sulfonamides have been evaluated in terms of biological activity and this synthesis area remains a very active one. For example, within the last two years (2007-2008) over fifty patent applications have been filed relating to the utility of sulfonamides synthesized from trans-β-styrene-sulfonyl chloride [(E)-2-phenylethenesulfonyl chloride, 3].
Despite the widespread application of nucleophilic substitution reactions of sulfonyl chlorides, there has been considerable uncertainty regarding the detailed mechanism of the process [1]. Such knowledge would be useful in the selection of optimum reaction conditions. Solvolytic reactions afford a good prototype for these substitutions [2].
The original Grunwald-Winstein equation was developed in 1948 [3] so as to give a linear free energy relationship treatment of the specific rates (first-order rate coefficients) for solvolysis of initially neutral substrates reacting by an ionization (SN1 + E1) mechanism. The equation can be
| (1) |
expressed as in equation 1 where k and ko are the specific rates of solvolysis in a given solvent and in the standard solvent (arbitrary chosen as 80% ethanol), respectively, m represents the sensitivity to changes in the solvent ionizing power Y (arbitrary set at unity for tert-butyl chloride solvolyses in the early studies), and c is a constant (residual) term. It has been demonstrated [3] that different Y scales are needed for different leaving groups and that the use of adamantyl derivatives minimizes nucleophilic solvation effects at the α-carbon, leading to a purer ionization process than for t-butyl chloride.
For bimolecular (SN2 and/or E2) reactions, in which the solvent also acts as a nucleophile or base, the correlation can be extended [4] to include a term governed by the sensitivity (l) to changes in solvent nucleophilicity (N), as shown in equation 2.
| (2) |
Initially, methyl p-toluenesulfonate was used as the standard substrate to arrive at a solvent nucleophilicity scale termed NOTs [5]. This required making an estimate of the sensitivity of the solvolysis of methyl p-toluenesulfonate towards changes in solvent ionizing power (the m value of equation 2), which cannot be obtained directly. More recently, to minimize leaving group effects upon specific rate variation as the solvent is varied, a scale, labeled NT, has been developed [6] based upon the solvolyses of the S-methyldibenzothiophenium ion. In these solvolyses, the leaving group is a neutral molecule and the appreciable solvent-leaving group interaction, when the leaving group is anionic, is avoided. The development of solvent nucleophilicity scales has been reviewed [2, 7].
It is generally accepted that, for most sulfonyl chlorides, the mechanism of solvolysis is bimolecular in character [1, 2, 8–22]. There have, however, been claims of SN1 (ionization) reactions for a few substrates. The solvolyses of N,N-dimethylsulfamoyl chloride were considered by Hall [8] to be unimolecular, and this view was supported by Robertson [23].
Convincing evidence has been presented that the solvolysis are SN2 in character [21, 24, 25]. Similarily, a claim that reactions of alkanesulfonyl chlorides solvolyze, at least in part, by SN1 reactions in polar solvents [26] is no longer tenable [1, 2]. Also, a claim that 2,4,6-trimethylbenzene-sulfonyl chloride solvolyzes by the SN1 mechanism [16, 27, 28] has been shown to be incorrect [1, 2, 17, 18]. At the present time, there does not seem to be any convincing evidence for SN1 reaction in the solvolysis of a sulfonyl chloride.
Although the solvolyses are established as bimolecular, other more detailed aspects remain to be considered. There has been considerable discussion as to whether the mechanism involves a concerted SN2 pathway or an addition-elimination (SAN) pathway and, irrespective of which of these pathways operates, whether attack by one solvent molecule is assisted by a general base catalysis from the involvement of a second solvent molecule. The SAN mechanism has largely been proposed for reactions with added nucleophiles under what otherwise would be solvolytic conditions [1] and the mechanism has only occasionally been suggested for solvolytic reactions. In proposing a duality of reaction channels for solvolyses of electron-rich benzenesulfonyl chlorides, it was suggested that one channel involves SN2 and the other SAN or a base catalyzed SN2 reaction [18]. Subsequent publication by the authors, favor the base catalyzed SN2 reaction as the second channel [29, 30], without totally ruling out the possibility of the addition-elimination pathway [31].
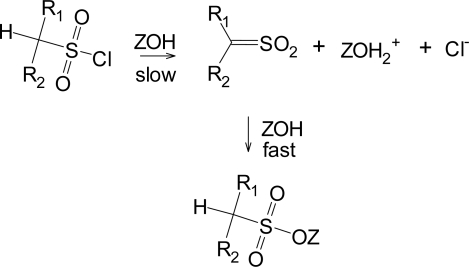
Although the addition-elimination pathway appears to be disfavored for sulfonyl chlorides, the elimination-addition reaction (Scheme 1) is well established for suitable substrates, primarily those which have a hydrogen plus electron-withdrawing groups on the α-carbon [11, 32]. The sulfene intermediate formed in the slow step then readily adds solvent (ZOH) to give (in the absence of isotopic labeling) a product identical to the direct substitution product.
Scheme 1.
Formation of a sulfene intermediate from substrates having a hydrogen plus electron-withdrawing groups on the α-carbon.
Another pathway has been observed when the R group of RSO2Cl forms a relatively stable carbocation, such as a tertiary-butyl group [33]. This pathway shown in Scheme 2, leads to the products typically formed from a tertiary carbocation, including tert-butyl chloride. This solvolysis-decomposition reaction is closely related to that observed [34] with similarily constituted tert-alkyl chloroformates (ROCOCl), but with loss of SO2 rather than CO2.
Scheme 2.
Solvolysis-decomposition pathway for tert-butylsulfonyl chloride.
The three substrates of the present study would not be expected to proceed by the ionization, elimination-addition, or solvolysis-decomposition routes and one can concentrate on whether the rate-determining nucleophilic attack by the solvent involves a concerted or stepwise substitution pathway, and on whether general base catalysis is assisting the process.
2. Results and Discussion
The solvolyses lead to the shown products (Scheme 3) at rates which are convenient for measurement by the titration of the developed acid. Both the sulfonic acid and the hydrochloric acid titrate as strong acids.
Scheme 3.
Solvolysis for benzenesulfonyl chloride (1), p-nitrobenzenesulfonyl chloride (2), and trans-β-styrenesulfonyl chloride [(E)-2-phenylethenesulfonyl chloride] (3).
The specific rates already available for 1 and 2 in the literature were complemented by the determination of additional values. For 1 values were already available in aqueous acetone [35], aqueous dioxane [28], water [28, 36], and 97% 2,2,2-trifluoroethanol (TFE) [22]. Values were also available for ethanol and mixtures of ethanol with water or TFE [37] but a repeat of these determinations led to specific rates some 20 - 35% lower; the lower values were used in the correlations. Additional values were obtained in aqueous methanol, 80% acetone, 80 – 50% TFE, TFE-ethanol, and aqueous 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP). All of the specific rate values at 35.0 °C for solvolyses of 1, as used in the correlation, are shown in Table 1.
Table 1.
Specific rates of solvolysis (k) of benzenesulfonyl chloride (1) and p–nitrobenzenesulfonyl chloride (2) in pure and binary solvents at 35.0 ºC and the solvent nucleophilicity (NT) and solvent ionizing power (YCl) values for the solvents.
| Solvent (%)a | 1; 105k(s−1)b | 2; 105k(s−1)b | NTc | YCld |
|---|---|---|---|---|
| 100% EtOH | 3.60±0.34e | (10.7)f | 0.37 | −2.52 |
| 90% EtOH | 13.6±0.6e | (83.9)f | 0.16 | −0.94 |
| 80% EtOH | 19.6±1.1e | 117±5e | 0.00 | 0.00 |
| 70% EtOH | 32.4±1.2e | (221)f | −0.20 | 0.78 |
| 60% EtOH | (285)f | −0.39 | 1.38 | |
| 100% H2O | (751)g (756)h,i | (759)h | −1.38 | 4.57 |
| 100% MeOH | 15.1±0.8 | 21.8±1.4 | 0.17 | −1.17 |
| 90% MeOH | 27.9±1.4 | −0.01 | −0.18 | |
| 80% MeOH | 20.1±0.8 | −0.06 | 0.67 | |
| 70% MeOH | 27.6±0.9 | −0.40 | 1.46 | |
| 90% Acetone | 16.9±0.7 | −0.35 | −2.39 | |
| 87% Acetone | (23.3)j | −0.34 | −1.81 | |
| 80% Acetone | 3.64±0.20 | 65.7±2.9 | −0.37 | −0.83 |
| 75% Acetone | (5.57)j | (72.1)j | −0.39k | −0.28 k |
| 63% Acetone | (14.0)j | (135)j | −0.54k | 0.62k |
| 52% Acetone | (27.9)j | (219)j | −0.68k | 1.45k |
| 42% Acetone | (62.5)j | (414)j | −0.81k | 2.21k |
| 34% Dioxane | (216)h | (686)h | −0.92k | 2.75k |
| 18% Dioxane | (419)h | (844)h | −1.13k | 3.76k |
| 10% Dioxane | (592)h | (786)h | −1.23k | 4.23k |
| 100% TFE | 0.0247±0.0011 | −3.93 | 2.81 | |
| 97% TFE (w/w) | (0.0774)l | 0.0450±0.0018m | −3.30 | 2.83 |
| 90% TFE (w/w) | 0.562±0.021 | −2.55 | 2.85 | |
| 80% TFE (w/w) | 2.57±0.09 | −2.19 | 2.90 | |
| 70% TFE (w/w) | 5.48±0.22 | −1.98 | 2.96 | |
| 50% TFE (w/w) | 19.0±0.5 | −1.73 | 3.16 | |
| 80T-20E | 1.70±0.05 | −1.76 | 1.89 | |
| 60T-40E | 3.33±0.08 | −0.94 | 0.63 | |
| 50T-50E | 3.88±0.18 | −0.64 | 0.16 | |
| 40T-60E | 5.48±0.21 | (7.61)f | −0.34 | −0.48 |
| 30T-70E | (9.84)f | −0.11k | −0.95k | |
| 20T-80E | 4.73±0.10e | (10.5)f | 0.08 | −1.42 |
| 97% HFIP(w/w) | 0.0196±0.0011 | −5.26 | 5.17 | |
| 70% HFIP(w/w) | 2.40±0.06 | −2.94 | 3.83 | |
| 50% HFIP(w/w) | 7.52±0.22 | −2.49 | 3.80 |
On volume-volume basis at 25.0 ºC, except when indicated as weight-weight (w/w) basis.
With associated standard deviations; values in parentheses are obtained (directly or by an Arrhenius treatment of specific rates) from the literature, as indicated.
From ref. [7].
These values are 20-35% lower than values reported in ref. [37].
From ref. [37].
From Arrhenius treatment of values in ref. [36].
Calculated using the activation parameters reported in ref. [28].
A value of 825 is calculated from the rate data of ref. [10].
These values are calculated from specific rates reported in ref. [35].
Obtained by interpolation.
Estimated from values in ref. [22].
Arrhenius equation treatment of specific rates in ref. [22] leads to a value of 0.0411.
Also shown in Table 1 are the specific rates of solvolysis for 2 used in the correlations. Values were available for ethanol [37], water [28] and their mixtures [37], for several aqueous acetone [35] and aqueous dioxane [28] compositions, and for TFE-ethanol mixtures [37]. Additional determinations were made in methanol, 80% ethanol, 90% and 80% acetone, and in 100%, 97% and 90% TFE. The NT [6, 7] and YCl [3, 38, 39] values needed for the application of equation 2 to the specific rate values for 1 and 2 (Figures 2 and 3) are also listed in the Table.
Figure 2.
The plot of log (k/ko) vs. (1.26 NT + 0.65 YCl) for the solvolyses of benzene-sulfonyl chloride (1) in pure and binary solvents at 35.0 ºC.
Figure 3.
The plot of log (k/ko) vs. (1.54 NT + 0.69 YCl) for the solvolyses of p-nitro-benzenesulfonyl chloride (2) in pure and binary solvents at 35.0 ºC. Four acetone-water points (90% - 75%) were not included in the correlation but are added to show the extent of their deviations from the plot.
Specific rates of solvolysis of trans-β-styrenesulfonyl chloride (3) have not been previously determined and a study was carried out, at 45.0 °C, in terms of increases in conductivity observed as the strong acids are produced (Scheme 3) in ethanol, methanol, and water, and the full range of aqueous ethanol and methanol compositions. These values are reported in Table 2. A value obtained in deuterium oxide could be combined with the water value to give a kinetic solvent isotope effect (kH2O/kD2O) of 1.46 ± 0.02. Similarly the values in methanol and methanol-d give a KSIE value (kMeOH/kMeOD) of 1.76 ± 0.02. In Table 3, two additional NT values and one additional YCl value for dioxane-water mixtures are abstracted from the literature [18, 40, 41] and specific rates are tabulated for a series of aqueous acetone and aqueous dioxane compositions. Table 4 contains the specific rates obtained in TFE-water, HFIP-water, and TFE-ethanol. The ratio of the specific rates in 40% ethanol (Table 2) and 97% TFE (Table 4), two solvents of similar ionizing power but very different solvent nucleophilicities, (k40EtOH/k97TFE) is 846, indicating that solvent nucleophilicity is an important rate-controlling factor [3, 22, 42]. The correlation of the specific rates of solvolysis of 3, in all 43 solvents and using the extended Grunwald-Winstein equation, is presented in Figure 4.
Table 2.
Specific rates of solvolysis (k) of trans-β-styrenesulfonyl chloride (3)a in pure ethanol, methanol, and water, and in alcohol-water binary mixtures at 45.0 ºC.
| Solventb | k(s−1) | nc | NTd | YCld |
|---|---|---|---|---|
| 100 EtOH | (2.18±0.03)×10−4 | 3 | 0.37 | −2.52 |
| 90 EtOH | (5.40±0.01)×10−4 | 1 | 0.16 | −0.94 |
| 80 EtOH | (8.83±0.02)×10−4 | 1 | 0.00 | 0.00 |
| 70 EtOH | (1.20±0.01)×10−3 | 1 | −0.20 | 0.78 |
| 60 EtOH | (1.76±0.01)×10−3 | 1 | −0.38 | 1.38 |
| 50 EtOH | (2.40±0.01)×10−3 | 1 | −0.58 | 2.02 |
| 40 EtOH | (3.95±0.02)×10−3 | 1 | −0.74 | 2.75 |
| 30 EtOH | (6.35±0.01)×10−3e | 1 | −0.93 | 3.53 |
| 20 EtOH | (8.23±0.03)×10−3e | 1 | −1.16 | 4.09 |
| 10 EtOH | (1.07±0.07)×10−2f | 4 | −1.31 | 4.40 |
| H2O | (1.21±0.05)×10−2f | 6 | −1.38 | 4.57 |
| D2Og | (8.28±0.20)×10−3f | 5 | ||
| 100 MeOH | (6.33±0.08)×10−4 | 5 | 0.17 | −1.17 |
| 100 MeODh | (3.59±0.08)×10−4 | 4 | ||
| 90 MeOH | (1.44±0.01)×10−3 | 1 | −0.01 | −0.18 |
| 80 MeOH | (2.43±0.01)×10−3 | 3 | −0.06 | 0.67 |
| 70 MeOH | (3.61±0.01)×10−3 | 1 | −0.40 | 1.46 |
| 60 MeOH | (5.12±0.01)×10−3 | 1 | −0.54 | 2.07 |
| 50 MeOH | (7.04±0.01)×10−3 | 1 | −0.57 | 2.70 |
| 40 MeOH | (9.43±0.01)×10−3 | 3 | −0.87 | 3.25 |
| 30 MeOH | (1.10±0.01)×10−2e | 1 | −1.06 | 3.73 |
| 20 MeOH | (1.29±0.01)×10−2f | 1 | −1.23 | 4.10 |
| 10 MeOH | (1.31±0.01)×10−2f | 1 | −1.36 | 4.39 |
Determined conductimetrically and typically injected 4 μL of 3% (w/w) substrate in dry acetonitrile into the kinetic apparatus containing 2 mL of solvent (concentration of ca. 2.4 × 10−4 mol dm−3); errors accompanying the specific rates are standard deviations.
Percentage by volume at 25.0 ºC of organic component (v/v%).
Number of runs.
Injected 4 μL of 1.0% (w/w) substrate in dry acetonitrile (concentration of ca. 8 × 10−5 mol dm−3).
Injected 4 μL of 0.5% (w/w) substrate in dry acetonitrile (concentration of ca. 4 × 10−5 mol dm−3).
Kinetic Solvent Isotope Effect (KSIE), kH2O/kD2O = 1.46 (±0.02) at 45.0 ºC (average from five paired values).
KSIE, kMeOH/kMeOD = 1.76 (±0.02) at 45.0 ºC (average from four paired values).
Table 3.
Specific rates of solvolysis (k) of trans-β-styrenesulfonyl chloride (3)a in aqueous acetone and aqueous dioxane at 45.0 ºC.
| Solventb | k(s−1) | nc | NTd | YCld |
|---|---|---|---|---|
| 90 Acetone | (2.12±0.01)×10−5 | 1 | −0.35 | −2.39 |
| 80 Acetone | (8.53±0.01)×10−5 | 1 | −0.37 | −0.83 |
| 70 Acetone | (2.10±0.01)×10−4 | 1 | −0.42 | 0.17 |
| 60 Acetone | (4.33±0.05)×10−4 | 1 | −0.48 | 0.95 |
| 50 Acetone | (9.14±0.01)×10−4 | 1 | −0.52 | 1.73 |
| 40 Acetone | (1.74±0.02)×10−3 | 1 | −0.70 | 2.46 |
| 30 Acetone | (3.29±0.01)×10−3e | 1 | −0.83 | 3.21 |
| 20 Acetone | (5.36±0.01)×10−3e | 1 | −0.96 | 3.77 |
| 10 Acetone | (9.01±0.06)×10−3f | 3 | 1.23 | 4.28 |
| 80 Dioxane | (7.58±0.01)×10−5 | 1 | −0.46 | |
| 50 Dioxane | (1.13±0.01)×10−3 | 1 | −0.66g | |
| 30 Dioxane | (3.75±0.04)×10−3e | 1 | −0.98h | 2.97i |
Table 4.
Specific rates of solvolysis (k) of trans-β-styrenesulfonyl chloride (3)a in binary mixtures of water with 2, 2, 2-trifluoroethanol (TFE) and 1, 1, 1, 3, 3, 3-hexafluoro-2-propanol (HFIP) and in TFE-ethanol (T-E) mixtures at 45.0 ºC.
| Solventb | k(s−1) | nc | NTd | YCld |
|---|---|---|---|---|
| 97 TFEe | (4.67±0.01)×10−6 | 1 | −3.30 | 2.83 |
| 90 TFE | (2.22±0.01)×10−5 | 1 | −2.55 | 2.85 |
| 80 TFE | (6.53±0.01)×10−5 | 1 | −2.19 | 2.90 |
| 70 TFE | (1.57±0.01)×10−4 | 1 | −1.98 | 2.96 |
| 50 TFE | (5.12±0.07)×10−4 | 2 | −1.73 | 3.16 |
| 90 HFIP | (8.33±0.02)×10−6 | 1 | −3.84 | 4.31f |
| 70 HFIP | (1.06±0.01)×10−4 | 1 | −2.94 | 3.83f |
| 50 HFIP | (2.61±0.01)×10−4 | 1 | −2.49 | 3.80 |
| 80T-20Eg | (5.18±0.02)×10−5 | 1 | −1.76 | 1.89 |
| 60T-40Eg | (1.21±0.01)×10−4 | 1 | −0.94 | 0.63 |
| 50T-50Eg | (1.53±0.01)×10−4 | 2 | −0.64 | 0.16 |
| 40T-60Eg | (1.89±0.01)×10−4 | 1 | −0.34 | −0.48 |
| 20T-80Eg | (1.97±0.02)×10−4 | 2 | 0.08 | −1.42 |
Figure 4.
The plot of log (k/ko) vs. (1.24 NT + 0.58 YCl) for the solvolyses of trans-β-styrenesulfonyl chloride (3) in pure and binary solvents at 45.0 ºC.
For nine representative solvents, specific rates were also determined at 25.0, 35.0, and 55.0 °C (Table 5) and the determinations at the four temperatures (45.0 °C values from Tables 2–4) were analyzed using the Eyring equation, so as to obtain enthalpies and entropies of activation. The entropies of activation were all appreciably negative (-13 to -34 k cal mol-1 K-1), consistent with a bimolecular (or higher molecularity) solvolytic process.
Table 5.
Specific rates (k/s−1) of solvolysis for trans-β-Styrenesulfonyl Chloride at temperature other than 45.0 ºC and activation parameters.
| Solvent | T, ºC | k/s−1 | ΔH≠298/(kcal/mol)a | ΔS≠298/(cal/mol K)a |
|---|---|---|---|---|
| 100 EtOH | 25 | (2.72±0.02)×10−5 | 19.0±0.2 | −15.9±0.8 |
| 35 | (8.32±0.01)×10−5 | |||
| 55 | (5.64±0.01)×10−4 | |||
| 80 EtOH | 25 | (1.47±0.03)×10−4 | 16.0±0.3 | −22.3±1.0 |
| 35 | (3.51±0.01)×10−4 | |||
| 55 | (1.88±0.03)×10−3 | |||
| 30 EtOH | 25 | (1.25±0.04)×10−3 | 14.3±0.2 | −23.8±0.9 |
| 35 | (2.94±0.01)×10−3 | |||
| 55 | (1.25±0.04)×10−2 | |||
| 100 MeOH | 25 | (1.14±0.01)×10−4 | 14.9±0.5 | −26.6±1.6 |
| 35 | (2.67±0.01)×10−4 | |||
| 55 | (1.22±0.01)×10−3 | |||
| 70 MeOH | 25 | (6.84±0.01)×10−4 | 15.0±0.1 | −22.7±0.4 |
| 35 | (1.65±0.04)×10−3 | |||
| 55 | (7.66±0.04)×10−3 | |||
| 50 HFIP | 25 | (3.65±0.02)×10−5 | 17.9±0.2 | −18.8±0.8 |
| 35 | (9.61±0.01)×10−5 | |||
| 55 | (6.27±0.01)×10−4 | |||
| 80 Acetone | 25 | (1.77±0.01)×10−5 | 14.0±0.4 | −33.4±1.4 |
| 35 | (4.30±0.02)×10−5 | |||
| 55 | (1.71±0.01)×10−4 | |||
| 80 Dioxane | 25 | (1.52±0.01)×10−4 | 14.8±0.3 | −31.0±0.9 |
| 35 | (3.35±0.03)×10−5 | |||
| 55 | (1.63±0.01)×10−4 | |||
| 30 Dioxane | 25 | (4.98±0.01)×10−4 | 17.9±0.3 | −13.4±0.9 |
| 35 | (1.41±0.01)×10−3 | |||
| 55 | (8.66±0.01)×10−3 |
The new values for the specifc rates of solvolysis of 1 and 2, in conjunction with earlier values, allow for correlations using equation 2 for a good selection of solvent types, including the important fluoroalcohol solvents. Similarly, within this study, the specific rates of solvolysis of 3 have been determined in 43 well chosen solvents. The sensitivity values, the residual (intercept) value, and the associated standard errors are reported within Table 6, together with the multiple correlation coefficient and the F-test values. With 1 and 3, all of the available values are included in the correlation. For 2, (Figure 3) it is found that two data points, for 90% and 87% acetone, lie appreciably off the correlation line and two data points (80% and 75% acetone) lie somewhat off the plot. In the table, correlation values are given for all solvents and also with the two and four data points obtained in the acetone-rich solvents omitted. The goodness-of-fit parameters show a progressive improvement for the limited analyses. Since the specific rate in 87% acetone is from an independent source [35] and the values for 90% and 87% are consistent with each other, the deviations appear to be real and not due to experimental error. The observation that these deviations appear only when the p-nitro group is present suggests a specific interaction between the group and the acetone-rich solvents. It has previously been observed that the Hammett σ values for a p-nitro group are somewhat dependent on the solvent [43, 44]. The benefits of a good mix of solvent type are well illustrated by the previous approximate l and m values [20] for 16 solvents for the solvolyses of 2 of 1.39±0.50 for l, and 0.65±0.13 for m improving to values of 1.44±0.11 and 0.57±0.06 for the full 23 solvents now available (Table 6).
Table 6.
Correlation of the specific rates of solvolytic nucleophilic displacement at the sulfur of sulfonyl chlorides using the extended Grunwald-Winstein equation (equation 2)
| Substrate | T ºC | na | lb | mb | c | Rc | Fd | l/m |
|---|---|---|---|---|---|---|---|---|
| 1 | 35.0 | 29 | 1.26±0.05 | 0.65±0.03 | 0.13±0.05 | 0.979 | 304 | 1.94 |
| 2 | 35.0 | 23 | 1.44±0.11 | 0.57±0.06 | 0.20±0.11 | 0.945 | 83 | 2.53 |
| 21e | 1.52±0.09 | 0.66±0.05 | 0.10±0.09 | 0.968 | 134 | 2.30 | ||
| 19f | 1.54±0.08 | 0.69±0.04 | 0.01±0.08 | 0.981 | 209 | 2.23 | ||
| 3 | 45.0 | 43 | 1.24±0.04 | 0.58±0.02 | 0.07±0.04 | 0.982 | 542 | 2.14 |
| p-MeOC6H4SO2Cl | 25.0 | 38g | 1.07±0.08 | 0.60±0.03 | 0.22±0.06 | 0.967 | 254 | 1.78 |
| p-MeC6H4SO2Cl | 25.0 | 34g | 1.19±0.07 | 0.61±0.02 | 0.20±0.05 | 0.975 | 305 | 1.95 |
| 3,4- diMeOC6H3SO2Cl | 25.0 | 40g | 1.24±0.07 | 0.64±0.03 | 0.14±0.06 | 0.967 | 264 | 1.94 |
| MeSO2Cl | 45.0 | 39g | 1.17±0.04 | 0.49±0.02 | 0.23±0.05 | 0.981 | 454 | 2.39 |
| i-PrSO2Cl | 45.0 | 19g | 1.28±0.05 | 0.64±0.03 | 0.18±0.06 | 0.988 | 333 | 2.00 |
| C6H5CH2SO2Cl | 45.0 | 25g | 0.80±0.06 | 0.39±0.04 | 0.21±0.06 | 0.947 | 95 | 2.05 |
| (CH3) 2NSO2Cl | 25.0 | 32g | 1.20±0.04 | 0.72±0.03 | 0.11±0.04 | 0.985 | 478 | 1.67 |
| 2-thiopheneSO2Cl | 25.0 | 34g | 1.35±0.05 | 0.70±0.02 | 0.28±0.05 | 0.983 | 455 | 1.93 |
Number of data points.
With associated standard error.
Multiple correlation coefficient.
F-test value.
Omitting 90% and 87% acetone.
Omitting 90-75% acetone.
From tabulation in ref. [45]; where appropriate, references to earlier sources are given in this reference.
Also included in Table 6 are the corresponding sensitivity and goodness-of-fit parameters from previous correlations of sulfonyl chlorides [45] using the extended Grunwald-Winstein equation. With the exception of low values for both l and m for the solvolyses of α-toluenesulfonyl chloride, the l values vary from a low of 1.07±0.08 for p-methoxybenzenesulfonyl chloride to a value of 1.54±0.07 for 2, with the values for 1 (1.26±0.05) and 3 (1.24±0.04) being almost identical and towards the middle of the range. The l/m ratios are very close in value for all of the entries in the table, varying from a low of 1.67 for N,N-dimethylsulfamoyl chloride to 2.39 to methanesulfonyl chloride. Restricting to arenesulfonyl chlorides, the lowest value is 1.78 for p-MeOC6H4SO2Cl and the highest is 2.23 for p-NO2C6H4SO2Cl. The l value and the l/m value variations for the arenesulfonyl chlorides are consistent with a situation in which all members of the family solvolyze by an SN2 mechanism but with the detailed pathways involving a looser transition state in the presence of the electron supplying p-MeO group, relative to 1, and varying to a tighter transition state with the electron-withdrawing p-NO2 group as the substituent [37, 46].
A major consideration over the past two decades involves the extent to which the bimolecular nucleophilic substitution reactions of sulfonyl chlorides may be proceeding with general base catalysis. Evidence comes mainly from two sources: the successful analysis in binary water-alcohol solvents in terms of the four possible specific rates expected to contribute if the pathway is termolecular (first-order in substrate and second-order in “solvent” (alcohol and/or water), as was observed for the solvolyses of 2 [30], and consideration of the magnitude of KSIEs, using the rationale that higher values would be expected with the involvement of two solvent molecules (as general base catalysis and as nucleophile) compared to the involvement of only one as nucleophile [18, 19, 29, 31, 47–49]. Initially, water and deuterium oxide were used as the solvents [47] but solubility problems can arise with organic substrates and methanol and methanol-d (MeOD) have been found to afford a useful alternative [19]. The KSIE values are usually slightly higher in value in the methanol solvents [19, 31, 49] but the solvolyses of methanesulfonyl chloride provide an exception to this generalization [48].
The KSIE values (kMeOH/kMeOD) for 1-3, are tabulated in Table 7, where they are compared with values for other sulfonyl chlorides. The values are all appreciably above unity (1.45 to 2.54), consistent with the nucleophilic attack by solvent indicated by the treatment using equation 2. The monosubstituted arenesulfonyl chlorides have KSIEs which vary from 1.58 for the p-MeO-derivative to 1.72 for the p-Me-derivative to 1.79 for the parent benzenesulfonyl chloride (1) to 2.31 for the p-NO2-derivative (2) [19]. These increases parallel increases in the k40EtOH/k97TFE ratio (a simplified treatment for determining the sensitivity to solvent nucleophilicity, which works well provided there is no intervening change in mechanism), with values of 300, 450, 2900, and 15000, respectively. That care must be taken in interrelating the two types of entry in Table 7 is indicated, however, by very similar (k40EtOH/k97TFE) ratios (2010 and 2790) for MeSO2Cl [49,50] and i-PrSO2Cl [21], but very different (kMeOH/kMeOD) ratios of 1.62 and 2.54 at 25 oC and 1.51 and 2.41 at 35 oC, respectively. It has been proposed that the KSIE values in Table 7 are sufficiently large to justify the proposal of the substitution reactions being assisted by general base catalysis (Scheme 4) [48, 49]. For a series of para-substituted benzenesulfonyl chlorides, it has been suggested that both assisted and unassisted SN2 pathways operate, with the presence of an electron-withdrawing substituent strongly favoring the pathway assisted by general-base catalysis [19]. At the other extreme, it has been proposed that, in TFE [17] or in highly ionizing aqueous acetic acid [51], the solvolyses of p-(dimethylamino)benzenesulfonyl chloride occur through a concerted bimolecular mechanism with bond breaking running ahead of bond formation in the transition state. A problem with the evaluation of the relevance of general-base catalysis effects in the solvolyses of sulfonyl chlorides is that, although the possibility of analyzing in terms of third-order rate coefficients in the solvolyses of para-nitrobenzenesulfonyl chloride (2) is suggestive [30], there are no substrates for which one can say with a high degree of confidence that the bimolecular solvolyses are either assisted or unassisted by general base catalysis. This leads to a situation in which there are no firmly established reference (standard) KSIE values for solvolyses involving nucleophilic attack at the sulfur atom of a sulfonyl chloride.
Table 7.
The kinetic solvent isotope effect (KSIE) for solvolyses in methanol and methanol-d (kMeOH/kMeOD) and the ratios of specific rates for solvolyses in 40% (v/v) ethanol-water relative to 97% (w/w) TFE-water.
| Substrate | kMeOH/kMeOD (T ºC) | k40EtOH/k97TFEa (T ºC) |
|---|---|---|
| 1 | 1.79 (25º)b | 2900 (25º)c |
| 2 | 2.31 (25º)b | 15000 (25º)c |
| 3 | 1.76 (45°) | 846 (45°) |
| 4-MeOC6H4SO2Cl | 1.58 (25º)b | 300 (25º)c |
| 4-MeC6H4SO2Cl | 1.72 (25º)b | 450 (25º)c |
| MeSO2Cl | 1.62 (25º); 1.51 (35º)d | 2010 (45º)e |
| i-PrSO2Cl | 2.54 (25º); 2.41 (35º)f | 2790 (45º)g |
| (CH3)2NSO2Cl | 359 (25º)h | |
| 4-MeO-2,6- diMeC6H2SO2Cl | 1.58 (25º)b,i | 89 (25º)i |
| 2,4,6-triMeC6H2SO2Cl | 1.68 (25º)b,j | 202 (25º)j |
| 3,4-diMeOC6H3SO2Cl | 1.45 (25º)k | 386 (25º)k |
a Solvents with similar YCl but very different NT values.
Values from ref. [19].
Values from ref. [22]; references for values determined elsewhere are given.
Values from ref. [48].
Values from ref. [49].
Values from ref. [21], value for 40% EtOH of 108 × 10−6 s−1 obtained by interpolation.
Value for 40% EtOH of 733 × 10−6 s−1 obtained from Arrhenius plots of data in ref. [25] and value for 97% TFE from ref. [21].
Values from ref. [29].
Values from ref. [18].
Values from ref. [31].
Scheme 4.
Substitution being assisted by general base catalysis.
3. Conclusions
Additional specific rates of solvolysis, mainly in solvents rich in fluoroalcohol, have been determined at 35.0 °C for benzenesulfonyl chloride (1) and for p-nitrobenzenesulfonyl chloride (2). Correlations, using equation 2, previously carried out with 15 or 16 solvents [20] and handicapped by a shortage of solvents rich in fluoroalcohol, are considerably improved in the present study, with 29 and 23 solvents, respectively, now available. For 2, there is noticeable deviation from the correlation line for solvents rich in acetone and it is suggested that this could be a consequence of specific interactions between the nitro-group of 2 and the solvent.
A comprehensive study of the specific rates of solvolysis of trans-β-styrenesulfonyl chloride (3) in 43 solvents showed that a very good extended Grunwald-Winstein equation treatment of the data (equation 2) can be carried out with all solvents included. Values typical for solvolyses of sulfonyl chlorides (l = 1.24; m = 0.58) were obtained. The KSIEs (at 45.0 °C) of 1.76 in methanol and methanol-d and of 1.46 in H2O and D2O are also typical values for the solvolyses of sulfonyl chlorides.
Indeed, a comparison (Table 6) of the results from the correlations using equation 2, for all sulfonyl chlorides studied in this way leads, with one exception, to l values in the range 1.07 – 1.54 and m values in the range of 0.49 – 0.72. The one exception, α-toluenesulfonyl chloride, has low values for both l and m, which lead to a typical l/m ratio of 2.05 (range of value 1.67 – 2.53).
It appears that all of the sulfonyl chlorides so far investigated in terms of equation 2 are solvolyzing by a common mechanism, generally believed to be SN2. In support of a bimolecular pathway, very recent calculations [52] indicate that most sulfonyl chlorides solvolyze with relatively little development of cationic character. The trends of values seen for l, m, and the l/m ratio (Table 6) plus the trends in the k40EtOH/k97TFE ratio (a simplified measure of the sensitivity towards changes in solvent nucleophilicity) of Table 7 and, to some extent, the trends in KSIE values for solvolyses in methanol and methanol-d support a mechanism with variations in detailed transition state structure within a conventional SN2 process and/or variations in the extent of general base catalysis (Scheme 4) towards a fundamentally SN2 process.
4. Experimental Section
The benzenesulfonyl chloride (1, Sigma-Aldrich, 99%), p-nitrobenzenesulfonyl chloride (2, Sigma-Aldrich, 97%), and trans-β-styrenesulfonyl chloride (3, Sigma-Aldrich, 97%) were used as received. Solvents were purified as described previously [6]. The specific rates of solvolysis of 1 and 2 were determined by titration of the acid developed [6]. The calculation of the specific rates of solvolysis (first-order rate coefficients) were obtained when the conventional Guggenheim treatment [53] was modified [54] so as to give the infinity titer, which was then used to calculate for each run a series of integrated rate coefficients. For faster reactions, experimental values for the infinity titer could be obtained and these were in good agreement with the estimated values. The specific rates and associated standard deviations, as presented in Table 1, are obtained by averaging all of the values from, at least, duplicate runs. For the solvolysis of 3, the specific rates were determined using an apparatus that allows a rapid response to changes in conductivity [55]. The details of the apparatus have been reported previously [56]. Typical, the determinations followed the injection of 4 μL of a 0.5 to 3.0% (w/w) solution of the substrate in dry acetonitrile into 2 mL of the appropriate solvent. Specific rates, with associated standard deviations, were determined [53] from a computer analysis (Guggenheim Method) of the plots of conductivity against time. The multiple regression analyses were carried out using commercially available statistical packages.
Figure 1.
Molecular structures of benzenesulfonyl chloride (1), p-nitrobenzenesulfonyl chloride (2), and trans-β-styrenesulfonyl chloride (3).
Acknowledgments
This research was supported by grant number 2 P2O RR016472-08 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. The grant was obtained under the leadership of the Delaware Biotechnology Institute, University of Delaware, and the authors sincerely appreciate their efforts. Z. H. R. thanks Dong-Eui University for support of this work by grant number 2007AA114.
References and Notes
- 1.Gordon IM, Maskill H, Ruasse M-F. Sulfonyl Transfer Reactions. Chem. Soc. Rev. 1989;18:123–151. [Google Scholar]
- 2.Kevill DN, D’Souza MJ. Sixty Years of the Grunwald-Winstein Equation: Development and Recent Applications. J. Chem. Res. 2008:61–66. [Google Scholar]
- 3.Bentley TW, Llewellyn G. YX Scales of Solvent Ionizing Power. Prog. Phys. Org. Chem. 1990;17:121–158. [Google Scholar]
- 4.Winstein S, Grunwald E, Jones HW. The Correlation of Solvolyses Rates and the Classification of Solvolysis Reactions into Mechanistic Categories. J. Am. Chem. Soc. 1951;73:2700–2707. [Google Scholar]
- 5.Schadt FL, Bentley TW, Schleyer PvR. The SN2-SN1 Spectrum. 2. Quantitative Treatments of Nucleophilic Solvent Assistance. A Scale of Solvent Nucleophilicities. J. Am. Chem. Soc. 1976;98:7667–7674. [Google Scholar]
- 6.Kevill DN, Anderson SW. An Improved Scale of Solvent Nucleophilicity Based on the Solvolysis of the S-Methyldibenzothiophenium Ion. J. Org. Chem. 1991;56:1845–1850. [Google Scholar]
- 7.Kevill DN. Development and Uses of Scales of Solvent Nucleophilicity. In: Charton M, editor. Advances in Quantitative Structure-Property Relationships. Vol. 1. JAI Press; Greenwich, CT: 1996. pp. 81–115. [Google Scholar]
- 8.Hall HK., Jr Kinetics of Reactions of Acyl Chlorides. II. Mechanisms of Hydrolysis of Sulfonyl Chlorides. J. Am. Chem. Soc. 1956;78:1450–1454. [Google Scholar]
- 9.Jenkins FE, Hambly AN. Solvolysis of Sulfonyl Halides. I. The Hydrolysis of Aromatic Sulfonyl Chlorides in Aqueous Dioxane and Aqueous Acetone. Aust. J. Chem. 1961;14:190–204. [Google Scholar]
- 10.Robertson RE, Rossall B. Sulfonyl Chloride Kinetics. Part II. Solvolysis of a Series of Benzenesulfonyl Chlorides: An Unexpected Heat Capacity Variation. Can. J. Chem. 1971;49:1441–1450. [Google Scholar]
- 11.Seifert R, Zbirovsky M, Sauer M. Hydrolysis of Methane-, Chloromethane-, and Dichloromethanesulfonyl Chlorides. Collect. Czech. Chem. Commun. 1973;38:2477–2483. [Google Scholar]
- 12.Lee I, Kim WK. Nucleophilic Displacement at a Sulfur Center (X). Solvolysis of Phenylmethanesulfonyl Chloride. J. Korean Chem. Soc. 1978;22:111–116. [Google Scholar]
- 13.Nair MR, Jayaraman PO. Nucleophilic Substitution at Sulphonyl Sulfur — Kinetics of Hydrolysis of Methanesulphonyl Chloride in the Presence of Added Salts. Indian J. Chem. 1983;22A:63–64. [Google Scholar]
- 14.Beck H, Doerffel K. Untersuchungen zur Verseifung aromatischer Sulfochloride. J. prakt. Chemie. 1983;325:36–40. [Google Scholar]
- 15.Arcoria A, Ballistreri EP, Spina E, Tomaselli GA, Maccarone E. Nucleophilic Substitution at Sulphonyl Sulfur. Part 4. Hydrolysis of Thiophenesulphonyl Halides in Water-Acetone Mixtures. J. Chem. Soc. Perkin Trans. 2. 1988:1793–1798. [Google Scholar]
- 16.Vizgert RV, Rubleva LI, Maksimenko NN. Reactivity of Sterically Hindered Derivatives of Aromatic Sulfonic Acids. I. Effects of Substituents on Rate and Mechanism of Hydrolysis of Some Substituted Benzenesulfonyl Chlorides. J. Org. Chem. (USSR) 1989;25:727–730. [Google Scholar]
- 17.Forbes RM, Maskill H. Sulphonyl Transfer Reactions: Solvolysis of Arenesulphonyl Chlorides in Aqueous Trifluoroethanol. J. Chem. Soc. Chem. Commun. 1991:854–856. [Google Scholar]
- 18.Koo IS, Bentley TW, Kang DH, Lee I. Limitations of the Transition State Variation Model. Part 2. Dual Reaction Channels for Solvolyses of 2,4,6-Trimethylbenzenesulphonyl Chloride. J. Chem. Soc. Perkin Trans. 2. 1991:175–179. [Google Scholar]
- 19.Koo IS, Lee I, Oh J, Yang K, Bentley TW. Substituent Effects on the Kinetic Solvent Isotope Effect in Solvolyses of Arenesulphonyl Chlorides. J. Phys. Org. Chem. 1993;6:223–227. [Google Scholar]
- 20.Kevill DN, D’Souza MJ. Application of the NT Solvent Nucleophilicity Scale to Attack at Sulfur: Solvolyses of Benzenesulfonyl Chlorides. Collect. Czech. Chem. Commun. 1999;64:1790–1796. [Google Scholar]
- 21.Kevill DN, Park B-C, Park K-H, D’Souza MJ, Yaakoubd L, Mlynarski SL, Kyong JB. Rate and Product Studies in the Solvolyses of N,N-Dimethylsulfamoyl and 2-Propanesulfonyl Chlorides. Org. Biomol. Chem. 2006;4:1580–1586. doi: 10.1039/b518129a. [DOI] [PubMed] [Google Scholar]
- 22.Bentley TW, Jones RO. Substituent Effects on the Formation of Sulfonyl Cations from Sulfonyl Chlorides: Comparison of Solvolytic Kinetic Data with Calculated Gas Phase Energies. J. Phys. Org. Chem. 2007;20:1093–1101. [Google Scholar]
- 23.Ko ECF, Robertson RE. The Hydrolysis of Sulfamoyl Chlorides. I. Hydrolysis of Dimethylsulfamoyl Chloride. Heat Capacity of Activation, the Secondary γ-Deuterium Isotope Effect, and Solvent Isotope Effect. J. Am. Chem. Soc. 1972;94:573–575. [Google Scholar]
- 24.Rogne O. Solvolysis of Dimethylsulphamoyl Chloride in Water and Aqueous Acetone. J. Chem. Soc. B. 1969:663–665. [Google Scholar]
- 25.Lee BC, Lee I. Nucleophilic Displacement at Sulfur Center. XII. Solvolysis of Dimethylsulfamoyl Chloride in Aqueous Binaries. J. Korean Chem. Soc. 1980;24:342–346. [Google Scholar]
- 26.Geiseler G, Asinger F. Ueber die Abhaengigkeit der Reactionsfaehigkert funktioneller Gruppen in der Paraffinkohlenwasserstoffen von ihrer Stellung in der Molekel. II. Mitteil: Der Einfluss der Kettenlaenge des Alkylrestes auf die Hydrolysegeschwindigkeit primaerer und sekundaerer Mono-and Disulfochloride. Ber. dtsch. chem.. Ges. 1956;89:1100–1111. [Google Scholar]
- 27.Vizgert RV. The Mechanism of Hydrolysis of Aromatic Sulfonyl Chlorides. XII. J. Gen. Chem. (USSR) 1962;32:619–623. [Google Scholar]
- 28.Tonnet ML, Hambly AN. Solvolysis of Sulfonyl Halides. VII. Hydrolysis of Some Aromatic Sulfonyl Chlorides in Aqueous Dioxane. Aust. J. Chem. 1971;24:703–712. [Google Scholar]
- 29.Koo IS, Bentley TW, Llewellyn G, Yang K. Limitations of the Transition-state Variation Model. Part 3. Solvolyses of Electron-rich Benzenesulphonyl Chlorides. J. Chem. Soc. Perkin Trans. 2. 1991:1175–1179. [Google Scholar]
- 30.Bentley TW, Jones RO, Koo IS. Stoichiometric Solvation Effects. Part 2. A New Product-Rate Correlation for Solvolyses of p-Nitrobenzenesulfonyl Chloride in Alcohol-Water Mixtures. J. Chem. Soc. Perkin Trans. 2. 1994:753–759. [Google Scholar]
- 31.Koo IS, Kwon E, Choi H, Yang K, Park JK, Lee JP, Lee I, Bentley TW. Limitations of the Transition State Variation Model. Part 8. Dual Reaction Channels for Solvolyses of 3,4-Dimethoxybenzenesulfonyl Chloride. Bull. Korean Chem. Soc. 2007;28:2377–2381. [Google Scholar]
- 32.King JF. The Return of Sulfenes. Acc. Chem. Res. 1975;8:10–17. [Google Scholar]
- 33.King JF, Lam JYL, Dave V. tert-Butyl Cation Formation in the Hydrolysis of 2-Methyl-2-propanesulfonyl Chloride, the Simplest Tertiary Alkanesulfonyl Chloride. J. Org. Chem. 1995;60:2831–2834. [Google Scholar]
- 34.Kevill DN, Kyong JB, Weitl FL. Solvolysis-Decomposition of 1-Adamantyl Chloroformate: Evidence for Ion-Pair Return in 1-Adamantyl Chloride Solvolysis. J. Org. Chem. 1990;55:4304–4311. doi: 10.1021/jo0207426. [DOI] [PubMed] [Google Scholar]
- 35.Kim WK, Lee I. Nucleophilic Displacement at Sulfur Center. II. Solvolysis of Benzenesulfonyl Chloride in Acetone-Water Mixtures. J. Korean Chem. Soc. 1973;17:163–168. [Google Scholar]
- 36.Rogne O. Kinetics of the Neutral and Alkaline Hydrolysis of Aromatic Sulfonyl Chlorides in Water. J. Chem. Soc. (B) 1968:1294–1296. [Google Scholar]
- 37.Lee I, Koo IS, Kang HK. SN2 Transition State Variation in the Benzenesulfonyl Chloride Solvolysis. Bull. Korean Chem. Soc. 1981;2:41–45. [Google Scholar]
- 38.Kevill DN, D’Souza MJ. Additional YCl Values and Correlation of the Specific Rates of Solvolysis of tert-Butyl Chloride in Terms of NT and YCl Scales. J. Chem. Res. Synop. 1993:174–175. [Google Scholar]
- 39.Lomas JS, D’Souza MJ, Kevill DN. Extremely Large Acceleration of the Solvolysis of 1-Adamantyl Chloride Upon Incorporation of a Spiro Adamantane Substituent: Solvolysis of 1-Chlorospiro[adamantane-2,2′-adamantane] J. Am. Chem. Soc. 1995;117:5891–5892. [Google Scholar]
- 40.Kevill DN, Carver JS. Rate and Product Studies with Dimethyl Phosphorochloridate and Phosphorochloridothionate Under Solvolytic Conditions. Org. Bimol. Chem. 2004;2:2040–2043. doi: 10.1039/b402093f. [DOI] [PubMed] [Google Scholar]
- 41.Kevill DN, D’Souza MJ. Correlation of the Rates of Solvolysis of Phenyl Chloroformate. J. Chem. Soc., Perkin Trans. 2. 1997:1721–1724. [Google Scholar]
- 42.Cafferata LFR, Desvard OE, Sicre JE. Solvolysis of Some Alkyl Esters of Fluorosulphuric Acid in Water and Aqueous Solvents. J. Chem. Soc. Perkin Trans. 2. 1981:940–943. [Google Scholar]
- 43.Gutbezahl B, Grunwald E. The Effect of Solvent on Equilibrium and Rate Constants. II. The Measurement and Correlation of Acid Dissociation Constants of Anilinium and Ammonium Salts in the System Ethanol-Water. J. Am. Chem. Soc. 1953;75:559–565. [Google Scholar]
- 44.Kevill DN, Kolwyck KC, Shold DM, Kim C-B. Correlation of the Ethanolysis Rates of Adamantyl Arenesulfonates. J. Am. Chem. Soc. 1973;95:6022–6027. [Google Scholar]
- 45.D’Souza MJ, Yaakoubd L, Mlynarski SL, Kevill DN. Concerted Solvent Processes for Common Sulfonyl Chloride Precursors Used in the Synthesis of Sulfonamide-based Drugs. Int. J. Mol. Sci. 2008;9:914–925. doi: 10.3390/ijms9050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ballistreri FP, Cantone A, Maccarone E, Tomaselli GA. Tripolone, M. Nucleophilic Substitution at Sulphonyl Sulfur. Part 2. Hydrolysis and Alcoholysis of Aromatic Sulphonyl Chlorides. J. Chem. Soc. Perkin Trans. 2. 1981:438–441. [Google Scholar]
- 47.Robertson RE, Rossall B, Sugamori SE, Treindl L. Heat Capacity of Activation for the Hydrolysis of Methanesulfonyl Chloride and Benzenesulfonyl Chloride in Light and Heavy Water. Can. J. Chem. 1969;47:4199–4206. [Google Scholar]
- 48.Koo IS, Yang K, An SK, Lee C-K, Lee I. Stoichiometric Solvation Effects. Solvolysis of Methanesulfonyl Chloride. Bull Korean Chem. Soc. 2000;21:1011–1014. [Google Scholar]
- 49.Koo IS, Yang K, Shin HB, An SK, Lee JP, Lee I. Stoichiometric Solvation Effects. Solvolysis of Isopropylsulfonyl Chloride. Bull. Korean Chem. Soc. 2004;25:699–703. [Google Scholar]
- 50.Kevill DN, Ryu ZH, Niedermeyer MA, Koyoshi F, D’Souza MJ. Rate and Product Studies in the Solvolyses of Methanesulfonic Anhydride and a Comparison with Methanesulfonyl Chloride Solvolysis. J. Phys. Org. Chem. 2007;20:431–438. [Google Scholar]
- 51.Thea S, Carpanelli C, Cevasco G. Sulfonyl Transfer Reactions — A Kinetic Study on the Solvolysis of p-(Dimethylamino)benzenesulfonyl Chloride in Aqueous Acetic Acid. Eur. J. Org. Chem. 2001:151–154. [Google Scholar]
- 52.Bentley TW. Structural Effects on the Solvolytic Reactivity of Carboxylic and Sulfonic Acid Chlorides. Comparisons with Gas-Phase Data for Cation Formation. J. Org. Chem. 2008;73:6251–6257. doi: 10.1021/jo800841g. [DOI] [PubMed] [Google Scholar]
- 53.Frost AA, Pearson RG. Kinetics and Mechanism-a Study of Homogeneous Chemical Reactions. 2nd Ed. Wiley; New York: 1961. pp. 49–50. [Google Scholar]
- 54.Kevill DN, Abduljaber MH. Correlation of the Rates of Solvolysis of Cyclopropylcarbinyl and Cyclobutyl Bromides Using the Extended Grunwald-Winstein Equation. J. Org. Chem. 2000;65:2548–2554. doi: 10.1021/jo991904+. [DOI] [PubMed] [Google Scholar]
- 55.Bentley TW, Jones RO. Stoichiometric Solvation Effects. Part 1. New Equations Relating Product Selectivities to Alcohol-Water Solvent Compositions for Hydrolyses of p-Nitrobenzoyl Chloride. J. Chem. Soc. Perkin Trans. 2. 1993:2351–2357. [Google Scholar]
- 56.Lee I, Lee HW, Uhm TS, Sung DD, Ryu ZH. Solvation in Mixed Solvents (VII). Solvolysis of t-Butyl Halide in Isodielectric Solvents. J. Korean Chem. Soc. 1988;32:85–93. [Google Scholar]