Abstract
Polyphenols, occurring in fruit and vegetables, wine, tea, extra virgin olive oil, chocolate and other cocoa products, have been demonstrated to have clear antioxidant properties in vitro, and many of their biological actions have been attributed to their intrinsic reducing capabilities. However, it has become clear that, in complex biological systems, polyphenols exhibit several additional properties which are yet poorly understood. Apoptosis is a genetically controlled and evolutionarily conserved form of cell death of critical importance for the normal embryonic development and for the maintenance of tissue homeostasis in the adult organism. The malfunction of the death machinery may play a primary role in various pathological processes, since too little or too much apoptosis can lead to proliferative or degenerative diseases, respectively. Cancer cells are characterized by a deregulated proliferation, and/or an inability to undergo programmed cell death. A large body of evidence indicates that polyphenols can exert chemopreventive effects towards different organ specific cancers, affecting the overall process of carcinogenesis by several mechanisms: inhibition of DNA synthesis, modulation of ROS production, regulation of cell cycle arrest, modulation of survival/proliferation pathways. In addition, polyphenols can directly influence different points of the apoptotic process, and/or the expression of regulatory proteins. Although the bulk of data has been obtained in in vitro systems, a number of clinical studies suggesting a preventive and therapeutic effectiveness of polyphenols in vivo is available. However, a deeper knowledge of the underlying mechanisms responsible for the modulation of apoptosis by polyphenols, and their real effectiveness, is necessary in order to propose them as potential chemopreventive and chemotherapeutic candidates for cancer treatment.
Keywords: polyphenols, carcinogenesis, apoptosis
1. Introduction
Polyphenols are the most abundant antioxidants in human diet and are widespread constituents of fruit, vegetables, cereals, extra virgin olive oil, dry legumes, chocolate and beverages, such as tea, coffee and wine [1]. Despite their wide distribution, the healthy effects of dietary polyphenols have come to the attention of nutritionists only in the last years. One of the main factors responsible for delayed research on polyphenols is the diversity and the complexity of their chemical structure which influences the antioxidant power. As antioxidants, polyphenols may protect cell constituents against oxidative damage. Therefore, they can limit the risk of various degenerative diseases associated with oxidative stress, such as cardiovascular diseases, type 2 diabetes and cancer [2–4]. However, accumulating evidence [5–7] indicates that, in complex biological systems, polyphenols exhibit several additional properties which may be independent of conventional antioxidant activities. This is also suggested by at least two considerations. Firstly, phenolic compounds are metabolized in vivo, giving rise to compounds that lose the original antioxidant potential [8,9]. Secondly, the concentrations of polyphenols and their metabolites, in plasma or tissues, are lower than those of other antioxidants such as ascorbic acid and α-tocopherol, which renders their competition unlikely [10–13]. Such novel mechanisms of action of dietary polyphenols might entail their interaction with cell signalling consequently influencing gene expression and modulating several cell activities [14].
Dietary polyphenols have attracted a great deal of interest because of their perceived ability to act as highly effective chemopreventive agents [15–17]. In addition, the low toxicity and the very few adverse side effects linked to polyphenols consumption give them potential advantages with respect to the traditional chemotherapeutic agents [18].
Several well-accepted mechanisms may, at least partially, explain the effectiveness of these compounds as chemopreventive agents in cancer cells. In fact they are able to: 1. suppress the over-expression of pro-oxidant enzymes implicated in cancer development; 2. inhibit the transcription factor activation, thus regulating target genes involved in cell survival and proliferation; 3. induce apoptosis; 4. inhibit matrix metalloproteinases (MMPs) and vascular endothelial growth factor (VEGF) counteracting angiogenesis which is involved in metastasis development [17,19–26].
The present review will focus the molecular basis of chemopreventive activity of polyphenols, addressing to their effects on the induction of apoptosis in cancer cells.
2. Apoptosis and cancer
Apoptosis is a genetically controlled and evolutionarily conserved form of cell death of critical importance for the normal embryonic development and for the maintenance of tissue homeostasis in the adult organism. Cancer cells are characterized by a deregulated proliferation, and/or an inability to undergo programmed cell death. The machinery responsible for killing and degradation of the cell via apoptosis is constitutively expressed and becomes activated through various stimuli.
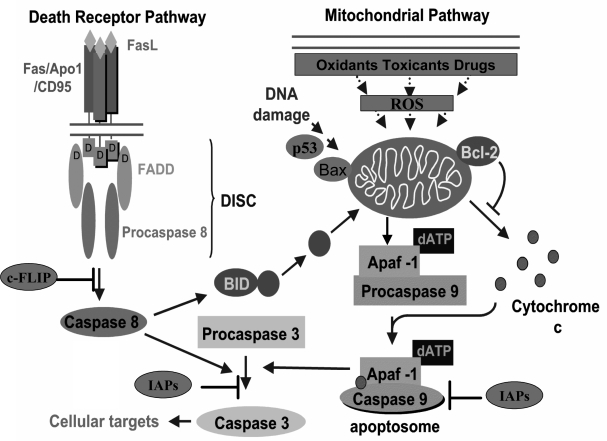
Apoptosis, characterized by a set of morphologic changes, can occur in mammalian cells by the extrinsic or intrinsic pathways [27]. The extrinsic or death receptor pathway is activated when a specific ligand binds its corresponding cell-surface death receptor, such as tumour necrosis factor (TNF) receptor, TNF-related apoptosis-inducing ligand (TRAIL) receptor and Fas receptor. In particular, the well-characterized Fas receptor (also called APO-1or CD95) is activated by binding Fas ligand that leads to its trimerization and to the recruitment of Fas-Associated protein with Death Domain (FADD). The consequent conformational changes result in the binding of procaspases-8 to a supramolecular complex called Death-Inducing Signalling Complex (DISC) [28]. Caspase-8 activation can be blocked by cellular FADD-Like interleukin-1ß-converting enzyme Inhibitory Protein (c-FLIP). Conversely, caspase-8 can also activate Bcl-2 interacting domain (Bid), a pro-apoptotic member of the Bcl-2 family of proteins, described below, which, in turn, can directly affect the mitochondrial membrane potential, thus interacting with the intrinsic pathway (Figure 1).
Figure 1.
Apoptosis pathways: The extrinsic or death receptor pathway (left) is triggered by members of the death receptor superfamily such as Fas. Binding of Fas-L to Fas induces trimerization of the receptor, recruitment of specific adaptor proteins (FADD) and consequently recruitment of procaspase 8 molecules. The multi-molecular complex (DISC) results in the activation of caspase-8, which can be blocked by c-FLIP. Active caspase-8 can in turn activate Bid, a pro-apoptotic member of Bcl-2 family proteins, which represents a crosstalk between extrinsic and intrinsic pathways. Oxidants, toxicants, drugs or ionizing radiation, which all induce ROS overproduction and the stress signalling, can activate the intrinsic pathway (right). The intrinsic or mitochondrial pathway is also triggered by DNA damage via p53 activities. The death stimuli result in loss of mitochondrial membrane integrity and release of cythocrome c, Apaf-1 and other pro-apoptotic factors in the cytoplasm. Maintenance or perturbation of mitochondrial membrane potential depends on the ratio between pro-apoptotic (Bax) and anti-apoptotic (Bcl-2) members of Bcl-2 family, by causing or preventing cythocrome c release. Multiple molecules of cythocrome c, Apaf-1, dATP and procaspase-9 associate to form a supramolecular complex termed ‘apoptosome’, that activates caspase-9 through autocatalysis. Both the activated caspase-9 and caspase-8 cleave procaspase-3 generating the active caspase-3 that, in turn, activates other executor caspases and cleaves cellular targets. Caspase activity is controlled by Inhibitors of Apoptosis Protein (IAPs) family.
The intrinsic or mitochondrial pathway is activated by different agents, such as oxidants, toxicants, drugs or ionizing radiations, all of which induce ROS overproduction and the onset of oxidative stress. The activation of this pathway is accompanied by the translocation of cytochrome c from the mitochondrial intermembrane space into the cytoplasm. Cytochrome c and Apoptotic protease-activating factor 1 (Apaf-1), are released from mitochondria and function as proapoptotic factors. Cytochrome c, Apaf-1, dATP and procaspase-9 form a supramolecular complex termed ‘apoptosome’, that activates caspase-9 through autocatalysis.
Both the pathways lead to the activation of caspase-3 that, in turn, activates other executor caspases, cleaves cytoskeleton and activates specific DNAses (Figure 1).
Among the molecules that exert their regulatory effect in determining cell fate, the proteins of the Bcl-2 family represent important checkpoints which control the main steps of the apoptotic cell death [29]. The maintenance or perturbation of mitochondrial membrane potential depends, in fact, on the ratio between pro-apoptotic (Bax, Bad, Bak, Bid, Bcl-Xs) and anti-apoptotic (Bcl-2, Bcl-XL, Bag-1, Bcl-W) members of Bcl-2 family [30,31].
Another key protein is the transcription factor p53, also known as tumour protein 53, that regulates cell cycle and apoptosis and hence functions as a tumour suppressor. In the presence of DNA damage, p53 protein arrests cell cycle allowing time for cells to repair DNA. When the damage cannot be successfully repaired, p53 acts as pro-apoptotic signal. The loss of p53, or its mutations, decreases caspase activation and therefore the occurrence of apoptosis. p53 down-regulates several anti-apoptotic genes and/or can directly activate the apoptotic pathways [32–35]. In addition p53 can displace Bax or Bid from pre-existing complexes with Bcl-XL, by binding to Bcl-XL itself, thus triggering apoptosis [36].
3. Carcinogenesis and modulation of apoptosis by polyphenols
Carcinogenesis is the result of an imbalance of the tissue homeostasis. In a stable mature tissue the rates of replication and cell death are balanced. In certain circumstances, the sustained rate of cell replication can exceed the rate of apoptosis, resulting in hyperplasia which in itself does not imply tumour development. In fact, cell proliferation is regulated by checkpoint molecules at the major stages of the cell cycle. If anyone of these checkpoints is overruled, cell can be prone to natural or induced mutations. Apoptosis eliminates genetically damaged cells or cells that may be inappropriately induced to proliferate, representing thus a protective mechanism against neoplastic transformation and development of tumours. The mutated cells which escape the apoptotic control can, in fact, become the progeny of neoplastic cell population. The multistage process of carcinogenesis could be divided in three main stages: initiation, promotion and progression [18,37]. In the initiation stage cells can opposite to carcinogens by the activation of different detoxifying enzymes such as the phase I and II enzymes. However, phase I enzymes (e.g. cytocrome P450), by reacting with carcinogens or xenobiotics, form potent electrophile, mutagenic compounds which are able to interact with the DNA triggering, in turn, nucleic acid damage and mutations. Although phase II enzymes (e.g. glutathione transferase) can detoxify these compounds by forming water-soluble glutathione or sulfate conjugates which are easily eliminated by the body, this defence mechanism is often inadequate. The stage of tumour promotion is characterized by cell proliferation which is induced by the activation and/or over-expression of enzymes involved in the synthesis of nucleotides and DNA (ornithine decarboxylase), and in the regulation of the differentiation process (DNA polymerase or topoisomerase II). Moreover, during the promotion stage, ROS overproduction occurs, mainly due to the over-expression of pro-oxidant enzymes (e.g. cyclo-oxygenase, lypoxygenase), which leads to cell damages and further DNA mutations. In the progression stage, the final stage of carcinogenesis, the mutated cells proliferate in uncontrolled manner and acquire a metastatic potential.
Polyphenols can affect the overall process of carcinogenesis by several mechanisms. In particular, polyphenols contribute to counteract oxidative stress occurrence [38,39] and, in so doing, they can contribute to the prevention of cancer onset and development. In fact by modulating oxidative stress in cancer cells, polyphenols can affect the signal transduction, the activation of redox-sensitive transcription factors and the expression of specific genes that influence cell proliferation and apoptosis [40,41]. In addition, a growing body of evidence indicates that polyphenols can directly modulate different points of the apoptotic process and/or the expression of regulatory proteins, such as the release of cytochrome c with subsequent activation of caspases-9 and caspases-3 [42–45], the increase of caspases-8 and t-Bid levels [44], the down-regulation of Bcl-2 and Bcl-XL expression, the enhanced expression of Bax and Bak [44,46,47] and the modulation of transcription factor NF-κB [48]. Among the food-derived polyphenols screened for the chemopreventive effectiveness, resveratrol, a stilbene present in high amounts in grapes [49], is particularly interesting because of its ability to affect a broad range of intracellular mediators involved in the initiation, promotion and progression of cancer [50–52].
Resveratrol could prevent or delay the onset of various cancers because of its ability to regulate multiple cellular events associated with carcinogenesis. In particular, it inhibits cell proliferation and induces apoptosis [25]. The induction of apoptosis by resveratrol has been reported to be associated with increased caspase activity [53–56], cell cycle dysregulation [57–59], decreased Bcl-2 and Bcl-XL levels, and increased Bax levels [56,60]. Interestingly, these pro-apoptotic actions have been reported to be frequently associated with the activation of p53 in different cancer cells [60–62]. A recent paper, which reviewed in detail the most convincing studies on resveratrol, pointed out this compound as a promising candidate for chemoprevention and chemotherapy [63]. However, the efficacy of resveratrol is still debated because of the multiplicity of affected targets and the contradictory effects related to the dose and the time of treatment as well as to the cellular phenotype.
The anticarcinogenic activity of tea polyphenols has been shown in vitro in different cancer cells (skin, prostate, colon, breast and lung) [26,64]. Green and black tea polyphenols, such as the flavonoid epigallocatechin-3-gallate (EGCG), have been demonstrated to regulate cancer cell growth and transformation, apoptosis, angiogenesis and metastasis, through the regulation of multiple signalling pathways probably depending on the cell type. Specifically, EGCG influences the expression of members of the Bcl-2 family of proteins, as well as of VEGF, MMPs and cell cycle inhibitors [26,65,66]. The hydroxybenzoic acid protocatechuic acid, one of the main metabolites of anthocyanins [67], also found in olives [68], brown rice [69] and tea [70], has been recently shown to induce apoptosis in human gastric adenocarcinoma cells through the Fas/FasL pathway, by activating JNK/p38 kinases [71]. Caffeic acid, a hydroxycinnamic acid found in many types of fruit and in high concentration in coffee [1], induced apoptosis in human breast cancer cells [72] by activating pro-apoptotic factors such as Fas, Bax and caspases. Likewise, it increased caspase-3 activity in stomach cancer, colon cancer and pro-myelocytic leukemia cells [73]. Furthermore, the treatment of glioma cells with caffeic acid induced the release of cytocrome c from mitochondria into cytosol and enhanced the expression of p53, Bax and Bak [74]. Pro-apoptotic activity, mediated by caspase-3 dependent mechanism, has been observed also in oral squamous carcinoma cells exposed to different phenolic compounds derived from ginger, a common condiment for various foods and beverages [75,76]. In Jurkat human T-cell leukemia cells, similar compounds activated mitochondrial pathway and altered the balance between the pro-and anti-apoptotic proteins, down regulating the anti-apoptotic Bcl-2 protein and enhancing the expression of the pro-apoptotic Bax [77]. Also curcumin, a polyphenolic compound derived from the rhizome of the plant Curcuma longa, induced apoptosis by suppressing the constitutive expression of Bcl-2 and Bcl-XL, and by activating caspase-7 and caspase-9 in mantle cell lymphoma [78] and multiple myeloma [79] cell lines. Recently it has been demonstrated that curcumin induced apoptosis in prostate cancer cells, by down-regulating the expression of Bcl-2 and Bcl-XL and up-regulating the expression of p53, Bax, Bak, and Bim [80].
As mentioned above, cancer cells constitutively generate large, but tolerable, amounts of ROS. Consequently, this suggests that a certain level of oxidative stress may be required to maintain a balance between proliferation and apoptosis [81]. ROS apparently function as signalling molecules in the mitogen-activated protein kinase (MAPKs) pathways [82] leading to the activation of redox-sensitive transcription factors and responsive genes which are involved in the survival and proliferation of cancer cells. The MAPK signalling cascades include extracellular signal-related protein kinases (ERKs), JNKs/stress-activated protein kinases (SAPKs), and p38 kinases [83]. The ERK pathway has been associated with the regulation of cell proliferation since it transmits signals initiated by growth promoters, and may ultimately foster cell growth and survival [15]. In contrast, the activation of JNK and p38 kinases is controlled by stress signalling, such as oxidative stress, and has been associated with the induction of apoptosis [84,85]. The balance between ERK and JNK activation is a key factor for cell survival since both, the decrease of ERK and the increase of JNK, are required to induce apoptosis. The activated MAPKs translocate to the nucleus, where they phosphorylate a number of substrates, including the transcription factors AP-1 and NF-κB which are linked to carcinogenesis and tumour promotion [15]. The activation of AP-1 and NF-κB promotes, in fact, survival and cellular proliferation, while their down-regulation sensitizes cells to apoptosis [81]. The excess of ROS can be scavenged by phenolic compounds. Consequently, the oxidative stress-responsive genes can be suppressed and cancer cell proliferation inhibited. On the other hand, polyphenols can also induce the formation of ROS to achieve an intolerable level of oxidative stress in cancer cells [81]. When the critical threshold for cancer cells to cope with oxidative stress has been reached, key cellular components, such as DNA, are irreparably damaged. In addition, genes involved in initiating cell cycle arrest and/or apoptosis are activated. Therefore polyphenols can either scavenge the constitutive ROS or, paradoxically, generate additional amounts of ROS to inhibit the proliferation of cancer cells. Both the mechanism of action seem to be strictly linked to the phenolic concentration and the experimental conditions. It has been observed, in fact, that low or high concentrations of the same phenolic compound are responsible for antioxidant and pro-oxidant activity, respectively [81,86]. Several studies suggest that polyphenols can scavenge the constitutively high amounts of H2O2 in different cancer cells, such as human epidermal keratinocytes, U-937 cells, Jurkat cells, HeLa cells and glioma cells [87–89]. Consequently, they were able to block the MAPK signalling, the activation of NF-κB and AP-1, and the induction of responsive genes that stimulate cancer cell proliferation [88]. In particular, resveratrol prevented NF-κB activation induced by phorbol myristate acetate, lipopolysaccaride, okadaic acid, ceramide and, most importantly, H2O2. Resveratrol had similar effects on the events which lead to the activation of transcription factors, as AP-1 in HeLa cells exposed to either PMA or ultraviolet radiation [88]. The flavone apigenin, abundantly present in fruit and vegetables, induced growth inhibition of human anaplastic thyroid carcinoma cells, probably by directly inhibiting the phosphorylation of MAPK, or alternatively, by scavenging H2O2 that activates the protein kinases [90].
On the other hand, polyphenols such as EGCG, quercetin, and gallic acid, can have pro-oxidant effects generating H2O2 in a time- and concentration-dependent manner when added to cell culture media consequently provoking stressful and/or cytotoxic effects [91]. Likewise, the apoptosis induced in Ha-ras gene-transformed human bronchial epithelial cells by a 24hr-treatment with 25 μM EGCG, or related tea catechins [92], was attributed to ROS production. In fact, the catechins induced the formation of H2O2 and the addition of catalase prevented the apoptosis. In addition, the tea catechins decreased c-jun protein phosphorylation, and consequently AP-1 activity needed to activate some genes which promote cancer cell viability. Similarly, the apoptosis induced in human oral squamous carcinoma cells by EGCG was attributed to the generation of H2O2 in the cell culture medium [93]. Finally, it has been recently demonstrated that the 3,4 dihydroxybenzoic acid induced apoptosis in human gastric carcinoma cells by ROS overproduction which is able to activate JNK/p38 MAPKs [94].
It is worth of note that cancer cells, compared to normal cells, are more susceptible to be killed by anticancer drugs and polyphenols as well. This is probably because cancer cells are already close to a threshold for tolerating ROS. In fact, by using the same concentration, phenolic compounds induced apoptosis in cultured cancer cells, but not in their normal counterparts [95–98]. In agreement with these findings, internucleosomal DNA fragmentation was detected in A431 (human epidermoid carcinoma cells), HaCaT (human carcinoma keratinocytes), DU145 (human prostate carcinoma cell line) and L5178Y cell lines (mouse lymphoma cells), but not in normal human epidermal keratinocytes [99] after treatment with EGCG. In addition Hsu et al. [100] demonstrated the influence of the age of the cells studied (differentiated / undifferentiated) by showing that the ECGC induced differentiation of immature normal human keratinocytes after 24 h of treatment, while stimulate cellular proliferation was stimulated in aged keratinocytes (15–25 days). It should be also taken in account that polyphenols can elicit different cellular responses depending on concentration used for the experiment. The dose-dependent effect of EGCG on the inhibition of cell proliferation was demonstrated in neuroblastoma SH-SY5Y cells, where low flavanol concentration (1 μmol/L) induced an anti-apoptotic response, while higher concentration (50 μmol/L) caused a pro-apoptotic effect [101]. In addition EGCG enhanced proliferation at 0.5 μmol/L and did not affect cell growth at 50 μmol/L in normal keratinocytes, while it decreased cell proliferation at both concentrations in squamous carcinoma cells [95]. In conclusion, EGCG seems to possess a dual mechanism of action depending on the concentration, although it appears that this flavanol exerts its action in a selective manner in normal and cancer cells.
4. Conclusion
Dietary polyphenols have attracted a great deal of interest because of their perceived ability to act as highly effective chemopreventive and chemotherapeutic agents. In fact they are able to affect the overall process of carcinogenesis by suppressing the over-expression of pro-oxidant enzymes, by inhibiting targets genes involved in cell proliferation and by inducing apoptosis.
Apoptosis represents a protective mechanism against neoplastic transformation and development of tumours by eliminating genetically damaged cells or cells that may be inappropriately induced to proliferate by mitogenic and proliferative stimuli.
A growing body of evidence provides new insight in the comprehension of the cellular and molecular mechanisms responsible for the induction of apoptosis by polyphenols. The experimental data suggest a multifaceted action of polyphenols on the modulation of cell signals and biochemical pathways involved in cell survival and cell death. Moreover these effects depend on the concentration of polyphenols, the cell system, the cell age and the type or stage of the degenerative process.
Although some studies well address the effectiveness of polyphenols in humans [102–104], in our opinion a deeper knowledge of the mechanisms responsible for the induction of apoptosis by polyphenols, and their real effectiveness in vivo, is necessary in order to propose them as potential chemopreventive and chemotherapeutic candidates for cancer treatment.
References
- 1.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 2.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. DOI 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 3.Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res. 1995;22(4):375–383. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 4.Scalbert A, Manach C, Morand C, Remesy C, Jimenez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45(4):287–306. doi: 10.1080/1040869059096. DOI 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 5.Casalini C, Lodovici M, Briani C, Paganelli G, Remy S, Cheynier V, Dolara P. Effect of complex polyphenols and tannins from red wine (WCPT) on chemically induced oxidative DNA damage in the rat. Eur J Nutr. 1999;38(4):190–195. doi: 10.1007/s003940050061. DOI 10.1007/s003940050061. [DOI] [PubMed] [Google Scholar]
- 6.Giovannelli L, Testa G, De Filippo C, Cheynier V, Clifford MN, Dolara P. Effect of complex polyphenols and tannins from red wine on DNA oxidative damage of rat colon mucosa in vivo. Eur J Nutr. 2000;39(5):207–212. doi: 10.1007/s003940070013. DOI 10.1007/s003940070013. [DOI] [PubMed] [Google Scholar]
- 7.Takagi A, Sai K, Umemura T, Hasegawa R, Kurokawa Y. Inhibitory effects of vitamin E and ellagic acid on 8-hydroxydeoxyguanosine formation in liver nuclear DNA of rats treated with 2-nitropropane. Cancer Lett. 1995;91(1):139–144. doi: 10.1016/0304-3835(95)03734-e. DOI 10.1016/0304-3835(95)03734-E. [DOI] [PubMed] [Google Scholar]
- 8.Huber WW, McDaniel LP, Kaderlik KR, Teitel CH, Lang NP, Kadlubar FF. Chemoprotection against the formation of colon DNA adducts from the food-borne carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in the rat. Mutat. Res. 1997;376:115–122. doi: 10.1016/s0027-5107(97)00033-x. [DOI] [PubMed] [Google Scholar]
- 9.Lodovici M, Casalini C, De Filippo C, Copeland E, Xu X, Clifford M, Dolara P. Inhibition of 1,2-dimethylhydrazine-induced oxidative DNA damage in rat colon mucosa by black tea complex polyphenols. Food Chem Toxicol. 2000;38(12):1085–1088. doi: 10.1016/s0278-6915(00)00109-5. DOI 10.1016/S0278-5 6915(00)00109-5. [DOI] [PubMed] [Google Scholar]
- 10.Boyle SP, Dobson VL, Duthie SJ, Kyle JA, Collins AR. Absorption and DNA protective effects of flavonoid glycosides from an onion meal. Eur J Nutr. 2000;39(5):213–223. doi: 10.1007/s003940070014. DOI 10.1007/s003940070014. [DOI] [PubMed] [Google Scholar]
- 11.Lampe JW. Health effects of vegetables and fruit: assessing mechanisms of action in human experimental studies. Am. J. Clin. Nutr. 1999;70:475S–490S. doi: 10.1093/ajcn/70.3.475s. [DOI] [PubMed] [Google Scholar]
- 12.Lean ME, Noroozi M, Kelly I, Burns J, Talwar D, Sattar N, Crozier A. Dietary flavonols protect diabetic human lymphocytes against oxidative damage to DNA. Diabetes. 1999;48(1):176–181. doi: 10.2337/diabetes.48.1.176. DOI 10.2337/diabetes.48.1.176. [DOI] [PubMed] [Google Scholar]
- 13.Leighton F, Cuevas A, Guasch V, Perez DD, Strobel P, San Martin A, Urzua U, Diez MS, Foncea R, Castillo O, Mizon C, Espinoza MA, Urquiaga I, Rozowski J, Maiz A, Germain A. lasma polyphenols and antioxidants, oxidative DNA damage and endothelial function in a diet and wine intervention study in humans. P. Drugs Exp Clin Res. 1999;25:133–141. [PubMed] [Google Scholar]
- 14.Masella R, Di Benedetto R, Vari R, Filesi C, Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem. 2005;16(10):577–586. doi: 10.1016/j.jnutbio.2005.05.013. DOI 10.1016/j.jnutbio.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Bode AM, Dong Z. Targeting signal transduction pathways by chemopreventive agents. Mutat. Res. 2004;555:33–51. doi: 10.1016/j.mrfmmm.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Kandaswami C, Lee LT, Lee PP, Hwang JJ, Ke FC, Huang YT, Lee MT. The antitumor activities of flavonoids. In Vivo. 2005;19(5):895–909. [PubMed] [Google Scholar]
- 17.Thomasset SC, Berry DP, Garcea G, Marczylo T, Steward WP, Gescher AJ. Dietary polyphenolic phytochemicals--promising cancer chemopreventive agents in humans? A review of their clinical properties. Int. J. Cancer. 2007;120(3):451–458. doi: 10.1002/ijc.22419. DOI 10.1002/ijc.22419. [DOI] [PubMed] [Google Scholar]
- 18.Bode AM, Dong Z. Molecular and cellular targets. Mol Carcinog. 2006;45:422–430. doi: 10.1002/mc.20222. DOI 10.1002/mc.20222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006;71(10):1397–1421. doi: 10.1016/j.bcp.2006.02.009. DOI 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Na HK, Surh YJ. Intracellular signaling network as a prime chemopreventive target of (−)-epigallocatechin gallate. Mol. Nutr. Food Res. 2006;50(2):152–159. doi: 10.1002/mnfr.200500154. DOI 10.1002/mnfr.200500154. [DOI] [PubMed] [Google Scholar]
- 21.Prescott SM, Fitzpatrick FA. Cyclooxygenase-2 and carcinogenesis. Biochim Biophys Acta. 2000;1470:M69–M78. doi: 10.1016/s0304-419x(00)00006-8. [DOI] [PubMed] [Google Scholar]
- 22.Subbaramaiah K, Dannenberg AJ. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol Sci. 2003;24(2):96–102. doi: 10.1016/S0165-6147(02)00043-3. DOI 10.1016/S0165-6147(02)00043-3. [DOI] [PubMed] [Google Scholar]
- 23.Turini ME, DuBois RN. Cyclooxygenase-2: a therapeutic target. Annu Rev Med. 2002;53:35–57. doi: 10.1146/annurev.med.53.082901.103952. DOI 10.1146/annurev.med.53.082901.103952. [DOI] [PubMed] [Google Scholar]
- 24.Oak MH, El Bedoui J, Schini-Kerth VB. Antiangiogenic properties of natural polyphenols from red wine and green tea. J Nutr Biochem. 2005;16:1–8. doi: 10.1016/j.jnutbio.2004.09.004. DOI 10.1016/j.jnutbio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Shankar S, Singh G, Srivastava RK. Chemoprevention by resveratrol: molecular mechanisms and therapeutic potential. Front Biosci. 2007;12:4839–4854. doi: 10.2741/2432. DOI 10.2741/2432. [DOI] [PubMed] [Google Scholar]
- 26.Shankar S, Ganapathy S, Srivastava RK. Green tea polyphenols: biology and therapeutic implications in cancer. Front Biosci. 2007;12:4881–4899. doi: 10.2741/2435. DOI 10.2741/2435. [DOI] [PubMed] [Google Scholar]
- 27.Khan N, Afaq F, Mukhtar H. Apoptosis by dietary factors: the suicide solution for delaying cancer growth. Carcinogenesis. 2007;28(2):233–239. doi: 10.1093/carcin/bgl243. DOI 10.1093/carcin/bgl243. [DOI] [PubMed] [Google Scholar]
- 28.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281(5381):1305–1308. doi: 10.1126/science.281.5381.1305. DOI 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 29.Antonsson B, Martinou JC. The Bcl-2 protein family. Exp Cell Res. 2000;256(1):50–57. doi: 10.1006/excr.2000.4839. DOI 10.1006/excr.2000.4839. [DOI] [PubMed] [Google Scholar]
- 30.Del Principe MI, Del Poeta G, Venditti A, Buccisano F, Maurillo L, Mazzone C, Bruno A, Neri B, Irno Consalvo M, Lo Coco F, Amadori S. Apoptosis and immaturity in acute myeloid leukemia. Hematology. 2005;10(1):25–34. doi: 10.1080/10245330400020454. DOI 10.1080/10245330400020454. [DOI] [PubMed] [Google Scholar]
- 31.Roset R, Ortet L, Gil-Gomez G. Role of Bcl-2 family members on apoptosis: what we have learned from knock-out mice. Front Biosci. 2007;12:4722–4730. doi: 10.2741/2421. DOI 10.2741/2421. [DOI] [PubMed] [Google Scholar]
- 32.Baptiste N, Friedlander P, Chen X, Prives C. The proline-rich domain of p53 is required for cooperation with anti-neoplastic agents to promote apoptosis of tumor cells. Oncogene. 2002;21(1):9–21. doi: 10.1038/sj.onc.1205015. DOI 10.1038/sj.onc.1205015. [DOI] [PubMed] [Google Scholar]
- 33.Bennett M, Macdonald K, Chan SW, Luzio JP, Simari R, Weissberg P. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science. 1998;282(5387):290–293. doi: 10.1126/science.282.5387.290. DOI 10.1126/science.282.5387.290. [DOI] [PubMed] [Google Scholar]
- 34.Matas D, Sigal A, Stambolsky P, Milyavsky M, Weisz L, Schwartz D, Goldfinger N, Rotter V. Integrity of the N-terminal transcription domain of p53 is required for mutant p53 interference with drug-induced apoptosis. Embo J. 2001;20(15):4163–4172. doi: 10.1093/emboj/20.15.4163. DOI 10.1093/emboj/20.15.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuler M, Bossy-Wetzel E, Goldstein JC, Fitzgerald P, Green DR. p53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. J Biol Chem. 2000;275(10):7337–7342. doi: 10.1074/jbc.275.10.7337. DOI 10.1074/jbc.275.10.7337. [DOI] [PubMed] [Google Scholar]
- 36.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303(5660):1010–1014. doi: 10.1126/science.1092734. DOI 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 37.Trosko JE. Dietary modulation of the multistage, multimechanisms of human carcinogenesis: effects on initiated stem cells and cell-cell communication. Nutr Cancer. 2006;54:102–110. doi: 10.1207/s15327914nc5401_12. DOI 10.1207/s15327914nc5401_12. [DOI] [PubMed] [Google Scholar]
- 38.Porrini M, Riso P, Brusamolino A, Berti C, Guarnieri S, Visioli F. Daily intake of a formulated tomato drink affects carotenoid plasma and lymphocyte concentrations and improves cellular antioxidant protection. Br J Nutr. 2005;93(1):93–99. doi: 10.1079/bjn20041315. DOI 10.1079/BJN20041315. [DOI] [PubMed] [Google Scholar]
- 39.Yao LH, Jiang YM, Shi J, Tomas-Barberan FA, Datta N, Singanusong R, Chen SS. Flavonoids in food and their health benefits. Plant Foods Hum Nutr. 2004;59(3):113–122. doi: 10.1007/s11130-004-0049-7. DOI 10.1007/s11130-004-0049-7. [DOI] [PubMed] [Google Scholar]
- 40.Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006;72:1439–1452. doi: 10.1016/j.bcp.2006.07.004. DOI 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Sang S, Hou Z, Lambert JD, Yang CS. Redox properties of tea polyphenols and related biological activities. Antioxid Redox Signal. 2005;7:1704–1714. doi: 10.1089/ars.2005.7.1704. DOI 10.1089/ars.2005.7.1704. [DOI] [PubMed] [Google Scholar]
- 42.Ong CS, Tran E, Nguyen TT, Ong CK, Lee SK, Lee JJ, Ng CP, Leong C, Huynh H. Quercetin-induced growth inhibition and cell death in nasopharyngeal carcinoma cells are associated with increase in Bad and hypophosphorylated retinoblastoma expressions. Oncol Rep. 2004;11(3):727–733. [PubMed] [Google Scholar]
- 43.Shimizu M, Deguchi A, Lim JT, Moriwaki H, Kopelovich L, Weinstein IB. (−)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin Cancer Res. 2005;11:2735–2746. doi: 10.1158/1078-0432.CCR-04-2014. [DOI] [PubMed] [Google Scholar]
- 44.Selvendiran K, Koga H, Ueno T, Yoshida T, Maeyama M, Torimura T, Yano H, Kojiro M, Sata M. Luteolin promotes degradation in signal transducer and activator of transcription 3 in human hepatoma cells: an implication for the antitumor potential of flavonoids. Cancer Res. 2006;66(9):4826–4834. doi: 10.1158/0008-5472.CAN-05-4062. DOI 10.1158/0008-5472.CAN-05-4062. [DOI] [PubMed] [Google Scholar]
- 45.Michels G, Watjen W, Niering P, Steffan B, Thi QH, Chovolou Y, Kampkotter A, Bast A, Proksch P, Kahl R. Pro-apoptotic effects of the flavonoid luteolin in rat H4IIE cells. Toxicology. 2005;206(3):337–348. doi: 10.1016/j.tox.2004.07.022. DOI 10.1016/j.tox.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 46.Kuo PL, Lin CC. Green tea constituent (−)-epigallocatechin-3-gallate inhibits Hep G2 cell proliferation and induces apoptosis through p53-dependent and Fas-mediated pathways. J Biomed Sci. 2003;10(2):219–227. doi: 10.1007/BF02256057. [DOI] [PubMed] [Google Scholar]
- 47.Lee HJ, Wang CJ, Kuo HC, Chou FP, Jean LF, Tseng TH. Induction apoptosis of luteolin in human hepatoma HepG2 cells involving mitochondria translocation of Bax/Bak and activation of JNK. Toxicol Appl Pharmacol. 2005;203(2):124–131. doi: 10.1016/j.taap.2004.08.004. DOI 10.1016/j.taap.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 48.Gong L, Li Y, Nedeljkovic-Kurepa A, Sarkar FH. Inactivation of NF-kappaB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene. 2003;22(30):4702–4709. doi: 10.1038/sj.onc.1206583. DOI 10.1038/sj.onc.1206583. [DOI] [PubMed] [Google Scholar]
- 49.Wenzel E, Somoza V. Metabolism and bioavailability of trans-resveratrol. Mol Nutr Food Res. 2005;49(5):472–481. doi: 10.1002/mnfr.200500010. DOI 10.1002/mnfr.200500010. [DOI] [PubMed] [Google Scholar]
- 50.Gusman J, Malonne H, Atassi G. A reappraisal of the potential chemopreventive and chemotherapeutic properties of resveratrol. Carcinogenesis. 2001;22:1111–1117. doi: 10.1093/carcin/22.8.1111. DOI 10.1093/carcin/22.8.1111. [DOI] [PubMed] [Google Scholar]
- 51.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 52.Kelloff GJ, Crowell JA, Steele VE, Lubet RA, Malone WA, Boone CW, Kopelovich L, Hawk ET, Lieberman R, Lawrence JA, Ali I, Viner JL, Sigman CC. Progress in cancer chemoprevention: development of diet-derived chemopreventive agents. J Nutr. 2000;130:467S–471S. doi: 10.1093/jn/130.2.467S. [DOI] [PubMed] [Google Scholar]
- 53.Wolter F, Akoglu B, Clausnitzer A, Stein J. Downregulation of the cyclin D1/Cdk4 complex occurs during resveratrol-induced cell cycle arrest in colon cancer cell lines. J Nutr. 2001;131(8):2197–2203. doi: 10.1093/jn/131.8.2197. [DOI] [PubMed] [Google Scholar]
- 54.Kim YA, Lee WH, Choi TH, Rhee SH, Park KY, Choi YH. Involvement of p21WAF1/CIP1, pRB, Bax and NF-kappaB in induction of growth arrest and apoptosis by resveratrol in human lung carcinoma A549 cells. Int J Oncol. 2003;23(4):1143–1149. [PubMed] [Google Scholar]
- 55.Gao X, Xu YX, Divine G, Janakiraman N, Chapman RA, Gautam SC. Disparate in vitro and in vivo antileukemic effects of resveratrol, a natural polyphenolic compound found in grapes. J Nutr. 2002;132(7):2076–2081. doi: 10.1093/jn/132.7.2076. [DOI] [PubMed] [Google Scholar]
- 56.Billard C, Izard JC, Roman V, Kern C, Mathiot C, Mentz F, Kolb JP. Comparative antiproliferative and apoptotic effects of resveratrol, epsilon-viniferin and vine-shots derived polyphenols (vineatrols) on chronic B lymphocytic leukemia cells and normal human lymphocytes. Leuk Lymphoma. 2002;43(10):1991–2002. doi: 10.1080/1042819021000015952. DOI 10.1080/1042819021000015952. [DOI] [PubMed] [Google Scholar]
- 57.Joe AK, Liu H, Suzui M, Vural ME, Xiao D, Weinstein IB. Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin Cancer Res. 2002;8(3):893–903. [PubMed] [Google Scholar]
- 58.Clement MV, Hirpara JL, Chawdhury SH, Pervaiz S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood. 1998;92(3):996–1002. [PubMed] [Google Scholar]
- 59.Surh YJ, Hurh YJ, Kang JY, Lee E, Kong G, Lee SJ. Resveratrol, an antioxidant present in red wine, induces apoptosis in human promyelocytic leukemia (HL-60) cells. Cancer Lett. 1999;140(1–2):1–10. doi: 10.1016/s0304-3835(99)00039-7. [DOI] [PubMed] [Google Scholar]
- 60.Kim YA, Choi BT, Lee YT, Park DI, Rhee SH, Park KY, Choi YH. Resveratrol inhibits cell proliferation and induces apoptosis of human breast carcinoma MCF-7 cells. Oncol Rep. 2004;11(2):441–446. [PubMed] [Google Scholar]
- 61.Alkhalaf M. Resveratrol-Induced Apoptosis Is Associated with Activation of p53 and Inhibition of Protein Translation in T47D Human Breast Cancer Cells. Pharmacology. 2007;80(2–3):134–143. doi: 10.1159/000103253. [DOI] [PubMed] [Google Scholar]
- 62.Shih A, Davis FB, Lin HY, Davis PJ. Resveratrol induces apoptosis in thyroid cancer cell lines via a MAPK- and p53-dependent mechanism. J Clin Endocrinol Metab. 2002;87(3):1223–1232. doi: 10.1210/jcem.87.3.8345. DOI 10.1210/jc.87.3.1223. [DOI] [PubMed] [Google Scholar]
- 63.Signorelli P, Ghidoni R. Resveratrol as an anticancer nutrient: molecular basis, open questions and promises. J Nutr Biochem. 2005;16:449–466. doi: 10.1016/j.jnutbio.2005.01.017. DOI 10.1016/j.jnutbio.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 64.Lambert JD, Hong J, Yang GY, Liao J, Yang CS. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am J Clin Nutr. 2005;81:284S–291S. doi: 10.1093/ajcn/81.1.284S. [DOI] [PubMed] [Google Scholar]
- 65.Chen C, Shen G, Hebbar V, Hu R, Owuor ED, Kong AN. Epigallocatechin-3-gallate-induced stress signals in HT-29 human colon adenocarcinoma cells. Carcinogenesis. 2003;24(8):1369–1378. doi: 10.1093/carcin/bgg091. DOI 10.1093/carcin/bgg091. [DOI] [PubMed] [Google Scholar]
- 66.Hastak K, Gupta S, Ahmad N, Agarwal MK, Agarwal ML, Mukhtar H. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene. 2003;22(31):4851–4859. doi: 10.1038/sj.onc.1206708. DOI 10.1038/sj.onc.1206708. [DOI] [PubMed] [Google Scholar]
- 67.Aura AM, Martin-Lopez P, O'Leary KA, Williamson G, Oksman-Caldentey KM, Poutanen K, Santos-Buelga C. In vitro metabolism of anthocyanins by human gut microflora. Eur J Nutr. 2005;44(3):133–142. doi: 10.1007/s00394-004-0502-2. DOI 10.1007/s00394-004-0502-2. [DOI] [PubMed] [Google Scholar]
- 68.Masella R, Cantafora A, Modesti D, Cardilli A, Gennaro L, Bocca A, Coni E. Antioxidant activity of 3,4-DHPEA-EA and protocatechuic acid: a comparative assessment with other olive oil biophenols. Redox Rep. 1999;4(3):113–121. doi: 10.1179/135100099101534792. DOI 10.1179/135100099101534792. [DOI] [PubMed] [Google Scholar]
- 69.Hudson EA, Dinh PA, Kokubun T, Simmonds MS, Gescher A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol Biomarkers Prev. 2000;9(11):1163–1170. [PubMed] [Google Scholar]
- 70.Yen GC, Hsieh CL. Reactive oxygen species scavenging activity of Du-zhong (Eucommia ulmoides oliv.) and its active compounds. J Agric Food Chem. 2000;48:3431–3436. doi: 10.1021/jf000150t. [DOI] [PubMed] [Google Scholar]
- 71.Lin HH, Chen JH, Huang CC, Wang CJ. Apoptotic effect of 3,4-dihydroxybenzoic acid on human gastric carcinoma cells involving JNK/p38 MAPK signaling activation. Int J Cancer. 2007;120(11):2306–2316. doi: 10.1002/ijc.22571. DOI 10.1002/ijc.22571. [DOI] [PubMed] [Google Scholar]
- 72.Watabe M, Hishikawa K, Takayanagi A, Shimizu N, Nakaki T. Caffeic acid phenethyl ester induces apoptosis by inhibition of NFkappaB and activation of Fas in human breast cancer MCF-7 cells. J Biol Chem. 2004;279(7):6017–6026. doi: 10.1074/jbc.M306040200. DOI 10.1074/jbc.M306040200. [DOI] [PubMed] [Google Scholar]
- 73.Kurata R, Adachi M, Yamakawa O, Yoshimoto M. Growth suppression of human cancer cells by polyphenolics from sweetpotato (Ipomoea batatas L.) leaves. J Agric Food Chem. 2007;55:185–190. doi: 10.1021/jf0620259. DOI 10.1021/jf0620259. [DOI] [PubMed] [Google Scholar]
- 74.Lee YJ, Kuo HC, Chu CY, Wang CJ, Lin WC, Tseng TH. Involvement of tumor suppressor protein p53 and p38 MAPK in caffeic acid phenethyl ester-induced apoptosis of C6 glioma cells. Biochem Pharmacol. 2003;66(12):2281–2289. doi: 10.1016/j.bcp.2003.07.014. DOI 10.1016/j.bcp.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 75.Thatte U, Bagadey S, Dahanukar S. Modulation of programmed cell death by medicinal plants. Cell Mol Biol (Noisy-le-grand) 2000;46(1):199–214. [PubMed] [Google Scholar]
- 76.Keum YS, Kim J, Lee KH, Park KK, Surh YJ, Lee JM, Lee SS, Yoon JH, Joo SY, Cha IH, Yook JI. Induction of apoptosis and caspase-3 activation by chemopreventive [6]-paradol and structurally related compounds in KB cells. Cancer Lett. 2002;177(1):41–47. doi: 10.1016/s0304-3835(01)00781-9. DOI 10.1016/S0304-3835(01)00781-9. [DOI] [PubMed] [Google Scholar]
- 77.Miyoshi N, Nakamura Y, Ueda Y, Abe M, Ozawa Y, Uchida K, Osawa T. Dietary ginger constituents, galanals A and B, are potent apoptosis inducers in Human T lymphoma Jurkat cells. Cancer Lett. 2003;199(2):113–119. doi: 10.1016/s0304-3835(03)00381-1. DOI 10.1016/S0304-3835(03)00381-1. [DOI] [PubMed] [Google Scholar]
- 78.Shishodia S, Amin HM, Lai R, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol. 2005;70(5):700–713. doi: 10.1016/j.bcp.2005.04.043. DOI 10.1016/j.bcp.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 79.Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101(3):1053–1062. doi: 10.1182/blood-2002-05-1320. DOI 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 80.Shankar S, Srivastava RK. Involvement of Bcl-2 family members, phosphatidylinositol 3′-kinase/AKT and mitochondrial p53 in curcumin (diferulolylmethane)-induced apoptosis in prostate cancer. Int J Oncol. 2007;30(4):905–918. [PubMed] [Google Scholar]
- 81.Loo G. Redox-sensitive mechanisms of phytochemical-mediated inhibition of cancer cell proliferation (review) J Nutr Biochem. 2003;14(2):64–73. doi: 10.1016/s0955-2863(02)00251-6. DOI 10.1016/S0955-2863(02)00251-6. [DOI] [PubMed] [Google Scholar]
- 82.Sun Y, Oberley LW. Redox regulation of transcriptional activators. Free Radic Biol Med. 1996;21(3):335–348. doi: 10.1016/0891-5849(96)00109-8. DOI 10.1016/0891-5849(96)00109-8. [DOI] [PubMed] [Google Scholar]
- 83.Martin KR. Targeting apoptosis with dietary bioactive agents. Exp Biol Med (Maywood) 2006;231(2):117–129. doi: 10.1177/153537020623100201. [DOI] [PubMed] [Google Scholar]
- 84.Cowan KJ, Storey KB. Mitogen-activated protein kinases: new signaling pathways functioning in cellular responses to environmental stress. J Exp Biol. 2003;206(7):1107–1115. doi: 10.1242/jeb.00220. [DOI] [PubMed] [Google Scholar]
- 85.Cross TG, Scheel-Toellner D, Henriquez NV, Deacon E, Salmon M, Lord JM. Serine/threonine protein kinases and apoptosis. Exp Cell Res. 2000;256(1):34–41. doi: 10.1006/excr.2000.4836. DOI 10.1006/excr.2000.4836. [DOI] [PubMed] [Google Scholar]
- 86.Ramos S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J Nutr Biochem. 2007;18(7):427–442. doi: 10.1016/j.jnutbio.2006.11.004. DOI 10.1016/j.jnutbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 87.Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164(12):6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 88.Yu R, Hebbar V, Kim DW, Mandlekar S, Pezzuto JM, Kong AN. Resveratrol inhibits phorbol ester and UV-induced activator protein 1 activation by interfering with mitogen-activated protein kinase pathways. Mol Pharmacol. 2001;60(1):217–224. doi: 10.1124/mol.60.1.217. [DOI] [PubMed] [Google Scholar]
- 89.Katiyar SK, Afaq F, Azizuddin K, Mukhtar H. Inhibition of UVB-induced oxidative stress-mediated phosphorylation of mitogen-activated protein kinase signaling pathways in cultured human epidermal keratinocytes by green tea polyphenol (minus;)-epigallocatechin-3-gallate. Toxicol Appl Pharmacol. 2001;176(2):110–107. doi: 10.1006/taap.2001.9276. DOI 10.1006/taap.2001.9276. [DOI] [PubMed] [Google Scholar]
- 90.Yin F, Giuliano AE, Van Herle AJ. Signal pathways involved in apigenin inhibition of growth and induction of apoptosis of human anaplastic thyroid cancer cells (ARO) Anticancer Res. 1999;19:4297–4303. [PubMed] [Google Scholar]
- 91.Long LH, Clement MV, Halliwell B. Artifacts in cell culture: rapid generation of hydrogen peroxide on addition of (−)-epigallocatechin(−)-epigallocatechin gallate(+)-catechin, and quercetin to commonly used cell culture media. Biochem Biophys Res Commun. 2000;273(1):50–53. doi: 10.1006/bbrc.2000.2895. DOI 10.1006/bbrc.2000.2895. [DOI] [PubMed] [Google Scholar]
- 92.Yang GY, Liao J, Li C, Chung J, Yurkow EJ, Ho CT, Yang CS. Effect of black and green tea polyphenols on c-jun phosphorylation and H(2)O(2) production in transformed and non-transformed human bronchial cell lines: possible mechanisms of cell growth inhibition and apoptosis induction. Carcinogenesis. 2000;21(11):2035–2039. doi: 10.1093/carcin/21.11.2035. DOI 10.1093/carcin/21.11.2035. [DOI] [PubMed] [Google Scholar]
- 93.Sakagami H, Arakawa H, Maeda M, Satoh K, Kadofuku T, Fukuchi K, Gomi K. Production of hydrogen peroxide and methionine sulfoxide by epigallocatechin gallate and antioxidants. Anticancer Res. 2001;21:2633–2641. [PubMed] [Google Scholar]
- 94.Chen C, Yu R, Owuor ED, Kong AN. Activation of antioxidant-response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch Pharm Res. 2000;23(6):605–612. doi: 10.1007/BF02975249. [DOI] [PubMed] [Google Scholar]
- 95.Chung JH, Han JH, Hwang EJ, Seo JY, Cho KH, Kim KH, Youn JI, Eun HC. Dual mechanisms of green tea extract (EGCG)-induced cell survival in human epidermal keratinocytes. Faseb J. 2003;17:1913–1915. doi: 10.1096/fj.02-0914fje. [DOI] [PubMed] [Google Scholar]
- 96.Brusselmans K, De Schrijver E, Heyns W, Verhoeven G, Swinnen JV. Epigallocatechin-3-gallate is a potent natural inhibitor of fatty acid synthase in intact cells and selectively induces apoptosis in prostate cancer cells. Int J Cancer. 2003;106(6):856–862. doi: 10.1002/ijc.11317. DOI 10.1002/ijc.11317. [DOI] [PubMed] [Google Scholar]
- 97.Chen ZP, Schell JB, Ho CT, Chen KY. Green tea epigallocatechin gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Lett. 1998;129(2):173–179. doi: 10.1016/s0304-3835(98)00108-6. DOI 10.1016/S0304-3835(98)00108-6. [DOI] [PubMed] [Google Scholar]
- 98.Kawai K, Tsuno NH, Kitayama J, Okaji Y, Yazawa K, Asakage M, Sasaki S, Watanabe T, Takahashi K, Nagawa H. Epigallocatechin gallate induces apoptosis of monocytes. J Allergy Clin Immunol. 2005;115(1):186–191. doi: 10.1016/j.jaci.2004.10.005. DOI 10.1016/j.jaci.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 99.Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Natl Cancer Inst. 1997;89(24):1881–1886. doi: 10.1093/jnci/89.24.1881. DOI 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- 100.Hsu S, Bollag WB, Lewis J, Huang Q, Singh B, Sharawy M, Yamamoto T, Schuster G. Green tea polyphenols induce differentiation and proliferation in epidermal keratinocytes. J Pharmacol Exp Ther. 2003;306(1):29–34. doi: 10.1124/jpet.103.049734. DOI 10.1124/jpet.103.049734. [DOI] [PubMed] [Google Scholar]
- 101.Tan X, Hu D, Li S, Han Y, Zhang Y, Zhou D. Differences of four catechins in cell cycle arrest and induction of apoptosis in LoVo cells. Cancer Lett. 2000;158:1–6. doi: 10.1016/s0304-3835(00)00445-6. [DOI] [PubMed] [Google Scholar]
- 102.Pantuck AJ, Leppert JT, Zomorodian N, Aronson W, Hong J, Barnard RJ, Seeram N, Liker H, Wang H, Elashoff R, Heber D, Aviram M, Ignarro L, Belldegrun A. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin Cancer Res. 2006;12:4018–4026. doi: 10.1158/1078-0432.CCR-05-2290. DOI 10.1158/1078-0432.CCR-05-2290. [DOI] [PubMed] [Google Scholar]
- 103.Seeram NP, Aronson WJ, Zhang Y, Henning SM, Moro A, Lee RP, Sartippour M, Harris DM, Rettig M, Suchard MA, Pantuck AJ, Belldegrun A, Heber D. Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J Agric Food Chem. 2007;55:7732–7737. doi: 10.1021/jf071303g. [DOI] [PubMed] [Google Scholar]
- 104.Siddiqui IA, Saleem M, Adhami VM, Asim M, Mukhtar H. Tea beverage in chemoprevention and chemotherapy of prostate cancer. Acta Pharmacol Sin. 2007;28:1392–1408. doi: 10.1111/j.1745-7254.2007.00693.x. DOI 10.1111/j.1745-7254.2007.00693.x. [DOI] [PubMed] [Google Scholar]