Abstract
Various etiological factors contributing to the development of mycotoxic nephropathy in farm animals and humans are reviewed. The possible synergistic effect between ochratoxin A (OTA) and other mycotoxins, as penicillic acid (PA) and fumonisin B1 (FB1), contributing to this nephropathy is also considered and discussed. The most convenient ways of prophylaxis and various preventive measures against OTA contamination of feeds or foods are reviewed. A reference is made concerning the most successful methods of veterinary hygiene control in the slaughterhouses in order to prevent the entering of OTA in commercial channels with a view to human health. The economic efficacy of these prophylactic procedures is also considered. An evaluation of human exposure to OTA is made.
Keywords: mycotoxic nephropathy, Balkan endemic nephropathy, ochratoxin A, penicillic acid, fumonisin B1, synergism, preventive measures, hygiene control, pathogenesis
Abbreviations: MN - mycotoxic nephropathy, MPN - mycotoxic porcine nephropathy, MAN - mycotoxic avian nephropathy, MNFA - mycotoxic nephropathy in farm animals, BEN - Balkan endemic nephropathy, OTA - ochratoxin A, OTα - ochratoxin α, PA - penicillic acid, FB1 - fumonisin B1
Introduction
The mycotoxic nephropathy (MN) is a renal disorder caused by alimentary ingestion of secondary fungal metabolites possessing nephrotoxic properties and encountered in feeds/foods/forages made mainly from cereals or fibrous plants, and kept in storehouse conditions and increased humidity. Since its discovering the disease has been described using various names: nephrosis provoked by moulds, chronic interstitial fibrosis of kidneys, chronic interstitial nephritis, etc. Recently, the terms “ochratoxicosis” and “mycotoxic nephropathy” are the most frequently used ones for describing this nephropathy.
During the last years this kidney disease has gained high publicity in some countries with well developed stock-breeding (especially pig-breeding) as Denmark, Sweden, Bulgaria, etc. In order to prevent human exposure to various nephrotoxic mycotoxins, mainly ochratoxin A (OTA), via consuming the meat of animals with nephropathy, the timely diagnosis of disease during the meat inspection at slaughterhouses is very important. In such a way the exposure of humans to this very hazardous and relatively heat stable toxin from chicken/pigs meat can be prevented [1].
There are some variances in the manifestation of the disease, especially in the clinicomorphological picture, which in a lot of the cases is influenced by the secondary bacterial infections [2] as a result of the pronounced immunosuppression in the affected animals [2, 3, 4, 5, 6]. Therefore, this review paper could contribute to timely diagnostics of mycotoxic nephropathy in farm animals (MNFA) as well as could help the organization of the prophylactic measures against this disease. In addition, some information is given how to make an evaluation of human exposure to OTA on the base of the found concentrations of OTA in human serum via calculating the daily OTA intake of humans in endemic areas.
1. Complex Etiology of MN
Although many nephrotoxic fungal compounds such as fumonisins are known [7], only OTA and partly citrinin can be firmly associated with spontaneous cases of MN. Ochratoxins are a group of structurally related compounds. Between them, the first and the most toxic compound discovered is OTA, which was isolated from Aspergillus ochraceus and chemiclly defined in 1965 by several South African scientists [8–11]. OTA consists of a dihydroisocoumarin moiety linked through its 7-carboxyl group by an amide bond to one molecule of L-β phenylalanine (7-carboxy-5-chloro-8-hydroxy-3-4-dihydro-3R-methylisocoumarin). Citrinin was discovered in 1931 by Hetherington and Raistrick, who isolated the compound from several strains of Penicillium citrinum [12].
Fungi that produce OTA are widespread in nature and various foods or drinks [13, 14, 15], but commonly contaminate stored grain. Penicillium viridicatum, predominantly a storage fungus, is the mould that usually develops on grains at low temperatures and also is the major producer of OTA in the colder areas of the world (Northern Europe and Canada). On the other hand Aspergillus ochraceus probably is the most impotant producer of OTA in tropical and semitropical areas [16, 17]. However, the growth of A. ochraceus on natural substrates has not been associated with a natural occurrence of OTA. Not all isolates of P. viridicatum and A. ochraceus are OTA producers, but some of them produce other toxic compounds, e.g., citrinin, penicillic acid (PA), hydroxyaspergillic acid and secalonic acid [18].
A positive correlation has been observed between the frequency of spontaneous porcine nephropathy in Bulgaria and the rate of OTA in corresponding feed samples. However, the analysis for OTA of various feed samples from farms with high frequency of spontaneous mycotoxic porcine nephropathy (MPN) revealed that the values were substantially low and ranged from 38 to 552 (mean 207.1 ± 65.14) ng/g for 1993 and from 42 to 427 (mean 114.06 ± 35.79) ng/g for 1994 respectively. These farms usually had a history of incorrect feed storage. Sometimes the problem seemed to come from certain feed plants whose grains, collected during moist and rainly days, had not been properly dried. All farms supplied by these plants subsequently produced some pigs with nephropathy and growth depression, but after changing the certain suspected feeds the problems with poor growth of pigs disappeared. As a whole, the frequency and duration of the observed nephropathy in different batches of slaughtered pigs varied significantly (from 1–2% up to 80–90% frequency) and have depended on the duration of feeding of various suspected feeds stored for a long time in poor conditions and at high humidity [18–21].
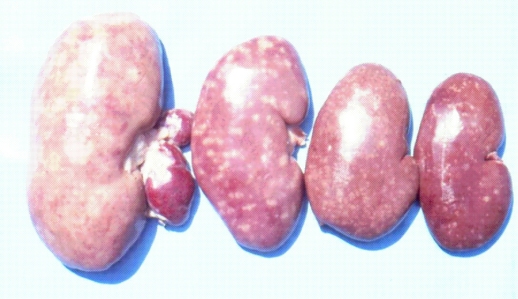
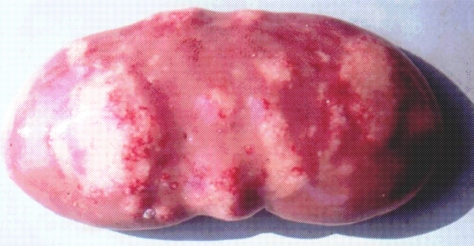
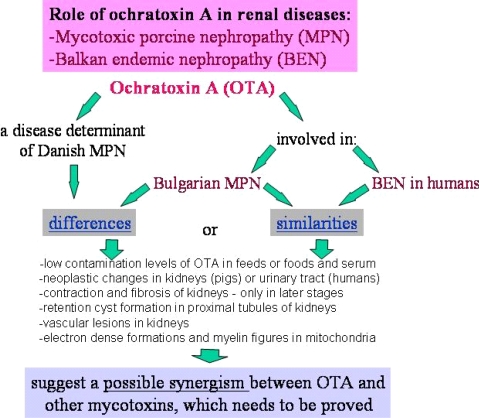
It is important to mention that kidney damages in spontaneous MPN in Bulgaria (Figure 1) are more similar to those in Balkan endemic nephropathy (BEN) in humans, than in Danish MPN. For example, the fibrotic changes and contraction of kidneys in the end-stages of MPN in Bulgaria are very comparable to those in BEN in humans [1], whereas such changes are not seen in the classical description of MPN as made in Denmark. In addition to the contraction of kidneys in later stages of Bulgarian MPN and BEN, there are various other similarities between BEN in humans and Bulgarian MPN as the low contamination levels of OTA in feeds or foods and serum, neoplastic changes in kidneys (pigs – Figure 2) or urinary tract (humans), retention cyst formation in proximal tubules of kidneys, vascular lesions in kidneys, electron dense formations and myelin-like figures in mitochondria of epithelial cells (Figure 3). However, these pathological changes have not been observed in classical MPN as described in Denmark or elsewhere. This circumstance, in addition to low feed/food levels of OTA in Bulgarian MPN and BEN, suggest a possible interaction between OTA and other mycotoxins, which needs to be proved (Figure 3). Recently, it was found that pathomorphological changes, including electron-dense formation and myelin-like figures, induced in pig's kidneys by combined exposure to such low contamination levels of OTA together with PA, are more similar to those in spontaneous MPN in Bulgaria than to classical Danish MPN [22].
Figure 1.
Range of enlargement and mottled or pale appearance of kidneys from pigs with MPN at age of 6–8 months taken at slaughtered time, including an example (left) with enlarged renal lymph nodes.
Figure 2.
Kidney with neoplastic tissue (fibroadenoma) - spontaneous case of MPN in Bulgaria.
Figure 3.
Role of OTA in the renal diseases MPN and BEN.
All feed samples originating from farms with high frequency of spontaneous avian nephropathy in Bulgaria were also positive for OTA and similarly to pig farms, the levels of OTA were substantially low (the values ranged from 90.8 to 310 ng/g (mean 196.2 ng/g ±45.9). The nephropathy in chickens was usually observed during the spring and summer, similarly to porcine nephropathy and in the same regions as well. The occurrence of nephropathy in different batches of slaughtered chickens varied from 1–2 per cent up to 90–100 per cent. The continuance of this nephropathy, also differed widely from one month to about 5–6 months or even throughout a year [23].
It is known that experimental pigs exposed to OTA contaminated feed at levels of about 200 ppb develop only microscopic lesions after a period of 3–4 months [24, 25]. The average contamination level of OTA in Bulgarian feeds for pigs in 1993 only exceeded 200 ppb OTA [18]. On the other hand, the low contamination levels of OTA in pigs/chicks feeds suggest a possible synergism between OTA and other compounds produced by the same ochratoxinogenic fungi. It is very likely that feeds contain other nephrotoxic mycotoxins or compounds which enhance the toxicity or produce a synergistic interaction with OTA [such as PA, fumonisin B1 (FB1), citrinin, viomelein, xanthomegnin etc]. Additive or synergistic interactions of these compounds have not been proved for all combinations.
Several years ago, it has been found that the toxicity of various strains of the same Aspergillus ochraceus group is very different, independently of the capacity of OTA production [22, 26, 27]. A potent synergistic effect was found between OTA and PA, mycotoxins produced by the same ochratoxinogenic fungi, when the same mycotoxins were given simultaneously to pigs and chickens [22, 26–30]. The production of multiple toxins by a single organism, such as Aspergillus ochraceus (which produces OTA and PA simultaneously), or by mixture of fungi presents a problem that has not been sufficiently investigated. Such mixtures of toxins may have additional or synergistic effects in farm animals. PA was suspected to be carcinogenic [31,32] and was found to have DNA-attacking ability in the rec assay [33] as well as to induce chromosome aberations [34]. Some data suggest that feed contamination levels of PA itself up to 4000 ppb have little toxicity (mostly hepatotoxicity) and do not adversely affect body or organ weights or biochemical parameters in chickens [35]. Only DNA breaks in mammalian cell lines [36, 37] and an inhibition of rat liver glutathione S-transferase activity in crude extracts in vitro [38] as well as PA-induced hepatobiliary excretory dysfunction [39] and liver hemorrhages [40] have been found so far. The higher toxicity of OTA in all above mentioned studies might be due to synergistic toxic effects between OTA and PA, as has been reported in mice [41, 42] and in poultry [28]. Previous studies have suggested an important role of the pancreatic enzyme carboxypeptidase A in the detoxification of OTA in the small intestine [43, 44]. Parker and colleagues [45] showed that PA inhibits carboxypeptidase activity in vitro and in vivo and such inhibition may significantly impair the primary detoxification of OTA in the intestinal tract and so be partly responsible for the enhanced toxicity of OTA observed in combination with PA [22, 26–30]. The PA-induced hepatobiliary excretory dysfunction [39] may also result in decreasing of hepatobiliary excretion of OTA. Such synergism between OTA and PA or other mycotoxins under field conditions may be responsible for the spontaneous mycotoxic nephropathy in Bulgaria, which can be caused by relatively low contamination levels of OTA in feed [18, 21, 23]. This could explain why the low levels of OTA in Bulgarian feeds for pigs [18, 21], chickens [23] or humans [1] have such high toxic effect on kidneys, when ingested with spontaneous contaminated feeds. The concentration of OTA in feeding forages is probably more important than that of PA, because the higher contamination levels of PA (60000 ppb PA in combine with 1000 ppb OTA) [46] have not been able to produce more significant toxic effects. This findings clearly suggest that the increase in OTA-toxicity is probably due to impaired detoxification of OTA via PA-inhibited carboxypeptidase activity in the intestinal tract.
Several years ago, Stoev and collaborators [22] induced macroscopic kidney damages in pigs fed on a mouldy diet containing low levels of OTA (180 ppb) in combination with PA for a 3 months feeding period, whereas microscopic lesions in kidneys in the same experiment were observed in pigs fed on a diet containing only 90 ppb OTA in combination with PA. The observed changes were similar to those caused in pigs at the end of the 4-month period of exposure to higher levels (markedly above 200 ppb) of pure OTA in feed [24]. It is therefore important to investigate the effect of combined administration of OTA and other mycotoxins, produced by the same ochratoxinogenic fungi as occur in the field.
In combined administration of OTA and PA much lower concentrations of OTA (about 100 ppb) are enough for a significant toxic effect in chickens, expressed by degenerative changes in internal organs, by a depletion of cells in lymphoid tissue and decrease of lymphoid organ's weight as well by known changes in various biochemical parameters [28]. Moreover, degenerative and weight changes in kidneys, liver and lymphoid organs as well as immunosuppression were seen recently in chickens at only 0.2 or 0.3 ppm OTA in combination with PA [26, 27, 29, 30, 47], whereas such changes had been seen in chickens exposed to significantly higher levels of pure OTA (about 4 ppm) in feed [48–51]. According to other authors [46] a similar but less pronounced synergistic toxic effect has been observed at higher contamination levels of OTA (1000 ppb). The mentioned above low experimental levels of OTA correspond very well to the feed levels of OTA found in spontaneous cases of mycotoxic avian nephropathy (MAN) in Bulgaria (0.09–0.31 ppm) [23], which suggests a possible multicausal nature of MAN in Bulgaria.
Furthermore, to ascribe the source of OTA to the A. ochraceus group of fungi is unwise because ochratoxinogenic forms are isolated so rarely [18, 52]. In such a way the source of OTA in Bulgaria is generally obscure. Therefore, it is already of great importance to carry out more mycological investigations in order to isolate the ochratoxinogenic fungi in Bulgarian feeds. In addition, the overall concentration of OTA in feed samples (from 0.38 to 0.552 ppm) were substantially lower than the 1 to 2 ppm required to reproduce MPN/MAN of severity similar to that observed in spontaneous cases [18, 21, 23, 53]. It seems, therefore, that the MPN/MAN in Bulgaria may have a multitoxin or multifactor etiology, because it cannot be explained by the concentration of OTA alone. The multimycotoxin etiology of MPN/MAN in Bulgaria is recently confirmed as high contamination levels of PA (838,6 ± 223,9 μg/kg - 88% positives) and FB1 (5564,1 ± 584 μg/kg - 96% positives) in Bulgarian feed samples from farms with mycotoxic porcine nephropathy were found, whereas the levels of OTA (188,8 ± 27,3 μg/kg - 100% positives) in the same feeds were consecutively lower (unpublished personal data). A similar multimycotoxin etiology was also found for South African MPN as the same mixture of mycotoxins (67,8 ± 39,2 μg/kg OTA - 83,3% positives; 149,2 ± 64,1 μg/kg PA - 41,7% positives, 5046,2 ± 1301 μg/kg FB1 - 80% positives) was found in South African feed samples from pig farms with nephropathy problems (unpublished personal data).
Some mycological investigations of OTA-contaminated feeds in Bulgaria revealed the common presence of P. aurantiogriseum complex [18], which is a potent producer of PA [54]. PA is produced by numerous species of Penicillium (especially P. aurantiogriseum) and Aspergillus at temperatures ranging from 4°C to 30°C, with the maximum rate of production occurring at about 25°C [55]. The production of PA decreased sharply at low oxygen concentrations, while fungal growth was not noticeably influenced [56]. PA progressively form complexes with compounds containing -SH radicals [57, 58]. The rate of toxin coupling with -SH radicals increases with pH as well as in high temperature [58]. The resulting complexes are much less toxic than the uncoupled molecules, resulting in actual detoxification. As a result, this toxin usually accumulates at relatively low temperatures during the winter at which detoxification is more reduced than toxin production [55]. That could explain why Bulgarian nephropathy in pigs/chickens was usually observed during the spring and summer.
It has recently been found that administration of P. polonicum extract (not containing OTA) to rats can provoke profound and persistent histopathological damages such as apoptic and karyomegalic or mitotic changes in the nuclei of tubular epithelium in kidneys of rats, including DNA-adducts formation [59]. The same P. polonicum strain, which is a common food/feed spoilage mould in warm temperate latitudes, has been found as a frequent contaminant in Bulgarian feeds, suspected of causing spontaneous porcine nephropathy [18, 52]. Moreover, the P. polonicum extract given to rats probably contained PA. It is known that the P. aurantiogriseum group (including P. polonicum strain) is a potent producer of PA, which can also provoke DNA breaks in mammalian cell lines as has been reported [36]. In such a way it could appear that the source of PA in Bulgarian feeds can be different from the source of OTA. Also, the same changes (apoptosis and karyomegaly in tubular epithelium), provoked by P. polonicum extract, could be induced by another unknown mycotoxin; this would be of interest, because the same mycotoxin could be partly responsible for the nephrotoxic damages described in Bulgarian nephropathy. Moreover, the quiet apoptosis, induced by P. polonicum nephrotoxin, could be a possible model for the cryptic and clinically-silent onset of renal atrophy in the idiopathic Balkan endemic nephropathy in humans [60]. On the other hand, it has been reported that OTA has a high potential to induce apoptosis and DNA-adducts in vitro [61, 62] and in rodent in vivo [63]. Also, Rahimtula et al. [64] showed that lipid peroxidation in kidney microsomes was enhanced by OTA and produced malondialdehyde. The same compound could give DNA adducts [61, 62]. In such a way, the extent to which OTA may interact with other components of commercial chicken/pig rations or human food compounded from agricultural produce may also influence the significance of the relatively lower doses of OTA that commercial chickens [23, 27], pigs [18, 21] or humans [1] may encounter in some feed/food.
2. Methods of Prophylaxis and Various Preventive Measures in Regard to the Main Etiological Agent of MN - OTA
Various different strategies can be employed to reduce human/animal exposure to OTA or to reduce its toxic effects when it is fed to animals. The same include the use of management practices to prevent the production of OTA by the storage fungi, the feeding of contaminated grain to animal species that are less susceptible to the toxic effects of OTA, such as ruminants, modification of the diet to promote enhanced hydrolysis of OTA in the gastrointestinal tract or reduced absorption, the use of a feeding regimen that can counteract the metabolic effects of OTA, the use of various procedures that can destroy OTA, such as physical and chemical methods for decontamination of commodities as well as the use of various feed additives, which can protect against the toxic effects of OTA etc. [65].
Gastrointestinal microorganisms have a large effect on the disposition of OTA because they promote the hydrolysis of OTA to its nontoxic form OTα. This is particularly important in ruminants [66] and therefore they are less susceptible to OTA-toxicity. In addition, the type of diet also affects the disposition of OTA in the rumen [67, 68]. Ruminal fluid obtained from hay-fed animals (pH 7.0) was able to hydrolyze OTA to ochratoxin α (OTα) in vitro five times faster than ruminal fluid obtained from grain-fed animals (pH 5.5). Disappearance of OTA from the rumen and the corresponding formation of OTα was also much faster for hay-fed than for grain-fed sheep. The bioavailability of OTA in sheep fed grain was 4.3 times greater than that in sheep fed hay. Thus, the rumen of the sheep has an important role in the detoxification of OTA and the type of diet affects the rate and extent of this process. As a result, the bioavailability of OTA, and probably the toxicity to ruminants, is decreased. In addition, the intestinal microflora of nonruminant animals, also affect the bioavailability of OTA, because the microorganisms in the intestines and particularly in the caecum and large intestine are also responsible for the hydrolysis of OTA to its nontoxic form OTα [66].
Another way to prevent the production of OTA is prevention of the growth of the storage fungi. The most important factors in safeguarding stored feedstuffs/foodstuffs from OTA prodution by moulds are their moisture content (water activity) and temperature [56]. Large quantities of OTA can be produced at intermediate and high ambient temperatures and high moisture contents by species belonging to the Penicillium and Aspergillus, whereas at lower ambient temperature the toxin can be produced by Penicillium spp. only. In addition, the moulds in the corn when stored at 21% moisture produced a large quantity of OTA (about 3.6 ppm), whereas none of this toxin was found in corn stored at 16% moisture [69]. A similar situation is seen in the barley stored at 16% and 20% moisture [70]. These and some other studies indicate that fungi in cereal grains stored at moisture contents lower than 15% generally do not produce OTA, which suggests that the moisture content in the cereal grains have to be decreased under 15% via various drying procedures before storage [71]. Storage at higher moisture levels requires that grain be maintained under anaerobic conditions, which prevent growth of fungi. Otherwise, a combination of mould inhibitors and sterilization techniques, possibly coupled with the inoculation of nontoxigenic competitive microflora, have to be applied to prevent the growth of OTA-producing fungi.
Chelack and collaborators [72, 73] have used another way to control the growth of OTA-producing fungi. They demonstrated that gamma or electron beam irradiation is a highly effective means of destroying spores from some OTA-producing fungi as A. alutaceus [72, 73]. Various other types of radiation (X-rays, ultraviolet light, microwaves) in addition to γ-irradiation are also used as a means for detoxification of mycotoxins as well as to control the growth of fungi [74, 75].
Leitao and collaborators [76] demonstrated that phosphine (PH3) was effective at inhibiting both fungal growth and sterigmatocystin production by A. versicolor, and this could be considered as another specific way to control the growth of OTA-producing fungi.
For foods with pH values from 5 to 6, such as the cereals or sorghum, the antimicrobial agent (food additive) that is able to prevent the growth and OTA-production by Aspergillus and Penicillium species, is methyl paraben or potassium sorbate. Small concentrations of these compounds are able completely to inhibite the growth by both genera of fungi and their OTA-production. At pH 4.5, as occurs in silage, fungal growth and OTA-production was completely inhibited by 0.02% potassium sorbate, 0.7% methyl paraben, and 0.2% sodium propionate [77].
The use of various procedures that can destroy OTA, such as physical and chemical methods for decontamination of commodities can be considered as another important way for safely utilizing of feedstuffs. Madsen and collaborators [78] reported that treatment of OTA-contaminated barley with 5% NH3 for 96 h at 70°C or warming of grain to 105°C in the presence of 0.5% NaOH as well as autoclaving at 132°C for 0.5 h can destroy the main part of OTA in the barley, but such treatments are not practical and are not able effectively reduce the concentration of OTA in grain.
Chelkowski and collaborators [79] reported that treatment of OTA-contaminated grain with ammonia reduced OTA concentrations to undetectable levels. Moreover, weight gains of chickens fed the OTA-contaminated grain were markedly decreased, whereas there was no reduction in weight gains when the chickens were fed the OTA-contaminated grain that had been ammoniated. They concluded that ammoniation of grain not only detoxifies several mycotoxins, including OTA, but also inhibits mould growth [79]. Feeding studies have shown no toxic effects related to the ammoniation process, but there are some changes in the nutritional quality of the feed, such as a decrease in lysine and sulfur containing amino acids [80]. In addition, adequate aeration after ammoniation is necessary for acceptance of the feed by animals. However, other than ammoniation, many of the techniques proposed to remove mycotoxins are currently perceived as impractical, ineffective, and/or potentially unsafe for largescale use [81].
Rotter and collaborators [82] demonstrated that inoculation of barley with a Lactobacillus species followed by ensiling (decreasing of pH) reduced the concentration of OTA by approximately 50% [82].
Also, Scott [83] reported that OTA is completely destroyed during malting of barley [83].
According to Laciakova and collaborators [84] formic acid of 0.25% concentration degraded OTA after 3 h exposure, propionic and sorbic acids in 1% concentration after 24 h exposure and benzoic acid in 0.5% concentration after 24 h exposure [84].
Several different approaches have been used to reduce OTA absorption, including the use of hydrated sodium calcium aluminosilicate (HSCAS), bentonite, charcoal, and cholestyramine. The addition of HSCAS (1%) and bentonite (1% and 10%) to a diet containing OTA had no effect on OTA concentration in swine blood, serum, tissues, and bile. The addition of 1% activated charcoal to the diet, in contrast, caused a slight decrease in the concentration of OTA in swine blood, whereas 10% charcoal decreased the concentration of OTA in blood, liver, kidney, spleen, and heart by 50% to 80% [85]. However, the supplementation of OTA-contaminated diet with activated charcoal was considered as an impractical method of reducing OTA toxicity in chicks or pigs that were continuously consuming OTA [86].
Cholestyramine, in contrast to the nonspecific absorbent discussed above, seems to be an effective absorbent of OTA in the gastrointestinal tract of nonruminant animals. Cholestyramine is a commercial anion exchange resin that has been shown to reduce blood OTA concentrations by 50%, when it was included in 0.5% in a rat diet containing 1 ppm OTA. In addition, the faecal excretion of OTA was significantly increased in rats given a single oral dose of OTA, which were fed on diet containing cholestyramine [85].
Another way to reduce OTA-toxicity supposed a good understanding of the mechanisms of OTA-toxicity: inhibition of phenylalanine metabolizing enzymes (having in mind a phenylalanine moiety of OTA), enhancing of lipid perooxidation, and inhibition of mitochondrial ATP production. This approach includes an addition of phenylalanine and protein to the diet. It is well known that the phenylalanine moiety of OTA competitively inhibits at least two enzymes: phenylalanyl-tRNA synthetase and phenylalanine hydroxylase, resulting in reduced protein synthesis and altered rates of tyrosine production from phenylalanine. The addition of phenylalanine to cell cultures containing OTA in vitro [87] or coadministration of phenylalanine with OTA [88, 89] reduced OTA-induced inhibition of protein synthesis. In vivo experiments show that injection of phenylalanine also prevented OTA-induced immunosuppression in mice [90, 91, 92] and partially reduced teratogenesis in rats [93]. The phenylalanine has to be used in 5:1 molar ratio towards OTA [94], because it had been found that phenylalanine given in higher doses as 10:1 molar ratio, provides only a slight protection against OTA, because of increasing the absorption of OTA from the stomach and intestine. Moreover, the higher supplementation of pure phenylalanine in chick diets contaminated with OTA also tended to create an amino acid imbalance, which reduced b. w. gain and feed conversion efficiencies [95, 96]. These studies suggest that little or no benefit is obtained by supplementing OTA-contaminated diets with phenylalanine.
The concentration of protein in the diet of growing chicks was also shown to ameliorate the toxicity of OTA [97, 98]. The consumption of high-protein diets (26%) by growing chicks over a three-week period compared to a diet containing a lower concentration of protein (14%) decreased the toxicity of 5 ppm OTA in diet as indicated by rate of growth, mortality and relative organ weights of chicks [97, 98]. This treatment, however, may not be practical because a large change in the protein concentration in diet may be costly and produces relatively small benefits
The ascorbic acid supplementation (300 mg/kg) of laying hen diet that contained 3 ppm OTA has been reported to have a good protective effect against OTA-action and can partially ameliorate OTA-toxicity (including the negative effect of OTA on the eggs production and on the weight of the eggs) [99, 100]. The mechanism of the protective effect is not clear but it is supposed to affect the production of lipid peroxides by OTA and to improve the membrane integrity of cell organelles, which is always affected by OTA-action [22].
The use of various feed additives can also protect against the toxic effects of OTA and can reduce farm losses from a decrease of weight gain in stock chicks avoiding the rejecttion or condemnation of OTA-contaminated feed [27, 30, 94]. Sesame seed (rich in proteins - about 20% and relatively rich in L-β phenylalanine - about 4.3%), given in 8% to the feed, offered a cheaper agronomic additive to supply animals with phenylalanine and thereby to protect against toxicity of OTA [94]. It might also avoid the increase of OTA-absorption from the stomach and intestine provoked by the pure phenylalanine in feeds [101]. Simultaneously, the increase of urinary excretion of OTA in presence of phenylalanine [101] could be preserved in a later stage. Moreover, the sesame seed (8% to the feed) increases the energy metabolism in animals [94], which usually is disturbed by OTA [102].
Roxazyme-G (Hofmann La Roche, Grenzach-Wyhlen, Austria) is a polyenzyme complement produced by fungi “Trichoderma”, which contains: cellulase, xylanase, endo-beta 1,3-1,4 glucanase, pectinase and amylase. Roxazyme-G, given in concentration 0.2g/kg feed, was reported to improve digestive dissimilation of polysaccharides to easily assimilated substances, that could improve utilization of feed by increasing digestible energy production by 8–13%, thereby counteracting OTA impairment of energy production in chicks [94].
Artichoke-extract is a complex containing compounds which might protect against OTA intoxication [26, 27, 30, 94]. The 5% total water-extract of artichoke (Cynara scolymus L), prepared as a steam infusion from dried leaves of artichoke and given to chicks in concentration 5 ml/kg.b.w. via the feed or water, usually contains various biologically active compounds: cynarine, flavonoids, cynaropicrin, dehydrocynaropicrin, grosheimin as well as a high content of calcium and ascorbic acid [103]. It has been recommended as a diuretic agent in cardiac and renal insufficiency, and might accelerate the urinary route of excretion of OTA [26, 27, 30]. Moreover, the cynarine content in such extract stimulates metabolism of cholesterol, decreases serum urea and lipids, improves diuresis and increases biliary secretion, which probably augment the hepatobiliary route of excretion of OTA (OTA is mainly eliminated via bile and urine) and thereby has been found to protect against OTA nephrotoxicity and hepatotoxicity [26, 27, 29, 30, 94] as well as against the immunosuppressive effect of OTA [27, 94, 104] or against OTA-induced anaemia [27]. Cynarine and flavonoids as well as some medical preparations prepared from artichoke-extract (chophytol and chophytamine) have a potent hepatoprotective effect against hepatotoxic damage [105–107]. Also, antipermeability and vasoconstrictive effects of water extract of artichoke could decrease oedema in various internal organs provoked by OTA [18, 27, 29, 30, 94]. The high content of calcium and vitamin C in the steam extract probably has also a protective effect against OTA-toxicity via improving membrane integrity of cell organelles and skeleton-strength of the chicks or via some other ways [27, 29, 94], since some authors reported that ascorbic acid supplementation (300 mg/kg) of laying hen diet that contained OTA (3.0 mg/kg) partially reduced OTA-toxicity [100].
Rosallsat, which is another plant extract (a polyextract of bulbus Alii Sativi and seminum Rosai canina), was seen also to have a protective effect against OTA-toxicity, when it was given per os in 0.6 ml per kg body mass daily as a supplement to the feed [26]. The Rosallsat is a new natural galenic phytosubstance of balanced polyvitamine, phytoncide and steroid-saponine composition - described completely in patent No 98915 BG / 1994, which contains biological active compound allicin (12.08 mg/g) as well as large quantities of vitamins A (0.0332 mg/g), F (0.8112 mg/g), E (0.1548 mg/g) and furostanic sapogenine of C57H96O30 [26, 29, 30]. The allicin in that study was formed in advance and included in a natural oil extract in commercial lots stabilized with natural antioxidants [26]. This is an advantage when compared with fresh or dried garlic cloves since thiosulphinate is hardly formed in the pH conditions of the stomach and intestine after eating fresh or dried garlic. The precise mechanism of protection of Rosallsat against OTA-toxicity has not been investigated in depth, but it is supposed that the biological active compound allicin and the high quantities of vitamins A, F and E in this plant extract probably are of great importance [26, 29, 30]. The Rosallsat is found to inhibit lipid peroxidation (unpublished personal data) and in such a way prevents one of the mechanism of OTA toxicity [64].
It was found that the intensity of macroscopical and histopathological changes, the deviations in relative organs’ weight and body weight (Table 1), the decrease of antibody titer (Table 2), as well as the intensity of changes in various haematological and biochemical parameters (Table 3) were slighter in chicks treated with some antidotes (5% artichoke-extract, 0.02% Roxazyme-G, 8% sesame seed, 0.0025% phenylalanine) in addition to 5 ppm OTA than in chicks only treated with 5 ppm OTA. In chicks of groups treated with sesame seed or artichoke-extract the described changes, especially the antibody titer against Newcastle disease, were more similar to those ones observed in chicks exposed to an approximately 5 times lower contamination levels of OTA (1 ppm) (Table 2). Moreover, it appeared that all of the mentioned above antidotes, used as supplements to the feeds, especially sesame seed and artichoke-extract, had a potent protective effect against OTA-induced toxicity and could be used as a practical approach for safely utilizing of OTA-contaminated feed [94]. In such a way the rejection or condemnation of such feed will be avoided as well as there will be no need to eliminate OTA from the feed, if its contamination levels are similar to these encountered in the practice (normally upto 1–2 ppm OTA). However, in the pigs which are more sensitive to OTA, the levels of 1 ppm OTA can be more dangerous [2, 22, 108] and this requires additional experimentation to clarify the antidote effect of the same additives in these animals.
Table 1.
Mean values of body weight (b.w.) after consecutive two-week periods in groups of chicks treated with OTA and various antidotes [94].
| OTA (ppm) | antidotes | 1 day b.w. (g) | 14 day b.w. (g) | 28 day b.w. (g) | 42 day b.w. (g) |
|---|---|---|---|---|---|
| 1 ppm | - | 80.0 ±1.9* | 253.0c ±9.5 | 428.53c ±15.2 | 727.02c ±22.1 |
| 5 ppm | - | 81.0 ±1.5 | 174.83 ±5.9 | 283.13 ±9.9 | 423.53 ±15.6 |
| 5 ppm | L-β phenylalanine | 81.5 ±6.7 | 233.51c ±7.6 | 374.03c ±11.2 | 623.53c ±14.8 |
| 5 ppm | Roxazyme G | 80.5 ±2.0 | 237.01c ±6.2 | 380.53c ±14.7 | 691.03c ±10.9 |
| 5 ppm | Sesame seed | 81.5 ±2.4 | 224.52c ±8.3 | 368.53c ±21.8 | 704.03c ±16.5 |
| 5 ppm | Artichoke-extract | 79.5 ±3.3 | 219.53c ±6.3 | 364.53c ±15.0 | 646.53c ±22.0 |
| Control
|
-
|
82.0 ±2.5
|
255.0c ±5.6
|
509.0c ±14.9
|
836.0c ±21.1
|
| Chicks number | - | 20 | 20 | 20 | 20 |
- SEM (standard error of the mean)
– Significant difference (p < 0.05)
– Very significant difference (p < 0.01)
– Extremely significant difference compared to the control group (p < 0.001)
- Extremely significant difference compared to the 5 ppm group without antidote (p < 0.001)
Table 2.
Mean values of haemagglutinin inhibiting antibody titers (HIAT) in groups of chicks treated with OTA and various antidotes - on day 21 after vaccination against Newcastle disease (28 days from the beginning of the experiment) [94].
| OTA (ppm) | Antidotes | HIAT (HU/ml) | Number of chicks |
|---|---|---|---|
| 1 ppm | - | 340.0c ±70.5* | 20 |
| 5 ppm | - | 7.13 ±1.0 | 20 |
| 5 ppm | L-β phenylalanine | 31.43c ±4.0 | 20 |
| 5 ppm | Roxazyme G | 16.43 ±4,8 | 20 |
| 5 ppm | Sesame seed | 280.0c ±40.0 | 20 |
| 5 ppm
|
Artichoke-extract
|
150.03c ±19.1
|
20
|
| Control | - | 353.3c ±57.9 | 20 |
- SEM (standard error of the mean)
– Extremely significant difference compared to the control group (p < 0.001)
- Extremely significant difference compared to the 5 ppm group without antidote (p < 0.001)
Table 3.
Mean serum values of total protein, urea and creatinine in groups of chicks treated with OTA and various antidotes (70th days) [94].
| OTA (ppm) | Antidotes | Total protein (g/l) | Urea (mmol/l) | Creatinine (μmol/l) | Number of chicks |
|---|---|---|---|---|---|
| 1 ppm | - | 31.271c ±1.60 | 2.463b ±0.061 | 124.0c ±3.5 | 10 |
| 5 ppm | - | 23.263 ±0.69 | 2.863 ±0.088 | 514.43 ±65.0 | 10 |
| 5 ppm | L-β phenylalanine | 25.963a ±1.00 | 1.88c ±0.149 | 309.53b ±16.8 | 10 |
| 5 ppm | Roxazyme G | 27.663c ±0.55 | 2.483c ±0.032 | 240.63c ±16.7 | 10 |
| 5 ppm | Sesame seed | 26.873c ±0.54 | 2.513b ±0.047 | 191.93c ±15.2 | 10 |
| 5 ppm
|
Artichoke-extract
|
26.493b ±0.62
|
2.523b ±0.022
|
275.13b ±20.4
|
10
|
| Control | - | 36.54c ±1.29 | 1.81c ±0.070 | 119.8c ±2.4 | 10 |
- SEM (standard error of the mean)
– Significant difference compared to the control group (p < 0.05)
– Very significant difference compared to the control group (p < 0.01)
– Extremely significant difference compared to the control group (p < 0.001)
– Significant difference compared to the 5 ppm group without antidote (p < 0.05)
– Very significant difference compared to the 5 ppm group without antidote (p < 0.01)
- Extremely significant difference compared to the 5 ppm group without antidote (p < 0.001)
A protective effect against OTA-induced decrease of total serum protein was found in chicks of all antidote-treated groups mentioned above, but a protection against the increase of serum glucose was only seen in groups treated with Roxazyme-G and artichoke-extract. Also, a protective effect against OTA-indiced increase of serum creatinine and urea was seen in all antidote-treated groups especially on day 70 [94]. In addition, the protective effect against the increase of serum values of uric acid and creatinine was strongest in the group treated with sesame seed (Table 3).
However, it appeared that the protective effect of phenylalanine was slighter than expected one in contrast to some of the other studied antidotes [94]. The main reason for that could be the circumstance that phenylalanine was found to increase the absorption of OTA from the stomach and intestine as well as the gastrointestinal transit of OTA. This resulted in eightfold and fourfold higher levels of OTA in serum and liver of mice, respectively, during the first 12 h [101], which could also increase its elimination. Therefore, phenylalanine has to be given after changing the feed source and stopping OTA-exposure of animals [109].
The strong protection of Roxazyme-G against OTA-induced increase of serum glucose could be due to the improved energy metabolism, whereas the protection of artichoke-extract against that increase could be due to an improvement of diuresis [27]. The protective effect of sesame seed and phenylalanine against OTA-provoked immunosuppression in humoral immune response and changes in differential WBC count [94] might be due to an improvement of protein synthesis, which usually is disturbed by OTA and subsequently, an improvement of OTA-induced delay of the division of the cells of the immune system.
It is known that OTA induces an increase of the weight of the organs taking part in its detoxification or elimination (kidneys and liver) as well as a decrease of the lymphoid organs’ weight and body weight, whereas some of the studied feed additives (5% artichoke-extract, 0.02% Roxazyme-G, 8% sesame seed, 0.0025% phenylalanine) significantly protects against these weight changes [94] as the protective effect of sesame seed and Roxazyme-G against these weight changes was stronger than the protective effect of artichoke-extract and phenylalanine.
In other experiments [26, 30, 110] it was also confirmed that Artichoke-extract has a significant protective effect against OTA-toxicity. In the same studies, another plant extract Rosallsat was also seen to protect against OTA-toxicity, expressed by statistically significant protection against the OTA-induced changes in serum levels of total protein, uric acid and cholesterol (Table 4). The serum levels of uric acid, which were most significantly influenced of the quantity of OTA in fed forages, usually give an exact assessment of the impairment of kidney function in chicks [49]. The absence of a confident increase in that parameters in Artichoke-extract and Rosallsat treated groups suggests a slight impairment in kidney function in these groups and confirms the protective effects of these feed additives against OTA-toxicity. On the other hand, the protective effect of Artichoke-extract and Rosallsat on the liver function may contribute to the higher cholesterol levels in the groups of chicks treated with the same antidotes [26]. In addition, the toxicological investigations in the same experiments revealed that the contamination levels of OTA in kidneys are lower in chicks treated with Artichoke or Rosallsat in addition to OTA than in chicks only treated with the same feed level of OTA [30].
Table 4.
Mean serum values of cholesterol and uric acid in groups of chicks treated with OTA (130, 305 and 790 ppb) and PA (1000–2000 ppb), and various antidotes (70th day) [26].
| OTA (ppb) | Antidotes | Cholesterol (mmol/l) | Uric acid (μmol/l) | Number of chicks |
|---|---|---|---|---|
| 130 ppb | - | 3.901b ±0.20 | 211.7c ±16.4 | 5 |
| 305 ppb | - | 3.752a ±0.17 | 458.03 ±23.3 | 5 |
| 790 ppb | - | 3.113 ±0.12 | 534.13 ±24.8 | 5 |
| 790 ppb | Artichoke-extract | 3.801a ±0.22 | 222.4c ±29.2 | 5 |
| 790 ppb
|
ROSALLSAT® |
4.63a ±0.51
|
182.2c ±28.8
|
5
|
| Control | - | 5.09c ±0.34 | 162.6c ±14.6 | 10 |
- SEM (standard error of the mean)
– Significant difference compared to the control group (p < 0.05)
– Very significant difference compared to the control group (p < 0.01)
– Extremely significant difference compared to the control group (p < 0.001)
– Significant difference compared to the 790 ppb group without antidote (p < 0.05)
– Very significant difference compared to the 790 ppb group without antidote (p < 0.01)
- Extremely significant difference compared to the 790 ppb group without antidote (p < 0.001)
3. A Possible Hygiene Control in Regard to OTA
By 1990, at least nine countries have regulations for OTA levels or had proposed and enforced official limits for OTA. Regulatory control of nephrotoxic mycotoxins in animal feeds in European countries has been summarized by van Egmond [111] and later by Boutrif and Canet [112]. On the other hand, European Union decided on an official limit for OTA in cereals designed for direct consuming of about 5 μg ÎÒÀ/kg [113]. The official limits for OTA in cereals in some countries can be seen in various FAO reports and commonly ranged between 2 μg/kg (Switzerland) and 20 μg/kg (Czech Republic), and rarely reached up to 50 μg/kg (Uruguay) [112].
In countries with wide spreading of MPN and MAN residues of OTA were often found in animal tissues samples during the meat inspection [18, 23, 114, 115, 116].
The contamination levels of OTA in liver, muscles and fat tissues of pigs can be easily found using the following experimental calculations [117]:
| liver | −0,7 ± 0,7 X |
| muscles | − 0,6 ± 0,4 X |
| fat tissue | −0,8 ± 0,3 X, |
| where “X” is the concentration of OTA in kidneys in μg/kg and X≥2 | |
By 1990, at least nine countries have regulations for OTA levels or had proposed and enforced official limits for OTA. Regulatory control of nephrotoxic mycotoxins in animal feeds in European countries has been summarized by van Egmond [111].
Because of the absence of correlation between macroscopic changes and concentration of OTA in pig kidneys it is very difficult to prevent the exposure of humans to the toxin from pork by toxicological investigations of “pale or mottled kidneys” as it is occurred in Denmark. The practice in Denmark of monitoring kidney tissues in only those pigs that have detectable kidney lesions could not provide a good basis for condemnation of carcases, because the intake of OTA may have not been of sufficient duration to produce detectable lesions. Therefore, the regulations in Denmark, according to which all enlarged and mottled kidneys are investigated for residues of OTA at slaughter time and all carcasses, whose kidneys contained OTA levels above 10 μg/kg [112] are condemned are not very safe and clever, because macrscopic changes in kidneys can be found only after 1–3 months OTA exposure via the feeds [22, 118]. Some investigations made by various authors revealed that at least 1–2 months are needed to develop characteristic macroscopical renal lesions in pigs exposed to natural or experimental OTA contaminated barley [24, 119]. Unfortunately, characteristic for MPN macroscopic changes in kidneys can not be found after a shorter period of OTA exposure via the feeds even with such high levels as 1000 ppb OTA [120] and animal products containing dangerous OTA contamination levels could easily pass meat inspection at slaughterhouses [22]. On the other hand the renal lesions in kidneys (mottled appearance) induced at an early age do not disappear when the pigs are fed on OTA-free diet, which also indicates the lack of efficiency of such control system and the implementation of some unnecessary toxicological investigations, which could be avoided [21, 22]. In spite of the toxicological investigations of such kidneys OTA-contaminated pork may enter the human food chain and thus represents a potential public health hazard [21]. Since the toxin is relatively heat stable [121], a limited occurrence of OTA might anticipated in prepared food from such animals.
Because of the assumption that mycotoxins and especially OTA are involved in etiology of Balkan endemic nephropathy [1, 122–124] the exposure of humans to this very hazardous toxin from pork or chicken meat (by the way “feed – pork/chicken - food”) need to be prevented. A much better procedure for preventing the exposure of humans to the toxin from meat would be a toxicological analysis of a few blood samples of pigs/chicken from farms, suspected of having MN, several weeks (in pigs) or several days (in chicken) before slaughter and a change in the feed source for a week (pigs) or for 2–3 days (chicken), if it is necessary. Also, the period of feed deprivation of pigs/chicken before slaughter could be prolonged [21, 23]. Because of the short half-life of OTA in pigs (72 – 120 hours) and especially in chickens (4 hours) [125] its concentration in blood and various tissues quickly decreases after changing the feed source or after prolonging the period of feed deprivation before slaughtering. Thus, the loss of condemnation of pig/chicken production would be prevented and a better procedure (than toxicological invesigations of “enlarged mottled kidneys” accepted in Denmark) would be realized for preventing the exposure of humans to OTA from meat [21–23, 114]. The preventive measures in already slaughtered chicks could include condemnation and removing of the kidneys and liver, where OTA is accumulated [23].
However, having in mind that a combined administration of OTA and other mycotoxins such a PA, often occurrs in the practice and synergistic effects between OTA and the same mycotoxins are reported, the lower contamination levels of OTA in food/feed would be also very dangerous for human and animal health [22, 30].
4. A Possible Involvement of Mycotoxins in Etiology of Balkan Endemic Nephropahy and Evaluation of Human Exposure to OTA
Balkan Endemic Nephropathy (BEN) in humans is another renal disorder having a similar pathological characteristics as MPN. It is most commonly observed in farmers at ages between 30 and 50 years, has oligosymptomatic clinical picture, silent onset and poor prognosis, progresses very slowly and manifests itself when the function of majority of nephrons have become impaired [126]. At this time there is agreement that the disease represents an unusual type of chronic interstitial nephropathy of unknown etiology, although there is an assumption that mycotoxins are involved [123]. The disease has a strict endemic character and is encountered in the rural populations in Bulgaria, Romania and ex-Yugoslavia. So far, at least 20,000 persons have been suffered from BEN. The total number of people at risk has been estimated to approximately 100,000 [126].
The high humidity and the poor food storage conditions in endemic villages, the low living and hygienic standard as well as the consumption of home prepared food from own crops - bread, bean and others [123, 127] correspond to the fact that the moulds producing mycotoxins contaminate mostly home produced grain feeds stored in bad conditions and high humidity, while the situation in the towns is markedly different - foodstuff supply is centralized and quality controlled (that explain the family character of BEN only in rural inhabitants). Also, the focality and zonality (characteristic for BEN) are typical for mycotoxicosis (i. e. endemic dissemination) and are determined mainly by the ecological conditions of the regions which are optimal for the development of the corresponding fungi and by the altitude [128]. The seasonal and yearly variations in incidence and prevalence of the disease and OTA-content in the human food as well as the positive correlation between the excess rainfall and the number of patients suffering from BEN, who died during the following two years [129], probably because of additional intake of moulded food (provoking new degenerative damages in kidneys and impairing the disease state), give additional support to the mycotoxin hypothesis of BEN [1]. However, the etiology of the disease is still unknown in spite of various etiological investigations including the search for heavy metals, trace elements, infectious agents and genetic factors as well as various bacteriological, toxicological, and hydrological investigations of water [123].
Nephrotoxic mycotoxin OTA has been suggested as one of possible disease determinants of BEN based on a comparison of morphological and functional kidney impairment between OTA-induced MPN and BEN [122]. In endemic areas, OTA has been found in human blood as well as in human food [123, 126, 130, 131, 132] and in animal feed in higher concentrations than in nonendemic areas [133] or the countries without BEN. It is important to mention that the feed levels of OTA in animal feeds from endemic for MPN regions (38–552 ppb) are approximately the same as in the foods from endemic for BEN regions (10–890 ppb), which additionally suggest a possible common etiology [1]. The mean concentration of OTA in human blood in Bulgarian-endemic areas ranges respectively from 20 μg/l for 1986 to 27.2 μg/l for 1989 [130, 132]. These high concentrations suggest for a possible role of OTA in a disease causation of BEN. However, OTA was detected in a concentration range of 5–100 μg/l only in 0.4 to 2.5% of a total 13,797 analysed blood samples from 14 endemic areas in Slavonski Brod (Croatia) during 9-year (1981–1989) period and in a concentration range 2–50 μg/l in 0.2 to 4.5% of a total 6,910 analysed serum samples from endemic village of Kaniza over the 10-year (1985–1994) period [126]. These concentrations of OTA in human blood are significantly lower towards those ones in Bulgaria [134], but correspond quite well to the prevalence of the disease (ranged between 1% and 4.5% during the last 15 years) and the incidennce of newly recorded BEN-suffering patients (ranged between 1.0 and 2.5 per thousand) in the same endemic areas.
The disease is also closely associated with a high frequency of carcinoma in the renal pelvis, ureter and urinary bladder [135, 136], which could be due to the carcenogenicity of nephrotoxic mycotoxin OTA [125, 137, 138], but the working group that evaluated the carcinogenicity of OTA to humans could not draw any conclusion [34]. Interestingly, some tumors in kidneys and bladders from patients living in areas associated with Balkan Endemic Nephropathy, have been found to contain DNA adducts similar to those obtained from the kidneys of mice exposed to OTA [139], which provides new evidence of the possible role of OTA in the development of tumors of the urinary tract. Moreover, 11 of 31 patients with urinary tract tumors in France exhibited a OTA-specific DNA adduct pattern similar to those found in Bulgarians suffering from BEN, OTA-treated rodent and Bulgarian pig suffering from nephropathy. OTA was present in the blood in the half of the same patients [140]. It was found that changes in debrisoquine 4-hydroxylation in vivo can occur in the rat in response to mouldy food [141] and patients suffering from BEN and/or upper urothelial tumors were more frequently extensive metabolizers of debrisoquine (DB) [142]. A recent study in Egypt revealed a positive correlation between OTA and the end stage renal disease (ESRD) or urothelial tumors in humans. Patients with ESRD as well as patients with nephrotic syndrom or urothelial tumors have significantly higher mean concentrations of OTA in blood (between 0.52 and 2.19 ng/ml) and urine (between 0.36 and 3.09 ng/ml) than patients from control groups (between 0 and 0.08 ng/ml in blood and between 0.01 and 0.26 ng/ml in urine) [143]. It is established that OTA decreases natural killer cell activity by the specific inhibition of endogenous interferon levels [144]. As natural killer cells are involved in the distribution of tumor cells, the ability of OTA to modulate the activity of these cells might contribute to its capacity to induce renal and hepatic carcinomas [2].
Recently, it has been found that OTA has a significantly longer plasma half-life in humans (33.55 days) than in animals [145]. A slow excretion of OTA suggests a tendency to an increase of toxin levels in the serum as well as assumes a very constant rate of OTA in human blood for a long period of time, even after repeated exposure to very low concentrations [146]. In such way the humans are more influenced by the dose and less influenced by the frequency, duration and quantity (if the quantity of the toxin ingested is comparatively low) of OTA exposure than other species, because in the humans OTA has a very constant and continuous effect on the proximal tubules even in little amounts, because of the very low unbound (free) fraction of OTA in blood. It can be supposed that only the free OTA in the macroorganism has a toxic effect on the tissues as well that continuous persistence of OTA in human blood and its slow excretion via kidneys may be influenced on the slow progression of BEN. Thus, consumption of food containing very low concentrations of OTA over a long period of time may become toxicologically significant [1].
A method is also presented for calculating the daily consumption of OTA through various food products on the basis of the measured content in plasma, even though the toxicokinetic constants are unknown. The renal filtration rate of OTA in humans, calculated from the glomerular filtration rate of inulin and the free fraction of OTA is 0.67 ml/kg body weight per day. The kidney filtration has been found to correspond to the total plasma clearance of OTA (almost 100%) in monkeys, but only to 10% in mice, rats and pigs. The equation: K0 = Clp.Cp/A shows the relationship between continuous intake (K0, ng/kg b. w. per day), the plasma clearance (Clp, ml/kg b. w. per day), the plasma concentration of OTA (Cp, ng/ml) and the bioavailability (A, proportion of toxin absorbed - about 50%). [147]. The same equation was used to calculate the daily intake of OTA from the concentration of the toxin in plasma of humans in endemic regions in Bulgaria. The daily intake of humans, calculated on the basis of the average plasma levels of OTA in endemic area in Bulgaria was found to be between 26.8 and 36.4 ng/kg b. w. as can be seen from Table 5 [1, 124]. It is important to mention, that this calculation should give an underestimate of the intake, since the value used for plasma clearance only involves glomerulal filtration. This underestimate could be as much as ten times, if the OTA clearance in man is more similar to those in rat, mouse and pig than that in monkey.
Table 5.
Calculation of the daily intake of OTA in patients suffering from BEN in endemic of BEN areas and in healthy patients from nonendemic areas in Bulgaria [1, 124].
| Areas | Year | Positive samples (%) | Cp (ng/ml) per day) | Reference | K0 (ng/kg b. w. |
|---|---|---|---|---|---|
| endemic of BEN | 1986 | 23.3 | 20.0 | [132] | 26.8 |
| 1989 | 29.2 | 27.2 | 36.4 | ||
| 1990 | 25.0 | 25.0 | 34.2 | ||
| nonendemic | 1986 | 7.7 | 10.0 | [132] | 13.4 |
| 1989 | 6.6 | 0 > 2 | 0 > 2.7 | ||
| 1990 | 6.6 | 8.0 | 10.7 |
Cp - average concentration of OTA in plasma
K0 - average daily intake
Because of the increase of scientific reports on OTA contamination in beverages and many kinds of food, the WHO/FAO Joint Expert Committee on Food Additives (JECFA) assessed the available information and proposed 112 ng/kg b.w. as a provisional tolerable weekly intake (PTWI) for OTA [148]. That corresponds to about 16 ng/kg body weight per day. Having in mind the strong carcinogenic effect of OTA its PTWI was later decreased to 100 ng/kg b.w., which corresponds to about 14 ng/kg body weight per day [149].
It can be concluded that the average daily intakes in the endemic areas in Bulgaria from 26.8 ng/kg b. w. for 1988 [1, 124], 36.4 for 1989 and 34.2 for 1990 respectively, exceeds strongly PTWI (100 ng/kg b. w. or 14 ng/kg b. w. per day), proposed by the JECFA [149]. JECFA bases its calculation of the tolerable intake mainly on the nephrotoxicity of OTA and does not address the question of the toxin's carcenogenic effect. Kuiper-Goodman and Scott, however, regard the carcinogenic effect as the most important effect and base their analysis on this. Tolerable daily intakes (TDIs), depending on the method used and calculated on the base of carcinogenic effect of OTA, range from 0.2 to 4,2 ng/kg b. w. It can be concluded that the maximum tolerable daily intake of OTA is 5 ng/kg b. w. [109]. It can be seen that the average daily intake of the endemic and in some extent nonendemic areas exceeds strongly the TDI calculated on the base of cancerogenic effect of OTA [1].
The performed calculations on TDIs show that OTA contamination of food and feed constitutes a public health problem of unknown dimensions. Therefore, it is quite relevant to investigate to what extent OTA is hazardous to humans. Because of the high thermal stability of this mycotoxin, ordinary cooking does not eliminate it. Thus, limited occurrence of OTA might be anticipated in prepared food.
Recently, however, more and more evidences appeared, that exposure to more than one mycotoxin may be an important factor in etiology of BEN [52] and MPN [18]. Moreover, the synergism between OTA and various other mycotoxins such a PA, citrinin or FB1 in field conditions may be responsible for an enhanced toxicity of OTA [22, 27, 30]. Due to the potent toxic and synergistic effects of OTA and PA or citrinin [22, 150] as well as between OTA and FB1 [151, 152], simultaneous exposure to those mycotoxins might be an important factor for development of chronic renal diseases in animals and humans, especially after long-term exposure. The same mycotoxins were recently found in high contamination levels (especially FB1 and PA) in most of the feeds originated from farms with mycotoxic porcine or avian nephropathy in Bulgaria (unpublished personal data). Because of that it is of great importance to investigate the combined effect between OTA and other mycotoxins produced by the same species as it is occurred in field conditions.
Acknowledgments
This research has been financially supported in part by Marie Curie Outgoing International Felowship within 6th European Community Framework Programme, Department of Science and Technology in South Africa, UK Royal Society Joint Project with Central and Eastern Europe, NATO grant and Foundation of Ministry of science and education of Bulgaria via 5 Research projects. The author also thanks Prof. Peter George Mantle (Department of Microbial Biochemistry, Imperial College of Science, Technology and Medicine, London, U.K.) for the supply of moulded shredded wheat substrate, containing high contamination levels of OTA, Prof. Piotr Golinski (Department of Chemistry, Academy of Agriculture, Poznan, Poland), S. W. Peterson (Microbial Genomics and Bioprocessing Research Unit, USDA, ARS, MWA, Peoria, U.S.A.) and Dr. Maja Peraica (Institute for Medical Research and Occupational Health, Croatia) for the supply of good producers of OTA and PA, Prof. Benedicte Hald (Department of Veterinary Microbiology, Royal Veterinary and Agricultural University, Frederiksberg, Denmark) and Prof. E. E. Creppy (University of Bordeaux II) for OTA analysis of feeds as well as all collaborators.
References
- 1.Stoev SD. The Role of Ochratoxin A as a Possible Cause of Balkan Endemic Nephropathy and its Risk Evaluation. Vet Human Toxicol. 1998;40(6):352–360. [PubMed] [Google Scholar]
- 2.Stoev SD, Goundasheva D, Mirtcheva T, Mantle P. Susceptibility to secondary bacterial infections in growing pigs as an early response in ochratoxicosis. Exp Toxicol Pathol. 2000;52:287–296. doi: 10.1016/S0940-2993(00)80049-4. [DOI] [PubMed] [Google Scholar]
- 3.Stoev SD, Koynarsky V, Mantle PG. Clinicomorphological studies in chicks fed ochratoxin A while simultaneously developing coccidiosis. Vet Res Commun. 2002;26:189–204. doi: 10.1023/a:1015201604241. [DOI] [PubMed] [Google Scholar]
- 4.Koynarski V, Stoev S, Grozeva N, Mirtcheva T, Daskalov H, Mitev J, Mantle P. Experimental coccidiosis provoked by Eimeria acervulina in chicks simultaneously fed on ochratoxin A contaminated diet. Res Vet Sci. 2007;82:225–231. doi: 10.1016/j.rvsc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Kumar A, Jindal N, Shukla CL, Pal Y, Ledoux DR, Rottinghaus GE. Effect of ochratoxin A on Escherichia coli-challenged broiler chicks. Avian Dis. 2003;47:415–424. doi: 10.1637/0005-2086(2003)047[0415:EOOAOE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Stoev SD, Grozeva N, Hald B. Ultrastructural and toxicological investigations in spontaneous cases of porcine nephropathy in Bulgaria. Vet Arhiv. 1998;68(2):39–49. [Google Scholar]
- 7.Dutton MF. Fumonisins, mycotoxins of increasing importance: their nature and their effects. Pharmacol Ther. 1996;70:137–161. doi: 10.1016/0163-7258(96)00006-x. [DOI] [PubMed] [Google Scholar]
- 8.van der Merwe KJ, Steyn PS, Fourie L, Scott DB, Theron JJ. Ochratoxin A, a toxic metabolite produced by Aspergillus ochraceus. Wilh Nature. 1965;205:1112–1113. doi: 10.1038/2051112a0. [DOI] [PubMed] [Google Scholar]
- 9.van der Merwe KJ, Steyn P, Fourie L. Mycotoxins. Part II. The constitution of ochratoxin A, B, and C, metabolites of Aspergillus ochraceus. Wilh J Chem Soc. 1965:7083–7088. doi: 10.1039/jr9650007083. [DOI] [PubMed] [Google Scholar]
- 10.Steyn PS. Wilh Thesis. University of South Africa; Pretoria: 1966. Chemical studies related to A. ochraceus. [Google Scholar]
- 11.Steyn PS, Holzapfel CW. The isolation of the methyl and ethyl esters of ochratoxin A and B, metabolites of Aspergillus ochraceus. Wilh J S Afr Chem Inst. 1967;20:186–189. [Google Scholar]
- 12.Hetherington AC, Raistrick R. Phil Trans Roy Soc London, Ser B. 1931;220:269–295. [Google Scholar]
- 13.Sage L, Krivobok S, Delbos E, Seigle-Murandi F, Creppy EE. Fungal flora and ochratoxin A production in grapes and musts from France. J Agric Food Chem. 2002;50:1306–1311. doi: 10.1021/jf011015z. [DOI] [PubMed] [Google Scholar]
- 14.Bau M, Bragulat RM, Abarca L, Minguez S, Cabañes F. Advances in Food Mycology, Book Series: Advances in Experimental Medicine and Biology. Vol. 571. Springer; New York, NY, USA: 2006. J. Ochratoxin a Producing Fungi from Spanish Vineyards; pp. 173–179. [DOI] [PubMed] [Google Scholar]
- 15.Gómez MR, Bragulat ML, Abarca S, Mínguez, Cabañes FJ. Ochratoxin A-producing fungi from grapes intended for liqueur wine production. Food Microbiol. 2006;23:541–545. doi: 10.1016/j.fm.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Cook WO, Osweiler GD, Anderson TD, Richard JL. Ochratoxicosis in Iowa swine. Am Vet Med Assn. 1986;188(12):1399–1402. [PubMed] [Google Scholar]
- 17.Pohland A, Nesheim S, Friedman L. Ochratoxin A, a review. Pure Appl Chem. 1992;64:1029–1046. [Google Scholar]
- 18.Stoev SD, Hald B, Mantle P. Porcine nephropathy in Bulgaria: a progressive syndrome of complex of uncertain (mycotoxin) etiology. Vet Rec. 1998;142:190–194. doi: 10.1136/vr.142.8.190. [DOI] [PubMed] [Google Scholar]
- 19.Stoev SD.Clinicomorphological, paraclinical and toxicological investigations in mycotoxic porcine nephropathy Ph.D. ThesisPublishing House of Science and Technics, Stara Zagora, Bulgaria.
- 20.Stoev S, Creppy EE, Hald B, Radic B. Examination of contamination levels of ochratoxin A in feed and serum from regions with high percentage of nephropathy in pigs. Proceedings of the 9th International Congress in Animal Hygiene; 17–21 August, 1997; Helsinki, Finland. pp. 840–843. [Google Scholar]
- 21.Stoev SD, Stoeva J, Anguelov G, Hald B, Creppy EE, Radic B. Haematological, biochemical and toxicological investigations in spontaneous cases with different frequency of porcine nephropathy in Bulgaria. J Vet Med Series A. 1998;45:229–236. doi: 10.1111/j.1439-0442.1998.tb00822.x. [DOI] [PubMed] [Google Scholar]
- 22.Stoev SD, Vitanov S, Anguelov G, Petkova-Bocharova T, Creppy EE. Experimental mycotoxic nephropathy in pigs provoked by a mouldy diet containing ochratoxin A and penicillic acid. Vet Res Commun. 2001;25:205–223. doi: 10.1023/a:1006433709685. [DOI] [PubMed] [Google Scholar]
- 23.Stoev S, Daskalov H, Radic B, Domijan A, Peraica M. Spontaneous mycotoxic nephropathy in Bulgarian chickens with unclarified mycotoxin aetiology. Vet Res. 2002;33(1):83–94. doi: 10.1051/vetres:2001008. [DOI] [PubMed] [Google Scholar]
- 24.Krogh P, Axelsen NH, Elling F, Gyrd-Hansen N, Hald B, Hyldgaard-Jensen J, Larsen AE, Madsen A, Mortensen HP, Moller T, Peterson OK, Ravnskov U, Rostgaard M, Aalund O.Experimental porcine nephropathy: Changes of renal function and structure induced by ochratoxin A-contaminated feed Acta Path Microb Scand, Sect A 1974. Suppl. 2461–21. [PubMed] [Google Scholar]
- 25.Krogh P. Advances in Veterinary Science and Comparative Medicine. Vol. 20. Academic Press; New York, NY, USA: 1976. Mycotoxic nephropathy; pp. 147–170. [PubMed] [Google Scholar]
- 26.Stoev S, Anguelov G, Pavlov D, Pirovski L. Some antidotes and paraclinical investigations in experimental intoxication with ochratoxin A and penicillic acid in chicks. Vet Arhiv. 1999;69:179–189. [Google Scholar]
- 27.Stoev SD, Anguelov G, Ivanov I, Pavlov D. Influence of ochratoxin A and an extract of artichoke on the vaccinal immunity and health in broiler chicks. Exp Toxicol Pathol. 2000;52:43–55. doi: 10.1016/S0940-2993(00)80014-7. [DOI] [PubMed] [Google Scholar]
- 28.Micco C, Miraglia M, Onori R, Libanori A, Brera C, Mantovani A, Macri C. Effect of combined exposure to ochratoxin A and penicillic acid on residues and toxicity in broilers. La Ravista della Societa Italiana di Scienza dell’Allimentazione. 1991;20(3):101–108. [Google Scholar]
- 29.Stoev SD. Some Metric, Antidote and Pathomorphological Investigations in Experimental Intoxication with Ochratoxin A and Penicillic Acid in Chicks. Bulg J Agric Sci. 1998;4:551–563. [Google Scholar]
- 30.Stoev SD, Stefanov M, Denev St, Radic B, Domijan A-M, Peraica M. Experimental mycotoxicosis in chickens induced by ochratoxin A and penicillic acid and intervention by natural plant extracts. Vet Res Commun. 2004;28(8):727–746. doi: 10.1023/b:verc.0000045960.46678.d3. [DOI] [PubMed] [Google Scholar]
- 31.Dickens F, Jones HEH. Carcinogenic activity of a series of reactive lactones and related substances. Br J Cancer. 1961;15:85–100. doi: 10.1038/bjc.1961.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmgren MS, Ciegler A. Toxicity and carcinogenicity of fungal lactones: patulin and penicillic acid. In: Keeler RF, Tu AT, editors. Handbook of Natural Toxins. Vol. 1. Marcel Dekker, Inc.; New Yoer, NY, USA: 1983. pp. 325–341. [Google Scholar]
- 33.Ueno Y, Kubota K. DNA-attacking ability of carcinogenic mycotoxins in recombination deficient mutant cells of Bacillus subtilis. Cancer Res. 1976;36:445–451. [PubMed] [Google Scholar]
- 34.IARC. IARC monographs on the evaluation of carcinogenic risk to humans: some naturally occurring substances; food items and constituents, heterocyclic aromatic amines and mycotoxins. Vol. 56. IARC; Lyon, France: 1993. Ochratoxin A; pp. 489–521. [Google Scholar]
- 35.Huff WE, Hamilton PB, Ciegler A. Evaluation of penicillic acid for toxicity in broiler chickens. Poultry Sci. 1980;59:1203–1207. doi: 10.3382/ps.0591203. [DOI] [PubMed] [Google Scholar]
- 36.Umeda M, Yamamoto T, Saito M. DNA-strand breakage of HeLa cells induced by several mycotoxins. Jpn J Exp Med. 1972;42:527–539. [PubMed] [Google Scholar]
- 37.Umeda M. Cytotoxicity of mycotoxins. In: Rodricks JV, Hesseltine CW, Mehlman MA, editors. Mycotoxins in Human and Animal Health. III. Pathotox Publ. Inc.; Park Forest South, USA: 1977. pp. 712–729. [Google Scholar]
- 38.Dierickx PJ, De Beer JO. Interaction of the mycotoxin penicillic acid with glutathione and rat liver glutathione S-transferases. Mycopathologia. 1984;86:137–141. doi: 10.1007/BF00441121. [DOI] [PubMed] [Google Scholar]
- 39.Chan PK, Hayes AW. Effect of penicillic acid on biliary excretion of indocyanine green in the mouse and rat. J Toxicol Environ Health. 1981;7:169–179. doi: 10.1080/15287398109529970. [DOI] [PubMed] [Google Scholar]
- 40.Hayes AW, Unger PP, Williams WL. Acute toxicity of penicillic acid and rubratoxin B in dogs. Ann Nutr Aliment. 1977;31:711–721. [PubMed] [Google Scholar]
- 41.Sansing GA, Lillehof EB, Detroy RW, Muller MA. Synergistic toxic effects of citrinin, ochratoxin A and penicillic acid in mice. Toxicon. 1976;14:213–220. doi: 10.1016/0041-0101(76)90009-x. [DOI] [PubMed] [Google Scholar]
- 42.Shepherd EC, Philips TD, Joiner GN, Kubena LF, Heidelbaugh ND. Ochratoxin A and penicillic acid interaction in mice. J Environ Sci Health B. 1981;16:557–573. doi: 10.1080/03601238109372279. [DOI] [PubMed] [Google Scholar]
- 43.Doster RC, Sinhuber RO. Compàrative rates of hydrolysis of ochratoxin A and B in vitro. Food Cosmet Toxicol. 1972;10:389–394. doi: 10.1016/s0015-6264(72)80257-8. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki S, Satoh T, Yamazaki M. The pharmacokinetics of ochratoxin A in rats. Jpn J Pharmacol. 1977;27:735–744. doi: 10.1254/jjp.27.735. [DOI] [PubMed] [Google Scholar]
- 45.Parker RW, Phillips TD, Kubena LE, Russell LH, Heidelbaugh ND. Inhibition of pancreatic carboxypeptidase A: a possible mechanism of interaction between penicillic acid and ochratoxin A. J Environ Sci Health. 1982;B17:77–91. doi: 10.1080/03601238209372304. [DOI] [PubMed] [Google Scholar]
- 46.Kubena LF, Phillips TD, Witzel DA. Heidelbaugh ND: Toxicity of ochratoxin A and penicillic acid to chicks. Bulletin Environ Contam Toxicol. 1984;32:711–716. doi: 10.1007/BF01607561. [DOI] [PubMed] [Google Scholar]
- 47.Stoev SD. Ultrastructural and antidote investigations into the experimental intoxication of chickens with ochratoxin A and penicillic acid. Folia Vet. 2000;44:85–90. [Google Scholar]
- 48.Dwivedi P, Burns RB. Pathology of ochratoxicosis A in young broiler chicks. Res in veter Sc. 1984;36(1):92–103. [PubMed] [Google Scholar]
- 49.Manning RO, Wyatt D. Toxicity of Aspergillus ochraceus contaminated wheat and different chemical forms of ochratoxin A in broiler chicks. Poultry Sci. 1984;63(3):458–465. doi: 10.3382/ps.0630458. [DOI] [PubMed] [Google Scholar]
- 50.Mohiudin SM, Warasi SMA, Reddy MV. Haematological and biochemical changes in broiler chicken. Indian Vet J. 1993;70:613–617. [Google Scholar]
- 51.Kozaczynski W. Experimental ochratoxicosis A in chickens. Histopathological and histochemical study. Archivum Veterinarium Polonicum. 1996;34(3–4):205–219. [PubMed] [Google Scholar]
- 52.Mantle PG, McHugh KM. Nephrotoxic fungi in foods from nephropathy households in Bulgaria. Mycological Res. 1993;97:205–212. [Google Scholar]
- 53.Krogh P. Causal associations of mycotoxic nephropathy. Acta Microb Scand. 1978;269:1–28. [PubMed] [Google Scholar]
- 54.Rubiales MV, Bragulat MR, Cabanes F. J. Mycotoxin production by Penicillium species in liquid medium. Revue Med Vet. 1998;149(6):526. [Google Scholar]
- 55.Le Bars J. Facteurs favorisant la production d’acide penicillique par le Penicillium verrucosum var.cyclopium dans les denrees alimentaires. Ann Rech Vet. 1980;11:321–326. [PubMed] [Google Scholar]
- 56.Northolt MD, van Egmond HP, Paulsch WE. Ochratoxin A production by some fungal species in relation to water activity and temperature. J Food Protect. 1979;42:485–490. doi: 10.4315/0362-028X-42.6.485. [DOI] [PubMed] [Google Scholar]
- 57.Lieu FY, Bullerman LB. Production and stability of aflatoxins, penicillic acid and patulin in several substrates. J Food Sci. 1977;42:1222–1224. [Google Scholar]
- 58.Lieu FY, Bullerman LB. Binding of patulin and penicillic acid to glutathione and cysteine and toxicity of the resulting adducts. Milchwissensch. 1978;33:16–20. [Google Scholar]
- 59.Miljkovic A, Pfohl-Leszkowicz A, Dobrota M, Mantle PG. Comparative responses to mode of oral administration and dose of ochratoxin A or nephrotoxic extract of Penicillium polonicum in rats. Exp Toxicol Pathol. 2003;54:305–312. doi: 10.1078/0940-2993-00262. [DOI] [PubMed] [Google Scholar]
- 60.Mantle P, Miljkovic A, Udupa V, Dobrota M. Does apoptosis cause renal atrophy in Balkan endemic nephropathy. The Lancet. 1998;352(9134):1118–1119. doi: 10.1016/S0140-6736(05)79758-0. [DOI] [PubMed] [Google Scholar]
- 61.Obrecht-Pflumio S, Dirheimer G. In vitro DNA and dGMP adducts formation caused by ochratoxin A. Chem Biol Interact. 2000;127:29–44. doi: 10.1016/s0009-2797(00)00169-1. [DOI] [PubMed] [Google Scholar]
- 62.Obrecht-Pflumio S, Dirheimer G. Horseradish peroxidase mediates DNA and deoxyguanosine 3’–monophosphate adduct formation in the presence of ochratoxin A. Arch Toxicol. 2001;75:583–590. doi: 10.1007/s00204-001-0289-3. [DOI] [PubMed] [Google Scholar]
- 63.Atroshi F, Biese I, Saloniemi H, Ali-Vehmas T, Saari S, Rizzo A, Veijalainen P. Significance of apoptosis and its relationship to antioxidants after ochratoxin A administration in mice. J Pharm Pharmaceut Sci. 2000;3:281–291. [PubMed] [Google Scholar]
- 64.Rahimtula AD, Bereziat JC, Eussacchini-Griot V, Bartsch H. Lipid peroxidation as a possible cause of ochratoxin A toxicity. Biochem Pharmacol. 1988;37:4469–4477. doi: 10.1016/0006-2952(88)90662-4. [DOI] [PubMed] [Google Scholar]
- 65.Stoev S. In: Mycotoxic Nephropathies in Farm Animals and Humans – Diagnostics, Risk Assessment and Preventive Measures. Bozhkov S, Diakov L, editors. Publishing House Contrast; Stara Zagora, Bulgaria: 2002. pp. 1–172. [Google Scholar]
- 66.Sreemannarayana O, Frohlich AA, Vitti TG, Marquardt RR, Abramson D. Studies of the tolerance and disposition of ochratoxin A in young calves. J Anim Sci. 1988;66:1703–1711. doi: 10.2527/jas1988.6671703x. [DOI] [PubMed] [Google Scholar]
- 67.Xiao H, Marquardt RR, Frohlich AA, Philips GD, Vitti TG. Effect of a hay and grain diet on the bioavailability of ochratoxin A in the rumen of sheep. J Anim Sci. 1991;69:3715. doi: 10.2527/1991.6993715x. [DOI] [PubMed] [Google Scholar]
- 68.Xiao H, Marquardt RR, Frohlich AA, Philips GD, Vitti TG. Effect of a hay and grain diet on the rate of hydrolysis of ochratoxin A in the rumen of sheep. J Anim Sci. 1991;69:3706. doi: 10.2527/1991.6993706x. [DOI] [PubMed] [Google Scholar]
- 69.Abramson D, Sinha RN, Mills JT. Mycotoxin formation and quality changes in granary-stored corn at 16 and 21% moisture content. Sciences des Aliments. 1985;5:653–663. [Google Scholar]
- 70.Abramson D, Mills J, Boycott B. Mycotoxins and mycoflors in animal feedstuffs in western Canada. Can J Comp Med. 1983;47:23–26. [PMC free article] [PubMed] [Google Scholar]
- 71.Frohlich AA, Marquardt RR, Ominski KR.Ochratoxin A as a contaminant in the human food chain: A Canadian perspective Mycotoxins, Endemic Nephropathy and Urinary Tract Tumours IARC; Lyon, France: 1991. Publication No. 115139–144. [PubMed] [Google Scholar]
- 72.Chelack WS, Borsa J, Marquardt RR, Frohlich AA. Role of competitive microbial flora in the radiation induced enhancement of ochratoxin production by Aspergillus alutaceus var alutaceus NRRL-3174. Appl Environ Microbiol. 1991;57:2492. doi: 10.1128/aem.57.9.2492-2496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chelack WS, Borsa J, Szekely JG, Marquardt RR, Frohlich AA. Variants of Aspergillus alutaceus var alutaceus formely Aspergillus ochraceus, with altered ochratoxin A production. Appl Environ Microbiol. 1991;57:2487. doi: 10.1128/aem.57.9.2487-2491.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Müller HM.A survey of methods of decontaminating mycotoxinsI. Physical methods. Anim Res Develop 19831870–96. [Google Scholar]
- 75.Samarajeewa U. In situ degradation of mycotoxins by physical methods. In: Smith JE, Henderson RS, editors. Mycotoxins and Animal Foods. CRC Press; Boca Raton, FL, USA: 1991. pp. 785–796. [Google Scholar]
- 76.Leitao J, Bailly J, Blanquant G. Action of phosphine (PH3) on production of sterimatocystin by various fungal strains isolated from foodstuffs. Food Addit Contam. 1990;7:26. doi: 10.1080/02652039009373839. [DOI] [PubMed] [Google Scholar]
- 77.Tong C, Draughon H. Inhibition by an antimicrobial food additives of ochratoxin A production by Aspergillus sulfureus and Penicillium viridicatum. Appl Environ Microbiol. 1985;49:1407. doi: 10.1128/aem.49.6.1407-1411.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Madsen A, Hald B, Mortensen H.Feeding experiments with ochratoxin A contaminated barley for bacon pigs-3Detoxification by ammoniation heating + NaOH, or autoclaving. Acta Agric Scand 198333171–175. [Google Scholar]
- 79.Chelkowski J, Golinski P, Godlewska B, Radomyska W, Szebiotko K, Wiewiorowska M. Mycotoxins in cereal grains. Part IV. Inactivation of ochratoxin A and other mycotoxins during ammoniation. Nahrung. 1981;25:631. doi: 10.1002/food.19810250705. [DOI] [PubMed] [Google Scholar]
- 80.Scott PM. Industrial and farm detoxification processes for mycotoxins. Revue Med Vet. 1998;149(6):543–548. [Google Scholar]
- 81.Council for Agricultural Science and Technology Mycotoxins: Economic and Health Risk Ames; Iowa: 1989. Task Force Report 1161–91. [Google Scholar]
- 82.Rotter RG, Marquardt RR, Frohlich AA, Abramson D. Ensiling as a means of reducing ochratoxin A concentrations in contaminated barley. J Sci Food Agric. 1990;50:155. [Google Scholar]
- 83.Scott PM. Mycotoxins transmitted into beer from contaminated grain during brewing. J AOAC Int. 1996;79:875–882. [PubMed] [Google Scholar]
- 84.Laciakova A, Cabadaj R, Conkova E, Pastorova B. Degradation of ochratoxin A by feed additives. Revue Med Vet. 1998;149(6):567. [Google Scholar]
- 85.Marquardt RR, Frolich AA. A review of recent advances in understanding ochratoxicosis. J Anim Sci. 1992;70:3968–3988. doi: 10.2527/1992.70123968x. [DOI] [PubMed] [Google Scholar]
- 86.Rotter RG, Frohlich AA, Marquardt RR. Influence of dietary charcoal on ochratoxin A toxicity in Leghorn chicks. Can J Vet Rec. 1989;53:449. [PMC free article] [PubMed] [Google Scholar]
- 87.Creppy EE, Stormer FC, Kern D, Roschenthaler R, Dirheimer G. Effects of ochratoxin A metabolites on yeast phenylalanyl-t-RNA syntetase and on the growth and “in vivo” protein synthesis of hepatoma cells. Chem Biol Interact. 1983;47:239–247. doi: 10.1016/0009-2797(83)90160-6. [DOI] [PubMed] [Google Scholar]
- 88.Creppy EE, Roschenthaler R, Dirheimer G. Inhibition of protein synthesis in mice by ochratoxin A and its prevention by phenylalanine. Food Chem Toxicol. 1984;22:883–886. doi: 10.1016/0278-6915(84)90170-4. [DOI] [PubMed] [Google Scholar]
- 89.Moroi K, Suzuki S, Kuga T, Yamazaki M, Kanisawa M. Reduction of ochratoxin A toxicity in mice treated with phenylalanine and phenobarbital. Toxicol Lett. 1985;25:1–5. doi: 10.1016/0378-4274(85)90092-x. [DOI] [PubMed] [Google Scholar]
- 90.Creppy EE, Lorkowski G, Roschenthaler R, Dirheimer G. Kinetics of the immunosuppressive action of ochratoxin A in mice. Proc. V Int. IUPAC Symp. Mycotoxins and Phycotoxins; Vienna, Austria: Austrian Chem. Soc.; 1982. pp. 289–292. [Google Scholar]
- 91.Haubeck HD, Lorkowski G, Kolsch E, Roschenthaler R. Immunosuppression by ochratoxin A and its prevention by phenylalanine. Appl Environ Microb. 1981;41:1040–1042. doi: 10.1128/aem.41.4.1040-1042.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Creppy EE, Chakor K, Fisher MJ, Dirheimer G. The mycotoxin ochratoxin A is a substrate for phenylalanine hydroxylase in isolated rat hepatocytes and in vivo. Arch Toxicol. 1990;64:279. doi: 10.1007/BF01972987. [DOI] [PubMed] [Google Scholar]
- 93.Mayura K, Parker R, Berndt WO, Phillips TD. Ochratoxin A - induced teratogenesis in rats: partial protection by phenylalanine. Appl Environ Microb. 1984;48:1186–1188. doi: 10.1128/aem.48.6.1186-1188.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stoev SD, Djuvinov D, Mirtcheva T, Pavlov D, Mantle P. Studies on some feed additives giving partial protection against ochratoxin A toxicity in chicks. Toxicol Lett. 2002;135:33–50. doi: 10.1016/s0378-4274(02)00234-5. [DOI] [PubMed] [Google Scholar]
- 95.Gibson RM, Bailey CA, Kubena LF, Huff WE, Harvey RB. Impact of L-phenylalanine supplementation on the performance of three-week-old broilers fed diets containing ochratoxin A. 1. Effects on body weight, feed conversion, relative organ weight, and mortality. Poult Sci. 1990;69:414–419. doi: 10.3382/ps.0690414. [DOI] [PubMed] [Google Scholar]
- 96.Bailey C, Gibson RM, Kubena LF, Huff WE, Harvey RB. Impact of L-phenylalanine supplementation on the performance of three-week-old broilers fed diets containing ochratoxin A. 2. Effects on haematology and clinical chemistry. Poultry Science. 1990;69:420–425. doi: 10.3382/ps.0690420. [DOI] [PubMed] [Google Scholar]
- 97.Bailey CA, Gibson RM, Kubena LF, Huff WE, Harvey RB. Ochratoxin A and Dietary Proteins. 2. Effects on Hematology and Various Clinical Chemistry Measurements. Poultry Sci. 1989;68:1664–1671. doi: 10.3382/ps.0681664. [DOI] [PubMed] [Google Scholar]
- 98.Gibson RM, Bailey CA, Kubena LF, Huff WE, Harvey RB. Ochratoxin A and dietary proteins. 1. Effects on body weight, feed conversion, relative organ weight, and mortality in three-week-old broilers. Poult Sci. 1989;68:1658–1663. doi: 10.3382/ps.0681658. [DOI] [PubMed] [Google Scholar]
- 99.Haazele FM. Univ. of Manitoba; Winnipeg, MB, Canada: 1992. Response to dietary ascorbic acid supplementation in laying hens: Effects of exposure to high temperature and ochratoxin ingestion. Ph. D. Thesis. [Google Scholar]
- 100.Haazele FM, Guenter W, Marquardt RR, Frohlich AA. Benefical effects of dietary ascorbic acid supplement on hens subjected to ochratoxin A toxicosis under normal and high ambient temperatures. Can J Anim Sci. 1993;73:149–157. [Google Scholar]
- 101.Roth A, Chakor K, Creppy EE, Kane A, Roschenthaler R, Dirheimer G. Evidence for an enterohepatic circulation of ochratoxin A in mice. Toxicology. 1988;48:293–308. doi: 10.1016/0300-483x(88)90110-2. [DOI] [PubMed] [Google Scholar]
- 102.Meisner H, Chan S. Ochratoxin A, an inhibitor of mitochondrial transport system. Biochemistry New York. 1974;13:2795–2799. doi: 10.1021/bi00711a002. [DOI] [PubMed] [Google Scholar]
- 103.Gahnian R, Asenov I. Medical plants and their relevance. In: Atanasov A, Georgiev C, Anastasova M, editors. Animal phytoterapy. Zemizdat; Sofia, Bulgaria: 1986. pp. 199–200. [Google Scholar]
- 104.Stoev S, Ivanov I, Pavlov D. Protective effect of artichoke extract on the ochratoxin A-induced immunosupression in broiler chicks;. Proceedings of the 8th World Conference on Animal Production; Seoul, Korea. June 28 - July 4, 1998; pp. 824–825. [Google Scholar]
- 105.Gahnian R, Mihailov G, Pavlov D, Panchev I, Nikolov A. Vet Medicine, Suppl 1. 1995. Influence of Artichoke (Cynara scolymus L.) leaves extract on development of experimental liver distrophy in sheep; pp. 104–105. [Google Scholar]
- 106.Cairela M, Nasta G, Vcci L, Veipari B. Cynarine effect on hepatic damages due to food excesses;. Proc. 2nd International Meeting on Globe Artichoke; Bari, Italy. November 21–24, 1973; p. 15. [Google Scholar]
- 107.Mantia G, Leone F, Hopps V, Consiglio D. Cynarine influence on hepatotoxic effects of some chemic antibiotics. Proc. 2nd International Meeting on Globe Artichoke; Bari, Italy. November 21–24, 1973; p. 10. [Google Scholar]
- 108.Stoev SD, Paskalev M, MacDonald S, Mantle P. Experimental one year ochratoxin A toxicosis in pigs. Exp Toxicol Pathol. 2002;53:481–487. doi: 10.1078/0940-2993-00213. [DOI] [PubMed] [Google Scholar]
- 109.Kuiper-Goodman T, Scott PM. Risk assessment of the mycotoxin ochratoxin A. Biomed Environ Sci. 1989;2:179–248. [PubMed] [Google Scholar]
- 110.Stoev S, Angelov G, Pavlov D. Some Paraclinical Investigations in Experimental Intoxication with Ochratoxin A and Penicillic Acid in Chicks. Bulg J Agric Sci. 1998;4:565–573. [Google Scholar]
- 111.Van Egmond HP. Current situation on regulations for mycotoxins: Overview of tolerances and status of standard methods of sampling and analysis. Food Addit Contam. 1989;6:139–188. doi: 10.1080/02652038909373773. [DOI] [PubMed] [Google Scholar]
- 112.Boutrif E, Canet C. Mycotoxin prevention and control: FAO programes. Revue Med Vet. 1998;149(6):681–694. [Google Scholar]
- 113.Rosner H. Mycotoxin Regulations: an Update. Revue Med Vet. 1998;149(6):679–680. [Google Scholar]
- 114.Stoev S, Creppy EE. Toxicological investigations of kidneys in spontaneous cases of nephropathy in pigs and necessary hygiene control. Proceedings of the 9th International Congress in Animal Hygiene; Helsinki, Finland. 17 – 21 August, 1997; pp. 949–952. [Google Scholar]
- 115.Krogh P. Ochratoxin A residues in tissues of slaughter pigs with nephropathy. Nord Vet Med. 1977;29:402–405. [PubMed] [Google Scholar]
- 116.Elling F, Hald B, Jacobsen C, Krogh P. Spontaneous toxic nephropathy in poultry associated with ochratoxin A. Acta Path Microb Scand, Sect, A. 1975;83:739–741. doi: 10.1111/j.1699-0463.1975.tb01406.x. [DOI] [PubMed] [Google Scholar]
- 117.Krogh P, Elling F, Hald B, Larsen AE, Lillehoy EB, Madsen A, Mortensen HP. Time-dependent disappearance of ochratoxin A residues in tissues of bacon pigs. Toxicology. 1976;6:235–242. doi: 10.1016/0300-483x(76)90025-1. [DOI] [PubMed] [Google Scholar]
- 118.Krogh P, Hald B, Pederson J. Occurrence of ochratoxin A and citrinin in cereals associated with mycotoxic porcine nephropathy. Acta path Microbiol Scand, Sect B. 1973;81:689–695. doi: 10.1111/j.1699-0463.1973.tb02261.x. [DOI] [PubMed] [Google Scholar]
- 119.Elling F. Feeding experiments with ochratoxin A-contaminated barley to bacon pigs. IV Renal lesions. Acta Agric Scand. 1983;33:153–159. [Google Scholar]
- 120.Krogh P, Elling F, Gyrd-Hansen N, Hald B, Larsen AE, Lillehoj EB, Madsen A, Mortensen HP, Ravnskov U. Experimental porcine nephropathy: Changes of renal function and structure perorally induced by cristalline ochratoxin A. Acta Path Microbiol Scand Sect A. 1976;84:429–434. [PubMed] [Google Scholar]
- 121.Trenk HL, Butz ME, Chu F. Production of ochratoxins in different cereal products by Aspergillus ochraceus. Appl Microbiol. 1971;21:1032–1035. doi: 10.1128/am.21.6.1032-1035.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Krogh P. Mycotoxic porcine nephropathy: a possible model for Balkan endemic nephropathy. In: Puchlev A, editor. Proceedings of the Second International Symposium on Endemic Nephropathy; Sofia, Bulgaria: Publishing House of the Bulgarian Academy of Sciences; 1972. pp. 266–270. [Google Scholar]
- 123.Plestina R. Some features of Balkan endemic nephropathy. Food Chem Toxicol. 1992;30(3):177–181. doi: 10.1016/0278-6915(92)90030-o. [DOI] [PubMed] [Google Scholar]
- 124.Stoev SD, Petkova-Bocharova TK. A possible role of ochratoxin A in a disease causation in connection with Balkan endemic nephropathy. Proceedings of the 8th International Congress on Animal Hygiene; St. Paul, MN, USA. September 12 – 16, 1994; pp. 61–64. [Google Scholar]
- 125.Nordic Working Group on Food Toxicology and Risk Evaluation (NNT). Health Evaluation of Ochratoxin A in Food Products; Nordic Working Group on Food Toxicology and Risk Evaluation: Copenhagen, Denmark, 1991; Nordic Semiar Workingpaper 545, pp. 1–29.
- 126.Fuchs R, Radic B. Endemic nephropathy. In: Cvoriscec D, Ceovic S, Stavljenic-Rukavina A, editors. Endemic Nephropathy in Croatia. Academia Croatica Scientiarum Medicarum; Zagreb, Croatia: 1996. pp. 31–38. [Google Scholar]
- 127.Ceovic S, Hrabar A, Saric M. Epidemiology of Balkan endemic nephropathy. Food Chem Toxicol. 1992;30:183–188. doi: 10.1016/0278-6915(92)90031-f. [DOI] [PubMed] [Google Scholar]
- 128.Ahmeteli M. Epidemologia endemi ceskoj nephropatii. Sovetskaja Medicina. 1973;5:123–128. [Google Scholar]
- 129.Austwick PKC. Comparative aspects of renal disease. Proceed Royal Soc Med. 1975;68:219–222. [Google Scholar]
- 130.Petkova-Bocharova T, Chernozemsky I, Castegnaro M. Ochratoxin A in human blood in relation to Balkan endemic nephropathy and urinary system tumours in Bulgaria. Food Addit Contam. 1988;5:299–301. doi: 10.1080/02652038809373707. [DOI] [PubMed] [Google Scholar]
- 131.Petkova-Bocharova T, Castegnaro M, Michelon J, Maru V.Ochratoxin A and other mycotoxins in cereals from an area of Balkan endemic nephropathy and urinary tract tumours in Bulgaria Mycotoxins, Endemic Nephropathy and Urinary Tract Tumours Castegnaro M, Plestina R, Dirheimer G, Chernozemsky IN, Bartsch H.IARC Lyon; France: 1991. Publication No. 115, pp. 83–87. [PubMed] [Google Scholar]
- 132.Petkova-Bocharova T, Castegnaro M.Ochratoxin A in human blood in relation to Balkan endemic nephropathy and urinary tract tumours in Bulgaria Mycotoxins, Endemic Nephropathy and Urinary Tract Tumours Castegnaro M, Plestina R, Dirheimer G, Chernozemsky IN, Bartsch H, IARC Lyon; France: 1991. Publication No. 115, pp. 135–137. [PubMed] [Google Scholar]
- 133.Krogh P, Hald B, Plestina R, Ceovic S. Balkan (endemic) nephropathy and foodborne ochratoxin A: Preliminary results of a survey of foodstuffs. Acta Pathol Microb Scand, Section B. 1977;85:238–240. doi: 10.1111/j.1699-0463.1977.tb01702.x. [DOI] [PubMed] [Google Scholar]
- 134.Peraica M, Radic B, Lucic A, Fuchs R, Bosanac I, Balija M, Grgicevic D. Ochratoxin A in blood of people from regions in Croatia with and without endemic nephropathy. Revue Med Vet. 1998;149(6):713. [Google Scholar]
- 135.Chernozemsky IN, Stoyanov IS, Petkova-Bocharova TK, Nikolov IG, Draganov IV, Stoichev I, Tanchev Yl, Naidenov D, Kalcheva ND. Geographic correlation between the occurrence of endemic nephropathy and urinary tract tumours in Vratza district, Bulgaria. Journal of Cancer. 1977;19:1–11. doi: 10.1002/ijc.2910190102. [DOI] [PubMed] [Google Scholar]
- 136.Radovanovic S, Jankovic S, Jeremovic J. Incidence of tumours of urinary organs in focus of Balkan endemic nephropathy. Kidney Int. 1991;40(Suppl. 34):75–77. [PubMed] [Google Scholar]
- 137.Kanisawa M, Suzuki S. Induction of renal and hepatic tumors in mice by ochratoxin A, a mycotoxin. Gann. 1978;69:599–600. [PubMed] [Google Scholar]
- 138.Bendele AM, Carlton MW, Krogh P, Lillehoj EB. Ochratoxin A carcinogenesis in the (C57GL/6J x C3H) F1 mouse. J Natl Cancer Inst. 1985;75:733–742. [PubMed] [Google Scholar]
- 139.Pfohl-Leszkowicz A, Grosse Y, Kane A, Creppy EE, Dirheimer G. Differential DNA adduct formation and disappearance in three mice tissues after treatment by the mycotoxin ochratoxin A. Mutation Res. 1993;289:265–273. doi: 10.1016/0027-5107(93)90077-s. [DOI] [PubMed] [Google Scholar]
- 140.Azemar B, Pinelli E, Plante P, Escourrou G, Petkova-Bocharova T, Pfohl-Leszkowicz A. Some human kidney tumours in France exhibited a specific ochratoxin A-DNA adduct pattern. Revue Med Vet. 1998;149(6):653. [Google Scholar]
- 141.Mantle P. Debrisoquine 4-Hydroxylation and Balkan Endemic Nephropathy. Nephron. 1998;752:139–141. doi: 10.1159/000045304. [DOI] [PubMed] [Google Scholar]
- 142.Nikolov IG, Chernozemsky IN, Idle JR.Genetic predisposition to Balkan endemic nephropathy: Ability to hydroxilate debrisoquine as a host risk factor Mycotoxins, Endemic Nephropathy and Urinary Tract Tumours Castegnaro M, Plestina R, Dirheimer G, Chernozemsky IN, Bartsch H, IARC Lyon; France: 1991. Publication No. 115289–296. [PubMed] [Google Scholar]
- 143.Wafa EW, Yahya RS, Sobh MA, Eraky I, El-Baz M, El-Gayar H, Betbeder AM, Creppy EE. Human Ochratoxicosis and Nephropathy in Egypt: A preliminary Study. Revue Med Vet. 1998;149(6):714. doi: 10.1177/096032719801700207. [DOI] [PubMed] [Google Scholar]
- 144.Luster MI, Germolec DR, Burleson GR, Jameson CW, Ackermann MF, Lamm KR, Hayes HT. Selectiv immunosuppression in mice of natural killer cell activity by ochratoxin A. Cancer Res. 1987;47:2259–2263. [PubMed] [Google Scholar]
- 145.Studer-Rohr I, Schlatter J, Dietrich DR. Kinetic parameters and intraindividual fluctuations of ochratoxin A plasma levels in humans. Arch Toxicol. 2000;74(9):499–510. doi: 10.1007/s002040000157. [DOI] [PubMed] [Google Scholar]
- 146.Fuchs R, Hult K. Ochratoxin A in blood and its pharmacokinetic properties. Food Chem Toxicol. 1992;30:201–204. doi: 10.1016/0278-6915(92)90034-i. [DOI] [PubMed] [Google Scholar]
- 147.Klaassen CD. Distribution, excretion and absorbtion of toxicants. In: Klaassen CD, Amdur MO, Doull J, editors. Casarett and Doull's Toxicology. 3rd. Ed. Macmillian; New York, NY, USA: 1986. pp. 33–66. The Basic Science of Poinsons. [Google Scholar]
- 148.WHO Evaluation of certain food additives and contaminants Thirty-seventh report of the joint FAO/WHO Expert Committee on Food Additives World Health Organization; Geneva, Switzerland: 1991. WHO Technical Report Series 80629–31. [PubMed] [Google Scholar]
- 149.JECFA. Toxicological evaluation of certain food additives. Proc. 49th Meeting of JECFA; Geneva, Switzerland: WHO Food Additives Series; 1997. [Google Scholar]
- 150.Bernhoft A, Keblys M, Morrison E, Larsen HS, Flåmyen A. Combined effects of selected Penicillium mycotoxins on “in vitro” proliferation of porcine lymphocytes. Mycopathologia. 2004;158:441–450. doi: 10.1007/s11046-004-2843-z. [DOI] [PubMed] [Google Scholar]
- 151.Klaric MS, Rumora L, Ljubanovic D. Cytotoxicity and apoptosis induced by fumonisin B1, beauvericin and ochratoxin A in porcine kidney PK15 cells: effects of individual and combined treatment. Arch Toxicol. 2008;82(4):247–255. doi: 10.1007/s00204-007-0245-y. [DOI] [PubMed] [Google Scholar]
- 152.Creppy EE, Chiarappa P, Baudrimont I, Borracci P, Moukha S, Carrate MR. Synergistic effects of fumonisin B1 and ochratoxin A: are “in vitro” cytotoxicity data predictive of “in vivo” acute toxicity. Toxicology. 2004;201:115–123. doi: 10.1016/j.tox.2004.04.008. [DOI] [PubMed] [Google Scholar]