Abstract
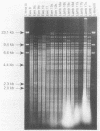
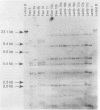
Pneumonia has been identified as a major cause of poor lamb survival in indigenous herds of Rocky Mountain bighorn sheep (Ovis canadensis canadensis) in central Idaho. Pasteurella haemolytica was isolated from five adult Rocky Mountain bighorn ewes captured from a free-ranging herd in central Idaho. The lambs from two of these ewes delivered by cesarean section were free of P. haemolytica until 40 days of age and after repeated contact with their dams. The lambs subsequently developed signs of pneumonia, and P. haemolytica was isolated from nasal, pharyngeal, and transtracheal wash samples from each lamb. All P. haemolytica biotype T isolates from the ewes and lambs, as well as those from a 9-month-old lamb of the same herd from which samples for culture were obtained 2 years earlier, were subjected to HaeIII restriction enzyme analysis (REA) and ribotyping. Two ribotypes and seven REA patterns were visually distinguishable by these procedures. Similarity coefficients (SAB) of 0.09 to 0.95 were calculated for the seven REA patterns. The REA patterns of the isolates from the lambs were identical (SAB = 1.0). The isolates from the lambs also had SAB values of 1.0, which was indicative of identity with one of the seven isolates cultured from the ewes at the time of capture and with the organism isolated from the 9-month-old lamb. These procedures have the discriminatory capabilities necessary to monitor the transmission of specific strains of bacteria within and between animal populations.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Sultan I. I., Aitken I. D. The tonsillar carriage of Pasteurella haemolytica in lambs. J Comp Pathol. 1985 Apr;95(2):193–201. doi: 10.1016/0021-9975(85)90005-2. [DOI] [PubMed] [Google Scholar]
- BIBERSTEIN E. L. CROSS-REACTIONS BETWEEN TYPES OF PASTEURELLA HEMOLYTICA. Cornell Vet. 1965 Jul;55:495–499. [PubMed] [Google Scholar]
- BIBERSTEIN E. L., GILLS M., KNIGHT H. Serological types of Pasteurella hemolytica. Cornell Vet. 1960 Jul;50:283–300. [PubMed] [Google Scholar]
- Biberstein E. L., Thompson D. A. Epidemiological studies on Pasteurella Haemolytica in sheep. J Comp Pathol. 1966 Jan;76(1):83–94. doi: 10.1016/0021-9975(66)90050-8. [DOI] [PubMed] [Google Scholar]
- Bisgaard M., Mutters R. Re-investigations of selected bovine and ovine strains previously classified as Pasteurella haemolytica and description of some new taxa within the Pasteurella haemolytica-complex. Acta Pathol Microbiol Immunol Scand B. 1986 Jun;94(3):185–193. doi: 10.1111/j.1699-0463.1986.tb03040.x. [DOI] [PubMed] [Google Scholar]
- Bradbury W. C., Pearson A. D., Marko M. A., Congi R. V., Penner J. L. Investigation of a Campylobacter jejuni outbreak by serotyping and chromosomal restriction endonuclease analysis. J Clin Microbiol. 1984 Mar;19(3):342–346. doi: 10.1128/jcm.19.3.342-346.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler R. L., Bird R. G., Smith M. D., Anger H. S., Turfrey B. A. Scanning electron microscope studies on preparations of bovine cornea exposed to Moraxella bovis. J Comp Pathol. 1983 Jan;93(1):1–8. doi: 10.1016/0021-9975(83)90038-5. [DOI] [PubMed] [Google Scholar]
- Cleary P. P., Kaplan E. L., Livdahl C., Skjold S. DNA fingerprints of Streptococcus pyogenes are M type specific. J Infect Dis. 1988 Dec;158(6):1317–1323. doi: 10.1093/infdis/158.6.1317. [DOI] [PubMed] [Google Scholar]
- Cox N., Johnston J., Szarka Z., Wright D. J., Archard L. C. Characterization of an rRNA gene-specific cDNA probe: applications in bacterial identification. J Gen Microbiol. 1990 Aug;136(8):1639–1643. doi: 10.1099/00221287-136-8-1639. [DOI] [PubMed] [Google Scholar]
- Fox M. L., Thomson R. G., Magwood S. E. Pasteurella haemolytica of cattle: serotype, production of beta-galactosidase and antibacterial sensitivity. Can J Comp Med. 1971 Oct;35(4):313–317. [PMC free article] [PubMed] [Google Scholar]
- Frank G. H. Pasteurella haemolytica and respiratory disease in cattle. Proc Annu Meet U S Anim Health Assoc. 1979;(83):153–160. [PubMed] [Google Scholar]
- Frank G. H. Serotypes of Pasteurella haemolytica in sheep in the midwestern United States. Am J Vet Res. 1982 Nov;43(11):2035–2037. [PubMed] [Google Scholar]
- Frank G. H., Smith P. C. Prevalence of Pasteurella haemolytica in transported calves. Am J Vet Res. 1983 Jun;44(6):981–985. [PubMed] [Google Scholar]
- Frank G. H., Wessman G. E. Rapid plate agglutination procedure for serotyping Pasteurella haemolytica. J Clin Microbiol. 1978 Feb;7(2):142–145. doi: 10.1128/jcm.7.2.142-145.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhart C. J., Ward G. E., Murtaugh M. P. Species-specific cloned DNA probes for the identification of Campylobacter hyointestinalis. J Clin Microbiol. 1989 Dec;27(12):2717–2723. doi: 10.1128/jcm.27.12.2717-2723.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour N. J. Pasteurella haemolytica infections in sheep. Tijdschr Diergeneeskd. 1980 Oct 15;105(20):191–198. [PubMed] [Google Scholar]
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- HOERLEIN A. B., SAXENA S. P., MANSFIELD M. E. Studies on shipping fever of cattle. II. Prevalence of Pasteurella species in nasal secretions from normal calves and calves with shipping fever. Am J Vet Res. 1961 May;22:470–472. [PubMed] [Google Scholar]
- Howard C. J., Taylor G. Immune responses to mycoplasma infections of the respiratory tract. Vet Immunol Immunopathol. 1985 Oct;10(1):3–32. doi: 10.1016/0165-2427(85)90037-6. [DOI] [PubMed] [Google Scholar]
- Kristiansen B. E., Sørensen B., Bjorvatn B., Falk E. S., Fosse E., Bryn K., Frøholm L. O., Gaustad P., Bøvre K. An outbreak of group B meningococcal disease: tracing the causative strain of Neisseria meningitidis by DNA fingerprinting. J Clin Microbiol. 1986 Apr;23(4):764–767. doi: 10.1128/jcm.23.4.764-767.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince D. V., Clarke J. K., Alley M. R. Serotypes of Pasteurella haemolytica from the respiratory tract of sheep in New Zealand. N Z Vet J. 1985 May;33(5):76–77. doi: 10.1080/00480169.1985.35171. [DOI] [PubMed] [Google Scholar]
- SMITH G. R. Isolation of two types of Pasteurella haemolytica from sheep. Nature. 1959 Apr 18;183(4668):1132–1133. doi: 10.1038/1831132a0. [DOI] [PubMed] [Google Scholar]
- Schmid J., Voss E., Soll D. R. Computer-assisted methods for assessing strain relatedness in Candida albicans by fingerprinting with the moderately repetitive sequence Ca3. J Clin Microbiol. 1990 Jun;28(6):1236–1243. doi: 10.1128/jcm.28.6.1236-1243.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snipes K. P., Kasten R. W., Wild M. A., Miller M. W., Jessup D. A., Silflow R. L., Foreyt W. J., Carpenter T. E. Using ribosomal RNA gene restriction patterns in distinguishing isolates of Pasteurella haemolytica from bighorn sheep (Ovis canadensis). J Wildl Dis. 1992 Jul;28(3):347–354. doi: 10.7589/0090-3558-28.3.347. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thompson D. A., Fraser J., Gilmour N. J. Serotypes of Pasteurella haemolytica in ovine pasteurellosis. Res Vet Sci. 1977 Jan;22(1):130–131. [PubMed] [Google Scholar]
- Wesley I. V., Bryner J. H. Antigenic and restriction enzyme analysis of isolates of Campylobacter fetus subsp venerealis recovered from persistently infected cattle. Am J Vet Res. 1989 Jun;50(6):807–813. [PubMed] [Google Scholar]
- Wild M. A., Miller M. W. Detecting nonhemolytic Pasteurella haemolytica infections in healthy Rocky Mountain bighorn sheep (Ovis canadensis canadensis): influences of sample site and handling. J Wildl Dis. 1991 Jan;27(1):53–60. doi: 10.7589/0090-3558-27.1.53. [DOI] [PubMed] [Google Scholar]