Abstract
Neurons are highly polarized cells with morphologically and molecularly distinct axonal and dendritic compartments. It is not well understood how postsynaptic receptors are selectively enriched in dendrites in vivo. We investigated the molecular mechanisms of dendritically polarized localization of a glutamate receptor, an acetylcholine receptor, and a ROR-type receptor tyrosine kinase in the interneuron RIA in C. elegans. We found that the clathrin adaptor AP-1 complex μ1 subunit UNC-101 functions cell autonomously to maintain the correct localization of these receptors in a dynamin-dependent manner. In unc-101 mutants, instead of being dendritically enriched, all 3 receptors are evenly distributed in the axonal and dendritic compartments. Surprisingly, UNC-101 predominantly localizes to the axonal compartment, suggesting a possible transcytosis model for the dendritic targeting of neurotransmitter receptors.
Keywords: polarity, trafficking, glutamate receptor, dendrite
Neurons are polarized cells that receive, process, and transmit information. These different functions are performed by morphologically and functionally distinct subcellular compartments—dendrites and axons—that contain proteins specialized for signal input or output. In principle, many different cellular mechanisms could contribute to molecular polarization in neurons (1, 2). Proteins destined for the axon or the dendrite could be selectively sorted into different transport vesicles at the transGolgi network (TGN) and selectively delivered to their proper subcellular compartment. Alternatively, steady-state differences in axonal and dendritic protein distribution could be achieved by selective stabilization of proteins by scaffolding proteins at certain areas of the plasma membrane, as well as by selective sorting and redistribution following endocytosis.
What is currently known about sorting and trafficking of axonal and dendritic proteins to their proper compartments? Three axonally localized proteins, NgCAM, Nav1.2, and VAMP, are delivered to both axons and dendrites in neurons and achieve polarization following selective endocytosis from the dendritic plasma membrane (3–6). In contrast, dendritically localized transferrin receptor appears to be directly transported to the dendrite (3). For a number of dendritically targeted proteins, structure-function analysis has revealed C-terminal tyrosine-based or dileucine-based cytoplasmic motifs that mediate dendritic targeting (7, 8).
Interesting parallels have been made between dendritic sorting in neurons and basolateral sorting in epithelia (9). A number of basolaterally sorted proteins localize to the somatodendritic region in cultured hippocampal neurons and rely on the same sorting sequences in both cell types (10). One of the key players involved in basolateral sorting in polarized epithelial cells is μ1B, the medium subunit of clathrin adaptor AP-1 complex (11). AP complexes are cytosolic tetramers that mediate sorting in secretory and endocytic pathways by promoting budding of clathrin-coated vesicles (12, 13). μ subunits are particularly important for sorting as they recognize sorting signals within cytoplasmic regions of transmembrane protein cargo (12). In epithelial cells, AP-1 medium subunit μ1B acts in recycling endosomes to mediate both targeting from TGN via the transendosomal route and postendocytic recycling to the basolateral membrane (14, 15).
AP complexes are important for sorting in neurons as well. A study by Dwyer et al. (16) found that AP-1 medium subunit μ1/unc-101 is required for the localization of odorant receptors to the olfactory cilia in Caenorhabditis elegans, because in the absence of UNC-101, these proteins are distributed uniformly on the plasma membrane. A similar phenotype was observed for the polycystin TRPP2/PKD-2, which normally localizes to the cilia of male sensory neurons and is delocalized in unc-101 mutants (17). A recent study showed that a disruption of another adaptor complex, AP-4, leads to the accumulation of AMPA-type glutamate receptors in axonal autophagosomes in mice (18).
Here we show that, in addition to sorting proteins to cilia, μ1/UNC-101 plays a more general role in the sorting of postsynaptic receptors to dendrites in C. elegans. These postsynaptic proteins include AMPA-type glutamate receptor GLR-1, α7-type nicotinic acetylcholine receptor ACR-16, and the receptor tyrosine kinase CAM-1/ROR. We show that the cytosolic domains of these postsynaptic receptors are both necessary and sufficient for their dendritic localization in a manner dependent on UNC-101. Furthermore, we find that UNC-101 localizes presynaptically, where it may be involved in the retrieval of postsynaptic proteins following endocytosis.
Results
AP-1 Subunit μ1/UNC-101 Is Required for Polarized Localization of Glutamate Receptor GLR-1 in RIA Neurons.
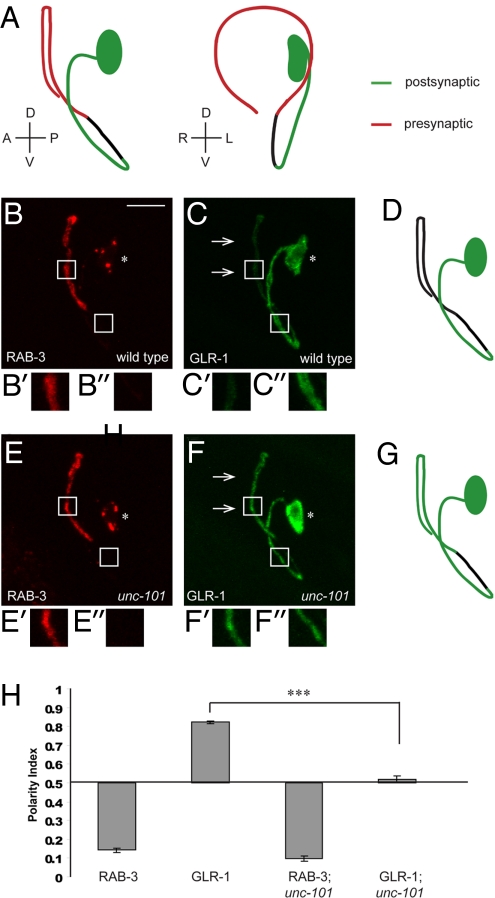
RIA interneurons are a pair of bilaterally symmetric neurons located in the head of the worm. Each RIA neuron has a single process that extends anteriorly and then ventrally, subsequently reentering the large bundle of neuronal processes known as the nerve ring (19). The EM reconstruction revealed that the RIA process is polarized (19): the proximal region of the RIA neurite is exclusively postsynaptic (Fig. 1A, green), whereas the distal region is mainly presynaptic (Fig. 1A, red). RIA neurons express a number of postsynaptic receptors (20, 21) and are involved in several C. elegans behaviors, including thermotaxis (22).
Fig. 1.
AP-1 subunit μ1/unc-101 is required for polarized localization of glutamate receptor GLR-1 in RIA neurons. (A) Schematic diagrams of the left RIA neuron as viewed from the left and the anterior side of the worm, respectively. RIA neurite is polarized, with a proximal region that is exclusively postsynaptic (green), followed by an asynaptic isthmus region (black), and a distal, mainly presynaptic region (red). (B and C) Representative wild-type animal expressing synaptic vesicle marker mCherry::RAB-3 (B) and AMPA-type glutamate receptor subunit GLR-1::GFP (C). For this and all subsequent images, asterisk denotes the RIA cell body; arrows point to the presynaptic region of the RIA neurite. Anterior is to the left, ventral is down. (Scale bar: 10 μm.) High-magnification views of RIA presynaptic region are shown in B′ and C′, and of postsynaptic region in B′′ and C′′. (D) A diagram depicting localization of GLR-1::GFP in a wild-type RIA neuron. (E and F) Representative unc-101(m1) animal expressing mCherry::RAB-3 (E) and GLR-1::GFP (F). GLR-1::GFP is mislocalized to the presynaptic region of the RIA neurite in unc-101 mutants (arrows). High-magnification views of RIA presynaptic region are shown in E′ and F′, and of postsynaptic region in E′′ and F′′. (G) A diagram depicting localization of GLR-1::GFP in an unc-101 RIA neuron. (H) Polarity index quantification for mCherry::RAB-3 and GLR-1::GFP in wild-type and unc-101 mutants. ***P < 0.001; error bars, SEM.
We visualized the presynaptic specializations in RIA neurons in vivo by expressing synaptic vesicle-associated protein, RAB-3, fused to mCherry (23), under a RIA-specific glr-3 promoter (20). As predicted by the EM reconstruction, this construct localized to the distal, presynaptic segment of the RIA process (Fig. 1B). The postsynaptic region of RIA was visualized using GLR-1, an AMPA-type glutamate receptor subunit (24, 25). glr-1 is expressed in RIA, because electrophysiological studies have found that the majority of glutamate-induced current in RIA is abolished in glr-1 mutants (V. Maricq, personal communication). The functional GLR-1::GFP construct (26) localized predominantly to the proximal, postsynaptic segment of RIA (Fig. 1 C and D). We next investigated the localization of RAB-3 and GLR-1 in various C. elegans mutants. In particular, we chose to focus on the AP-1 subunit μ1/UNC-101 because of its importance for the localization of odorant receptors to cilia in C. elegans (16) and the involvement of μ1B in epithelial basolateral sorting (11, 15). We found that in animals mutant for unc-101(m1), a strong loss-of-function allele (27), GLR-1::GFP was uniformly distributed throughout postsynaptic and presynaptic compartments (Fig. 1 F and G), whereas mCherry::RAB-3 localization was unaffected (Fig. 1E). We quantified this finding by comparing average fluorescence intensities in the postsynaptic and presynaptic regions of RIA and deriving a polarity index, PI (see supporting information (SI) Methods). A completely axonal protein has a PI of 0, whereas an exclusively dendritically localized protein has a PI of 1. As shown in Fig. 1H, the PI of mCherry::RAB-3 was not affected by the unc-101 mutation, whereas GLR-1::GFP distribution became unpolarized. Thus, μ1/UNC-101 is required for the localization of GLR-1::GFP to the postsynaptic compartment in RIA neurons. We were unable to test the requirement for the other 3 subunits of the AP-1 complex because their RNAi leads to embryonic lethality (28).
μ1/unc-101 Is Required for Localization of Multiple Postsynaptic Receptors in Several Polarized C. elegans Neurons.
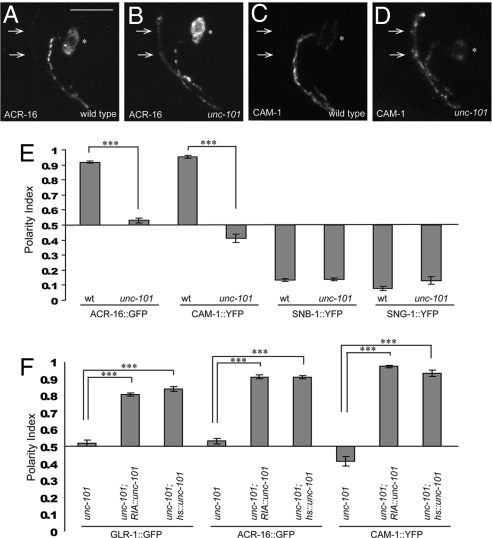
We next investigated whether UNC-101 is required for dendritic localization of other postsynaptic receptors in RIA in addition to GLR-1. ACR-16 is a nicotinic acetylcholine receptor (nAChR) subunit (29), homologous to the vertebrate nAChR subunit α7, which localizes dendritically in cultured hippocampal neurons (30). In RIA, the functional ACR-16::GFP construct (31) reproducibly localizes to the postsynaptic segment of the RIA process and is excluded from the presynaptic segment (Fig. 2 A and E). ACR-16::GFP distribution was dramatically affected in unc-101 mutants, in which it localizes both pre- and postsynaptically (Fig. 2 B and E).
Fig. 2.
μ1/unc-101 is required for dendritic localization of 2 additional postsynaptic receptors in RIA neurons, and acts cell autonomously to maintain polarized receptor distribution. (A and B) α7-type nicotinic acetylcholine receptor subunit ACR-16::GFP localizes selectively to the RIA postsynaptic region in wild-type animals (A), but is mislocalized to the presynaptic region (arrows) in unc-101(m1) mutants (B). (C and D) ROR-type receptor tyrosine kinase CAM-1::YFP localizes selectively to the RIA postsynaptic region in wild-type animals (C) but is mislocalized to the presynaptic region (arrows) in unc-101(m1) mutants (D). (E) Polarity index quantification for ACR-16::GFP and CAM-1::YFP in wild-type and unc-101(m1) animals. In addition, polarity indices for 2 presynaptic markers, SNB-1::YFP and SNG-1::YFP are also shown. (F) Polarity indices quantification for GLR-1::GFP, ACR-16::GFP, and CAM-1::YFP in unc-101(m1) mutants expressing unc-101 cDNA under the control of either RIA-specific glr-3 promoter or heat-shock promoter. ***P < 0.001; error bars, SEM.
We also examined the localization of another dendritic construct, CAM-1::YFP (Fig. 2 C and D). CAM-1 is a receptor tyrosine kinase that localizes somatodendrically in a number of C. elegans neurons (32, 33). Its mammalian homologs, Ror1 and Ror2, also localize somatodendritically in cultured hippocampal neurons (34). As expected, CAM-1::YFP localized to the postsynaptic region of RIA neurons (Fig. 2 C and E) and became unpolarized in unc-101 mutants (Fig. 2 D and E). Importantly, unlike at the worm neuromuscular junction (31), RIA ACR-16::GFP localization was not affected in cam-1(gm122) mutants (data not shown). Thus, ACR-16 and CAM-1 can be treated as 2 independent dendritic markers in RIA neurons.
To confirm specificity of unc-101 phenotype for postsynaptic markers, we examined the localization of 2 additional presynaptic markers (35), synaptobrevin (SNB-1::YFP) and synaptogyrin (SNG-1::YFP). The localization of these integral membrane synaptic proteins was not affected in unc-101 mutants (Fig. 2E and Fig. S1 A–D), suggesting that UNC-101 is selectively required for postsynaptic protein localization in RIA neurons. Furthermore, to investigate if unc-101 phenotype is selective for RIA neurons, we observed postsynaptic marker localization in 2 other polarized neurons in C. elegans, the motor neuron DA9 and the interneuron AVE. CAM-1::YFP localization to the dendrite and proximal axon of DA9 (32) and GLR-1::YFP localization to the postsynaptic region of AVE were both disrupted in unc-101 mutants (Fig. S2). Together, these findings show that unc-101 affects localization of postsynaptic receptors in a number of polarized C. elegans neurons.
μ1/unc-101 Acts Cell Autonomously in RIA Neurons to Maintain Polarized Distribution of Postsynaptic Receptors.
The mislocalization of postsynaptic receptors in RIA and other neurons could result from the loss of UNC-101 function in the neurons themselves or be a consequence of disruptions in their cellular environment. To distinguish between these 2 possibilities, we expressed unc-101 cDNA under the control of the RIA-specific promoter Pglr-3 (20) in unc-101(m1) mutants. This construct was able to fully rescue GLR-1::GFP, ACR-16::GFP, and CAM-1::YFP localization defects of unc-101 mutants (Fig. 2F and Fig. S1 E, G, and I), suggesting that UNC-101 plays a cell-autonomous role in determining RIA polarity.
A priori, unc-101 phenotype could be explained by a developmental defect in RIA polarization, in which the dendritic fate of the RIA proximal segment is not correctly specified or postsynaptic terminals are not appropriately formed. Alternatively, UNC-101 may be required to maintain ongoing trafficking of postsynaptic receptors to the dendrite, in which case we would predict that its phenotype could be rescued even after development has been completed. To distinguish between these possibilities, we expressed unc-101 cDNA under the control of inducible heat-shock promoter (36). The transgenic animals carrying the Phs::unc-101 construct were heat shocked for 2 h at the L1/L2 stage (at which point RIA polarity is already established), and the degree of rescue was assessed 24 h later. Phs::unc-101 construct was able to fully rescue the GLR-1, ACR-16, and CAM-1 mislocalization defects in unc-101 animals (Fig. 2F and Fig. S1 F, H, and J). Thus, UNC-101 is required for maintenance of postsynaptic receptor polarized distribution in RIA neurons.
Cytosolic Domains of Postsynaptic Receptors Are Necessary and Sufficient for Dendritic Localization in RIA Neurons.
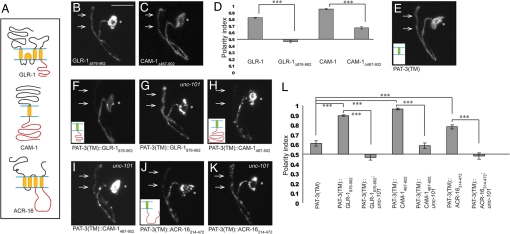
μ subunits of AP complexes classically mediate cargo recognition by binding tyrosine- or dileucine-based sorting motifs in the cytoplasmic portions of their targets (12). To elucidate how μ1/UNC-101 regulates postsynaptic localization of its targets, we performed structure-function experiments on GLR-1, CAM-1, and ACR-16 to identify sequences that mediate their dendritic enrichment. We hypothesized that the cytoplasmic C-terminus regions of GLR-1 and CAM-1, and the prominent cytoplasmic loop between the third and the fourth transmembrane domain of ACR-16, contain sorting information responsible for their postsynaptic enrichment (Fig. 3A).
Fig. 3.
Cytosolic domains of postsynaptic receptors are necessary and sufficient for dendritic localization in RIA neurons, in a manner dependent on unc-101. (A) Schematic representation of GLR-1, CAM-1, and ACR-16. Transmembrane regions are shown in yellow, key cytosolic regions in red. (B) Localization of the truncated GLR-1 lacking its C-terminal cytoplasmic domain, GLR-1Δ876–962::YFP, in wild-type RIA neurons. (C) Localization of truncated CAM-1 lacking its C-terminal cytoplasmic domain, CAM-1Δ467–902::YFP, in wild-type RIA neurons. (D) Polarity index quantification. (E) PAT-3(TM)::YFP is unpolarized in RIA neurons. Inset in this and subsequent images shows the schematic representation of the construct. (F and G) RIA localization of PAT-3(TM)::GLR-1876–962::YFP in wild-type (F) and unc-101(m1) (G) animals. (H and I) RIA localization of PAT-3(TM)::CAM-1467–902::YFP in wild-type (H) and unc-101 (I) animals. (J and K) RIA localization of PAT-3(TM)::ACR-16314–472::YFP in wild-type (J) and unc-101 (K) animals. (L) Polarity index quantification for PAT-3 gain-of-function constructs. ***P < 0.001; error bars, SEM.
To test this hypothesis, we made truncated GLR-1 and CAM-1 constructs, GLR-1Δ876–962 and CAM-1Δ467–902, which lack entire cytoplasmic C termini. We found that these truncated constructs were significantly less dendritically enriched than the full-length constructs (Fig. 3 B–D). Thus, cytoplasmic C termini of GLR-1 and CAM-1 are necessary for postsynaptic localization in RIA neurons. To test whether the same sequences are also sufficient for postsynaptic localization, we fused them in frame with the transmembrane domain of an integrin, PAT-3 (37). When tagged with YFP, PAT-3(TM) localizes in a nonpolarized fashion to both pre- and postsynaptic domains of RIA neurons (Fig. 3 E and L). However, when GLR-1 and CAM-1 C-terminus regions and ACR-16 cytoplasmic loop were fused to PAT-3(TM), these constructs displayed a striking postsynaptic localization (Fig. 3 F, H, J, and L). Thus, cytoplasmic sequences of GLR-1, CAM-1, and ACR-16 are sufficient for postsynaptic targeting in RIA neurons.
Finally, if μ1/UNC-101 is recognizing these cytoplasmic sequences and sorting them to the postsynaptic domain of RIA, we would expect that the PAT-3 gain-of-function constructs would be mislocalized in unc-101 mutants. Indeed, when crossed into unc-101, PAT-3(TM)::GLR-1876–962, PAT-3(TM)::CAM-1467–902, and PAT-3(TM)::ACR-16314–472 become unpolarized (Fig. 3 G, I, K, and L).
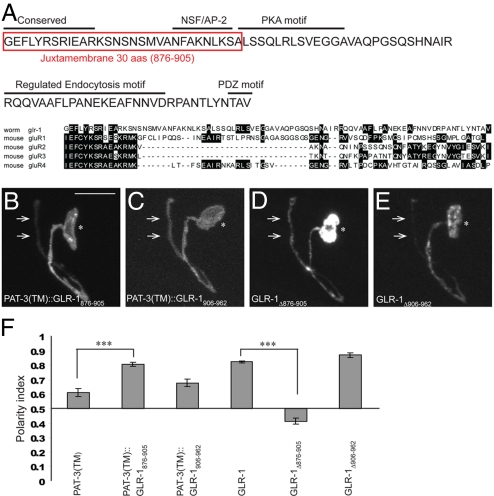
Juxtamembrane 30 aa of GLR-1 Are Necessary and Sufficient for RIA Postsynaptic Localization.
Given the importance of glutamate receptors for basal neurotransmission and plasticity in the vertebrate nervous system (38), we sought to identify more specific dendritic targeting motifs within the GLR-1 C terminus. Alignment with vertebrate glutamate receptors had previously revealed a highly conserved stretch of amino acids that immediately follow the last transmembrane domain (37) (Fig. 4A). We hypothesized that these amino acids may be important for postsynaptic targeting of GLR-1 in RIA neurons.
Fig. 4.
Juxtamembrane 30 aa of GLR-1 are necessary and sufficient for RIA postsynaptic localization. (A) Amino acid sequence of GLR-1 cytoplasmic C terminus and alignment with vertebrate glutamate receptor C termini. Sequence boxed in red is necessary and sufficient for GLR-1 dendritic localization. (B) PAT-3 fused to juxtamembrane 30 aa of GLR-1 (PAT-3(TM)::GLR-1876–905::YFP) is dendritically enriched in RIA neurons. (C) PAT-3 fused to distal 56 aa of GLR-1 (PAT-3(TM)::GLR-1906–962::YFP) is more uniformly distributed in RIA neurons. (D) GLR-1 lacking juxtamembrane 30 aa (GLR-1Δ876–905::YFP) is unpolarized in RIA neurons. (E) GLR-1 lacking the distal 56 aa of its C terminus (GLR-1Δ906–962::YFP) localizes to the RIA dendrite. (F) Polarity index quantification. ***P < 0.001; error bars, SEM.
To test this hypothesis, we performed further structure-function experiments and found that 30 juxtamembrane amino acids (876–905) of GLR-1 C terminus are critical for its dendritic localization in RIA neurons. PAT-3(TM)::GLR-1876–905 construct preferentially localized to the postsynaptic domain of RIA neurons (Fig. 4 B and F), whereas PAT-3(TM)::GLR-1906–962 localized less well (Fig. 4 C and F). Conversely, GLR-1Δ876–905, which lacks the juxtamembrane 30 aa, was unpolarized (Fig. 4 D and F), whereas GLR-1Δ906–962, which lacks the distal portion of the C terminus tail, localized well to the RIA dendrite (Fig. 4 E and F). These findings suggest that juxtamembrane 30 aa of GLR-1 are both necessary and sufficient for GLR-1 localization to the RIA postsynaptic region. Because μ subunits classically recognize tyrosine-based sorting motifs, we mutated a conserved tyrosine in the juxtamembrane region of GLR-1 into alanine (Y880A). This alteration was not sufficient to mislocalize the full-length GLR-1::GFP (data not shown), suggesting that the specific sorting motif responsible for dendritic localization of GLR-1 may be unconventional or redundant.
μ1/UNC-101 Localizes to Presynaptic Sites, Where It May Be Involved in the Retrieval of Postsynaptic Receptors.
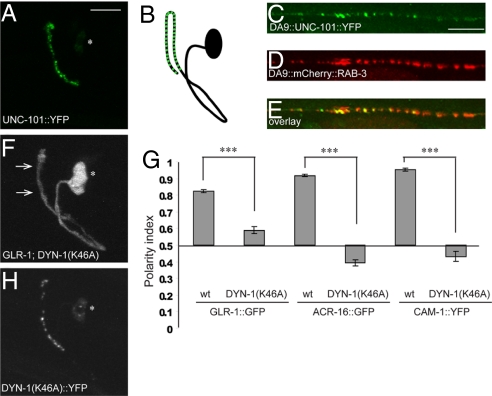
To determine the subcellular site of action of UNC-101, we expressed fluorophore-tagged UNC-101 in RIA neurons. This construct was functional, as it was able to rescue GLR-1::GFP mislocalization in unc-101 mutants (data not shown). Interestingly, we found that UNC-101::YFP localized to the presynaptic domain of the RIA process (Fig. 5 A and B). To determine if UNC-101::YFP is indeed localizing to presynaptic sites, we expressed UNC-101::YFP in the DA9 neuron, in which presynaptic sites are well segregated from each other and thus one can distinguish between axonal vs. presynaptic localization. In the DA9 neuron, UNC-101::YFP colocalized with the synaptic vesicle marker mCherry::RAB-3 (Fig. 5 C–E), suggesting that UNC-101 is acting at the presynaptic sites.
Fig. 5.
μ1/UNC-101 localizes to presynaptic sites, where it may be involved in retrieval of postsynaptic receptors. (A and B) Localization of UNC-101::YFP to the presynaptic region of RIA neurons (A), and the accompanying schematic (B). (C–E) DA9 localization of UNC-101::YFP (C), mCherry::RAB-3 (D), and the overlay (E). (F) GLR-1::GFP is mislocalized to the presynaptic region of RIA (arrows) in the presence of dynamin DYN-1(K46A). (G) Polarity index quantification for GLR-1::GFP, ACR-16::GFP, and CAM-1::YFP in the absence and presence of DYN-1(K46A). ***P < 0.001; error bars, SEM. (H) DYN-1(K46A)::YFP localizes to the presynaptic region of RIA neurons.
Localization of μ1/UNC-101 to the presynaptic sites raised the possibility that UNC-101 acts postendocytically to retrieve postsynaptic receptors from the “inappropriate” axonal compartment. To test this hypothesis, we sought to disrupt endocytosis in RIA neurons and see if this leads to mislocalization of postsynaptic receptors. Dynamin is a mechanoenzyme that acts as a key regulator of endocytosis by pinching off clathrin-coated vesicles from the membrane (39). To test if dynamin is required for the polarized distribution of GLR-1, we expressed in RIA neurons a dominant negative version of C. elegans dynamin, dyn-1, in which the key lysine residue in the GTP-binding site was replaced by alanine (K46A, equivalent to vertebrate K44A) (40). DynaminK44A has been broadly used to potently interfere with endocytosis in a variety of cell types (41). We observed a striking mislocalization of GLR-1, ACR-16, and CAM-1 proteins to the RIA presynaptic region in DYN-1(K46A) transgenic animals (Fig. 5 F and G and Fig. S3 A and B), consistent with the idea that endocytosis is required for proper postsynaptic protein localization. We also measured the absolute intensity of GLR-1::GFP in RIA pre- and postsynaptic segments of wild-type, unc-101(m1), and DYN-1(K46A) animals, and found that the average intensity in RIA dendrite was not elevated in mutant animals compared with the wild type (Fig. S3C). These findings are consistent with the idea that the observed phenotypes are not a result of an overall lack of receptor turnover in the dendrite resulting in “spillover” into the axon.
We also investigated the consequences of absence of UNC-101 and presence of dominant negative dynamin in the same cell. unc-101 animals carrying DYN-1(K46A) transgene had polarity indices that were significantly different from wild-type animals and qualitatively similar to each of the mutants alone (Fig. S3D). Although the interpretation of this finding is complicated by the fact that DYN-1(K46A) is a dominant-negative construct and not a null loss-of-function allele, these findings are consistent with dyn-1 and unc-101 acting in the same genetic pathway. Interestingly, DYN-1(K46A) tagged with YFP localized to the presynaptic domain in RIA in a pattern similar to that of UNC-101::YFP (Fig. 5H), suggesting that it acts mainly at the presynaptic sites to block endocytosis of postsynaptic receptors. This is consistent with previous reports that DYN-1 localizes presynaptically in C. elegans (42).
Our attempts at genetic loss-of-function block of endocytosis were less successful. A temperature-sensitive allele of dynamin, dyn-1(ky51), did not display a defect in postsynaptic receptor localization in RIA neurons at the restrictive temperature (data not shown). However, animals carrying this hypomorphic allele regain the ability to move after initial uncoordination at the restrictive temperature, which is never observed in the Drosophila shibire mutants (43). This suggests that the endocytosis block in dyn-1(ky51) animals is not complete and may explain the discrepancy between our dominant negative and loss-of-function data.
We also sought to interfere with endocytosis by examining 2 additional mutants, AP-2 subunit μ2 dpy-23(e840) and AP180 unc-11(e47). The vertebrate AP-2 complex has been shown to interact with the AMPA-type GluR2 receptor and mediate endocytosis of GluR2 at the postsynaptic terminals (44, 45). A mutation in the homologue of AP-2 μ2 subunit, dpy-23(e840), had no obvious effect on receptor localization in RIA neurons (data not shown). However, the role of dpy-23 in postsynaptic receptor endocytosis in C. elegans has not been documented, and its role in synaptic vesicle endocytosis remains to be clarified (46). Finally, clathrin adaptor AP180 unc-11(e47) mutants also displayed no GLR-1 polarization phenotype in RIA neurons (data not shown). This finding is surprising given the importance of unc-11 for regulating GLR-1 abundance in the C. elegans ventral nerve cord (47–49), and may reflect cell-specific differences in the reliance on particular components of the endocytosis machinery.
Discussion
Information flow in neural circuits is subserved by anatomical and functional polarization of neurons into axons and dendrites. Though much progress has been made in understanding local cycling and dynamics of postsynaptic receptors (in particular, AMPA-type glutamate receptors) at the postsynaptic membrane (38), less is known about global mechanisms that specify enrichment of postsynaptic receptors in dendrites in the first place. Here we use the transparent nematode C. elegans to examine postsynaptic receptor sorting in vivo and with single-cell resolution. We find that μ1/UNC-101 subunit of the AP-1 adaptor complex is required for polarized localization of several postsynaptic receptors to dendrites in C. elegans. Furthermore, we present evidence showing that UNC-101 may be acting postendocytically at presynaptic sites to remove ectopic postsynaptic receptors from the axonal compartment, thus leading to their steady-state dendritic enrichment.
Our data, however, do not exclude a model in which UNC-101 also functions at the TGN to sort GLR-1-, CAM-1-, and ACR-16-carrying vesicles. Indeed, a previous study that examined the role of UNC-101 in targeting of olfactory receptors to cilia in sensory neurons proposed that UNC-101 acts as a dendritic sorter at the TGN (16). This conclusion was based on 2 lines of evidence: first, the authors observed a lack of phenotype in the hypomorphic dyn-1(ky51) mutants and in dpy-23(e840) mutants, which is consistent with our findings using the same alleles. It is possible that endocytosis needs to be severely compromised for a receptor localization phenotype to become apparent. Second, the authors performed live imaging experiments, convincingly showing that odorant receptors are trafficked from the cell body to the cilia in wild-type animals. This trafficking was abolished in unc-101 mutants, suggesting that UNC-101 plays a role in the formation of “dendritic vesicles” in the cell body. It is possible that UNC-101 plays a role at both of these locales, to initially direct receptors to the right location and also to maintain their enrichment there. Because it has not been reported that C. elegans neurons have a diffusion barrier between axons and dendrites comparable to the axon initial segment in vertebrate neurons (50), active removal of postsynaptic receptors from the axonal segment would be necessary to combat lateral diffusion even in the presence of initial, TGN-based targeting. In epithelial cells, AP-1 μ1B subunit acts in recycling endosomes to mediate both targeting from TGN via the transendosomal route and postendocytic recycling to the basolateral membrane (14, 15). Thus, there is precedent for a μ1 subunit playing a sorting role along both secretory and endocytic pathways in the same cell type.
μ subunits classically mediate cargo recognition by binding to motifs in the cytosolic sequences of target proteins (12). In agreement with this, we found that cytosolic sequences of the tested postsynaptic receptors are both necessary and sufficient for dendritic targeting in RIA neurons. Interestingly, the equivalent cytosolic sequences of their vertebrate homologues (GluR1 C-terminus region and α7 nAChR M3-M4 loop) are sufficient for dendritic localization in cultured hippocampal neurons (30, 51). These findings suggest that the mechanisms of dendritic targeting may be evolutionarily conserved, which is remarkable given that C. elegans neurons are morphologically quite different from vertebrate hippocampal neurons. In addition to being smaller and simpler, 2 of the neurons used in this study (RIA and AVE) have a pseudounipolar morphology, with a single neurite that is functionally partitioned into an axon and a dendrite. Furthermore, vertebrate neurons contain the axon initial segment, which acts as a diffusion barrier between axon and dendrites and helps maintain neuronal polarity (50, 52); this feature is likely not found in C. elegans neurons. The evolutionary conservation even in such morphologically different cell types highlights the fundamental importance of this mechanism of dendritic targeting.
Further structure-function analysis of GLR-1 C terminus showed that the juxtamembrane 30 aa are necessary and sufficient for dendritic localization. This result is in agreement with the finding that the proximal 39 aa of GluR1 tail are sufficient for dendritic targeting in vertebrate neurons (51). The juxtamembrane 30 aa of GLR-1 contain a proximal, highly conserved 12 aa sequence that is present in vertebrate glutamate receptors (37). This highly conserved sequence has been found to be critical for AMPA-stimulated GluR2 endocytosis in HEK cells (53), and for endocytosis and endocytic sorting of NMDA receptors (54). In addition, the juxtamembrane tail portions of glutamate receptors are known to interact with actin-associated protein 4.1N (GluR1) (55), fusion-associated ATPase NSF (GluR2) (56, 57), and the adaptor complex AP-2 (GluR2) (45). Thus, this region of glutamate receptor C termini appears crucial for various endocytic and exocytic processes, as well as interactions with the cytoskeleton. Interestingly, distal C-terminus PDZ motifs of AMPA-type glutamate receptors have been implicated in their synaptic clustering/retention in both vertebrates (58, 59) and in C. elegans (26, 37). Yet, these PDZ motifs are not required for glutamate receptor dendritic targeting (51, and this study), suggesting that molecular mechanisms specifying “global targeting” (to the dendrite) and “local targeting” (to the synapse) are at least partly distinct.
Materials and Methods
Strains and Genetics.
Worms were raised on OP50 E. coli seeded NGM plates at 22 °C. Wild-type animals were N2 Bristol strain. The following strains were provided by the Caenorhabditis Genetics Center: DR1 unc-101(m1) I; NG2615 cam-1(gm122) II; CB840 dpy-23(e840) X; CB47 unc-11(e47) I; and CX51 dyn-1(ky51) X.
Cloning and Constructs.
Plasmids and transgenic strains were generated using standard techniques (60). Transgenic strain list and detailed subcloning information are included in SI Methods.
Supplementary Material
Acknowledgments.
We thank M. Patel and B. Winckler for helpful comments on the manuscript; C. Maeder for help with microscopy; A. Fire, J. Kaplan, V. Maricq, and C. Rongo for sharing strains and reagents; and the Caenorhabditis Genetics Center and NBP Japan for providing strains. K.S. is supported by the Howard Hughes Medical Institute, Human Frontier Science Foundation, and the W.M. Keck Foundation. M.A.M is supported by the Stanford Medical Scientist Training Program and National Institutes of Health Grant GM007365. G.J.W. is supported by a National Science Foundation Graduate Research Fellowship and a Stanford Graduate Fellowship.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812078106/DCSupplemental.
References
- 1.Horton AC, Ehlers MD. Neuronal polarity and trafficking. Neuron. 2003;40:277–295. doi: 10.1016/s0896-6273(03)00629-9. [DOI] [PubMed] [Google Scholar]
- 2.Winckler B, Mellman I. Neuronal polarity: Controlling the sorting and diffusion of membrane components. Neuron. 1999;23:637–640. doi: 10.1016/s0896-6273(01)80021-0. [DOI] [PubMed] [Google Scholar]
- 3.Burack MA, Silverman MA, Banker G. The role of selective transport in neuronal protein sorting. Neuron. 2000;26:465–472. doi: 10.1016/s0896-6273(00)81178-2. [DOI] [PubMed] [Google Scholar]
- 4.Wisco D, et al. Uncovering multiple axonal targeting pathways in hippocampal neurons. J Cell Biol. 2003;162:1317–1328. doi: 10.1083/jcb.200307069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrido JJ, et al. Identification of an axonal determinant in the C-terminus of the sodium channel Na(v) 1.2. EMBO J. 2001;20:5950–5961. doi: 10.1093/emboj/20.21.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sampo B, Kaech S, Kunz S, Banker G. Two distinct mechanisms target membrane proteins to the axonal surface. Neuron. 2003;37:611–624. doi: 10.1016/s0896-6273(03)00058-8. [DOI] [PubMed] [Google Scholar]
- 7.West AE, Neve RL, Buckley KM. Identification of a somatodendritic targeting signal in the cytoplasmic domain of the transferrin receptor. J Neurosci. 1997;17:6038–6047. doi: 10.1523/JNEUROSCI.17-16-06038.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivera JF, Ahmad S, Quick MW, Liman ER, Arnold DB. An evolutionarily conserved dileucine motif in Shal K+ channels mediates dendritic targeting. Nat Neurosci. 2003;6:243–250. doi: 10.1038/nn1020. [DOI] [PubMed] [Google Scholar]
- 9.Dotti CG, Simons K. Polarized sorting of viral glycoproteins to the axon and dendrites of hippocampal neurons in culture. Cell. 1990;62:63–72. doi: 10.1016/0092-8674(90)90240-f. [DOI] [PubMed] [Google Scholar]
- 10.Jareb M, Banker G. The polarized sorting of membrane proteins expressed in cultured hippocampal neurons using viral vectors. Neuron. 1998;20:855–867. doi: 10.1016/s0896-6273(00)80468-7. [DOI] [PubMed] [Google Scholar]
- 11.Folsch H, Ohno H, Bonifacino JS, Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99:189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- 12.Nakatsu F, Ohno H. Adaptor protein complexes as the key regulators of protein sorting in the post-Golgi network. Cell Struct Funct. 2003;28:419–429. doi: 10.1247/csf.28.419. [DOI] [PubMed] [Google Scholar]
- 13.Boehm M, Bonifacino JS. Genetic analyses of adaptin function from yeast to mammals. Gene. 2002;286:175–186. doi: 10.1016/s0378-1119(02)00422-5. [DOI] [PubMed] [Google Scholar]
- 14.Ang AL, et al. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J Cell Biol. 2004;167:531–543. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gan Y, McGraw TE, Rodriguez-Boulan E. The epithelial-specific adaptor AP1B mediates post-endocytic recycling to the basolateral membrane. Nat Cell Biol. 2002;4:605–609. doi: 10.1038/ncb827. [DOI] [PubMed] [Google Scholar]
- 16.Dwyer ND, Adler CE, Crump JG, L'Etoile ND, Bargmann CI. Polarized dendritic transport and the AP-1 mu1 clathrin adaptor UNC-101 localize odorant receptors to olfactory cilia. Neuron. 2001;31:277–287. doi: 10.1016/s0896-6273(01)00361-0. [DOI] [PubMed] [Google Scholar]
- 17.Bae YK, et al. General and cell-type specific mechanisms target TRPP2/PKD-2 to cilia. Development. 2006;133:3859–3870. doi: 10.1242/dev.02555. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda S, et al. Accumulation of AMPA receptors in autophagosomes in neuronal axons lacking adaptor protein AP-4. Neuron. 2008;57:730–745. doi: 10.1016/j.neuron.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 19.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 20.Brockie PJ, Madsen DM, Zheng Y, Mellem J, Maricq AV. Differential expression of glutamate receptor subunits in the nervous system of Caenorhabditis elegans and their regulation by the homeodomain protein UNC-42. J Neurosci. 2001;21:1510–1522. doi: 10.1523/JNEUROSCI.21-05-01510.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsalik EL, et al. LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Dev Biol. 2003;263:81–102. doi: 10.1016/s0012-1606(03)00447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mori I, Ohshima Y. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature. 1995;376:344–348. doi: 10.1038/376344a0. [DOI] [PubMed] [Google Scholar]
- 23.Mahoney TR, et al. Regulation of synaptic transmission by RAB-3 and RAB-27 in Caenorhabditis elegans. Mol Biol Cell. 2006;17:2617–2625. doi: 10.1091/mbc.E05-12-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hart AC, Sims S, Kaplan JM. Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor. Nature. 1995;378:82–85. doi: 10.1038/378082a0. [DOI] [PubMed] [Google Scholar]
- 25.Maricq AV, Peckol E, Driscoll M, Bargmann CI. Mechanosensory signalling in C. elegans mediated by the GLR-1 glutamate receptor. Nature. 1995;378:78–81. doi: 10.1038/378078a0. [DOI] [PubMed] [Google Scholar]
- 26.Rongo C, Whitfield CW, Rodal A, Kim SK, Kaplan JM. LIN-10 is a shared component of the polarized protein localization pathways in neurons and epithelia. Cell. 1998;94:751–759. doi: 10.1016/s0092-8674(00)81734-1. [DOI] [PubMed] [Google Scholar]
- 27.Lee J, Jongeward GD, Sternberg PW. unc-101, a gene required for many aspects of Caenorhabditis elegans development and behavior, encodes a clathrin-associated protein. Genes Dev. 1994;8:60–73. doi: 10.1101/gad.8.1.60. [DOI] [PubMed] [Google Scholar]
- 28.Shim J, Sternberg PW, Lee J. Distinct and redundant functions of mu1 medium chains of the AP-1 clathrin-associated protein complex in the nematode Caenorhabditis elegans. Mol Biol Cell. 2000;11:2743–2756. doi: 10.1091/mbc.11.8.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Touroutine D, et al. acr-16 encodes an essential subunit of the levamisole-resistant nicotinic receptor at the Caenorhabditis elegans neuromuscular junction. J Biol Chem. 2005;280:27013–27021. doi: 10.1074/jbc.M502818200. [DOI] [PubMed] [Google Scholar]
- 30.Xu J, Zhu Y, Heinemann SF. Identification of sequence motifs that target neuronal nicotinic receptors to dendrites and axons. J Neurosci. 2006;26:9780–9793. doi: 10.1523/JNEUROSCI.0840-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Francis MM, et al. The Ror receptor tyrosine kinase CAM-1 is required for ACR-16-mediated synaptic transmission at the C. elegans neuromuscular junction. Neuron. 2005;46:581–594. doi: 10.1016/j.neuron.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Klassen MP, Shen K. Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in C. elegans. Cell. 2007;130:704–716. doi: 10.1016/j.cell.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 33.Sieburth D, et al. Systematic analysis of genes required for synapse structure and function. Nature. 2005;436:510–517. doi: 10.1038/nature03809. [DOI] [PubMed] [Google Scholar]
- 34.Paganoni S, Ferreira A. Expression and subcellular localization of Ror tyrosine kinase receptors are developmentally regulated in cultured hippocampal neurons. J Neurosci Res. 2003;73:429–440. doi: 10.1002/jnr.10674. [DOI] [PubMed] [Google Scholar]
- 35.Nonet ML. Visualization of synaptic specializations in live C. elegans with synaptic vesicle protein-GFP fusions. J Neurosci Methods. 1999;89:33–40. doi: 10.1016/s0165-0270(99)00031-x. [DOI] [PubMed] [Google Scholar]
- 36.Stringham EG, Dixon DK, Jones D, Candido EP. Temporal and spatial expression patterns of the small heat shock (hsp16) genes in transgenic Caenorhabditis elegans. Mol Biol Cell. 1992;3:221–233. doi: 10.1091/mbc.3.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang HC, Rongo C. Cytosolic tail sequences and subunit interactions are critical for synaptic localization of glutamate receptors. J Cell Sci. 2005;118:1945–1956. doi: 10.1242/jcs.02320. [DOI] [PubMed] [Google Scholar]
- 38.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 39.McNiven MA, Cao H, Pitts KR, Yoon Y. The dynamin family of mechanoenzymes: Pinching in new places. Trends Biochem Sci. 2000;25:115–120. doi: 10.1016/s0968-0004(99)01538-8. [DOI] [PubMed] [Google Scholar]
- 40.van der Bliek AM, et al. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Damke H. Dynamin and receptor-mediated endocytosis. FEBS Lett. 1996;389:48–51. doi: 10.1016/0014-5793(96)00517-0. [DOI] [PubMed] [Google Scholar]
- 42.Labrousse AM, Shurland DL, van der Bliek AM. Contribution of the GTPase domain to the subcellular localization of dynamin in the nematode Caenorhabditis elegans. Mol Biol Cell. 1998;9:3227–3239. doi: 10.1091/mbc.9.11.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark SG, Shurland DL, Meyerowitz EM, Bargmann CI, van der Bliek AM. A dynamin GTPase mutation causes a rapid and reversible temperature-inducible locomotion defect in C. elegans. Proc Natl Acad Sci USA. 1997;94:10438–10443. doi: 10.1073/pnas.94.19.10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carroll RC, et al. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc Natl Acad Sci USA. 1999;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SH, Liu L, Wang YT, Sheng M. Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron. 2002;36:661–674. doi: 10.1016/s0896-6273(02)01024-3. [DOI] [PubMed] [Google Scholar]
- 46.Dittman JS, Kaplan JM. Factors regulating the abundance and localization of synaptobrevin in the plasma membrane. Proc Natl Acad Sci USA. 2006;103:11399–11404. doi: 10.1073/pnas.0600784103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burbea M, Dreier L, Dittman JS, Grunwald ME, Kaplan JM. Ubiquitin and AP180 regulate the abundance of GLR-1 glutamate receptors at postsynaptic elements in C. elegans. Neuron. 2002;35:107–120. doi: 10.1016/s0896-6273(02)00749-3. [DOI] [PubMed] [Google Scholar]
- 48.Grunwald ME, Mellem JE, Strutz N, Maricq AV, Kaplan JM. Clathrin-mediated endocytosis is required for compensatory regulation of GLR-1 glutamate receptors after activity blockade. Proc Natl Acad Sci USA. 2004;101:3190–3195. doi: 10.1073/pnas.0306156101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dreier L, Burbea M, Kaplan JM. LIN-23-mediated degradation of beta-catenin regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans. Neuron. 2005;46:51–64. doi: 10.1016/j.neuron.2004.12.058. [DOI] [PubMed] [Google Scholar]
- 50.Winckler B, Forscher P, Mellman I. A diffusion barrier maintains distribution of membrane proteins in polarized neurons. Nature. 1999;397:698–701. doi: 10.1038/17806. [DOI] [PubMed] [Google Scholar]
- 51.Ruberti F, Dotti CG. Involvement of the proximal C terminus of the AMPA receptor subunit GluR1 in dendritic sorting. J Neurosci. 2000;20:RC78. doi: 10.1523/JNEUROSCI.20-11-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hedstrom KL, Ogawa Y, Rasband MN. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J Cell Biol. 2008;183:635–640. doi: 10.1083/jcb.200806112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin JW, et al. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat Neurosci. 2000;3:1282–1290. doi: 10.1038/81814. [DOI] [PubMed] [Google Scholar]
- 54.Scott DB, Michailidis I, Mu Y, Logothetis D, Ehlers MD. Endocytosis and degradative sorting of NMDA receptors by conserved membrane-proximal signals. J Neurosci. 2004;24:7096–7109. doi: 10.1523/JNEUROSCI.0780-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen L, Liang F, Walensky LD, Huganir RL. Regulation of AMPA receptor GluR1 subunit surface expression by a 4.1N-linked actin cytoskeletal association. J Neurosci. 2000;20:7932–7940. doi: 10.1523/JNEUROSCI.20-21-07932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nishimune A, et al. NSF binding to GluR2 regulates synaptic transmission. Neuron. 1998;21:87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- 57.Song I, et al. Interaction of the N-ethylmaleimide-sensitive factor with AMPA receptors. Neuron. 1998;21:393–400. doi: 10.1016/s0896-6273(00)80548-6. [DOI] [PubMed] [Google Scholar]
- 58.Xia J, Zhang X, Staudinger J, Huganir RL. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron. 1999;22:179–187. doi: 10.1016/s0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 59.Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW. SAP97 is associated with the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J Biol Chem. 1998;273:19518–19524. doi: 10.1074/jbc.273.31.19518. [DOI] [PubMed] [Google Scholar]
- 60.Mello C, Fire A. DNA transformation. Methods Cell Biol. 1995;48:451–482. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.