Abstract
Bone digestion occurs when osteoclasts adhere onto bone surfaces and polarize to form acidic, hydrolase-rich resorption lacunae. For this process, they condense their actin-rich podosomes in tight belts to establish sealing zones, which segregate their basal membranes from those facing resorption lacunae. This polarization process remains poorly understood. Here, we combined quantitative proteomics and gene silencing to identify new substrates of the Src tyrosine kinase, a key regulator of osteoclast function. We now report that a depletion of the ARF GTPase-activating protein GIT2, which localizes to sealing zones upon Src phosphorylation, or a lack of GTP hydrolysis on ARF6 impairs sealing zone formation and polarized membrane traffic. Surprisingly, the Rho guanine nucleotide exchange factors α and β PIX, which usually coordinate ARF and Rho signaling, were found to be dispensable. We conclude that the Src-dependent localization of GIT2 is essential for down-regulating ARF6 activity at sealing zones, and thus for maintaining osteoclast polarity.
Keywords: polarity, GIT2, podosomes, quantitative proteomics
Osteoclasts are specialized, multinucleated cells derived from the monocyte/macrophage hematopoietic lineage that digest the bone matrix when they adhere and polarize onto bone surfaces. For this process, they form specific adhesive, actin-rich structures, referred to as podosomes, consisting of small columns of F-actin surrounded by proteins such as paxillin, talin, and integrins (1). On contact with bone surfaces, osteoclasts condense their podosomes into a compact, highly dynamic podosomal belt or ring that determines a sealing zone (2, 3). The sealing zone encircles a specialized, highly convoluted membrane domain facing the bone surface, i.e., the ruffled border, thereby forming a sealed resorption lacuna between this membrane domain and the bone. The ruffled border is enriched in lysosomal membrane proteins and ion transporters, like the V-type H+-ATPase needed to acidify the resorption lacuna and dissolve the bone matrix (4).
When bone digestion is complete, osteoclasts disassemble their sealing zone and depolarize to move to another resorption site. Throughout their life span, osteoclasts achieve multiple cycles of bone resorption and migration (4, 5). How these different events of resorption and migration, which are linked to sealing zone assembly and disassembly, are coordinated is not understood yet. A first clue came from the targeted disruption of the Src gene in mice leading to osteopetrosis due to nonfunctional osteoclasts (6). This phenotype is Src specific because the disruption of any other Src kinase family member failed to reproduce such a phenotype (6). Src interacts with a plethora of binding partners and substrates, which involves this tyrosine-kinase in several fundamental cellular processes including cytoskeletal remodeling, mitogenic signaling, cell cycle control, and survival (7). The fact that Src−/− osteoclasts are unable to organize their actin cytoskeleton points at a particular involvement of Src in this process (8, 9). Recent data suggest that Src regulates rearrangements in the actin cytoskeleton by stimulating the Rac GTPase via a protein complex comprising the Src and Syk kinases, the αvβ3 integrin, and the Rac guanine nucleotide exchange factor Vav3 (10). Although this study highlights the importance of Rac signaling, it neglects the impact of other small GTPases or factors regulated by Src to coordinate the extensive actin remodeling in osteoclasts. Therefore, we set out to identify new Src substrates or binding partners by using quantitative proteomics. We now report that Src also down-regulates ARF6 activity via the GTPase activating protein GIT2 to maintain sealing zones and osteoclast polarity during bone degradation.
Results
As a cell-model system allowing biochemical analyses of bone-digesting cells, we used Raw 264.7 cells that differentiate in vitro toward osteoclasts upon treatment with the osteoclastogenic cytokine receptor activator of NF-κB ligand (RANKL) (11). Raw 264.7 cell-derived osteoclasts conform to conventional assays for osteoclast function as primary osteoclasts isolated from bones (2, 12, 13). Upon transfer onto bone or hydroxyapatite-coated bone-mimicking surfaces, these osteoclasts polarize by establishing sealing zones, as seen in primary osteoclasts grown on natural-bone matrices. Src expressed as GFP-tagged protein using a recombinant adenovirus was highly enriched on the ruffled border and at the circumference of osteoclast sealing zones (Fig. 1A), whereas tyrosine-phosphorylated proteins localized to the circumference and the core of the sealing zones (Fig. 1B). Consistent with the established Src function in osteoclasts, treatment with the Src inhibitors SU6656 (Fig. 1B) and PP2 (data not shown) abolished the formation of actin-rich structures enriched in tyrosine-phosphorylated proteins. Accordingly, the recovery of tyrosine-phosphorylated proteins in lysates and anti-phosphotyrosine immunoprecipitates was drastically reduced after Src inhibition by SU6656 (Fig. 1C) and PP2.
Fig. 1.
Identification of candidate Src targets in osteoclasts. (A) Raw 264.7 cell-derived osteoclasts were seeded on osteologic discs and infected with recombinant adenoviruses encoding for Src-GFP (green). Twenty-four hours after viral infection, cells were fixed, stained with phalloidin (red) and DAPI (blue), and analyzed by confocal microscopy. (B) Osteoclasts grown on osteologic discs were fixed, double-stained with anti-phosphotyrosine antibody (green) and phalloidin (red), and analyzed by confocal microscopy. Note that after treatment with the Src inhibitor SU6656 (10 μM), both the sealing zone itself and the tyrosine-phosphorylated proteins enriched at the sealing zone disappear. (Scale bars, 20 μm.) (C) Osteoclasts were treated with 10 μM SU6656 (+) or left untreated (−), cell lysates were prepared, and tyrosine-phosphorylated proteins were immunoprecipitated with anti-phosphotyrosine antibody. Cell lysates (Left) and immunoprecipitates (Right) were probed by Western blotting for tyrosine-phosphorylated proteins. (D) Strategy. Osteoclasts were differentially labeled by growing them in medium containing either light (Arg-0) or heavy (Arg-6) arginine during their RANKL-induced differentiation before treatment of one sample with SU6656. After immunoprecipitation with anti-phosphotyrosine antibodies, precipitated proteins were digested with trypsin and analyzed by mass spectrometry. (E) Abundance ratios of proteins identified in the anti-phosphotyrosine immunoprecipitate. An isotope ratio of 0.8 or below was considered to be decreased protein abundance. (F) Cell lysates (Left) and immunoprecipitates (Right) were prepared as in C and probed by Western blotting for the indicated proteins.
Identification of Src Substrates and Src-Dependent Binding Partners.
To identify Src substrates and binding partners, we applied a mass-spectrometry-based proteomic approach using the stable isotope labeling with amino acids in cell culture (SILAC) method (14), which allows the quantification of changes in protein abundance after Src inhibition. Raw 264.7 cells were grown in medium containing either normal 12C6 arginine (Arg-0) or the isotopic variant 13C6 arginine (Arg-6) and differentiated toward osteoclasts. Arg-6-labeled cells were treated with SU6656, and Arg-0-labeled cells served as the control. Combined cell lysates were incubated with anti-phosphotyrosine antibodies. The purified phosphotyrosine-containing protein complexes were resolved by SDS/PAGE and analyzed by nano LC-MS/MS (Fig. 1D). MS/MS spectra of tryptic peptides were used for protein identification, whereas the relative intensities of the corresponding Arg-0- and Arg-6-containing peptides in the MS spectra were used to calculate abundance ratios (Fig. 1E). An isotope ratio of 0.8 or below was considered as decreased protein abundance. Among the 117 phosphotyrosine-containing proteins identified, of which 45 are known core elements of podosomes (1), we detected 25 proteins with altered abundance in phosphotyrosine-containing complexes after Src inhibition, of which 13 were quantified with at least 2 peptides (Table 1 and Table S1). Among those proteins, we found Src itself, most likely because of decreased autophosphorylation, and several known Src interaction partners such as the Src substrate p130Cas (Crk-associated substrate) (15), which interacts with Pyk2 (focal adhesion kinase 2) (15); the Cas-binding protein Bcar3 (breast cancer anti-estrogen resistance protein 3) (16); and the Rho-GEF Vav, which associates with the phosphorylated Hcls1 required for proper Vav localization (17). Most notably, we identified potential new Src substrates and/or interaction partners, namely the adaptor protein Dok3 (docking protein 3), the F-BAR protein PSTPIP1 (proline-serine-threonine phosphatase-interacting protein 1), the Rho-GEF α PIX, and the ARF-GAPs GIT1 and GIT2, which usually form complexes with the Rho-GEF α or β PIX (18).
Table 1.
Effect of Src inhibition on protein phosphorylation
| Protein | SwissProt accession no. | Peptides (MS/MS) | Quantified peptides (peptide ratio) | Normalized ratio |
|---|---|---|---|---|
| Tyrosine kinases | ||||
| FAK1 | P34152 | 22 | 8 | 0.78 |
| Pyk2 (FAK2) | Q9QVP9 | 7 | 3 | 0.68 |
| Src | P05480 | 3 | 2 | 0.80 |
| Known tyrosine kinase effectors | ||||
| p130Cas (Bcar1) | Q61140 | 6 | 2 | 0.68 |
| Bcar3 | Q9QZK2 | 2 | 2 | 0.58 |
| Hcls1 (HS1) | P49710 | 4 | 2 | 0.71 |
| Dok3 | Q9QZK7 | 3 | 3 | 0.38 |
| Cytoskeletal proteins | ||||
| Desmoplakin-3 | Q02257 | 5 | 2 | 0.17 |
| Abp1 | Q62418 | 2 | 2 | 0.74 |
| PSTPIP1 | P97814 | 5 | 3 | 0.47 |
| GTPase-interacting proteins | ||||
| GIT1 | Q68FF6 | 7 | 3 | 0.51 |
| GIT2 | Q9JLQ2 | 2 | 2 | 0.64 |
| α PIX | Q8K4I3 | 3 | 2 | 0.63 |
Shown are proteins with altered abundance in phosphotyrosine-containing complexes after treatment of osteoclasts with the Src inhibitor SU6656.
To verify the MS data, we first performed Western blot experiments using specific antibodies after immunoprecipitation with anti-phosphotyrosine antibodies (Fig. 1F). In this analysis, we also considered paxillin, an Src substrate (19) and core component of podosomes (1) that we could identify but not quantify because of missing arginine-containing peptides. Whereas we did not detect differences in the protein amounts in cell lysates, the abundance of all tested proteins was reduced in phosphotyrosine-containing complexes after Src inhibition. Because chemical inhibitors of kinases are not monospecific, we also used siRNA-mediated depletion of Src. Recombinant adenoviruses expressing short hairpin RNAs or Stealth siRNA oligonucleotide duplexes targeting Src were applied to osteoclasts, which led to a ≈90% reduction of the Src protein (Fig. S1). Accordingly, sealing zones and phosphotyrosine-containing proteins could not be detected after Src knockdown (Fig. S1A). The amount of Src, FAK1, paxillin, Dok3, and GIT2 that was immunoprecipitated with anti-phosphotyrosine antibodies was reduced (63%, 97%, 70%, 54%, and 35% reductions, respectively), thereby validating the quantitative proteomic analysis of drug-treated osteoclasts (Fig. S1C). Pyk2 and Vav phosphorylation was not significantly affected, possibly because of a lack of stimulated integrin signaling in osteoclasts grown under conditions allowing proteomic analyses. Together, these results confirm recent findings identifying Pyk2 (20), paxillin (19), and the Rho-GEF Vav (21) as Src substrates and point at a role of Src in the phosphorylation of the ARF-GAPs GIT1/GIT2 and the Rho-GEF α PIX.
ARF-GAP GIT2 Is a Key Component Regulating Sealing Zone Formation.
Our subsequent functional analysis essentially focused on GIT-family ARF-GAPs. Because known Src substrates such as paxillin and FAK1 are enriched along the sealing zone, we first tested whether GIT proteins could be found at the same location (Fig. 2). Indeed, GIT1 and GIT2 localized to the condensed podosomal belts of the sealing zone and to some extent to internal punctate structures (Fig. 2). This localization of GIT proteins to podosomal belts, like that of paxillin and FAK1, was lost after Src inhibition (Fig. 2). We then reasoned that a loss of GIT or Src function should result in similar phenotypes. Therefore, we used siRNAs to reduce the expression of either protein, which yielded an 80–90% depletion at the mRNA and protein level (Fig. 3 A and B). As shown in Fig. 3 C and D, the depletion of GIT2 or Src resulted in a loss of sealing zones in ≈75% of the osteoclasts examined, which exhibited numerous membrane protrusions as typically observed in spreading or moving cells, thus resembling Src−/− osteoclasts (8, 9). The efficient siRNA-mediated depletion of GIT1 remained without any effect on sealing-zone integrity. Similarly, the knockdown of the Rho GEF α PIX or β PIX, the other PIX member that was not detected as a Src target, remained without any effect, although podosomal belts appeared less compact, consistent with the previous report (22) (Fig. S2 A and B). Also, the α and β PIX double knockdown did not modify sealing-zone formation (Fig. S2 A and B). Thus, GIT2 is essential for sealing zone formation in polarized osteoclasts, whereas GIT1 and α and β PIX are dispensable for this function.
Fig. 2.
Src-dependent localization of GIT proteins to the sealing zone of osteoclasts. Osteoclasts were seeded on osteologic discs; treated with Src inhibitor or left untreated; fixed; and stained with phalloidin (red), DAPI (blue), and with antibodies for the indicated proteins (green). Cells were analyzed by confocal microscopy. (Scale bars, 20 μm.)
Fig. 3.
RNAi-mediated depletion of GIT2 and Src impairs sealing zone formation in osteoclasts. (A) Osteoclasts were either mock-electroporated or electroporated with stealth RNAi duplexes targeting the indicated genes or with scrambled RNAi duplexes. Electroporated osteoclasts were grown for an additional 43 h, and mRNA was extracted, reverse transcribed, and analyzed by quantitative PCR using the comparative ct method. Shown are relative mRNA levels of Src and GIT2 normalized to GAPDH mRNA levels. (B). Electroporated osteoclasts were grown for an additional 43 h, and total cell lysates were prepared and probed by Western blotting for Src, GIT2, and Tubulin. (C and D) Osteoclasts were electroporated as in A. Electroporated osteoclasts were grown for an additional 43 h on osteologic discs, fixed, stained with phalloidin (red) and DAPI (blue), and analyzed by confocal microscopy. Sealing zones were counted (C). Values are mean ± SD from 3 different experiments (n = 300 osteoclasts/experiment). Representative images are shown (D). *, P < 0.001. (Scale bars, 20 μm.)
GIT2 Down-Regulates ARF6 at Sealing Zones of Osteoclasts.
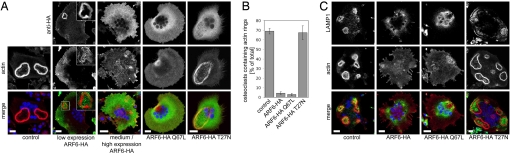
GIT2 stimulates hydrolysis of GTP bound to the different ARF-GTPase subfamily members, including ARF6 (18). Because ARF6 is the only known ARF member operating at the plasma membrane (23, 24), we speculated that GIT2 might inactivate ARF6 with respect to sealing-zone formation. To test this possibility, we characterized the effects of ectopically expressed ARF6. ARF6 was largely excluded from podosomal belts of osteoclasts expressing low levels of this GTPase (Fig. 4A), being found at the circumference of the sealing zone, the ruffled border, and basal membranes of osteoclasts. At higher expression levels, which were the case in most of the osteoclasts, ARF6 was found at the plasma membrane of spreading cells devoid of actin rings. Because ARF6 over-expression could override its tightly controlled regulation, we reasoned that the expression of ARF6Q67L, a mutant blocked in GTP hydrolysis, should show that active ARF6 destabilizes sealing-zone formation and results in a lack of osteoclast polarization. Indeed, Fig. 4 A and B show that, like adenovirus-mediated over-expression of wild-type ARF6, mutant ARF6Q67L impairs the assembly of sealing zones in osteoclasts. Consequently, osteoclasts were unable to segregate their membrane components, as exemplified by the lysosomal glycoprotein LAMP1 (Fig. 4C). LAMP1 normally concentrates within the ruffled border of digesting osteoclasts and in membrane-bound structures located above the ruffled border. In contrast, LAMP1 was detected in perinuclear structures, presumably lysosomes, in ARF6- or ARF6Q67L-expressing osteoclasts. The expression of an ARF6 mutant defective in GTP loading (ARF6T27N) or the efficient knockdown of ARF6 (Fig. S2 C–E) remained without any effect. Thus, these results suggest that the ARF-GAP GIT2 mediates the down-regulation of ARF6, which is essential to stabilize sealing zones and osteoclast polarity.
Fig. 4.
Active ARF6 impairs sealing zone formation and osteoclasts polarization. (A and C) Osteoclasts were seeded on osteologic discs and infected with recombinant adenoviruses encoding for HA-tagged ARF6, ARF6Q67L, and ARF6T27N or mock-infected (control). Forty-two hours after viral infection, cells were fixed; stained with anti-HA or with anti-LAMP1 antibody (green), phalloidin (red), and DAPI (blue); and analyzed by confocal microscopy. (Scale bars, 20 μm.) (B) Sealing zones were counted; values are mean ± SD from 3 different experiments (n = 250 osteoclasts/experiment). *, P < 0.001.
Discussion
Our study demonstrates the importance of GIT2 and ARF6 in the dynamics of condensed podosomal belts forming sealing zones and the maintenance of membrane components at the ruffled border.
GITs represent hubs that coordinate ARF and Rho signaling, thereby regulating various cellular functions, including membrane trafficking, scaffolding of signaling cascades, and actin remodeling at adhesion sites (18). The recruitment of GIT proteins to adhesion sites relies on their interaction with paxillin (18, 25). In nonpolarized cells, GIT2 binds to paxillin upon phosphorylation by FAK and/or Src (26). Thus, it is more than likely that the proper localization of GIT2 to sealing zones of polarized osteoclasts is determined by Src-dependent phosphorylation. Indeed, Src inhibition in osteoclasts abolishes the proper localization of GIT2 and, interestingly, that of GIT1, too. The facts, however, that GIT2 binds more tightly with paxillin than GIT1 (25) and that the knockdown of GIT2, but not of GIT1, dramatically affects sealing zone formation support the idea that GIT2 is the principal mediator of adhesion-site stability (25, 27). GITs usually form complexes with the Rac1/Cdc42-GEFs α and β PIX (18). Although α PIX was found as an Src target in osteoclasts, α PIX is dispensable for GIT2 function in sealing zone dynamics. Furthermore, α and β PIX were found redundant in function, a finding that strongly suggests that the coordination between ARF6 and Rac1/Cdc42 signaling most likely involves other members of the Rho-GEF family, potentially the Vav proteins (10).
ARF6 is emerging as a common regulator of various actin-rich, adhesive structures, i.e., focal adhesions in nonpolarized cells, adherens junctions in polarized epithelial cells, invadopodia in cancer cells (23, 25), and podosomes in osteoclasts, which are believed to represent distinct actin-rich supramolecular structures (1, 28, 29). In this respect, ARF6 function seems to be versatile but always following a mechanistic principle, namely supporting membrane mass supply for membrane extension. In tumor cells, this basic feature of active ARF6 is thought to promote their invasive capacities, probably by plasma membrane extension to facilitate invadopodia formation (30, 31). In polarized epithelial cells, active ARF6 facilitates the turnover of adherens junctions by promoting endocytosis of E-cadherin or β1-integrins, which leads to depolarization and cell spreading, as seen during epithelial-to-mesenchymal transition (32–34). In polarized osteoclasts, active ARF6 also destabilizes condensed podosome belts, which form the sealing zone; influences the polarized sorting of proteins, as exemplified here by LAMP1; and appears to favor cell spreading. Thus, ARF6 repression leading to a down-regulation of endocytosis at specific adhesive sites would be required to stabilize adhesive structures, whereas ARF6 activation would lead to membrane extension, which results either in the destabilization of certain adhesive structures as seen in polarized cells or in the generation of protrusions for invadopodia formation. The regulation of ARF6 could, however, differ according to the actin-rich structure or the cell type considered. Src-dependent phosphorylation destabilizes adherens junctions of polarized epithelial cells (34), whereas it promotes, together with GIT2, sealing-zone formation in polarized osteoclasts. These observations suggest that Src phosphorylates different ARF6-interacting proteins or effectors in these different cell types. The experimental strategy used in this study could potentially be applied to gain further insight into the regulation of different adhesive structures and the molecules coordinating ARF6 and Rho signaling.
Bone digestion occurs through several, repeated cycles of sealing-zone formation allowing osteoclasts to adhere and polarize onto bone surfaces, and sealing zone destabilization allowing osteoclasts to migrate to other areas to digest (4). Our study strongly suggests that these cycles could involve a tight equilibrium between cycles of GIT2-dependent ARF6 down-regulation and ARF6 activation, a process that needs to be coordinated with Rho down-regulation and activation. To elucidate this regulatory mechanism, it will be important to clearly identify the effector molecules coordinating Rho and ARF6 signaling. We took complementary approaches to identify genes whose expression is drastically modified during osteoclastogenesis (35). Besides Vav proteins, this analysis highlighted several other regulators of Rho GTPases. Therefore, further studies are needed to find the molecular links coordinating ARF6/GIT2 and Rho signaling during these regulated cycles of osteoclast adhesion and migration required for bone digestion.
Materials and Methods
Cell Culture, SILAC, and Osteoclastogenesis.
Production of RANKL, cultivation of Raw 264.7 cells (American Type Culture Collection), and differentiation toward osteoclasts were performed as previously described (35). At day 4 of differentiation, cells were transferred to BD BioCoat osteologic discs (BD Bioscience) for microscopy. For SILAC experiments, cells were cultured in DMEM lacking arginine (PAN Biotech) supplemented with 10% (vol/vol) dialyzed FCS. Arg-6 and Arg-0 SILAC media were prepared by adding l-arginine-U-13C6 (Cambridge Isotope Laboratories) or the corresponding nonlabeled amino acid, respectively, and cells were cultured for 5 days in SILAC media.
Gene Transduction and RNAi in Osteoclasts.
Adenoviral vectors and recombinant adenoviruses carrying Src-GFP, ARF6-HA, ARF6-HAQ67L, ARF6-HAT27N, and short hairpin RNAs targeting Src or GFP were generated according to the manufacturer's protocol (AdEasy Adenoviral System, Stratagene). Raw 264.7-cell-derived osteoclasts were incubated with medium containing recombinant adenoviruses for 24 to 35 h. Experiments were performed between 43 h postinfection for ectopic gene expression and 60 h postinfection for RNAi experiments. Stealth RNAi duplexes were obtained from Invitrogen. Predesigned stealth RNAi or scrambled stealth RNAi duplexes (each 800 nM) were electroporated into osteoclasts by using a CytoPulse PA-4000 electroporator (CytoPulse Sciences). Electroporated cells were resuspended in medium supplemented with RANKL and allowed to recover for 43 h. Then cells were processed for subsequent analysis. Detailed information regarding stealth RNAi duplexes and short hairpin RNAs are given in SI Text.
Quantitative PCR.
Total RNA was isolated by using the RNeasy Kit (Qiagen). DNase-I-treated total RNA was reverse transcribed by using the SuperScript II First Strand Synthesis system (Invitrogen). Quantitative PCR was performed with a Stratagene Mx4000 QPCR system and the Brilliant SYBR Green QPCR kit according to the manufacturer's instructions (Stratagene). QPCR analyses were done in triplicate, and cycle threshold (ct) values were normalized against the internal control gene GAPDH. Fold differences in expression levels were calculated according to the comparative ct method (36). Primer sequences are given in SI Text.
Immunofluorescence, Confocal Laser Scanning Microscopy, and Image Analysis.
Sample preparation and image acquisition were done as described previously (37). Images were processed with Adobe Photoshop v7.0 (Adobe Systems). For quantification, more than 300 osteoclasts per condition and per experiment were counted and analyzed for their ability to form sealing zones. Means and standard deviations (SD) were calculated, and data were analyzed for statistical significance by using one-way ANOVA.
Immunoprecipitation and Western Blotting.
For drug-induced inhibition of Src, osteoclasts were treated with 10 μM SU6656 or its vehicle for 90 min. Drug- or RNAi-treated cells were processed according to Kratchmarova et al. (38). Then cell extracts were either subjected to SDS/PAGE or immunoprecipitated by incubation with anti-phosphotyrosine antibodies (25 μg of 4G10/mg of lysate and 10 μl of P-Tyr-100/mg of lysate) for 6 h at 4 °C. Proteins were eluted, resolved by SDS/PAGE, blotted, and subsequently incubated with primary antibodies. After incubation with secondary antibodies conjugated with horseradish peroxidase (Jackson Immuno Research), bands were detected with enhanced chemiluminescence Western blotting detection reagents (Amersham). Densitometric quantification of Western blots was performed by using ImageJ software (National Institutes of Health).
Mass Spectrometry and Data Analysis.
Tryptic digest of proteins and nanoLC-MS/MS experiments were done as described previously (37). Tryptic peptides were separated by a reversed-phase Micromass capillary liquid chromatography system connected to a Z-spray nanoelectrospray ion source and a quadruple orthogonal acceleration time-of-flight mass spectrometer Q-TOF Ultima (Micromass). MS/MS data analysis was performed by using MASSLYNX software Version 4.0 (Micromass), and resulting data files were searched using MASCOT Search Engine Version 2.0 (Matrix Science) against the SwissProt database version 240206 (211104 sequences). Quantification was carried out by using the open-source software MSQUANT (Peter Mortensen and Matthias Mann, http://msquant.sourceforge.net/). Further details are provided in SI Text.
Online SI.
Further information, including the RNAi-mediated depletion of Src impairing protein tyrosine phosphorylation in osteoclasts (Fig. S1), a demonstration that RNAi-mediated depletion of GIT1, α PIX, β PIX, and ARF6 does not impair sealing zone formation in osteoclasts (Fig. S2), and a list of all quantified proteins of the SILAC approach (Table S1), is available in the supporting information. Table S2 lists the oligonucleotides used to silence murine Src, GIT2, GIT1, and α PIX. Table S3 lists the short hairpin oligonucleotide sequences used in this study. Table S4 lists the primers used in this study.
Supplementary Material
Acknowledgments.
We thank D. Thiel and J. Lehmann for technical assistance, M. Schümann for help with the MS measurements, R.T. Premont (Duke University, Durham, NC) for his generous gift of anti-GIT antibodies, M. Zerial (Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany) for the Src-GFP construct, and K. Simons and M. Zerial for their critical reading of the manuscript. This work was supported in part by Dresden University of Technology Grant HWP-1207; Sachsisches Ministerium fur Wissenschaft und KunstEuropaische Fond fur Regionale Entwicklung Grant 1203; Bundesministerium für Bildung und Forschung Grant 0313815B; and Deutsche Forschungsgemeinschaft Grants TRR13/2-08, HO 254/2-1, HO 2584/1-1, HO 2584/6-1, and HO 2584/8-1.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804464106/DCSupplemental.
References
- 1.Linder S, Aepfelbacher M. Podosomes: Adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 2.Luxenburg C, et al. The architecture of the adhesive apparatus of cultured osteoclasts: From podosome formation to sealing zone assembly. PLoS ONE. 2007;2:e179. doi: 10.1371/journal.pone.0000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jurdic P, Saltel F, Chabadel A, Destaing O. Podosome and sealing zone: Specificity of the osteoclast model. Eur J Cell Biol. 2006;85:195–202. doi: 10.1016/j.ejcb.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Vaananen HK, Zhao H, Mulari M, Halleen JM. The cell biology of osteoclast function. J Cell Sci. 2000;113(Pt 3):377–381. doi: 10.1242/jcs.113.3.377. [DOI] [PubMed] [Google Scholar]
- 5.Saltel F, Destaing O, Bard F, Eichert D, Jurdic P. Apatite-mediated actin dynamics in resorbing osteoclasts. Mol Biol Cell. 2004;15:5231–5241. doi: 10.1091/mbc.E04-06-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lowell CA, Soriano P. Knockouts of Src-family kinases: Stiff bones, wimpy T cells, and bad memories. Genes Dev. 1996;10:1845–1857. doi: 10.1101/gad.10.15.1845. [DOI] [PubMed] [Google Scholar]
- 7.Martin GS. The hunting of the Src. Nature Reviews. 2001;2:467–475. doi: 10.1038/35073094. [DOI] [PubMed] [Google Scholar]
- 8.Schwartzberg PL, et al. Rescue of osteoclast function by transgenic expression of kinase-deficient Src in src−/− mutant mice. Genes Dev. 1997;11:2835–2844. doi: 10.1101/gad.11.21.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyazaki T, et al. Src kinase activity is essential for osteoclast function. J Biol Chem. 2004;279:17660–17666. doi: 10.1074/jbc.M311032200. [DOI] [PubMed] [Google Scholar]
- 10.Zou W, et al. Syk, c-Src, the alphavbeta3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J Cell Biol. 2007;176:877–888. doi: 10.1083/jcb.200611083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 12.Destaing O, Saltel F, Geminard JC, Jurdic P, Bard F. Podosomes display actin turnover and dynamic self-organization in osteoclasts expressing actin-green fluorescent protein. Mol Biol Cell. 2003;14:407–416. doi: 10.1091/mbc.E02-07-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toyomura T, et al. From lysosomes to the plasma membrane: Localization of vacuolar-type H+ -ATPase with the a3 isoform during osteoclast differentiation. J Biol Chem. 2003;278:22023–22030. doi: 10.1074/jbc.M302436200. [DOI] [PubMed] [Google Scholar]
- 14.Ong SE, et al. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics. 2002;1:376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 15.Lakkakorpi PT, et al. Stable association of PYK2 and p130(Cas) in osteoclasts and their co-localization in the sealing zone. J Biol Chem. 1999;274:4900–4907. doi: 10.1074/jbc.274.8.4900. [DOI] [PubMed] [Google Scholar]
- 16.Gotoh T, Cai D, Tian X, Feig LA, Lerner A. p130Cas regulates the activity of AND-34, a novel Ral, Rap1, and R-Ras guanine nucleotide exchange factor. J Biol Chem. 2000;275:30118–30123. doi: 10.1074/jbc.M003074200. [DOI] [PubMed] [Google Scholar]
- 17.Gomez TS, et al. HS1 functions as an essential actin-regulatory adaptor protein at the immune synapse. Immunity. 2006;24:741–752. doi: 10.1016/j.immuni.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoefen RJ, Berk BC. The multifunctional GIT family of proteins. J Cell Sci. 2006;119(Pt 8):1469–1475. doi: 10.1242/jcs.02925. [DOI] [PubMed] [Google Scholar]
- 19.Schaller MD. Paxillin: A focal adhesion-associated adaptor protein. Oncogene. 2001;20:6459–6472. doi: 10.1038/sj.onc.1204786. [DOI] [PubMed] [Google Scholar]
- 20.Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature. 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 21.Turner M, Billadeau DD. VAV proteins as signal integrators for multi-subunit immune-recognition receptors. Nat Rev Immunol. 2002;2:476–486. doi: 10.1038/nri840. [DOI] [PubMed] [Google Scholar]
- 22.Gringel A, et al. PAK4 and αPIX determine podosome size and number in macrophages through localized actin regulation. J Cell Phys. 2006;209:568–579. doi: 10.1002/jcp.20777. [DOI] [PubMed] [Google Scholar]
- 23.D'Souza-Schorey C, Chavrier P. ARF proteins: Roles in membrane traffic and beyond. Nature Reviews. 2006;7:347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 24.Heo WD, et al. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314:1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabe H, Onodera Y, Mazaki Y, Hashimoto S. ArfGAP family proteins in cell adhesion, migration and tumor invasion. Curr Opin Cell Biol. 2006;18:558–564. doi: 10.1016/j.ceb.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Brown MC, Cary LA, Jamieson JS, Cooper JA, Turner CE. Src and FAK kinases cooperate to phosphorylate paxillin kinase linker, stimulate its focal adhesion localization, and regulate cell spreading and protrusiveness. Mol Biol Cell. 2005;16:4316–4328. doi: 10.1091/mbc.E05-02-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frank SR, Adelstein MR, Hansen SH. GIT2 represses Crk- and Rac1-regulated cell spreading and Cdc42-mediated focal adhesion turnover. EMBO J. 2006;25:1848–1859. doi: 10.1038/sj.emboj.7601092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linder S. The matrix corroded: Podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17(3):107–117. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Buccione R, Orth JD, McNiven MA. Foot and mouth: Podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol. 2004;5:647–657. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto S, et al. Requirement for Arf6 in breast cancer invasive activities. Proc Natl Acad Sci USA. 2004;101:6647–6652. doi: 10.1073/pnas.0401753101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tague SE, Muralidharan V, D'Souza-Schorey C. ADP-ribosylation factor 6 regulates tumor cell invasion through the activation of the MEK/ERK signaling pathway. Proc Natl Acad Sci USA. 2004;101:9671–9676. doi: 10.1073/pnas.0403531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunphy JL, et al. The Arf6 GEF GEP100/BRAG2 regulates cell adhesion by controlling endocytosis of beta1 integrins. Curr Biol. 2006;16:315–320. doi: 10.1016/j.cub.2005.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palacios F, Price L, Schweitzer J, Collard JG, D'Souza-Schorey C. An essential role for ARF6-regulated membrane traffic in adherens junction turnover and epithelial cell migration. EMBO J. 2001;20:4973–4986. doi: 10.1093/emboj/20.17.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palacios F, Tushir JS, Fujita Y, D'Souza-Schorey C. Lysosomal targeting of E-cadherin: A unique mechanism for the down-regulation of cell–cell adhesion during epithelial to mesenchymal transitions. Mol Cell Biol. 2005;25:389–402. doi: 10.1128/MCB.25.1.389-402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Czupalla C, Mansukoski H, Pursche T, Krause E, Hoflack B. Comparative study of protein and mRNA expression during osteoclastogenesis. Proteomics. 2005;5:3868–3875. doi: 10.1002/pmic.200402059. [DOI] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 37.Czupalla C, et al. Proteomic analysis of lysosomal acid hydrolases secreted by osteoclasts: implications for lytic enzyme transport and bone metabolism. Mol Cell Proteomics. 2006;5:134–143. doi: 10.1074/mcp.M500291-MCP200. [DOI] [PubMed] [Google Scholar]
- 38.Kratchmarova I, Blagoev B, Haack-Sorensen M, Kassem M, Mann M. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science. 2005;308:1472–1477. doi: 10.1126/science.1107627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.