Abstract
Motor skills can take weeks to months to acquire and can diminish over time in the absence of continued practice. Thus, strategies that enhance skill acquisition or retention are of great scientific and practical interest. Here we investigated the effect of noninvasive cortical stimulation on the extended time course of learning a novel and challenging motor skill task. A skill measure was chosen to reflect shifts in the task's speed–accuracy tradeoff function (SAF), which prevented us from falsely interpreting variations in position along an unchanged SAF as a change in skill. Subjects practiced over 5 consecutive days while receiving transcranial direct current stimulation (tDCS) over the primary motor cortex (M1). Using the skill measure, we assessed the impact of anodal (relative to sham) tDCS on both within-day (online) and between-day (offline) effects and on the rate of forgetting during a 3-month follow-up (long-term retention). There was greater total (online plus offline) skill acquisition with anodal tDCS compared to sham, which was mediated through a selective enhancement of offline effects. Anodal tDCS did not change the rate of forgetting relative to sham across the 3-month follow-up period, and consequently the skill measure remained greater with anodal tDCS at 3 months. This prolonged enhancement may hold promise for the rehabilitation of brain injury. Furthermore, these findings support the existence of a consolidation mechanism, susceptible to anodal tDCS, which contributes to offline effects but not to online effects or long-term retention.
Keywords: long-term retention, motor cortex, motor learning, transcranial direct current stimulation (tDCS), transcranial magnetic stimulation (TMS)
Accurate motor performance is essential to almost everything we do, from typing, to driving, to playing sports. Having a motor skill implies a level of performance in a given task that is only achievable through practice (1). Evidence indicates that motor skill learning can continue over a prolonged time period (2–5). Within-session performance improvements (online effects) occur in the minutes or hours of a single training session and continue over days and weeks of repeated training sessions until performance nears asymptotic levels. Changes in performance can also occur between training sessions (offline effects), i.e., performance at the beginning of session n + 1 is different from performance at the end of session n (6, 7). We have intentionally chosen to avoid the use of the term “offline learning” because it has been used to refer to both a physiological process (consolidation) (6) and a particular measurement result (a positive offline effect) (8). Offline effects could also be negative, presumably because of forgetting processes (7). Skills can be retained to varying degrees over weeks to months after the completion of training (long-term retention) (5). Here we investigated the effect of noninvasive cortical stimulation on measurements of these 3 temporal components of skill learning (online effects, offline effects, and long-term retention). The principle underlying this approach is that if a perturbation has selective effects on these measures, then this would support the existence of distinct mechanistic processes corresponding to the 3 temporal components of skill learning (9).
Noninvasive brain stimulation methods have been used to modulate cortical excitability (10–12) and to perturb initial motor learning and consolidation (8, 13, 14). Anodal transcranial direct current stimulation (tDCS) delivered over the primary motor cortex (M1) increases motor cortical excitability without direct neuronal depolarization at the low intensities used in humans, whereas cathodal tDCS decreases cortical excitability (12). A single application of anodal tDCS over M1 has been shown to induce transient performance improvements in various motor tasks (15–18). Because these studies only examined the effects of tDCS within a single session, the relative impact of anodal tDCS on online effects, offline effects, and long-term retention is not known.
To examine the effect of tDCS on the different temporal components of motor skill learning, we devised a new sequential visual isometric pinch task (SVIPT) that is sufficiently difficult to ensure that performance continues to improve over 5 days of training (see supporting information SI Methods for full description of the task). The difficulty of the task made it comparable to real life skills, which often take weeks to months to acquire (3). To quantify skill we first empirically derived the speed–accuracy tradeoff function (SAF) for the SVIPT. This derivation is critically important, because otherwise it is not clear how to relate changes in speed and accuracy to a change in skill. We think that the formal consideration of changes in the SAF with training is a conceptual advance in the study of skill learning. For example, if a tennis player hits 125-mph serves but only gets 25% of the balls in the service box, is he more, less, or equally skilled in comparison to a player who hits the ball at 100 mph but gets 50% of the balls in? Answering this question in general requires the ability to distinguish between whether (i) the SAF has changed (which would mean that skill has changed) or (ii) performance has been sampled at a different place on the same SAF (which would mean that skill has not changed). Therefore, we developed a skill measure such that a change in it reflected a change in the SAF. We then determined whether anodal tDCS applied over M1 differentially modulates online effects, offline effects, and long-term retention.
Results
Characterization of the Speed–Accuracy Tradeoff Function (SAF) for the SVIPT and Derivation of a Skill Measure.
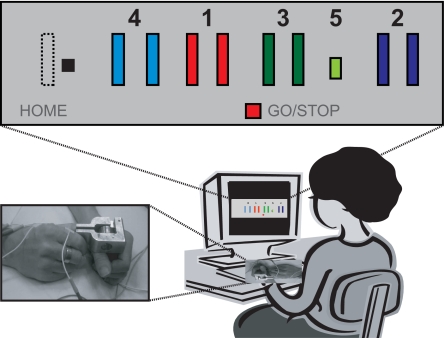
The SVIPT is shown in Fig. 1. Speed was captured by the average sequence movement time per training block (movement onset to stopping at gate 5). Accuracy was defined as the proportion of trials per block with hits to all 5 targets in the correct sequence order. The error rate was calculated as [1– accuracy rate]. Skill was defined as a practice-induced change in the SAF. We first established that the SAF of the SVIPT does indeed change over 5 days of training. We estimated the SAF in a sample of subjects who were either naïve to the task, or were trained on the task over a 5-day period. The 5-day training schedule was identical to that in the main experiment (Fig. 2), but without tDCS.
Fig. 1.
Sequential visual isometric pinch task. To control an on-screen cursor movement, subjects pinched a force transducer with thumb and index finger. The aim was to navigate the cursor quickly and accurately between a HOME position and 5 gates by alternating the pinch force exerted onto the transducer (see SI Methods for details). The practiced sequence was Home-1-Home-2-Home-3-Home-4-Home-5. Movement time (from movement onset to stopping at gate 5) and error rate (proportion of trials with at least 1 under- or overshooting movement) were used to determine a skill measure.
Fig. 2.
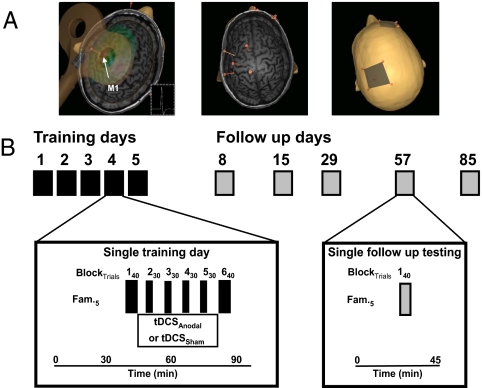
(A) Target region for cortical stimulation. Left M1 was determined by TMS targeting the optimal scalp position to elicit motor evoked potentials (MEPs) (inset) of the right first dorsal interosseus muscle. Neuronavigation revealed the precentral gyrus as the cortical target for tDCS. The anode/cathode was placed according to this landmark and the second electrode was placed on the right supraorbital area. (B) Study design. Subjects participated in 5 training and 5 follow-up sessions. During training of the SVIPT, 20 min of sham or anodal tDCS was applied to M1 in a double blind fashion. In the follow-ups, subjects performed 40 trials. Fam, familiarization with the experimental setting.
Subject performed 9 blocks of 10 trials each, with each block set at a different movement time (MT) imposed by a metronome. The order of the blocks was randomized and balanced within each group. There was a clear difference in the SAF between groups, with a given MT tending to be associated with a higher accuracy in the trained group compared to the naïve group (Fig. 3). We then empirically chose a 2-parameter model that fit these SAF data well and for which only 1 of the parameters (the so-called “skill parameter”; the larger the value of the parameter, the higher the skill) substantially changed with training. As we fit many functions to the pre- and posttraining SAFs, there was the possibility of a spuriously good fit. We therefore validated this model by acquiring another SAF data set in a separate set of trained subjects. The model fit this SAF well. Moreover, there was a near-perfect overlap of the SAFs for the original and validation groups (Fig. 3). Having validated the SAF model, we then estimated the skill parameter corresponding to each bivariate observation (per subject and per block) of error rate and movement time. We found that variability in the estimated skill parameter was multiplicative (data not shown). To validly perform parametric statistical analyses, we logarithmically transformed the skill parameter to homogenize the variances (Fig. 4). The natural logarithm of the skill parameter is referred to in the remainder of this report as the “skill measure,” and is used exclusively in all statistical analyses.
Fig. 3.
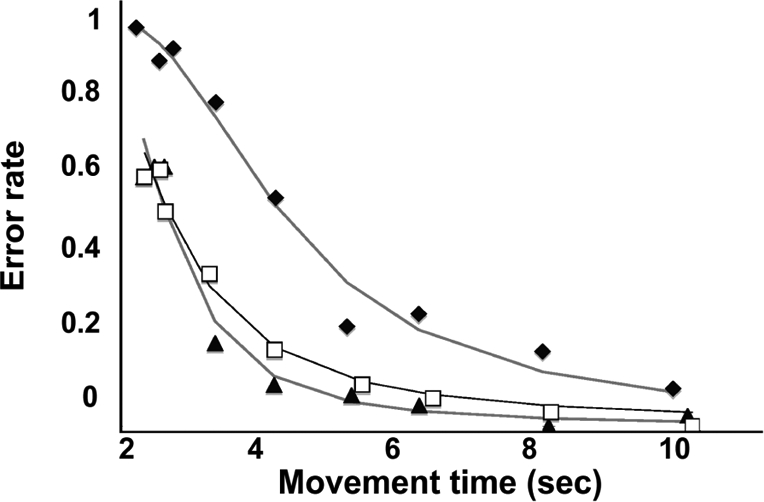
Speed–accuracy tradeoff function. Speed–accuracy tradeoff function data (black diamonds, pretraining initial data set; black triangles, posttraining initial data set; unfilled squares, posttraining validation data set) and the corresponding nonlinear least squares fits of Eq. 1 (SI Methods) (gray lines, initial data set fits; black line, validation data set fit). Data were obtained from 12 naïve subjects for the initial data set and 6 subjects per posttraining data set. The SAF is derived from the movement time (abscissa) and the error rate per block (ordinate).
Fig. 4.
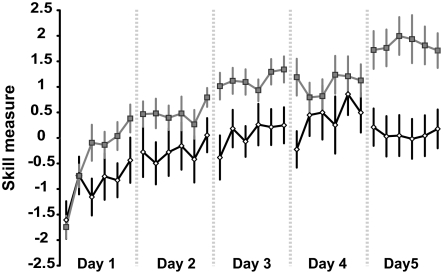
The learning curve for the sham (white diamonds) and anodal (gray squares) tDCS groups for the 30 training blocks over 5 days. Each block depicts the group mean of the averaged number of trials (40 in blocks 1 and 6; 30 in blocks 2–5). The dotted lines represent breaks between consecutive days. Both groups started with comparable skills at the beginning of day 1, but by day 5 the anodal tDCS group had acquired more skills than the sham tDCS group.
Application of Anodal tDCS over the Primary Motor Cortex Enhanced Skill Acquisition.
Having established that training on the SVIPT increases skill, we then determined whether anodal tDCS applied synchronously with motor training enhances the total amount of skill acquired over 5 days of training in comparison to sham tDCS. Two groups of 12 subjects each underwent 5 consecutive training sessions with the SVIPT (Fig. 1) under anodal tDCS or sham tDCS (Fig. 2, Table S1). As can be seen in Fig. 4, both groups started with the same level of skill in the first block of day 1 (P = 0.8199). As expected, the sham tDCS group exhibited positive total skill learning over the training course (P = 0.002). The anodal tDCS group exhibited greater total learning than the sham tDCS group (P = 0.005).
To determine whether this learning-enhancing effect of tDCS was polarity specific, a separate group of 12 subjects was trained over 5 days with synchronously applied cathodal tDCS. There was no significant difference in total learning between the cathodal and the sham tDCS groups (P = 0.494), but there was a significant difference in total learning between cathodal and anodal tDCS (P = 0.0055) (Fig. S1). Thus, the observed effect of tDCS on skill acquisition is indeed polarity specific.
Anodal tDCS Enhanced Acquisition Through Positive Offline Effects.
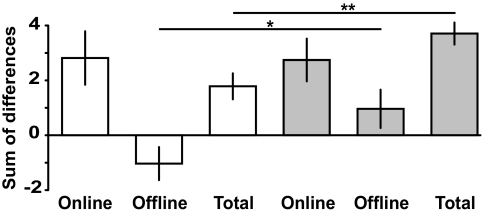
After establishing a difference in total learning between anodal tDCS and control conditions, we next determined the relative impact of anodal tDCS on online (within-day) and offline (between-day) effects. There was no significant difference in online effects between the 2 groups (P = 0.954) (Fig. 5), but a significant difference in offline effects (P = 0.04) (Fig. 5). In fact, the sham group showed negative offline effects (difference from zero: P = 0.05), as has been described in other skill learning studies (7, 19, 20). In contrast, the anodal tDCS group showed a trend toward positive offline effects (difference from zero: P = 0.091). Therefore, as total learning is simply the sum of online and offline effects, the improved total learning in the anodal tDCS group appears to be driven by the positive offline effect. We ruled out the possibility that the positive offline effect in the anodal tDCS group was because of an enhancement of learning rate within the first 40-trial block of each day by assessing offline effects with only 5 trials. There was a significant difference for offline effects between the anodal tDCS and sham tDCS groups using this higher resolution offline measure (P = 0.00065), with the anodal tDCS group showing a significant positive offline effect (P = 0.00048) and the sham tDCS group showing a significant negative offline effect (P = 0.019). We conclude that anodal tDCS applied synchronously with training induces offline gains, in contrast to an offline loss seen in the sham tDCS group.
Fig. 5.
Online and offline effects. Online (within-day) and offline (between-day) effects and total learning (online + offline) in the sham tDCS (white bars) and anodal tDCS (gray bars) groups are shown. Note that the significantly greater total learning in the anodal tDCS group (last gray bar) was predominantly driven by significantly greater offline effects compared to sham tDCS, in the absence of differences in online effects. Data show mean (bars) ± SEM. *, P < 0.05; **, P < 0.01.
Anodal tDCS Did Not Enhance Long-Term Retention.
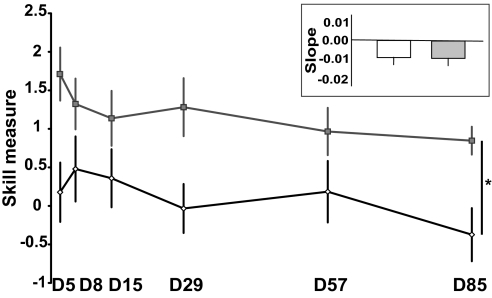
Skill retention was evaluated with a single testing block of 40 trials at 5 time points over a period of 3 months (Figs. 2B and 6). The 2 groups forgot at the same rate even though they started from different levels of skill on day 5 (Fig. 6). The slopes of forgetting across the 85 days between conditions were not significantly different (P = 0.971) (Fig. 6, inset). The persistence of greater skill in the anodal tDCS group compared to the sham tDCS group at all recall time points indicates the robustness of the tDCS effect. The skill measure was still significantly higher in the anodal tDCS group compared to the sham group at day 85 (P = 0.005). We conclude that anodal tDCS increased total skill learning over the 5 training days, but did not change the rate of forgetting after training.
Fig. 6.
Retention of skill. Skill at D5 and at follow-up sessions on D8, D15 ± 1, D29 ± 2, D57 ± 2, and D85 ± 2 is shown. Skill in the anodal tDCS group (gray squares) remained superior to the sham tDCS group (white diamonds) at all times, including D85. Small inset: retention, the time-weighed slope measure, calculated within single subjects over the follow-up period, did not differ between the sham (white bar) and anodal (gray bar) tDCS groups. Data show mean ± SEM. *, P < 0.01.
Discussion
We investigated the effect of anodal tDCS applied over M1 on motor skill acquisition over 5 consecutive days and its retention at several time points over a 3-month follow-up period. First, anodal tDCS in combination with training led to significantly greater total learning at the end of 5 days compared to sham. Second, the greater total learning in the anodal tDCS group was primarily mediated through induction of positive offline effects. Third, anodal tDCS did not affect the rate of forgetting over the 3-month follow-up period. The greater total learning in the anodal tDCS group at the end of day 5, however, meant that skill remained superior in this group compared to sham tDCS at 3 months.
Skill.
Previous studies of skill learning have reported speed and accuracy measures separately (5, 21, 22). As explained in the Introduction, these parallel measures can lead to ambiguity if they change in opposite directions (23) or if changes are subtle. Here we formally defined skill acquisition as a change in the SAF. We first derived a skill measure on the basis of empirical estimations of the SAF in separate groups of subjects. We were then able to use this skill measure for the main tDCS experiment without needing to generate SAFs at each time point.
The task used in this study was designed to assess skill rather than adaptation. Adaptation allows the motor system to regain former levels of performance in the setting of a perturbation, whereas skill is the acquisition of a higher level of performance (24, 25). Within the computational framework of optimal feedback control, it could be posited that adaptation is mediated through changes in a forward model, whereas skill represents a slower process of acquiring an optimal feedback control policy (26). This difference is apparent in the respective time course for these 2 kinds of motor learning. Adaptation to a perturbation—whether it is to prisms, visuomotor rotations, or force fields—reaches asymptote within 1 day (27, 28). In contrast, skills take much longer to acquire (3, 5, 22, 29). In this context, we consider the SVIPT a skill task because subjects improve their level of performance, reflected in a new SAF, over a prolonged period. Therefore, it shares psychophysical similarities with other skills such as sequential finger tapping or piano playing rather than with adaptation tasks or the serial reaction time task (SRTT), which measures acquisition of sequence order rather than performance accuracy (30). In the context of brain stimulation and localization, the distinction between adaptation and skill is particularly important because they appear to be mediated by separate neural substrates. For example, finger-tapping skill tasks typically show learning-related activation in contralateral M1 (22, 31), whereas adaptation tasks, such as visuomotor rotation, predominantly activate posterior parietal cortex and cerebellum (32–34).
Online Skill Acquisition.
Three previous studies have shown enhancement of motor learning in a single session using anodal tDCS in healthy subjects (15, 16, 18). Although our a priori hypotheses focused on net learning effects across 5 days of training, visual inspection of Fig. 4 suggests that there was indeed a greater day-1 within-session effect for anodal tDCS, consistent with these previous reports, supporting the idea that preexisting synaptic machinery is strengthened by tDCS. On the other hand, over the course of 5 training days there was no significant online effect for anodal tDCS compared to sham. These results suggest that the neural substrates underlying online effects, which are likely to include long-term potentiation (LTP)-like mechanisms (35, 36), may become saturated early on, manifesting as a ceiling for behavioral improvements within session (Fig. S2). Consistent with this interpretation, repeated motor training in rats led to occlusion of LTP expression in the motor cortex, typically paralleled by a ceiling in reaching skill gains (36). One might expect that polarity reversal would yield the opposite behavioral pattern (reduced consolidation). However, cathodal tDCS did not influence the learning process relative to sham (Fig. S1), a result consistent with previous findings on day 1 using a different task (16). Such results, in combination with the known downregulating effect of cathodal tDCS on motor-cortical excitability (12), suggest caution when using cortical excitability changes to predict behavioral consequences (15, 16).
Offline Consolidation.
Motor consolidation is understood to mean either resistance to interference or positive offline effects (“offline learning”) (6, 37, 38). Positive offline effects mediated the greater total learning in the anodal tDCS group: on average, performance at the beginning of day n + 1 was better than at the end of day n. The robustness of this offline effect was present whether derived from the initial 5 trials or 40 trials, arguing against an enhanced practice effect (savings) within the first training block. In contrast, the sham group showed an offline loss in skill. A decrease in performance after a rest period is well described for skill learning (7, 39, 40) and has been called the “warm-up decrement” (41). As we saw in our sham group, the warm-up decrement is small, insomuch that it does not reduce performance to naïve levels. Positive offline effects, which we observed only in the anodal tDCS group, are thus not a ubiquitous phenomenon in skill acquisition. Nevertheless, positive offline effects have garnered a great deal of attention in recent years as they have been reported in several influential studies of skill consolidation with finger-sequencing tasks (8, 42). To the best of our knowledge, there has been little comment about why some skill tasks show a warm-up decrement whereas others show consolidation. One possible explanation may relate to the distinction between continuous and discrete skill tasks (39). In a continuous task, as in our study, the behavior continues in an uninterrupted fashion during each trial. In contrast, finger-sequencing tasks, such as the SRTT, consist of separable discrete finger movements. Warm-up might not be needed in the latter case as one can explicitly call up each discrete element (finger movement). Support for a role of an explicit component in offline gains comes from the observation that sleep-dependent offline learning occurs only when subjects acquire explicit awareness of a sequence during training (43). Here we present the novel finding that tDCS induces consolidation in a continuous skill task, where an overnight decrement is the default occurrence (sham group). This finding implies that for a continuous skill the passage of time and/or sleep may be necessary but not sufficient for consolidation to occur. We cannot answer whether tDCS could induce consolidation in the absence of sleep because we did not perform a within-day, 2-session experiment.
What could the mechanism of tDCS-induced consolidation be? The effect of anodal tDCS on cortical excitability is known to outlast the stimulation period (44, 45). Animal data suggest that skill learning after day 1 depends on plasticity-related protein synthesis (46). Thus, one can postulate 2 possible mechanisms for anodal tDCS-induced positive offline learning: either anodal tDCS enhances protein synthesis directly during training, or its excitability effects during and after training interact downstream of learning-related protein synthesis.
Long-Term Retention.
Anodal tDCS did not affect the rate of forgetting over the ensuing 3 months relative to sham tDCS. The equal rate of forgetting led to a higher skill level at day 85 in the anodal tDCS group. This finding is important because a potential cost of faster learning over 5 days could have been faster forgetting. If there is an evolutionary reason why maximal potential levels of learning are not reached in the absence of stimulation, then there could be a hidden cost to learning enhancement that we do not currently appreciate. Our finding that anodal tDCS induced offline consolidation but did not hinder the rate of forgetting also suggests that the overnight warm-up decrement and forgetting of skill over weeks and months are distinct processes. Alternatively, we cannot rule out the possibility that the warm-up decrement seen in the sham group was not affected per se but overridden by an independent consolidation effect of anodal tDCS.
Role of M1 in Motor Learning.
In concordance with previous reports (13, 22, 47–50), our results suggest that M1 is a key structure in motor skill learning that can be purposefully modulated by noninvasive brain stimulation. That its role in the consolidation of motor skills can dissociate from initial acquisition was shown by a study in which low-frequency repetitive transcranial magnetic stimulation (rTMS) was applied over M1 between same-day training blocks of a ballistic pinch task (13). rTMS disrupted short-term retention of performance 15 min later, but not online learning or subsequent retention when applied 6 h after the end of training. Our results are consistent with this finding: anodal tDCS had, except for day 1, minimal effects on within-day acquisition and on long-term retention but had a marked effect on consolidation. Hence, decreasing motor cortical activity (using 1 Hz rTMS in ref. 13) and enhancing it (using anodal tDCS in our study) may have opposite effects on consolidation. It is therefore conceivable that M1 is involved in early consolidation of motor skills and that this consolidation can be enhanced or disrupted by noninvasive cortical stimulation (14, 51) either through a direct effect on M1 or indirectly through effects on other motor regions connected with M1. Whether there is a categorical difference when the between-session period includes sleep, as recently suggested by a study using rTMS and a variant of the SRTT (8), will require future investigation.
Conclusions.
The finding that the effect of anodal tDCS was specific for induction of consolidation, as opposed to enhancement of online effects or long-term retention, supports the view that motor skill learning comprises mechanistically and temporally distinct processes. The persistence of a beneficial effect of anodal tDCS at 3 months after the end of training may have promising implications for the design of motor learning protocols in healthy individuals and in patients undergoing neurorehabilitation.
Materials and Methods
Supplementary experimental and analytical details are available online. For a summary of the experimental groups and subject demographics, please see Table S1.
General.
All subjects gave written informed consent to participate in the study according to the declaration of Helsinki. The study was approved by the National Institute of Neurological Disorders and Stroke (NINDS) Institutional Review Board. All experiments were carried out at the laboratories of the Human Cortical Physiology Section, NINDS, National Institutes of Health. Participation required a normal general and neurological examination, dominant handedness, lack of chronic neurological or psychiatric disease or any severe medical condition, and lack of drug intake.
Training and Follow Up.
All subjects underwent ≈45 min of repeated task practice (200 trials) on 5 consecutive days (Fig. 2). Sessions took place between 8 a.m. and 2 p.m. and were separated by 24 h. Subjects received either sham or anodal tDCS for 20 min during training (Table S1, Fig. 2B). Training blocks 1 and 6 were always performed without stimulation. A third group served as active control in a separate experiment receiving cathodal tDCS during training. Retention of skill (40 trials of the SVIPT) was tested on day 8, day 15 ± 1, day 29 ± 2, day 57 ± 2, and day 85 ± 2 (Figs. 2B and 6). Time of day and experimental environment were kept constant in all sessions. In each session subjects underwent a brief psychophysical assessment and were asked to report potential side effects (SI Results and Table S2).
Transcranial Direct Current Stimulation.
tDCS was applied via 2 conducting 25 cm2 electrodes covered with a saline-soaked sponge. A bipolar electrode montage (left M1 and right supraorbital area) was used (Fig. 2A). The terminology “anodal” and “cathodal” refers to the electrode placed over the left M1. The M1 hand area was localized in all subjects with transcranial magnetic stimulation and in addition, in a subgroup of volunteers, using a neuronavigation device (Fig. 2A). A Phoresor II Auto (model PM850, IOMED, Salt Lake City, UT) device was used to apply tDCS. The stimulation was delivered at an intensity of 1 mA (current density 0.04 mA/cm2; total charge 0.048 C/cm2) for 20 min in the anodal and cathodal tDCS group and for up to 30 seconds in the Sham session according to a previously described method (52). Subjects and the investigator performing testing and data analysis were blinded to the type of tDCS.
Sequential Visual Isometric Pinch Task (SVIPT).
Subjects were seated in an armchair 60 cm in front of a 20 inch-screen monitor and held a force transducer between the thumb and the index finger of the right hand. Squeezing the force transducer moved a screen cursor horizontally to the right, while relaxing caused the cursor to move left. Upon presentation of a GO signal, the goal of the task was to move the cursor quickly and accurately between the start position (Home) and a numbered order of gates (Home-1-Home-2-Home-3-Home-4-Home-5). A STOP signal appeared when stopping at gate 5. To increase the difficulty of the task, we chose a logarithmic transduction of pinch force into cursor movement with the maximum rightward movement set to 35–45% of maximum force. The average movement time per training block was measured from movement onset to stopping at gate 5. The error rate was calculated as the proportion of trials with at least 1 over- or undershooting movement.
Determination of Skill.
Skill parameter.
The notion guiding our definition of skill was that a change in skill is equivalent to a change in the SAF (Fig. 3). Therefore, behavior reflecting a change in position along the curve of an unchanged SAF should not be interpreted as an authentic change in skill. We developed a parsimonious mathematical model for the SAF of the SVIPT such that training (which we confirmed indeed changes the SAF, see Fig. 3) is associated with a selective change in 1 model parameter, which we would therefore define as the skill parameter. We reasoned that if we could find such a model, then by using fixed estimates of the nonskill parameters, we would be able to estimate the skill parameter in each subject and time point during training simply from their corresponding bivariate observation of speed and accuracy.
Using a model (see SI Methods) that was validated in an independent sample of subjects, the proposed estimate of the skill parameter, a, was chosen to be:
where error rate and duration (movement time) are averages over some number of trials.
Multiplicative model of learning.
The noise in the skill parameter estimate was multiplicative, which required us to logarithmically transform it: we called the natural logarithm of the skill parameter the skill measure. Additive differences between skill measures at 2 different time points are proportional to the multiplicative difference (i.e., the ratio) of the skill parameters at those 2 time points. The online effects, offline effects, and total learning across the 5 days of training were then defined as:
total learning over 5 days of training:=online gains+offline gains
Supplementary Material
Acknowledgments.
We thank Dr. Steve Wise for helpful comments and Alana Bremers for help with analysis. This research was supported by the Intramural Research Program, National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH) (J.R., H.M.S., B.F., and L.G.C.), the Alexander von Humboldt Foundation Germany (J.R.), Graduate Partnerships Program Oxford-NIH PhD program (E.R.B.), the American Heart Association (0665347U, P.A.C.), National Institute of Child Health and Human Development (NICHD), NIH (R01HD053793, P.A.C.), the Rehabilitation Medicine Scientist Training Program (5K12HD001097, P.A.C.), and NINDS NIH R01–052804 (E.Z. and J.W.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805413106/DCSupplemental.
References
- 1.Schmidt RA, Lee TD. Motor Control and Learning: A Behavioral Emphasis. Champaign, Illinois: Human Kinetics; 2005. [Google Scholar]
- 2.Korman M, Raz N, Flash T, Karni A. Multiple shifts in the representation of a motor sequence during the acquisition of skilled performance. Proc Natl Acad Sci USA. 2003;100:12492–12497. doi: 10.1073/pnas.2035019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luft AR, Buitrago MM. Stages of motor skill learning. Mol Neurobiol. 2005;32:205–216. doi: 10.1385/MN:32:3:205. [DOI] [PubMed] [Google Scholar]
- 4.Shea CH, Kohl RM. Specificity and variability of practice. Res Q Exerc Sport. 1990;61:169–177. doi: 10.1080/02701367.1990.10608671. [DOI] [PubMed] [Google Scholar]
- 5.Savion-Lemieux T, Penhune VB. The effects of practice and delay on motor skill learning and retention. Exp Brain Res. 2005;161:423–431. doi: 10.1007/s00221-004-2085-9. [DOI] [PubMed] [Google Scholar]
- 6.Robertson EM, Pascual-Leone A, Miall RC. Current concepts in procedural consolidation. Nat Rev Neurosci. 2004;5:576–582. doi: 10.1038/nrn1426. [DOI] [PubMed] [Google Scholar]
- 7.Adams JA. Warm-up decrement in performance on the pursuit-rotor. Am J Psychol. 1952;65:404–414. [PubMed] [Google Scholar]
- 8.Robertson EM, Press DZ, Pascual-Leone A. Off-line learning and the primary motor cortex. J Neurosci. 2005;25:6372–6378. doi: 10.1523/JNEUROSCI.1851-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sternberg S. Process decomposition from double dissociation of subprocesses. Cortex. 2003;39:180–182. doi: 10.1016/s0010-9452(08)70097-2. [DOI] [PubMed] [Google Scholar]
- 10.Pascual-Leone A, Valls-Solé J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117(Pt 4):847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- 11.Rosenkranz K, Nitsche MA, Tergau F, Paulus W. Diminution of training-induced transient motor cortex plasticity by weak transcranial direct current stimulation in the human. Neurosci Lett. 2000;296:61–63. doi: 10.1016/s0304-3940(00)01621-9. [DOI] [PubMed] [Google Scholar]
- 12.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muellbacher W, et al. Early consolidation in human primary motor cortex. Nature. 2002;415:640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- 14.Richardson AG, et al. Disruption of primary motor cortex before learning impairs memory of movement dynamics. J Neurosci. 2006;26:12466–12470. doi: 10.1523/JNEUROSCI.1139-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antal A, et al. Facilitation of visuo-motor learning by transcranial direct current stimulation of the motor and extrastriate visual areas in humans. Eur J Neurosci. 2004;19:2888–2892. doi: 10.1111/j.1460-9568.2004.03367.x. [DOI] [PubMed] [Google Scholar]
- 16.Nitsche MA, et al. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cognit Neurosci. 2003;15:619–626. doi: 10.1162/089892903321662994. [DOI] [PubMed] [Google Scholar]
- 17.Boggio PS, et al. Enhancement of non-dominant hand motor function by anodal transcranial direct current stimulation. Neurosci Lett. 2006;404:232–236. doi: 10.1016/j.neulet.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 18.Vines BW, Nair DG, Schlaug G. Contralateral and ipsilateral motor effects after transcranial direct current stimulation. Neuroreport. 2006;17:671–674. doi: 10.1097/00001756-200604240-00023. [DOI] [PubMed] [Google Scholar]
- 19.Shea CH, Lai Q, Black C, Park J-H. Spacing practice sessions across days benefits the learning of motor skills. Hum Mov Sci. 2000;19:737–760. [Google Scholar]
- 20.Etnyre BR, Poindexter HB. Characteristics of motor performance, learning, warm-up decrement, and reminiscence during a balancing task. Percept Mot Skills. 1995;80:1027–1030. doi: 10.2466/pms.1995.80.3.1027. [DOI] [PubMed] [Google Scholar]
- 21.Walker MP, et al. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 22.Karni A, et al. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci USA. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheth BR, Janvelyan D, Khan M. Practice makes imperfect: restorative effects of sleep on motor learning. PLoS ONE. 2008;3:e3190. doi: 10.1371/journal.pone.0003190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallett M, Pascual-Leone A, Topka H. In: The Acquisition of Motor Behavior in Vertebrates. Bloedel JR, Ebner TJ, Wise SP, editors. Cambridge, MA: MIT Press; 1996. pp. 289–302. [Google Scholar]
- 25.Shadmehr R, Wise SP. The Computational Neurobiology of Reaching and Pointing: A Foundation for Motor Learning. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- 26.Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185:359–381. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nezafat R, Shadmehr R, Holcomb HH. Long-term adaptation to dynamics of reaching movements: a PET study. Exp Brain Res. 2001;140:66–76. doi: 10.1007/s002210100787. [DOI] [PubMed] [Google Scholar]
- 28.Kitazawa S, Kimura T, Uka T. Prism adaptation of reaching movements: specificity for the velocity of reaching. J Neurosci. 1997;17:1481–1492. doi: 10.1523/JNEUROSCI.17-04-01481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karni A. When practice makes perfect. Lancet. 1995;345:395. doi: 10.1016/s0140-6736(95)90386-0. [DOI] [PubMed] [Google Scholar]
- 30.Nissen MJ, Bullemer P. Attentional requirements of learning: evidence from performance measures. Cognit Psychol. 1987;19:1–32. [Google Scholar]
- 31.Karni A, et al. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- 32.Ghilardi M, et al. Patterns of regional brain activation associated with different forms of motor learning. Brain Res. 2000;871:127–145. doi: 10.1016/s0006-8993(00)02365-9. [DOI] [PubMed] [Google Scholar]
- 33.Krakauer JW, et al. Differential cortical and subcortical activations in learning rotations and gains for reaching: a PET study. J Neurophysiol. 2004;91:924–933. doi: 10.1152/jn.00675.2003. [DOI] [PubMed] [Google Scholar]
- 34.Diedrichsen J, Hashambhoy Y, Rane T, Shadmehr R. Neural correlates of reach errors. J Neurosci. 2005;25:9919–9931. doi: 10.1523/JNEUROSCI.1874-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenkranz K, Kacar A, Rothwell JC. Differential modulation of motor cortical plasticity and excitability in early and late phases of human motor learning. J Neurosci. 2007;27:12058–12066. doi: 10.1523/JNEUROSCI.2663-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- 37.Brashers-Krug T, Shadmehr R, Bizzi E. Consolidation in human motor memory. Nature. 1996;382:252–255. doi: 10.1038/382252a0. [DOI] [PubMed] [Google Scholar]
- 38.Krakauer JW, Shadmehr R. Consolidation of motor memory. Trends Neurosci. 2006;29:58–64. doi: 10.1016/j.tins.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Catalano JF. The effect of rest following massed practice of continuous and discrete motor tasks. J Mot Behav. 1978;10:63–67. doi: 10.1080/00222895.1978.10735136. [DOI] [PubMed] [Google Scholar]
- 40.Anshel MH. Examining warm-up decrement as a function of interpolated open and closed motor tasks: implications for practice strategies. J Sports Sci. 1995;13:247–256. doi: 10.1080/02640419508732234. [DOI] [PubMed] [Google Scholar]
- 41.Adams JA. The second facet of forgetting: a review of warmup decrement. Psychol Bull. 1961;58:257–273. doi: 10.1037/h0044798. [DOI] [PubMed] [Google Scholar]
- 42.Korman M, et al. Daytime sleep condenses the time course of motor memory consolidation. Nat Neurosci. 2007;10:1206–1213. doi: 10.1038/nn1959. [DOI] [PubMed] [Google Scholar]
- 43.Robertson EM, Pascual-Leone A, Press DZ. Awareness modifies the skill-learning benefits of sleep. Curr Biol. 2004;14:208–212. doi: 10.1016/j.cub.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 44.Bindman LJ, Lippold OC, Redfearn JW. Long-lasting changes in the level of the electrical activity of the cerebral cortex produced bypolarizing currents. Nature. 1962;196:584–585. doi: 10.1038/196584a0. [DOI] [PubMed] [Google Scholar]
- 45.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 46.Luft AR, et al. Motor skill learning depends on protein synthesis in motor cortex after training. J Neurosci. 2004;24:6515–6520. doi: 10.1523/JNEUROSCI.1034-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baraduc P, Lang N, Rothwell JC, Wolpert DM. Consolidation of dynamic motor learning is not disrupted by rTMS of primary motor cortex. Curr Biol. 2004;14:252–256. doi: 10.1016/j.cub.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 48.Ungerleider LG, Doyon J, Karni A. Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem. 2002;78:553–564. doi: 10.1006/nlme.2002.4091. [DOI] [PubMed] [Google Scholar]
- 49.Matsuzaka Y, Picard N, Strick PL. Skill representation in the primary motor cortex after long-term practice. J Neurophysiol. 2007;97:1819–1832. doi: 10.1152/jn.00784.2006. [DOI] [PubMed] [Google Scholar]
- 50.Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning. Curr Opin Neurobiol. 2002;12:217–222. doi: 10.1016/s0959-4388(02)00307-0. [DOI] [PubMed] [Google Scholar]
- 51.Hadipour-Niktarash A, Lee CK, Desmond JE, Shadmehr R. Impairment of retention but not acquisition of a visuomotor skill through time-dependent disruption of primary motor cortex. J Neurosci. 2007;27:13413–13419. doi: 10.1523/JNEUROSCI.2570-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.