Abstract
Pulmonary tissue damage resulting from influenza virus infection is caused by both the cytolytic activity of the virus and the host immune response. Immune-mediated injury results from T cell-mediated destruction of virus-infected cells and by release of cytokines and chemokines that attract polymorphonuclear leukocytes (PML) and macrophages to the infected site. The cytokines/chemokines potentiate dendritic cell (DC) activation and T cell expansion, which further enhances local damage. Here we report that immune modulation by local administration to the respiratory tract of sphingosine analog AAL-R significantly dampens the release of cytokines and chemokines while maintaining protective neutralizing antibody and cytotoxic T cell responses. As a result there was a marked reduction of infiltrating PML and macrophages into the lung and resultant pulmonary tissue injury. DC maturation was suppressed, which limited proliferation of specific antiviral T cells in the lung and draining lymph nodes. Further, AAL-R was effective in controlling CD8+ T cell accumulation in the lungs even when given 4 days after initiation of influenza virus infection. These data indicate that sphingosine analogs display useful potential for controlling the immunopathology caused by influenza virus.
Keywords: immunopathology, dendritic cells, cytokine storm, T cells
The antiviral host response evolved to limit the spread of infection at the cost of causing tissue injury. There is a balance between the protective and injurious responses that leads either to the purging of infectious virus and host recovery or to severe disease and even death. Thus, strategies to balance the antiviral immune response in favor of host outcome need to be developed.
T cell response elicited early in the course of infection recognizes, attacks and lyses virus-infected cells to eliminate potential factories of progeny viruses (1–5). The protective influenza antibody response is elicited later and plays a role in controlling re-infection (6, 7). The innate and adaptive immune systems release wide varieties of cytokines and chemokines that activate and attract inflammatory cells to the site of infection (2, 8, 9). However, these molecules can also cause the host harm by a phenomenon known as cytokine storm. Cytokine storm has been convincingly documented both in experimental animals infected with the 1918/1919 and H5N1 influenza viruses (2, 10–13) as well as in humans (14–18) succumbing to H5N1 infection. Although antiviral drugs can be used to treat the virus, a strategy to balance the resultant cytokine release and lung injury while maintaining benefits of the antiviral protective immune response is needed (19). Influenza virus replication is most often limited primarily to the respiratory tract but the systemic signs and symptoms of disease, e.g., fever, muscle pain and shakes, intestinal tract involvement, are related to cytokine effects (20, 21).
We reported that intratracheal (i.t.) delivery of the sphingosine analog AAL-R or its phosphate ester inhibited virus-specific T cell responses to influenza-virus infection whereas, in contrast, neither intraperitoneal (i.p.) delivery of AAL-R nor i.t. administration of the non-phosphorylable stereoisomer AAL-S were effective (22). Here, we extend those findings by determining the mechanism of AAL-R down-modulation of the antiviral T cell response. Using a mouse model of influenza virus infection, three findings are made. First, local administration of sphingosine analog AAL-R to the respiratory tract down-modulates the anti-influenza virus T cell response and inhibits cytokine/chemokine release without altering the formation, kinetics or titer of anti-influenza-neutralizing antibodies. Limiting the navigation signals of cytokines and chemokines reduces the infiltration of PML and macrophages into the lung. Second, AAL-R acts on pulmonary and draining lymph node dendritic cells (DC). AAL-R significantly down-regulates major histocompatibility (MHC) class I and II molecules, as well as co-stimulatory B7–2 molecule from the surfaces of DC in the lung and draining lymph nodes, leading to a significant decrease in accumulation of virus-specific T cells in the lung. Third, AAL-R therapy is effective when administered 4 days after initiation of influenza virus infection. Thus, short-term modulation of S1P receptors in lungs interferes with antigen presentation by DC and provides an additional therapeutic approach in treating aberrant cytokine production in influenza and likely other infectious respiratory diseases.
Results
An In Vivo Model for Quantitating and Monitoring Migration of Influenza Virus-Specific T Cells into the Lung.
We established an in vivo model system to study influenza virus-mediated host immune responses by generating a recombinant influenza virus. A recombinant A/WSN/33 (WSN; H1N1) virus (FLU-LCMV) was engineered to bear the immunodominant H-2Db CD8 (GP 33–41) and I-Ab CD4 (GP 65–77) T cell receptor epitopes of lymphocytic choriomeningitis virus (LCMV) in its neuraminidase (NA) stalk using reverse genetics (23) (Fig. 1A). Availability of transgenic (tg) mice in which the T cell receptor (TCR) is specific for these LCMV immunodominant epitopes allowed in vivo quantification of T cell trafficking (24) and visualization of T cell immunobiology (25). Intranasal (i.n.) or i.t. inoculation of 1 × 105 PFU of FLU-LCMV resulted in expression of influenza viral antigens throughout the lungs. As shown in Fig. 1C, at 6 days post-infection (dpi), viral antigen is observed throughout the lung parenchyma. High power examination showed viral antigen in bronchioles and epithelial cells surrounding the airways (data not shown). Mice adoptively transferred with 2.5 × 104 Thy1.1+ GP 33–41-restricted CD8+ (GP33/CD8+) T cells and 2.5 × 104 GFP-expressing GP 65–77-restricted CD4+ (GP65/CD4+) T cells and subsequently inoculated i.n. with FLU-LCMV displayed pulmonary infiltration of virus-specific CD8+ and CD4+ T cells (Fig. 1D) at six dpi. As shown in Fig. 1 E and F, pulmonary accumulation of GFP+ GP33/CD8+ T cells occurred after 4 dpi, reached a plateau between 6 and 8 dpi, then dramatically decreased at 10 dpi, as revealed by both fluorescence imaging on whole lung sections (Fig. 1E) and on single-cell suspension from lung tissue analyzed by flow cytometry (Fig. 1F). This model system provided an opportunity for quantification of virus-specific T cells and a mechanistic study of the interplay between host immunity, influenza virus, and their modulation by chemical probes.
Fig. 1.
Generation of recombinant influenza virus and pulmonary accumulation of viral antigens and virus-specific T cells in vivo. (A) Reverse genetics strategy to insert CD4 and CD8 immunodominant epitopes of LCMV glycoprotein (GP), amino acids (aa) 33–41 (GP33) for CD8+ T cells, and GP amino acid 65–77 (GP65) for CD4+ T cells, into the neuraminidase (NA) stalk of A/WSN/33 virus. (B) Cartoon to display the administration of fluorochrome-labeled or phenotypically identifiable congenic LCMV-specific GP33/CD8+ T cells and GP65/CD4+ T cells from T cell receptor transgenic mice and instillation of influenza virus. (C) Distribution of influenza viral antigens (green) in the whole lung at day 6 postinfluenza virus i.n. administration. Nuclei appear in blue. (D) Simultaneous detection of adoptively transferred CD4+ (green) and CD8+ T cells (red) specific for FLU-LCMV at day 6 post-influenza i.n. inoculation. Nuclei appear in blue. (E) Analysis of virus-specific GFP+ TCR transgenic GP33/CD8+ T cells on day 4 (d4), d6, d8, and d10 after i.n. FLU-LCMV infection. GFP+ TCR transgenic GP33/CD8+ T cells appear in green and nuclei were stained with DAPI (blue). (F) By comparison to (E), flow cytometric analysis of GFP+ TCR transgenic GP33/CD8+ T cells after i.n. FLU-LCMV infection. The percentages of GFP+ TCR transgenic GP33/CD8+ T cells are depicted on scattergrams. E and F show representative samples of one mouse from a group of three.
Sphingosine Analog AAL-R Inhibits the Accumulation of Influenza Virus-Specific T Cells in the Lung but Does Not Interfere with the Production of Protective Neutralizing Antiiinfluenza Antibodies.
Earlier we reported that local administration of a single 0.1-mg/kg dose of AAL-R by an i.t., but not i.p. route, specifically down-modulated virus-specific T cell accumulation in the lung, whereas both routes of delivery induced T cell recirculation from the blood to secondary lymphoid tissues (22). This inhibition of T cell accumulation in the lung depended on phosphorylation of AAL-R as the chiral enantiomer molecule AAL-S that cannot be phosphorylated efficiently in vivo was impotent in restricting T cell infiltration in the lung.
We now assessed the effect of AAL-R treatment on the virus-specific immune responses. Treatment with 0.1 mg/kg AAL-R i.t. following FLU-LCMV inoculation inhibited the accumulation of total CD4+ and CD8+ T cells (Fig. 2A), as well as virus-specific T cells in the lung (Fig. 2B). However, AAL-R i.t. administration had no effect on the production of influenza virus-specific total immunoglobulins (Ig) (Fig. 2C) and IgM (data not shown) at 6, 10, 15, and 30 days following virus infection. Assays for influenza virus neutralizing antibodies revealed that the amount of immunoglobulins was not impaired by AAL-R administration when compared to AAL-S and vehicle-treated groups (Fig. 2D). Administration of AAL-R did not significantly increase viral burden within the lung, but slightly delayed viral clearance (Fig. 2E). By day 10 post-infection, both vehicle and AAL-R-treated mice were negative for infectious virus. These results indicate that AAL-R therapy given locally into the respiratory tract interferes with neither influenza virus replication nor the generation of protective antibodies but significantly down-regulated the numbers of virus-specific T cells in the lung. Although numbers of virus-specific CD8+ T cells were reduced, influenza virus infection was still controlled.
Fig. 2.
Effect of sphingosine analog on influenza virus-specific T cells and antibodies as well as virus titers in the lung. (A and B) Mice (n = 4 mice per group) were infected with 1 × 105 PFU of FLU-LCMV and administered i.t. with vehicle (VEH) or 0.1 mg/kg AAL-R, or in B, with VEH, 0.1 mg/kg of AAL-R, or isomer control AAL-S. At 6 dpi, lungs were processed for flow cytometric analysis to obtain numbers of CD4+ and CD8+ T cells in lungs (A) or percentage of virus-specific TCR transgenic GP33/CD8+ T cells among total CD8+ T cells in lungs (B). (C and D) Four to six mice per group were infected with mock or FLU-LCMV and treated with VEH, AAL-S or AAL-R (0.1 mg/kg) 1 hpi. (C) Influenza virus-specific total immunoglobulins in serum were assessed by ELISA at 6, 10, 15, and 30 dpi, mean ± SD. (D) Titers of virus neutralizing antibodies in serum were evaluated 30 dpi, mean ± SEM. (E) PFU/gram of lung tissue in normal, unaltered C57BL/6 mice following i.t. inoculation of 1 × 105 PFUs of FLU-LCMV and i.t. delivery of 0.1 mg/kg of AAL-R or vehicle 1hpi. At day 8 following AAL-R treatment, 2 of 4 mice had detectable levels of virus at 1 × 102 PFU/gram of lung. The level of detection is 1 × 102 PFU/g tissue. Four mice were used per group per timepoint. P > 0.23 for days 2, 4, and 6 after infection. ND: not detectable.
AAL-R Does Not Decrease FLU-LCMV Titers or Cytotoxic Activity of Remaining Virus-Specific CD8+ T Cells.
We next determined how AAL-R treatment would influence viral kinetics and ex vivo cytotoxic T lymphocyte (CTL) activity following FLU-LCMV infection. As expected, AAL-R reduced the pulmonary content of CD8+ T cells having the ability to produce IFN-γ in response to GP33 peptide stimulation in vitro 7 dpi, and this effect was maintained until 8 dpi (Table 1). Also, the kinetics of viral clearance in mice treated with AAL-R was not significantly altered compared to viral clearance observed in mice administered with VEH or AAL-S (Table 1), suggesting the remaining anti-viral T cell activity was sufficient to clear virus from the lungs. We further tested this idea by measuring the cell-based cytotoxic activity of GP33-specific CD8+ T cells extracted from lung and spleen after treatment with AAL-R, AAL-S or VEH (Fig. S1). CTL activity of virus-specific CD8+ T cells in the lung (Fig. S1A) or spleen (Fig. S1B) was not adversely affected by AAL-R when compared with VEH and AAL-S treatments. Intracellular cytokine staining revealed a significant reduction in the number of virus-specific CD8+ T cells in AAL-R treated mice (Fig. S1C). Calculation of cytolytic activity on a per cell basis revealed a significant increase in the CTL activity of GP33-specific splenocytes in AAL-R-treated mice when compared with AAL-S or vehicle treatments.
Table 1.
Effect of AAL-R on antigen-specific CD8+ T cells accumulation and viral titers
| Treatment | n | Day 5 after infection |
Day 7 after infection |
Day 8 after infection |
|||
|---|---|---|---|---|---|---|---|
| GP33-specific cells | Titer | GP33-specific cells | Titer | GP33-specific cells | Titer | ||
| VEH | 4 | 4.1 × 102 ± 1.1* | 14.3 × 104 ± 5.0‡ | 7.6 × 103 ± 2.0 | 3.9 × 102 ± 0.8 | 3.0 × 104 ± 1.3 | 1.3 × 102 (1/4)‡ |
| AAL-S | 4 | 3.3 × 102 ± 0.9 | 7.9 × 104 ± 2.0 | 4.7 × 103 ± 1.8 | 3.6 × 102 ± 0.9 | 3.8 × 104 ± 0.8 | 0.2 × 102 ± 0.03 (3/4) |
| AAL-R | 4 | 1.5 × 102 ± 0.5 | 6.9 × 104 ± 1.6 | 1.8 × 103 ± 0.2* | 26.0 × 102 ± 12.0 | 2.3 × 104 ± 0.8 | 10.1 × 102 ± 6.8 (2/4) |
Mice were infected with 1 × 105 PFU of FLU-LCMV and administered i.t. with VEH and or 0.1 mg/kg AAL-S or AAL-R 1 hpi. *, P ≤ 0.05 when compared to vehicle-treated mice.
*Values are presented as average number ± SEM.
†Values are presented as average PFU/g of tissue ± SEM.
‡Virus-positive mice/n.
Decreased Accumulation of Virus-Specific T Lymphocytes in the Lung Following AAL-R Treatment Is Associated with Suppression of Cytokine Synthesis and Alleviation of Immunopathology.
Next we assessed the ability of AAL-R to regulate influenza virus-induced cytokines/chemokines synthesis in vivo. We noted that upon FLU-LCMV infection there was a significant up-regulation of multiple cytokines/chemokines, most notably marked increase of interleukin (IL)-1α, IL-1β, IL-6, IL-10, IL-12, monocyte chemoattractant protein (MCP-1, CCL2), TNF-α, macrophage inflammatory protein (MIP-1α, CCL3), granulocyte macrophage colony stimulating factor (GM-CSF), and RANTES (CCL5) (Fig. 3A). Of interest IL-6 and MCP-1 levels were strikingly increased in our model, an event also observed in both H5N1-infected humans (16), as well as in macaques (11) and mice (10) infected with the 1918/1919 reconstructed influenza virus. Treatment with AAL-R significantly inhibited the production of IL-1α, IL-1β, IL-6, IL-10, MCP-1, TNF-α, and GM-CSF 2 dpi, when compared with mice treated with AAL-S (Fig. 3B). Histological examination of lungs revealed that AAL-R, but not AAL-S, dramatically decreased the accumulation of lymphocytes, leukocytes, monocytes and macrophages and injury in the distal airways (Fig. 3C) while modestly enhancing survival time (Fig. 3D). Thus, delivery of AAL-R i.t. diminished cytokine-mediated histopathologic injury of the lungs during influenza virus infection. Cytokine/chemokine suppression was associated with a decrease of GR-1+ PML and F4/80+ macrophages (Fig. 3E) into the lung which directly correlated with the diminished alveolar cytopathology and infiltration in the lung. However, despite AAL-R successfully down-modulating T cell-mediated injury and cytokine/chemokine release there was no change in lung A/WSN/33 viral titers even at 2 mg/kg of AAL-R compared with treatment with AAL-S or vehicle (Fig. 3F). This indicated that AAL-R neither disrupted viral replication nor the resultant protective antiviral T cell immune responses but did successfully downmodulate immunopathology.
Fig. 3.
AAL-R impairs cytokine release and leukocyte infiltration in the lungs resulting in diminished alveolar inflammation and congestion but without altering infectious influenza virus titer. (A and B) Multiplex ELISA was performed on lung homogenates obtained at 2 dpi from uninfected mice (n = 4) or mice infected with 1 × 105 PFU FLU-LCMV i.t. (n = 12). Virus-infected mice were treated with VEH or AAL-R (0.1 mg/kg) (n = 6). Cytokines detected at levels of more than 40 pg/ml in supernatant of lung homogenates and significanlty increased by at least two-fold by viral infection over uninfected mice are shown. (A) Fold increase of cytokines/chemokines from FLU-LCMV-infected mice over that of uninfected mice. (B) Percentage of inhibition caused by AAL-R (black bars) and AAL-S treatment (white bars) relatively to vehicle-treated mice. Mean ± SEM. *, P ≤ 0.05. (C) Naive C57BL/6 mice were (i) left untreated or (ii, iii, iv) infected i.t. with 1 × 105 PFU of FLU-LCMV. One hpi mice were either administered i.t. with VEH (ii), AAL-S (iii: 0.1 mg/kg) or AAL-R (iv: 0.1 mg/kg), and euthanized 6 days later. Lungs were processed for hematoxylin and eosin staining. Representative fields are shown. (Scale bars, 50 μm.) Results were equivalent in more than 20 fields analyzed per group (>5 fields/section, 2 sections/mouse and 2 mice/group). (D) Percent survival was observed with 13 mice per group from two combined experiments. Mice were infected i.t. with 5 × 103 PFU of A/WSN/33 virus. One hour later, mice were administered i.t. with vehicle or AAL-R (0.1 mg/kg) and monitored daily for mortality. (E) Decrease in PML (GR-1+) and macrophages (F4/80+) infiltrates in the lung. Mice were infected with 1 × 105 PFU of FLU-LCMV and then received i.t. either vehicle or AAL-R (0.1 mg/kg). Two dpi, Macrophages (F4/80) and PML (GR1) numbers were evaluated by flow cytometry. Pool of 2 experiments, n = 7–8, mean ± SEM; * significantly different from vehicle, P < 0.05. (F) Titers of A/WSN/33 in lung three days after i.n. infection with 1 × 105 PFU of A/WSN/33 and i.t. administration of VEH, or 2 mg/kg of AAL-S and AAL-R. Virus plaques were quantitated on MDCK cells. Mean ± SEM; * P < 0.05.
AAL-R Protects by Impairing the Antigen-Presenting Capacity of Pulmonary DC.
Upon pulmonary infection, influenza virus-specific T cells are rapidly induced and proliferate in mediastinal lymph node (MLN), then migrate to the infected sites, including the lungs (26, 27). Given i.t., AAL-R significantly reduced the number of virus-specific CD8+ (Fig. 4A) and CD4+ (Fig. 4B) T cells in the MLNs 5 and 6 dpi with FLU-LCMV, indicating that AAL-R inhibited clonal expansion of T cells upon influenza virus infection. The number of Annexin V-positive virus-specific T cells in both MLNs and lungs was not significantly altered by local treatment with AAL-R (data not shown), suggesting that AAL-R altered T cell stimulation by influencing antigen-presenting DC (28) rather than deleting T cells.
Fig. 4.
Sphingosine analog AAL-R inhibits both induction of influenza virus-specific T cells in MLN and activation of DC in the lung. (A) Number (left) and percentage (right) of Thy1.1+ TCR transgenic GP33/CD8+ T cells in MLN 5 dpi with mock or FLU-LCMV and i.t. administration of VEH or AAL-R (0.3 mg/kg) 1 hpi. Representative of two different experiments, both showed similar results, n = 3–4 mice per group, mean ± SEM; * P < 0.05. (B) Number (left) and percentage (right) of Thy1.1+ TCR transgenic GP65/CD4+ T cells in MLN 6 dpi with mock or FLU-LCMV and i.t. instillation with VEH or AAL-R (0.1 mg/kg) 1 hpi. n = 4 mice per group, mean ± SEM; * P = 0.07. Number of (C) CD11c+CD11b+CD103- cells and (D) CD11c+CD11b-CD103+ cells in the lungs at 2 dpi with mock or FLU-LCMV and i.t. treatment with VEH or 0.1 mg/kg AAL-R 1 hpi. (E) Number of annexin-V+ CD11c+cells in lungs 12 h after infection with mock or FLU-LCMV, and i.t. treatment with VEH or 0.1 mg/kg AAL-R 1 hpi. Representative of two different experiments, all showed similar results, n = 4 mice per group; * P < 0.05. (F and G) Mean fluorescence intensity (MFI) of MHC-I (H-2Kb), MHC-II (I-Ab) and B7–2 on (F) CD11b+ DC and (G) CD103+ DC was analyzed. Cells were isolated from lungs 2 dpi with FLU-LCMV and i.t. treatment with VEH or AAL-R (0.1 mg/kg) 1 hpi. n = 4 mice per group; mean ± SEM. *, P < 0.05. (H) Proliferation of T cells cultured alone (top); with DC from FLU-LCMV + VEH treated mice (center); with DC from FLU-LCMV + AAL-R-treated mice (bottom). See SI Materials and Methods for details. Representative of 3 different experiments, all showed similar results.
We then focused on pulmonary DC and found that i.t. delivery of AAL-R neither reduced the numbers of total DC (data not shown) nor of specific DC subsets (Fig. 4C and D). Moreover, AAL-R did not interfere with the viability of CD11c+ cells (Fig. 4E) in the lungs upon influenza virus infection. However, AAL-R strongly suppressed influenza virus-induced DC activation in the lungs as measured by down-regulation of MHC-I, MHC-II and co-stimulatory molecule B7–2 on the surfaces of CD11c+ cells. AAL-R impaired maturation of CD11c+ cell subsets (CD11b+CD103− CD11b+CD103+, CD11b−CD103+, and CD11b−CD103−) in the lung by reducing surface expression of MHC-I, MHC-II and B7–2, with suppression of the CD11b+CD103− subset being affected the most (Fig. 4F and G) (data not shown). Stimulatory capacity of CD11c+ cells was impaired by in vivo AAL-R treatment, as confirmed by inefficient induction of virus-specific CD8+ T cell proliferation in vitro (Fig. 4H).
We found that impaired activation of DC in the lungs resulted in subsequent inhibition of influenza virus-induced elevation of CD11c+ cell numbers (both CD11b+ and CD8α+) in the MLNs (Fig. S2A), as well as impaired activation of CD8α+ DCs present in the lymph nodes, which are efficient at priming CD8+ T cells (29). Intratracheal delivery of AAL-R also down-regulated MHC-I, MHC-II and B7–2 co-stimulatory molecules on DC in MLN (Fig. S2B). Thus, these results indicate that upon influenza virus infection, AAL-R locally administered to the respiratory tract disrupts antigen-presenting DC network by blocking DC-mediated signal transmission from the infection site to the MLNs, leading to a dramatic decrease in T cell expansion.
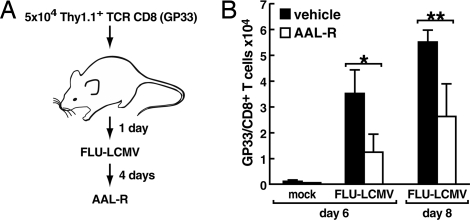
AAL-R Therapy Administered at a Later Time Point During Influenza Virus Infection Is Still Effective in Modulating the CD8+ T Cell Response to the Virus.
The last experiment determined whether administering 0.1 mg/kg of AAL-R locally into the respiratory tract at 4 dpi was effective in inhibiting T cell accumulation in the lung. As shown in Fig. 5, six and eight days after giving 1 × 105 PFU of FLU-LCMV the number of virus-specific CD8+ T cells was significantly reduced in the lung. In contrast, providing vehicle had no therapeutic effect.
Fig. 5.
Sphingosine analog AAL-R administered i.t. 4 days after FLU-LCMV infection is effective in limiting the accumulation of virus-specific CD8+ T cells in the lungs. (A) Cartoon of the experimental protocol. Mice, 4 per group, were infected with mock or 1 × 105 PFU of FLU-LCMV i.t. followed 4 days later by i.t. instillation of VEH or AAL-R (0.1 mg/kg). (B) Number of GP33/CD8+ T cells in lungs is shown. Mean ± SEM; *, P = 0.09; **, P = 0.07.
Discussion
Here we make three main points. First, a single low-dose administration of the sphingosine analog AAL-R locally into the respiratory airway, when provided at the time of influenza virus infection or after 4 days of infection, is able to effectively alter one of the two major arms of the antiviral immune response. Virus-specific T cell expansion in MLNs and accumulation in the lungs are significantly down-regulated whereas the production, kinetics and amounts of protective neutralizing antibodies are not altered. In addition, CD8+ T cell cytotoxic activity is not impaired. Second, AAL-R delivery in the respiratory tract can significantly decrease the release of a variety of cytokines and chemokines known to contribute to the cytokine storm effect, especially inhibition of IL-6 and MCP-1. The diminished synthesis of cytokines/chemokines following AAL-R treatment correlates directly with a decreased infiltration of PML and macrophages in the lung, resulting in less cytopathology of alveolar cells and inflammatory driven congestion of air spaces. Third, mechanistically, AAL-R acts on DC from both draining lymph nodes and the lung. The drug inhibits DC accumulation in the draining lymph nodes and down-regulates MHC class I and II molecules as well as co-stimulatory molecule B7–2. The decreased accumulation of DCs in the draining lymph nodes combined with the decreased expression of stimulatory and co-stimulatory molecules likely accounts for the diminished proliferation and expansion of T cells in draining lymph node and subsequent accumulation in the lung.
Injury to the lung accompanying influenza virus infection depends on both the virus and action of the host's immune response. The virus being cytopathic lyses cells. In addition, infected cells may apoptose thus removing the nidus of infection. Similarly, the cytotoxic function of antiviral T cells is to kill virus-infected cells, especially early in the infectious process, removing cells that make progeny virus. However, by virus or immune-induced lysis of cells, factors are released that attract scavenger PMLs and macrophages to the infected site. These cells, while removing cellular debris, also contribute to airway disease. Most important, the antiviral T cells release cytokines/chemokines at the site of infection via their specific recognition of infected targets. These factors then further act to provide navigation signals to attract the PML and macrophages thereby potentiating the pulmonary injury. In addition, the released cytokines/chemokines play the dominant role in causing the generalized systemic manifestations of influenza virus infection.
Administration of sphingosine analog AAL-R strikingly impaired accumulation of DC in the draining lymph nodes, as well as maturation of DC in the lung and MLN. There was a down-regulation of MHC and co-stimulatory molecules required to engage specific antiviral T cells. This led to diminished proliferation and clonal expansion of T cells in the MLN. These effects could also be shown with the phosphorylated S1P receptor agonist AFD-R (22), suggesting that targeting S1P receptors locally in the lung and on DC was sufficient to inhibit proinflammatory cytokine release and virus-specific T cells expansion. Others have reported that systemic delivery of sphingosine analog FTY720 can sequester normal expanding lymphocytes in lymphoid organs in various models (30–32) including during virus infection (33). However, this was unlikely a mechanism in our studies for the following reasons. First, elsewhere we documented that delivery of a single dose of the sphingosine analog AAL-R (0.1 mg/kg) in the airways did not cause sequestration of lymphocytes in draining lymphoid organs at 6 days after AAL-R treatment, but a decreased accumulation of virus-specific T cells does occur in the lung (22). The latter was not observed with systemic delivery of AAL-R. Further, i.p. delivery of AAL-R, the route used by others to show sequestering of lymphocytes in lymphoid organs, was not associated with inhibition of DC accumulation in draining lymph nodes (not shown). The fact that MLN DC numbers were significantly altered by i.t. but not i.p. treatment with AAL-R further indicates the likelihood of local effects of the AAL-R analog on DC maturation and function rather than changes of DC or lymphocyte migration from the blood to MLN (34, 35). Thus, the effects of local administration coupled with the failure of systemic dosing to induce suppression of DC molecules indicate that lymphopenia alone could not explain the immunomodulation we observed in the influenza model. Further, we noted a dramatic decrease in numbers of both CD11b+ and CD8α+ DC in MLN. Others have shown that CD8α+ DC are potent in stimulating T cells and do not originate from the lung after influenza virus infection (26). Our results thus suggest that the immunosuppressive effect of AAL-R is caused by modulation of DC function rather than by altering T cell trafficking.
Sphingosine analog did not inhibit the production of protective antibody (Fig. 2) even in the occurrence of significant T cell suppression. Generation of antibodies upon viral infection depends on multiple factors including infection route, duration of viral components' presence in specific locations, contribution of various anatomically distributed germinal centers (36), and dependency on T helper cells. Currently, the effect of systemic delivery of sphingosine analog on B cell response is controversial (33, 37), although no data are available for antibody response affected by local treatment of the analog. It is possible that local AAL-R-mediated split of B and T cell responses was caused by partial blockade of host immunity to influenza virus as: 1) the sphingosine analog was shown not to completely inhibit the release of cytokines upon influenza virus infection (Fig. 3B) (22); 2) remaining virus-specific CD4+ T cells (Fig. 4B) might be sufficient to reach the threshold for the generation of antibodies dependent on T helper cells; 3) there was sufficient effector T cell activity to balance influenza virus replication in the lung as infectious virus titers in the lung were equivalent with AAL-R, AAL-S or vehicle treatment (Fig. 2E, Fig. 3F, and Table 1). Recently, other investigators showed that CD11chi DC depletion upon influenza virus infection caused inhibition of T cell expansion but did not affect antibody production (38), which is consistent with our data. We are currently investigating local modulation of S1P receptors and the mechanism for separation of B cell response from T cell immunity.
Lastly, the observation that with AAL-R therapy virus titers in the lung were not significantly increased despite the down-modulation of numbers of antiviral-specific T cells, along with diminished cytokine/chemokine release and decreased injury to the lung suggest that combinatory therapy using antiviral drugs in concert with S1P analogs would be a preferred therapy. We are currently evaluating this possibility in mice models of virulent H5N1 influenza virus infection. Further, DC are known to contain at least four of the five S1P receptors. Dissection of the pulmonary DC S1P receptor(s) involved could suggest the design of more effective sphingosine analogs for treatment of lung inflammatory disorders.
Materials and Methods
Mice.
Mice were bred and maintained in a closed breeding facility at The Scripps Research Institute. C57BL/6, C57BL/6 Thy1.1+DbGP33–41 TCR-tg, C57BL/6 GFP+DbGP33–41 TCR-tg, C57BL/6 GFP+I-AbGP61–80 TCR-tg, and C57BL/6 Thy1.1+I-AbGP61–80 TCR-tg were used in this study. The handling of all mice conformed to the requirements of the National Institutes of Health and The Scripps Research Institute's Institutional Animal Care and Use Committee.
Cell Transfer.
Virus-specific CD8+ or CD4+ T cells were enriched by negative selection using Stemsep kit (Stemcell technologies), from the spleen of transgenic mice. Cells (5 × 104) were then injected in the tail vein of congenic mice 1 day before infection. When both CD8+ and CD4+ cells were transferred, 2.5 × 104 of each was used. To exclude any effect of transferred cells on the immunopathological response triggered by influenza virus infection, Figs. 2 C–E, 3 A–F, and 4 C–H were conducted without prior adoptive transfer of lymphocytes.
Viruses.
To generate a recombinant WSN mutant virus (FLU-LCMV), we inserted the LCMV immunodominant T cell-specific GP33 and GP65 tandem sequence AAGGCTGTCTACAATTTTGCCACCTGTGGGGGACGCACAAUGGGTCTTAAGGG-ACCCGACATTTACAAAGGAGTTTACCAATTTAAGTCAGTGGAGTTTGAT between nucleotides 145 and 146 of WSN NA gene. Insertion of up to 28 aa into the NA stalk does not impair NA function but insertion of more than 12 aa attenuates the virus. A/WSN/33 (WSN; H1N1) and FLU-LCMV were generated by using plasmid-based reverse genetics (23). Viruses were amplified and plaqued on Madin-Darby Canine Kidney (MDCK) cells.
Statistical Analysis.
Unless otherwise stated, bars represent means ± SEM and averages were compared using a bidirectional unpaired Student's t test with a 5% significance level. Star (*) was used to mark significant differences between 2 groups unless otherwise stated.
For specific information regarding the analytic assays performed in this study, please consult SI Text.
Supplementary Material
Acknowledgments.
This is Publication Number 19725 from the Department of Immunology and Microbial Science, Infectology and Chemical Physiology and Immunology; and The Scripps Research Institute Molecular Screening Center, The Scripps Research Institute (TSRI). This work was supported in part by USPHS grants AI074564 (MBAO, HR, YK, BH, DM, KW), AI009484 (MBAO), AI05509 (HR), AI069274 (YK), and NIMH-074404 (HR). YK and YH are also supported by Grants-in-Aid from the Ministries of Education, Culture, Sports, Science and Technology and of Health, Labor, and Welfare of Japan; HR is supported in part by a grant from Kyorin Pharmaceutical Company. DM is supported by Le Fonds de la Recherche en Sante du Quebec, Canada. The authors would like to acknowledge the technical assistance of Megan Welch and Nora Leaf. The authors thank the TSRI Flow Cytometry Core Facility; and Dusko Trajkovic for histological analyses.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812689106/DCSupplemental.
References
- 1.Whitton JL, Oldstone MB. In: Fields virology, 4th ed. Knipe DM, Howley PM, Griffin DE, Lamb RE, Martin MA, Rolzman B, Straus SE, editors. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 285–320. [Google Scholar]
- 2.La Gruta NL, Kedzierska K, Stambas J, Doherty PC. A question of self-preservation: immunopathology in influenza virus infection. Immunol Cell Biol. 2007;85:85–92. doi: 10.1038/sj.icb.7100026. [DOI] [PubMed] [Google Scholar]
- 3.Graham MB, Braciale TJ. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J Exp Med. 1997;186:2063–2068. doi: 10.1084/jem.186.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zinkernagel RM, Althage A. Antiviral protection by virus-immune cytotoxic T cells: infected target cells are lysed before infectious virus progeny is assembled. J Exp Med. 1977;145:644–651. doi: 10.1084/jem.145.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moskophidis D, Kioussis D. Contribution of virus-specific CD8+ cytotoxic T cells to virus clearance or pathologic manifestations of influenza virus infection in a T cell receptor transgenic mouse model. J Exp Med. 1998;188:223–232. doi: 10.1084/jem.188.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goulding J, et al. Respiratory infections: do we ever recover? Proc Am Thorac Soc. 2007;4:618–625. doi: 10.1513/pats.200706-066TH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerhard W. The role of the antibody response in influenza virus infection. Curr Top Microbiol Immunol. 2001;260:171–190. doi: 10.1007/978-3-662-05783-4_9. [DOI] [PubMed] [Google Scholar]
- 8.Biron CA, Sen GC. In: Fields Virology, 5th ed. Knipe DM, Howley PM, Griffin DE, Lamb RE, Martin MA, Rolzman B, Straus SE, editors. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 249–278. [Google Scholar]
- 9.Braciale TJ, Hahn YS, Burton DR. In: Fields Virology, 5th ed. Knipe DM, Howley PM, Griffin DE, Lamb RE, Martin MA, Rolzman B, Straus SE, editors. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 279–326. [Google Scholar]
- 10.Kash JC, et al. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature. 2006;443:578–581. doi: 10.1038/nature05181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobasa D, et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- 12.Cheung CY, et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 2002;360:1831–1837. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- 13.Tumpey TM, et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005;310:77–80. doi: 10.1126/science.1119392. [DOI] [PubMed] [Google Scholar]
- 14.Beigel JH, et al. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353:1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 15.Uiprasertkul M, et al. Apoptosis and Pathogenesis of Avian Influenza A (H5N1) Virus in Humans. Emerg Infect Dis. 2007;13:708–712. doi: 10.3201/eid1305.060572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Jong MD, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.To KF, et al. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J Med Virol. 2001;63:242–246. doi: 10.1002/1096-9071(200103)63:3<242::aid-jmv1007>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 18.Peiris JS, et al. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet. 2004;363:617–619. doi: 10.1016/S0140-6736(04)15595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearson H. Controlling immune response may cut bird flu death rate. Nat Med. 2006;12:1105. doi: 10.1038/nm1493. [DOI] [PubMed] [Google Scholar]
- 20.Hilleman MR. Realities and enigmas of human viral influenza: pathogenesis, epidemiology and control. Vaccine. 2002;20:3068–3087. doi: 10.1016/s0264-410x(02)00254-2. [DOI] [PubMed] [Google Scholar]
- 21.Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5:718–725. doi: 10.1016/S1473-3099(05)70270-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsolais D, et al. Local not systemic modulation of dendritic cell S1P receptors in lung blunts virus-specific immune responses to influenza. Mol Pharmacol. 2008;74:896–903. doi: 10.1124/mol.108.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neumann G, et al. Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci USA. 1999;96:9345–9350. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christen U, et al. A viral epitope that mimics a self antigen can accelerate but not initiate autoimmune diabetes. J Clin Invest. 2004;114:1290–1298. doi: 10.1172/JCI22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGavern DB, Christen U, Oldstone MB. Molecular anatomy of antigen-specific CD8(+) T cell engagement and synapse formation in vivo. Nat Immunol. 2002;3:918–925. doi: 10.1038/ni843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belz GT, et al. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc Natl Acad Sci USA. 2004;101:8670–8675. doi: 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumgarth N, Kelso A. Functionally distinct T cells in three compartments of the respiratory tract after influenza virus infection. Eur J Immunol. 1996;26:2189–2197. doi: 10.1002/eji.1830260934. [DOI] [PubMed] [Google Scholar]
- 28.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 29.Allan RS, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 30.Budde K, et al. FTY720 (fingolimod) in renal transplantation. Clin Transplant. 2006;20(Suppl 17):17–24. doi: 10.1111/j.1399-0012.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- 31.Mandala S, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 32.Sanna MG, et al. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279:13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- 33.Pinschewer DD, et al. FTY720 immunosuppression impairs effector T cell peripheral homing without affecting induction, expansion, and memory. J Immunol. 2000;164:5761–5770. doi: 10.4049/jimmunol.164.11.5761. [DOI] [PubMed] [Google Scholar]
- 34.Lan YY, et al. The sphingosine-1-phosphate receptor agonist FTY720 modulates dendritic cell trafficking in vivo. Am J Transplant. 2005;5:2649–2659. doi: 10.1111/j.1600-6143.2005.01085.x. [DOI] [PubMed] [Google Scholar]
- 35.Maeda Y, et al. Migration of CD4 T cells and dendritic cells toward sphingosine 1-phosphate (S1P) is mediated by different receptor subtypes: S1P regulates the functions of murine mature dendritic cells via S1P receptor type 3. J Immunol. 2007;178:3437–3446. doi: 10.4049/jimmunol.178.6.3437. [DOI] [PubMed] [Google Scholar]
- 36.Moyron-Quiroz JE, et al. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity. 2006;25:643–654. doi: 10.1016/j.immuni.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 37.Han S, et al. FTY720 suppresses humoral immunity by inhibiting germinal center reaction. Blood. 2004;104:4129–4133. doi: 10.1182/blood-2004-06-2075. [DOI] [PubMed] [Google Scholar]
- 38.GeurtsvanKessel CH, et al. Clearance of influenza virus from the lung depends on migratory langerin+CD11b- but not plasmacytoid dendritic cells. J Exp Med. 2008;205:1621–1634. doi: 10.1084/jem.20071365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.