Abstract
HIV protease inhibitors are a key component of anti-retroviral therapy, but their susceptibility to cytochrome P450 metabolism reduces their systemic availability and necessitates repetitive dosing. Importantly, failure to maintain adequate inhibitor levels is believed to provide an opportunity for resistance to emerge; thus, new strategies to prolong the lifetime of these drugs are needed. Toward this goal, numerous prodrug approaches have been developed, but these methods involve creating inactive precursors that require enzymatic processing. Using an alternative strategy inspired by the natural product FK506, we have synthetically modified an HIV protease inhibitor such that it acquires high affinity for the abundant, cytoplasmic chaperone, FK506-binding protein (FKBP). This modified protease inhibitor maintains activity against HIV-1 protease (IC50 = 19 nM) and, additionally, it is partitioned into the cellular component of whole blood via binding to FKBP. Interestingly, redistribution into this protected niche reduces metabolism and improves its half-life in mice by almost 20-fold compared with the unmodified compound. Based on these findings, we propose that addition of FKBP-binding groups might partially overcome the poor pharmacokinetic properties of existing HIV protease inhibitors and, potentially, other drug classes.
Keywords: AIDS, bifunctional molecules, chemical inducers of dimerization, pharmacology, prodrugs
Since its introduction over a decade ago, highly aggressive antiretroviral therapy (HAART) has proven to be effective at reducing viral loads and decreasing the morbidity and mortality associated with HIV-1 infection. However, adverse drug-drug interactions, poor adherence to antiviral regimens, and, importantly, the rapid emergence of multidrug resistance are posing new threats (1–3). To combat selection of resistant variants, maintaining inhibitor concentrations above critical thresholds is vitally important. However, the physiochemical properties of current antiretrovirals have made this a challenging task. For example, all 10 of the FDA-approved inhibitors of HIV-1 protease are good substrates for cytochrome P450 enzymes, especially CYP3A4, and they undergo extensive first-pass hepatic metabolism, leading to their rapid clearance (4). These properties necessitate the coadministration of P450 inhibitors, such as ritonavir, but this treatment often exacerbates side effects and leads to liver toxicity (5). Thus, there is a burgeoning need to improve the pharmacology of antiviral therapies.
A number of prodrug strategies have been evaluated in an attempt to improve existing HIV protease inhibitors (PIs) (6). For example, several groups have conjugated these molecules to amino acids and other nutrients to permit carrier-mediated absorption (7–9). Others have altered their distribution and persistence via covalent attachment of lipophilic groups (10), phosphates (11), polyethyleneglycol (12), or other modifications (13). In general, these strategies are intended to augment transepithelial delivery into systemic circulation and reduce binding to metabolic enzymes, with the hopes of improving absorption, distribution, metabolism, and excretion (ADME) profiles. In all cases, the modified drugs require enzymatic processing to release the active compound because the protected prodrug form has minimal activity.
Our group has been exploring a conceptually different approach to enhancing drug lifetimes that is inspired by the natural product, FK506. This compound is a potent immunosuppressive that forms a high-affinity complex with FK506-binding protein (FKBP) (14). Once bound to FKBP, FK506 engages in a ternary interaction with calcineurin through a nonoverlapping chemical domain (15–17). Thus, FK506 is a bifunctional molecule; it simultaneously binds two distinct proteins. To engage in both these interactions, the chemical structure of FK506 is quite large (Mr = 804) and rich in functional groups (>10 H-bond donors/acceptors). This complexity would be expected to adversely affect its pharmacological properties and, indeed, FK506 is an excellent substrate for CYP3A4 in vitro (18, 19). Despite these apparently suboptimal features, FK506 is used clinically and it has a surprisingly long half-life (between 8 and 18 h). Because of the unexpected persistence of FK506, we became interested in understanding the mechanism by which it avoids metabolism. One potential insight comes from the observation that FK506 accumulates within the cellular component of whole blood, which is a rich source of FKBP-expressing cells (20–22). In particular, lymphocytes express high levels of FKBP (22) and low levels of CYP3A4 (23). Therefore, we hypothesized that retention of FK506 within these cells might limit exposure to key metabolic enzymes. Consistent with this model, tethering FKBP-binding groups to otherwise unrelated small molecules reduces their affinity for CYP3A4 by up to 3.5-fold when coadministered with purified FKBP or FKBP-expressing cells in vitro (24).
Encouraged by these in vitro findings, we sought to explore whether the in vivo lifetime of an HIV PI could be modified by installation of an FKBP-binding group. Under this model, appending the FKBP-binding portion of FK506 to a known HIV protease inhibitor might be expected to impart some of the favorable cellular partitioning and lifetime properties of the natural product. In this report, we synthesize a bifunctional derivative of the FDA-approved PI amprenavir. Strikingly, we found that this compound is selectively partitioned into FKBP-expressing blood cells, which prolonged its lifetime by ≈20-fold in vivo. Because of the modular synthesis used, we anticipate that this nature-inspired, prodrug approach might have broad applications.
Results
Design and Synthesis of a Bifunctional Protease Inhibitor, SLFavir.
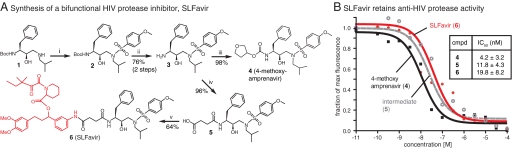
We hypothesized that binding to FKBP might alter the distribution and persistence of existing HIV PIs. To test this idea, we targeted a derivative of amprenavir (4; 4-methoxy amprenavir) (25, 26) for modification. Structure-activity relationships have demonstrated that chemical diversity may be introduced on the pseudoamine terminus of amprenavir without dramatic effects on antiviral activity (26). Therefore, we reasoned that an FKBP ligand might be coupled at this site. To generate these compounds, we followed a precedented route (26) from the known chiral alcohol, 1, to the key intermediate 3 (Fig. 1A). From this shared core, we either completed the 4-methoxy amprenavir scaffold, 4, by installation of hydroxytetrahydrofuran or we reacted the amine with succinic anhydride to provide intermediate 5. Carbodiimide-mediated coupling of 5 with an FKBP-binding group yielded the bifunctional compound 6. In this reaction, we took advantage of a well-known, synthetic ligand for FKBP (SLF) that has high affinity for FKBP (Kd ≈1 to 10 nM) (27) but does not interface with calcineurin; thus, installing this group provides tight binding to FKBP without concurrent anti-calcineurin activity (28–30). We refer to the bifunctional inhibitor as SLFavir because it incorporates chemical domains from both SLF and amprenavir.
Fig. 1.
Synthesis and functional analysis of a bifunctional HIV protease inhibitor. (A) The synthesis of the control inhibitor, 4-methoxy amprenavir, and the bifunctional derivative, SLFavir, are shown (with SLF in red). Reagents and conditions: (i) 4-methoxybenzenesulfonyl chloride, Et3N, DCM (ii) 1:1 TFA:DCM (iii) 2,5-dioxopyrrolidin-1-yl tetrahydrofuran-3-yl carbonate, Et3N, DCM (iv) succinic anhydride, DCM (v) SLF, DIC, DMAP, DMF. (B) Results of a FRET-based HIV protease assay. Compounds 4, 5, and 6 had measurable anti-HIV protease activity and the IC50 values are shown. Results are representative of 3 independent experiments and the error is SD.
SLFavir Retains Anti-HIV-1 Protease Activity In Vitro.
To evaluate the potency of compounds 4–6, we used a FRET-based assay in which inhibition of HIV-1 protease is quantified by the ability of a candidate to block cleavage of a quenched fluorescent substrate. Using this approach, we found that the unmodified parent PI, 4, had an IC50 of 4.2 nM (Fig. 1B), which agrees with the range of reported values for similar inhibitors (26, 31). Chemically coupling the SLF moiety resulted in a nominal decrease in potency, with the IC50 value of SLFavir remaining in the nanomolar range (19.8 nM). To explore the origins of this modestly diminished activity, we examined the precursor, 5. This molecule lacks the tetrahydrofuran and, consistent with a role for this group, compound 5 also had a slightly decreased potency (IC50 = 11.8 nM). These results are consistent with known structure-activity relationships (26) and they suggest that replacing the tetrahydrofuran with a short, flexible methylene linker attached to SLF does not drastically alter potency.
After confirming that SLFavir maintains anti-protease activity, we wanted to study whether binding to FKBP would impact this activity in vitro. In some systems, SLF conjugates have been found to bind both protein partners without a loss in affinity for either target (32), whereas in other examples, ternary binding is hindered by FKBP (33). These differences likely arise from variation in the spatial constraints imposed by different protein surfaces and the relative depth of the binding pockets. To empirically test this parameter for SLFavir, we premixed 6 (3 μM) with purified FKBP12 (1 μM). Under these conditions, FKBP did not block anti-HIV protease activity (Fig. S1A). This is an important result because it suggests that SLFavir can bind to FKBP without interrupting its association with the protease.
SLFavir Is Preferentially Localized Within the Cellular Component of Whole Blood Ex Vivo.
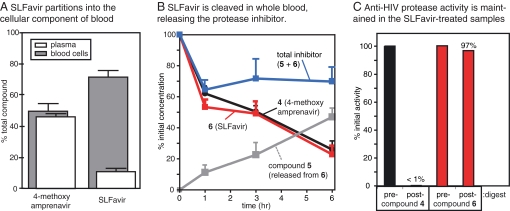
Because FKBP is abundantly expressed in the cytosol of erythrocytes and lymphocytes (22), we hypothesized that an FKBP-binding group might retain the bifunctional molecule in these cells. To test this idea, we treated mouse whole blood with 4 or SLFavir and analyzed the partitioning of the molecules between the plasma and hematocrit after a 60-min incubation at 37°C. We found that compound 4 was approximately equally distributed between the plasma and cells (Fig. 2A). However, SLFavir exhibited a striking 8-fold preference for the cellular compartment, suggesting that, similar to FK506 (20), binding to FKBP alters partitioning.
Fig. 2.
SLFavir is selectively partitioned into blood cells and is a prodrug ex vivo. (A) Whole blood treated with compound 4 or 6 was separated into plasma and blood cell fractions and compound levels were assayed by LC-MS. The results are the average of at least three experiments and the error is standard deviation. (B) Both SLFavir and compound 4 are metabolized in whole blood at 37°C, but the total amount of active product remains constant in the SLFavir treated samples because of the release of the active derivative, 5. (C) Whole blood samples after 6 h of incubation at 37°C were extracted, dried, and resuspended in DMSO for use in HIV protease assays. The activity of 4 was reduced ≈100-fold by incubation in whole blood whereas the activity of the bifunctional version 6 was largely unchanged.
SLFavir Is a Prodrug that Prolongs Anti-Protease Activity Ex Vivo.
To evaluate the functional consequences of this cellular distribution on the metabolism of the bifunctional product, we analyzed the levels of the inhibitors over a period of six hours ex vivo (Fig. 2B). At 37°C, 4 was slowly degraded and only ≈25% of the initial concentration remained after six hours. Interestingly, SLFavir had similar kinetics, but the LC-MS spectra revealed that the major cleavage product [m/z = 507] was 5, the oxobutanoic acid precursor of the bifunctional compound. As described in Fig. 1B, this intermediate retains activity against HIV-1 protease and it might be considered the released product of the prodrug. By adding the observed concentrations of 5 and 6, we calculated that the total amount of viable inhibitor remained elevated (within 70% of the initial value) throughout the experiment. To confirm the anti-protease activity of these samples, we subjected the extracted material to the HIV-1 protease FRET assay. These experiments confirmed that the SLFavir-treated sample retained nearly 100% of its inhibitory activity after 6 h whereas the activity of samples treated with compound 4 was reduced by ≈100-fold (Fig. 2C). Together, these results suggest that SLFavir is a prodrug and that it contributes to anti-protease activity longer than the parent compound.
SLFavir Is Sequestered into Blood Cells In Vivo.
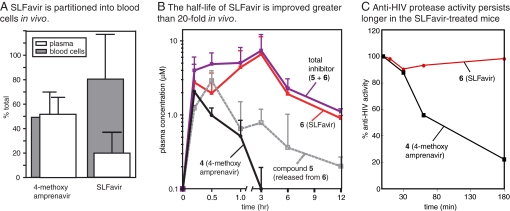
Encouraged by the ex vivo results, our next goal was to investigate the lifetime of the bifunctional molecule in a rodent model. To deliver molar equivalents, we used a 2.0-mM dosing solution and administered 10 μl/g i.p. to adult male C57BL/6 mice, equaling 0.4 μmols per gram or ≈10 mg/kg. One hour after administration, we confirmed that the compounds were accessible to the circulation by extracting with organic solvent and monitoring the parent ion by LC-MS. Analogous to the experiments performed on the ex vivo samples, we first examined the partitioning of the compounds between plasma and cellular components of whole blood. One hour after i.p. administration we found that 4 was distributed equally between the two portions, whereas SLFavir had a 5-fold preference for the cellular fraction; thus, binding to FKBP also alters the distribution of the bifunctional molecule in vivo. (Fig. 3A).
Fig. 3.
SLFavir is sequestered into blood cells and has a dramatically enhanced half-life in vivo. (A) After 60 min, whole blood was isolated from treated mice, separated into components and analyzed for drug content by LC-MS. Results are the average of at least three animals. (B) At time 0, adult male mice were injected i.p. with either compound 4 or 6. Whole blood was removed at the indicated times and the plasma drug levels determined. Total inhibitor level was calculated as the concentration of 5 + 6. All results are the average of at least three independent experiments and the error is standard deviation. (C) Plasma samples were dried and resuspended in DMSO for analysis in the HIV protease assay. Results are shown relative to a control (100% = 10 μM drug extracted immediately from whole blood).
SLFavir Has a Dramatically Enhanced Lifetime In Vivo.
To evaluate the functional consequences of enhanced cellular partitioning, we analyzed plasma levels of the inhibitors over a period of 12 h after i.p. injection. After 180 min, the levels of the unmodified parent compound, 4, were undetectable and this compound exhibited a half-life of ≈30 min (Fig. 3B). This value is consistent with studies of other protease inhibitors in rodents (34). In contrast, SLFavir had a half-life of ≈9 h and persisted in the plasma at levels >1 μM for 12 h. These results show that the bifunctional molecule has a significantly longer lifetime than the unmodified parent.
Importantly, SLFavir had an IC50 of ≈20 nM in vitro (see Fig. 1), which is 50 times lower than the observed plasma concentration. These results suggest that anti-HIV protease activity might also be maintained in these mice. To directly test this possibility, we subjected the plasma extracts to the HIV-1 protease assay. Using this method, we found that the activity of 4 was quickly lost (Fig. 3C), consistent with its rapid degradation in the rodent. However, the inhibitory activity of SLFavir remained relatively intact, retaining potency after 3 h. Consistent with our previous results, we found that compound 5 was released from the prodrug, which likely contributes to this improved persistence (see Fig. 3B). Together, these results suggest that the addition of an FKBP-binding group can significantly prolong the duration of anti-protease activity in mice.
Partitioning of a Fluorescent Bifunctional Molecule Is Reliant on Binding to FKBP.
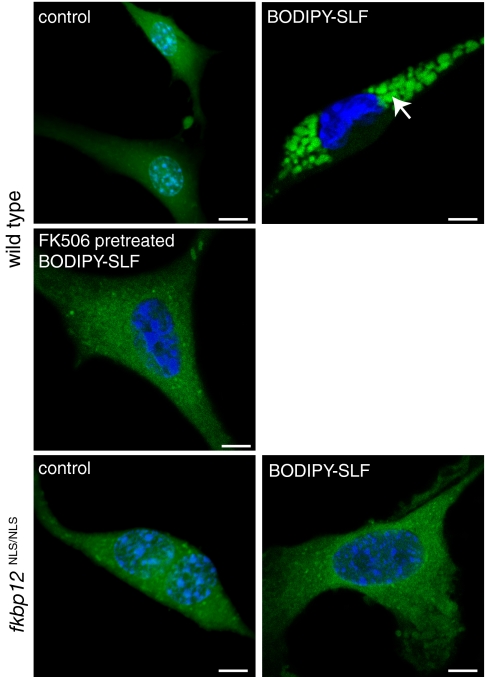
Although our strategy is designed to use FKBP-binding to alter partitioning, we wanted to further explore this requirement. Toward that goal, we synthesized a fluorescent probe (Bodipy-SLF) (Fig. S1B) that enables visualization of binding to this protein. Using confocal fluorescence microscopy, we found that mouse embryonic fibroblasts (MEFs) treated with the mock-conjugated, control probe exhibited a diffuse, nonspecific membrane staining (Fig. 4). Conversely, treatment of wild-type MEFs with the bifunctional probe resulted in prominent cytoplasmic fluorescence. To confirm an interaction with FKBP, we treated MEFs from a mutant cell line that fails to express FKBP12 (fkbpNLS/NLS) (35) and found that neither the control nor the Bodipy-SLF probe were retained. Next, we saturated the ligand-binding sites in wild-type MEFs by pretreating with FK506 (100 μM). When these cells were subsequently given the fluorescent probe, specific labeling was blocked. Together, these results and previous work (24) suggest that FKBP is responsible for the sequestering of SLF-bearing, bifunctional molecules.
Fig. 4.
A fluorescent SLF conjugate is selectively sequestered in FKBP-expressing cells. Wild-type or mutant MEFs (fkbp12NLS/NLS) were treated with the probe or a Bodipy control (100 μM). Only the wild-type MEFs had specific cytoplasmic staining. Size bars, 2 μm. Nuclei are stained with Hoescht.
Bifunctional Protease Inhibitor Has Superior Anti-HIV Activity in a Cultured Cell Model.
For this SLF-coupling strategy to produce an anti-viral, the modified compounds must retain activity against live virus. In vitro, addition of recombinant FKBP did not significantly influence anti-protease activity (see Fig. S1A) but this finding does not preclude the possibility that binding to FKBP might positively or negatively influence activity in cells through controlling the subcellular distribution of the modified inhibitor. To directly test this idea, we studied the infectivity of the T cell tropic HIV-1LAI strain in a CEM cell model by using a close chemical derivative of SLFavir, APX 23451. Under these conditions, unmodified amprenavir inhibited HIV maturation with an IC50 of 202 ± 136 nM whereas the bifunctional derivative had an IC50 of 2.5 ± 3.2 nM (Fig. 5). Thus, the SLF-modified compound was ≈80-fold more potent.
Fig. 5.
Bifunctional protease inhibitor has potent anti-viral activity in an HIV infectivity model. CEM lymphocytes were infected with T cell tropic HIV-1LA1 as described in Materials and Methods. The infected cells were treated with either amprenavir or a SLF-modified, protease inhibitor (APX 23451). Results are the average of triplicates and error bars are SE.
Discussion
A fundamental requirement in any drug treatment protocol is that levels of active compound remain above therapeutically effective concentrations. This feature is especially critical for HIV-1 therapy as constant antiviral pressure is required to prevent replication and suppress the emergence of multidrug resistant strains. Although current HAART regimens have been largely successful, significant problems persist. One of the major issues is that current protease inhibitors have limited lifetimes because of rapid metabolism by cytochrome P450 enzymes. In turn, suboptimal pharmacokinetics leads to high pill burdens, liver toxicity, and poor patient compliance, especially in developing countries. Thus, there is a continued need for improved derivatives.
In previous studies, we observed that FKBP significantly protected FK506, and bifunctional derivatives thereof, from interacting with CYP3A4 in vitro (24). Thus, we hypothesized that, in vivo, tethering FK506-like groups might redistribute the chimeric compounds into a protected biological niche, shielding them from metabolic enzymes, and potentially extending their lifetimes. To explicitly test this idea, we generated a bifunctional version of amprenavir and evaluated its lifetime and activity ex vivo and in vivo. Ex vivo, we found that SLFavir is converted to an active metabolite and that the overall anti-HIV protease activity in blood remains high (see Fig. 2). It is worth noting that, unlike other prodrug methods, both the precleaved and cleaved products are nanomolar inhibitors. The ability of whole blood to cleave SLFavir is currently not well understood but we are continuing to explore ways of controlling release kinetics. In infectivity studies with live HIV-1LA1 strain virions, the activity of SLF-modified amprenavir was enhanced ≈80-fold relative to the unmodified compound (IC50 ≈2.5 nM). This improvement might arise from FKBP-dependent concentration of the SLF-modified material in the cytoplasm or from increased persistence in the culture conditions. In vivo, we found that SLFavir has a half-life of >9 h in mice and its anti-protease activity persists unabated for at least 3 h (see Fig. 3). These parameters are ≈20-fold better than the unmodified compound and extrapolating these results to human patients would result in a half-life of >50 h (based on a 5.5-fold difference in glomerular filtration rates between humans and rodents). By comparison, the FDA-approved prodrug of amprenavir, Fosamprenavir, has a half-life of 7 to 8 h. Although this is a highly speculative analysis, such a difference would be predicted to have significant benefits in reducing pill burden, minimizing treatment costs, normalizing plasma drug levels, and possibly limiting side effects by lowering the requirement for coadministration of P450 inhibitors.
Although the present study focuses on proof-of-principle validation of an alternative mechanism, we propose that installation of FKBP-binding groups might constitute a strategy to improve therapeutic efficacy in AIDS patients. However, application of this strategy in the clinic will require extensive studies of oral bioavailability and a greater understanding of the design principles that control distribution and lifetime. One reason for this complexity is that the nature-inspired method combines elements of tissue targeting and cleavable prodrugs with a slow-release, in situ delivery scheme, by using FKBP as a natural reservoir. To highlight the fundamental differences between SLFavir and previous strategies, such as traditional prodrugs or other bifunctional molecules, we term these compounds “pharmacologically stabilized” derivatives. Finally, because the synthetic strategy is modular, other drug classes might be amenable to pharmacological stabilization by using parallel routes.
Materials and Methods
Chemical and Reagents.
Dry solvents were purchased from ThermoFisher and other starting materials were purchased from Sigma and used without further purification unless otherwise stated. SLF was obtained from Cayman Chemicals. Human FKBP12 was purified from Escherichia coli as described (36). The synthesis of protease inhibitors, the HIV protease assay, the LCMS protocol, and the confocal microscopy methods are described in the SI Text.
Animals.
The studies reported here adhere to the Stanford University principles of animal care. Mice were housed in groups of four to six at 22–24°C in a 12 h light/dark cycle and fed chow diet and water ad libitum. Male C57BL/6 mice weighing between 16 to 20 g were used for all experiments.
Ex Vivo Pharmacokinetic Studies in Whole Blood.
Stock solutions of 4 and 6 in dimethyl sulfoxide (10 mM) were diluted into freshly collected mouse blood at a final concentration of 100 μM. The samples were then incubated at 37°C for 0, 1, 3, and 6 h with shaking and processed as described below.
In Vivo Pharmacokinetic Studies in Mice.
4-Methoxy amprenavir and SLFavir dissolved in dimethyl sulfoxide (2.0 mM) were administered i.p. at an injection volume of 10.0 μl/g, equaling a dose of 0.4 μmol per gram animal weight. At least three animals were injected per time point per drug. Blood samples (≈250 μl) were obtained via cardiac puncture at 0, 10, 30, 60, 180, 360, and 720 min after injection, collected in K2EDTA microtainers, and immediately centrifuged at 3,706 rpm for 16 min to separate the plasma fraction from the cellular components. Each fraction was then processed immediately by using the extraction procedure described below.
HIV Infectivity Assay.
The T cell-tropic strain HIV-1LAI was used to infect CEM cells. CEM cells were grown in RPMI medium 1640 supplemented with 10% heat-inactivated FBS, penicillin (100 units/ml), streptomycin (100 μg/ml), and polybrene (2 μg/ml) at 37°C with 5% CO2. The titered virus was added to duplicate wells, which were pretreated with compounds for 1 h, at a low multiplicity of infection (MOI = 0.01) and incubated for 4 h at 37°C. The cells were washed three times with PBS (GIBCO/BRL), suspended in 2 ml culture medium containing the same concentration of compound as the initial preincubation conditions and further incubated at 37°C in 5% CO2. After 4 days of culture, media containing infected cells (200 μl) were used to measure production of HIV-1 p24 by antigen capture ELISA. Each duplicate well in the infectivity assay is split into triplicate wells for the p24 ELISA, and IC50 determined by using GraphPad PRISM. Results are expressed in relation to a solvent vehicle control. Compound APX 23451 is closely related to SLF-avir and a full description of this compound is forthcoming.
Supplementary Material
Acknowledgments.
We thank N. Stec for support, S. Corleone for advice, and K. Stankunas (Stanford University), L. Kallal (University of Michigan, Ann Arbor, MI), M. Molusky (University of Michigan), T. Eves (University of Michigan), and S. Lentz (University of Michigan) for reagents and technical assistance. We are indebted to D. Horejsh of Commonwealth Biotechnologies for performing the HIV infectivity experiments. This work was supported by the University of Michigan and a grant from ThermoFisher Scientific.
Footnotes
Conflict of interest statement: G.R.C. and J.E.G. have interests in Amplyx Pharmaceuticals, a biotechnology company that is developing bifunctional molecules.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805375106/DCSupplemental.
References
- 1.Flexner C. HIV drug development: The next 25 years. Nat Rev Drug Discov. 2007;6:959–966. doi: 10.1038/nrd2336. [DOI] [PubMed] [Google Scholar]
- 2.Clavel F, Hance AJ. HIV drug resistance. N Engl J Med. 2004;350:1023–1035. doi: 10.1056/NEJMra025195. [DOI] [PubMed] [Google Scholar]
- 3.Barbaro G, Scozzafava A, Mastrolorenzo A, Supuran CT. Highly active antiretroviral therapy: Current state of the art, new agents and their pharmacological interactions useful for improving therapeutic outcome. Curr Pharm Des. 2005;11:1805–1843. doi: 10.2174/1381612053764869. [DOI] [PubMed] [Google Scholar]
- 4.Turner SR. HIV protease inhibitors - the next generation. Curr Med Chem Anti-Infective Agents. 2002;1:141–162. [Google Scholar]
- 5.Dresser GK, Spence JD, Bailey DG. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin Pharmacokinet. 2000;38:41–57. doi: 10.2165/00003088-200038010-00003. [DOI] [PubMed] [Google Scholar]
- 6.Vierling P, Greiner J. Prodrugs of HIV protease inhibitors. Curr Pharm Des. 2003;9:1755–1770. doi: 10.2174/1381612033454441. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal S, et al. Peptide prodrugs: Improved oral absorption of lopinavir, a HIV protease inhibitor. Int J Pharm. 2008;359:7–14. doi: 10.1016/j.ijpharm.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rouquayrol M, Gaucher B, Roche D, Greiner J, Vierling P. Transepithelial transport of prodrugs of the HIV protease inhibitors saquinavir, indinavir, and nelfinavir across Caco-2 cell monolayers. Pharm Res. 2002;19:1704–1712. doi: 10.1023/a:1020913631309. [DOI] [PubMed] [Google Scholar]
- 9.Hamada Y, et al. Water-soluble prodrugs of dipeptide HIV protease inhibitors based on O–>N intramolecular acyl migration: Design, synthesis and kinetic study. Bioorg Med Chem. 2004;12:159–170. doi: 10.1016/j.bmc.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Furfine ES, et al. Preclinical pharmacology and pharmacokinetics of GW433908, a water-soluble prodrug of the human immunodeficiency virus protease inhibitor amprenavir. Antimicrob Agents Chemother. 2004;48:791–798. doi: 10.1128/AAC.48.3.791-798.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wire MB, Shelton MJ, Studenberg S. Fosamprenavir: Clinical pharmacokinetics and drug interactions of the amprenavir prodrug. Clin Pharmacokinet. 2006;45:137–168. doi: 10.2165/00003088-200645020-00002. [DOI] [PubMed] [Google Scholar]
- 12.Gunaseelan S, et al. Synthesis of poly(ethylene glycol)-based saquinavir prodrug conjugates and assessment of release and anti-HIV-1 bioactivity using a novel protease inhibition assay. Bioconjug Chem. 2004;15:1322–1333. doi: 10.1021/bc0498875. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto H, et al. Design, synthesis, and biological evaluation of anti-HIV double-drugs: Conjugates of HIV protease inhibitors with a reverse transcriptase inhibitor through spontaneously cleavable linkers. Bioorg Med Chem. 2001;9:1589–1600. doi: 10.1016/s0968-0896(01)00045-1. [DOI] [PubMed] [Google Scholar]
- 14.Van Duyne GD, Standaert RF, Karplus PA, Schreiber SL, Clardy J. Atomic structure of FKBP-FK506, an immunophilin-immunosuppressant complex. Science. 1991;252:839–842. doi: 10.1126/science.1709302. [DOI] [PubMed] [Google Scholar]
- 15.Ho S, et al. The mechanism of action of cyclosporin A and FK506. Clin Immunol Immunopathol. 1996;80:S40–45. doi: 10.1006/clin.1996.0140. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, et al. Inhibition of T cell signaling by immunophilin-ligand complexes correlates with loss of calcineurin phosphatase activity. Biochemistry. 1992;31:3896–3901. doi: 10.1021/bi00131a002. [DOI] [PubMed] [Google Scholar]
- 17.Griffith JP, et al. X-ray structure of calcineurin inhibited by the immunophilin-immunosuppressant FKBP12-FK506 complex. Cell. 1995;82:507–522. doi: 10.1016/0092-8674(95)90439-5. [DOI] [PubMed] [Google Scholar]
- 18.Takahara S. Efficacy of FK506 in renal transplantation. Ann N Y Acad Sci. 1993;696:235–244. doi: 10.1111/j.1749-6632.1993.tb17156.x. [DOI] [PubMed] [Google Scholar]
- 19.Fay JW, et al. FK506 (Tacrolimus) monotherapy for prevention of graft-versus-host disease after histocompatible sibling allogenic bone marrow transplantation. Blood. 1996;87:3514–3519. [PubMed] [Google Scholar]
- 20.Yura H, et al. Synthesis and pharmacokinetics of a novel macromolecular prodrug of Tacrolimus (FK506), FK506-dextran conjugate. J Control Release. 1999;57:87–99. doi: 10.1016/s0168-3659(98)00150-3. [DOI] [PubMed] [Google Scholar]
- 21.Galat A. Peptidyl cis/trans isomerases (immuniphilins): Biological diversity - targets - functions. Curr Topics Med Chem. 2003;3:1315–1347. doi: 10.2174/1568026033451862. [DOI] [PubMed] [Google Scholar]
- 22.Baughman G, Wiederrecht GJ, Chang F, Martin MM, Bourgeois S. Tissue distribution and abundance of human FKBP51, and FK506-binding protein that can mediate calcineurin inhibition. Biochem Biophys Res Commun. 1997;232:437–443. doi: 10.1006/bbrc.1997.6307. [DOI] [PubMed] [Google Scholar]
- 23.Nowakowski-Gashaw I, Mrozikiewicz PM, Roots I, Brockmoller J. Rapid quantification of CYP3A4 expression in human leukocytes by real-time reverse transcription-PCR. Clin Chem. 2002;48:366–370. [PubMed] [Google Scholar]
- 24.Marinec PS, Lancia JK, Gestwicki JE. Bifunctional molecules evade cytochrome P450 metabolism by forming protective complexes with FK506-binding protein. Mol Biosystems. 2008;4:571–578. doi: 10.1039/b720011k. [DOI] [PubMed] [Google Scholar]
- 25.Baker CT, et al. Design, synthesis, and conformational analysis of a novel series of HIV protease inhibitors. Bioorg Med Chem Lett. 1998;8:3631–3636. doi: 10.1016/s0960-894x(98)00669-6. [DOI] [PubMed] [Google Scholar]
- 26.Surleraux DL, et al. Discovery and selection of TMC114, a next generation HIV-1 protease inhibitor. J Med Chem. 2005;48:1813–1822. doi: 10.1021/jm049560p. [DOI] [PubMed] [Google Scholar]
- 27.Maynard-Smith LA, Chen LC, Banaszynski LA, Ooi AG, Wandless TJ. A directed approach for engineering conditional protein stability using biologically silent small molecules. J Biol Chem. 2007;282:24866–24872. doi: 10.1074/jbc.M703902200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amara JF, et al. A versatile synthetic dimerizer for the regulation of protein–protein interactions. Proc Natl Acad Sci USA. 1997;94:10618–10623. doi: 10.1073/pnas.94.20.10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gestwicki JE, Marinec PS. Chemical control over protein–protein interactions: Beyond inhibitors. Comb Chem High Throughput Screen. 2007;10:667–675. doi: 10.2174/138620707782507296. [DOI] [PubMed] [Google Scholar]
- 30.Clackson T. Controlling Protein–protein Interactions Using Chemical Inducers and Disrupters of Dimerization. Weinham, Germany: Wiley; 2007. [Google Scholar]
- 31.Koh Y, et al. Novel bis-tetrahydrofuranylurethane-containing nonpeptidic protease inhibitor (PI) UIC-94017 (TMC114) with potent activity against multi-PI-resistant human immunodeficiency virus in vitro. Antimicrob Agents Chemother. 2003;47:3123–3129. doi: 10.1128/AAC.47.10.3123-3129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gestwicki JE, Crabtree GR, Graef IA. Harnessing chaperones to generate small-molecule inhibitors of amyloid beta aggregation. Science. 2004;306:865–869. doi: 10.1126/science.1101262. [DOI] [PubMed] [Google Scholar]
- 33.Briesewitz R, Ray GT, Wandless TJ, Crabtree GR. Affinity modulation of small-molecule ligands by borrowing endogenous protein surfaces. Proc Natl Acad Sci USA. 1999;96:1953–1958. doi: 10.1073/pnas.96.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathias CV, et al. Safety of nelfinavir use during pregnancy: An experimental approach in rats. Clin Exp Obstet Gynecol. 2005;32:163–165. [PubMed] [Google Scholar]
- 35.Stankunas K, et al. Conditional protein alleles using knockin mice and a chemical inducer of dimerization. Mol Cell. 2003;12:1615–1624. doi: 10.1016/s1097-2765(03)00491-x. [DOI] [PubMed] [Google Scholar]
- 36.Stankunas K, et al. Rescue of Degradation-Prone Mutants of the FK506-Rapamycin Binding (FRB) Protein with Chemical Ligands. Chembiochem. 2007;8:1162–1169. doi: 10.1002/cbic.200700087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.