Abstract
Regulation of gene expression in response to mitogenic stimuli is a critical aspect underlying many forms of human cancers. The AP-1 complex mediates the transcriptional response to mitogens, and its deregulation causes developmental defects and tumors. We report that the coactivator CRTC1 cyclic AMP response element-binding protein (CREB)-regulated transcription coactivator 1 is a potent and indispensable modulator of AP-1 function. After exposure of cells to the AP-1 agonist 12-O-tetradecanoylphorbol-13-acetate (TPA), CRTC1 is recruited to AP-1 target gene promoters and associates with c-Jun and c-Fos to activate transcription. CRTC1 consistently synergizes with the proto-oncogene c-Jun to promote cellular growth, whereas AP-1–dependent proliferation is abrogated in CRTC1-deficient cells. Remarkably, we demonstrate that CRTC1-Maml2 oncoprotein, which causes mucoepidermoid carcinomas, binds and activates both c-Jun and c-Fos. Consequently, ablation of AP-1 function disrupts the cellular transformation and proliferation mediated by this oncogene. Together, these data illustrate a novel mechanism required to couple mitogenic signals to the AP-1 gene regulatory program.
Keywords: AP-1, CRTC1, Mect1-Maml2, Proliferation, Transformation
The cyclic AMP response element-binding protein (CREB)-regulated transcription coactivators (CRTCs, originally called TORCs) are a novel class of signal-dependent CREB coactivators identified using a high-throughput expression screen of a mammalian cDNA library (1, 2). CRTCs associate with the bZIP region of CREB via their N-terminal region and activate transcription through interactions with components of the basal transcriptional apparatus (1, 3). Under resting conditions, CRTCs are phosphorylated by sucrose nonfermenting1/AMP-activated protein kinase (AMP/SNF) kinases and sequestered in the cytoplasm (4, 5). When the intracellular levels of calcium or cAMP rise, CRTCs are dephosphorylated, travel to the nucleus and bind to CREB, thereby activating transcription. Consistent with their role as CREB activators, CRTCs have been shown to be key regulators of gluconeogenesis (5–8), adaptive mitochondrial biogenesis (9), β cell survival (10), and long-term synaptic plasticity (11).
Recent observations have suggested that CRTCs also promote activation of other transcription factors besides those of the CREB/ATF1 family. Indeed, the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) causes CRTC1 nuclear translocation in HeLa cells (12), and deletion of the TPA responsive element (TRE) from the IL-8 promoter abrogates CRTC1-mediated enhancement (2), suggesting that CRTC1 can stimulate transcriptional output of a TPA-regulated pathway. Recently, it was reported that CRTC1 can be phosphorylated and activated by MEKK1 (13), a critical kinase activated by several mitogenic stimuli, including TPA.
TPA is a tumor-promoting drug that activates transcription of a number of genes that typically contain a TPA response element (TRE = TGACTCA) in their promoter regions (14). In turn, the TRE is bound by the dimeric AP-1 transcription factor complex, comprising a large family of Fos (c-Fos, FosB, Fra1, Fra-2), Jun (c-Jun, JunB, JunD), and ATF bZIP proteins (15). The main AP-1 proteins in mammalian cells are c-Jun and c-Fos, with c-Jun the most potent transcriptional activator in this group.
Besides TPA, AP-1 also is activated by growth factors, cytokines, stress signals, and oncoproteins, and thus is involved in various cellular processes, including cell proliferation, differentiation, cell survival, apoptosis, and neoplastic transformation (14). Whereas the biological role of the AP-1 transcription factors is well established, the molecular mechanism through which these factors promote target gene activation remains incompletely understood. In this report, we demonstrate that CRTC1 is a potent, indispensable modulator of AP-1 activity that associates with c-Jun and c-Fos and promotes c-Jun–mediated cellular proliferation and transformation.
Results
CRTC1 Is an AP-1 Coactivator and Mediates the Transcriptional Response to TPA.
To evaluate the effect of CRTCs on TPA-induced transcription, we first tested the effect of CRTC1, CRTC2, and CRTC3 on the TPA-responsive human matrix metalloproteinase 1 (MMP1) and 3× AP-1 reporter constructs in HeLa cells. MMP1 contains a TRE (TGACTCA) located at −72 bp from the transcription start site (16). The 3× AP-1 construct has 3 TRE repeats upstream of the thymidine kinase minimal promoter. As shown in Fig. 1A, CRTC1 had a strong stimulatory effect on both promoters, whereas CRTC2 and CRTC3 displayed a weaker effect. Thus, we used CRTC1 in all subsequent experiments.
Fig. 1.
CRTC1 promotes the TPA response of AP-1 targets. (A) HeLa cells were transfected with MMP1-luc (Left) or 3× AP-1–Luc (Right) plasmids, TK renilla, and plasmids encoding CRTCs (C1, C2, C3: CRTC1–3) for 24 h, and luciferase assays were performed. *P < .05. (B) HeLa cells were transfected with 3× AP-1–Luc reporter, C1, A-Fos, or empty vector for 24 h and treated with 100 ng/mL of TPA for 10 h, where indicated. *C1, TPA, C1 + TPA versus control, P < .05; **C1 + A-Fos versus C1, P < .01; *** TPA + A-Fos versus TPA, P < .05; ****C1 + TPA + A-Fos versus C1 + TPA, P < .05. (C) MMP1-Luc (WT) or MMP1 ΔTRE-Luc reporters (ΔTRE) were transfected in HeLa cells with C1 or empty vector and treated with TPA as before. *C1, TPA, C1 + TPA versus control, P < .05; **C1, TPA, C1 + TPA ΔTRE versus C1, TPA, C1 + TPA WT, P < .01. (D) HeLa cells were transfected with siRNAs for CREB (CREBi), c-Jun (Juni), or nonspecific (SCR), MMP1-Luc, TK renilla, and CRTC1 plasmids for 72 h. The knockdown of c-Jun and CREB was verified by Western blot analysis (Bottom). *SCR CRTC1 versus SCR control, P < .01; **Juni CRTC1 versus SCR CRTC1, P < .01. (E) HeLa cells were treated with 100 ng/mL of TPA or 10 μM forskolin (FSK) + 40 μM IBMX (Left) or infected with the indicated adenoviruses (Right), and RT PCR was performed. (F) HeLa cells were transfected with 3× AP-1–Luc reporter and plasmids expressing C1 or its dominant negative (C1-DN) or empty vector and treated with TPA as above. *C1, TPA, C1 + TPA versus control, P < .05; **C1 + C1-DN versus C1, P < .01; ***TPA + C1-DN versus TPA, P < .01; ****C1 + TPA + C1-DN versus C1 + TPA, P < .05. (G) In Hela cells, MMP1-Luc reporter was transfected with siRNAs for C1 (C1i) or nonspecific (SCR) for 72 h and cells treated with TPA for 10 h, where indicated. The actual knockdown of C1 was verified by Western blot analysis (Bottom). *SCR TPA versus SCR control, P < .01; **C1i TPA versus SCR TPA, P < .05. (H) HeLa cells were transfected and treated as in image G, and Western blot analysis was performed with the specified antibodies.
The effect of CRTC1 was synergistic with TPA and was dependent on the AP-1 transcription factor complex, as demonstrated by the addition of the AP-1 dominant negative (DN) polypeptide A-Fos (17), which strongly reduced the response to TPA and CRTC1 both alone and in combination (Fig. 1B). The effect also depended on the TRE; the TRE mutant MMP1 promoter (ΔTRE) construct failed to respond to CRTC1 and TPA (Fig. 1C).
To rule out the involvement of CREB in this process, we performed knockdown experiments using RNA interference. Depletion of CREB using CREB siRNA did not affect CRTC1 inducibility of the MMP1 gene, whereas knockdown of the AP-1 member c-Jun abrogated the transcriptional response to CRTC1 (Fig. 1D). In contrast, ablation of CREB, but not c-Jun, disrupted the response to CRTC1 of the CREB target promoter EVX1 (18) (supporting information (SI) Fig. S1), thus demonstrating that CRTC1 regulation of AP-1 is functionally independent of CREB and vice versa.
To verify that the effect was detectable on endogenous AP-1–responsive genes, we performed RT-PCR on 3 different AP-1 targets: MMP1, MTIIA (19), and TIMP1 (20). As shown in Fig. 1E, all 3 of these genes were induced by exposure to TPA. Transduction of HeLa cells with adeno-CRTC1 increased MMP1, MTIIA, and TIMP1 mRNAs to levels comparable to those achieved by TPA treatment, demonstrating that CRTC1 induces endogenous AP-1 target genes. In contrast, none of the targets was activated by the cAMP agonists forskolin (FSK) and IBMX, further demonstrating that CREB is not involved in their regulation.
To determine whether abrogation of endogenous CRTC1 function suppresses the response to TPA, we used a DN CRTC1 construct, encoding the N-terminal region of CRTC1 (2). As shown in Fig. 1F, this construct disrupted the response to TPA and CRTC1 both alone and in combination. To further prove that CRTC1 is needed to confer the TPA response to AP-1 target genes, we performed knockdown experiments with siRNAs. Ablation of CRTC1 strongly decreased the TPA response of the MMP1 promoter compared with the nonspecific scrambled siRNA (Fig. 1G). In addition, the TPA induction of the endogenous AP-1 target TIMP1 was completely abrogated in CRTC1-deficient cells (Fig. 1H). These findings demonstrate that CRTC1 is indispensable for the transcriptional response to TPA.
The activation of CREB-mediated transcription by CRTC is achieved on binding of the N-terminal region of CRTC to the basic leucine-zipper domain (bZIP) of CREB (1). Because AP-1 family members also contain a bZIP DNA-binding domain, we hypothesized that CRTC1 might activate AP-1 through a similar transactivation mechanism. To test this hypothesis, we first performed co-immunoprecipitation studies. Both c-Jun and c-Fos were found in CRTC1 immunoprecipitates, whereas no binding was detected when the antibody was saturated with the specific immunizing peptide before immunoprecipitation (Fig. 2A). Furthermore, CRTC1 was readily detected in immunoprecipitates of c-Jun and c-Fos (Fig. 2B). Interestingly, whereas a diffuse CRTC1 band was detected in the total cell lysate, a discrete band with slower mobility was found to associate with AP-1, indicating that posttranslational modifications of CRTC1 may govern the interaction with AP-1.
Fig. 2.
CRTC1 interacts with the bZIP region of AP-1. (A and B) HeLa cells were transfected with Flag-tagged CRTC1, HA-c-Jun, and HA-c-Fos and immunoprecipitated with Flag (A) or HA (B) antibodies. Western blot analysis was performed with anti-HA or anti-Flag antibodies. For negative controls, Flag antibodies were blocked with 0.1 mg/mL of Flag peptide (A), or cells were transfected with an empty vector (B). (C) Co-immunoprecipitation of Flag-CRTC1 and HA-bZIP c-Jun or HA-bZIP c-Fos, followed by Western blot analysis with anti-HA antibody. (D) GST pulldown assay with GST CRTC11–142 or GST alone incubated with in vitro–translated CREB, c-Jun, or c-Fos. (E) HeLa cells were transfected for 24 h with Flag-tagged CRTC1 and treated for 6 h with TPA. Cell lysates were immunoprecipitated with Flag antibodies, and Western blot analysis was performed with c-Jun and Flag antibodies. (F) For ChIP, HeLa cells were treated for 6 h with TPA or vehicle, after which ChIP was performed as described above with the specific antibodies. The eluted DNA was PCR-amplified with primers encompassing the TRE site of MMP1 promoter (Bottom; schematic representation of MMP1 promoter and the primers used for the amplification). For a negative control, DNA was amplified with primers encompassing the coding region of the GAPDH gene.
In addition, both c-Jun and c-Fos bZIP domains were found in the immunocomplexes on immunoprecipitation of CRTC1 but not on immunoprecipitation performed with peptide-blocked antibodies (Fig. 2C), demonstrating CRTC1's ability to bind the bZIP domains of AP-1. To determine whether the interaction of CRTC1 and the AP-1 complex is direct, we expressed the N-terminal region (amino acid 1–142) of CRTC1 as a GST fusion protein; this region was previously shown to interact with CREB (1). As shown in Fig. 2D, incubation with 35S-labeled in vitro–translated c-Jun and c-Fos resulted in strong binding to GST-CRTC1 (aa 1–142) compared with the GST alone control, confirming that AP-1 can bind directly to the same region of CRTC1 that interacts with CREB.
We performed co-immunoprecipitation studies to determine whether TPA affects AP-1–CRTC1 complex formation. As shown in Fig. 1E, CRTC1 associated to endogenous c-Jun, and this binding was increased upon the addition of TPA to the cells. Furthermore, in chromatin immunprecipitation assays (ChIPs), we observed coincident MMP1 promoter occupancy of CRTC1 and c-Jun in response to TPA, demonstrating the formation of an AP-1–CRTC1 promoter complex induced by phorbol ester (Fig. 2F). Taken together, these findings demonstrate that CRTC1 mediates the mitogen-induced activation of AP-1 through direct binding between its N-terminal region and the bZIP domains of c-Jun and c-Fos.
CRTC1 Is Necessary for c-Jun–Dependent Cellular Proliferation.
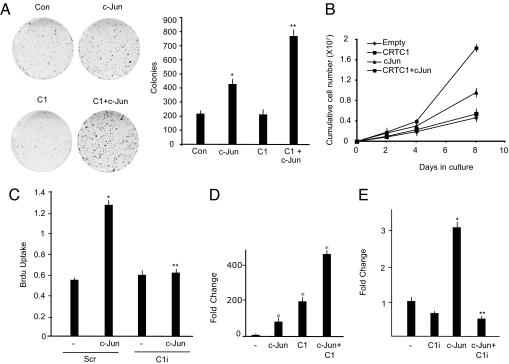
We performed colony-formation assays to investigate whether CRTC1 can modulate the AP-1–dependent cell proliferation. As shown in Fig. 3A, ectopic expression of c-Jun in HeLa cells caused a significant increase in the number of colonies compared with cells transfected with the empty vector. Remarkably, CRTC1 significantly increased c-Jun–induced colony formation compared with c-Jun alone, whereas expression of CRTC1 alone had no effect. Accordingly, cells expressing c-Jun and CRTC1 together grew faster than those expressing c-Jun alone, as determined by an analysis of cumulative cell numbers over time (Fig. 3B). To determine whether CRTC1 is required for AP-1–mediated cell proliferation, we performed BrdU incorporation assays in cells in which CRTC1 levels were reduced using specific siRNAs (Fig. 3C). Whereas the number of actively cycling cells more than doubled in the presence of ectopic c-Jun, this effect was completely abolished in CRTC1-deficient cells, confirming that CRTC1 is indispensable for this effect of c-Jun. Furthermore, CRTC1 strongly enhanced c-Jun–mediated promoter activation. Indeed, the MMP1 luciferase reporter displayed a synergistic activation by c-Jun and CRTC1 (Fig. 3D), and knockdown of CRTC1 strongly reduced c-Jun–induced MMP1 promoter activity (Fig. 3E). These findings support the hypothesis that CRTC1 is a critical modulator of c-Jun–dependent cell proliferation.
Fig. 3.
CRTC1 enhances c-Jun–mediated cell proliferation. (A) HeLa cells were transfected with the specific plasmids, grown with G418 for 2 weeks, fixed, and stained with Coomassie Blue (Con = pcDNA3). (Left) Representative image of the plates after staining. (Right) Quantitative analysis. Results represent the average ± SD of 3 separate experiments, each performed in duplicate. *c-Jun versus control, P < .05; **c-Jun + C1 versus c-Jun, P < .05. (B) Cells derived from duplicate plates from the colony assays in plot A were grown in culture as monolayers, and cell growth rates were determined by analyzing cumulative cell numbers over time. Cells were counted in triplicate; error bars represent SD. (C) HeLa cells were transfected for 72 h with the specified siRNA, and then retransfected with plasmids encoding c-Jun, GFP, or empty vector for 24 h. BrdU was then added to the cells for 5 h. After incubation, the cells were processed as described previously, and the percent incorporation of BrdU was assessed in the population of transfected cells. Results represent the average ± SD of 3 separate experiments, each performed in triplicate. *c-Jun versus control, P < .05; **C1 + c-Jun versus c-Jun, P < .05. (D) In HeLa cells, the MMP1-Luc reporter was transfected with c-Jun and C1 expression plasmids, alone or in combination, after which the luciferase assay was performed as described previously. *P < .05. (E) HeLa cells were transfected with MMP1-Luc reporter and plasmids expressing shRNA for C1 or nonspecific shRNA, c-Jun, or empty vector for 72 h. *c-Jun versus control P < .05; **c-Jun + C1i versus c-Jun P, < .01.
The CRTC1-MAML2 Oncoprotein Exerts Its Transforming Activity Through AP-1.
The effect of CRTC1 on cellular proliferation prompted us to evaluate its involvement in pathological processes in which aberrant regulation of cellular growth leads to neoplastic transformation. A role for CRTC1 in tumor formation was previously proposed for mucoepidermoid carcinomas (MECs). These tumors are associated with a chromosomal translocation t (11, 19) that generates a novel fusion protein, Mect1-Maml2 (M-M2), which contains a portion of the CRTC1 gene and exerts strong transforming activities (21). The M-M2 oncogene consists of the N-terminal portion of CRTC1 (originally called Mect1) with 981 aa of the C-terminal region of the Notch receptor coactivator Mastermind like 2 (Maml2).
Because our data demonstrate that AP-1 interacts with the N-terminal region of CRTC1, we reasoned that the transforming potential of M-M2 fusion could be related to its ability to activate inappropriate AP-1–mediated transcription. To test this hypothesis, we first performed co-immunoprecipitation studies. As shown in Fig. 4A, M-M2 formed complexes with both c-Jun and c-Fos that did not form in peptide-competed immunoprecipitation studies, demonstrating that M-M2 interacts with AP-1 in vivo. In addition, M-M2 caused a robust, 200-fold increase of MMP1 promoter activity and synergized with TPA, effects that were abrogated by the AP-1 DN A-Fos (Fig. 4B). Activation of MMP1 by M-M2 appeared to be dependent on the CRTC1 domain of M-M2, because the CRTC1 DN polypeptide suppressed the stimulatory effect on MMP1 transcription (Fig. 4C). Furthermore, ectopic expression of M-M2 enhanced the endogenous expression of the MMP1, MTIIA, and TIMP1 genes (Fig. 4D). Together, these findings demonstrate that M-M2 directly promotes AP-1–mediated transcription through CRTC1.
Fig. 4.
The M-M2 fusion oncoprotein enhances AP-1 function. (A) For co-immunoprecipitation, cell extracts of HeLa cells transfected with Flag–M-M2, HA–c-Jun, and HA–c-Fos were immunoprecipitated with Flag antibodies, after which Western blot analysis was performed using anti-HA and anti-Flag antibodies. (B) MMP1-Luc reporter was transfected in HeLa cells together with plasmids encoding M-M2, A-Fos, or empty vector. After 24 h, cells were exposed to TPA for 10 h where indicated, and luciferase assays were performed as described previously. *M-M2 versus control, P < .001; **M-M2 + A-Fos versus M-M2, P < .05. (C) HeLa cells were transfected with plasmids encoding M-M2 and C1-DN as indicated, and the luciferase assay was performed as described earlier. *M-M2 versus control, P < .001; **M-M2 + C1 DN versus M-M2, P < .05. (D) HeLa cells were transfected with plasmid encoding M-M2 or empty vector for 24 h, and RT-PCR was performed with primers amplifying MMP1, MTIIA, TIMP1, and GAPDH mRNAs. (E) In the focus formation assay, RK3E cells were transfected with the specific plasmids. After 3 weeks, cells were fixed, stained with crystal violet, and scored for foci formation. Results represent the average ± SD of 3 independent experiments, each performed in duplicate. *M-M2 + A-Fos versus M-M2, P < .01; **M-M2 + A-CREB versus M-M2, P < .05. (F) NCI-H292 cells were transfected with plasmids encoding A-Fos, A-CREB, C1-DN, GFP, or empty vector for 24 h. BrdU was then added to the cells for 5 h. After incubation, the cells were processed as described earlier, and the percent incorporation of BrdU was measured in the population of transfected cells. Results represent the average ± SD of 3 separate experiments, each performed in triplicate. *P < .05.
Previous studies have shown that the transforming potential of M-M2 is mediated by constitutive CREB activation (22, 23). To test whether the effect of the M-M2 fusion protein is also related to the ability to activate AP-1 target gene expression, we performed focus formation assays in RK3E cells, in which expression of M-M2 elicits focus formation (21). The presence of the AP-1 DN A-Fos strongly reduced the number of foci induced by M-M2 (Fig. 4E), indicating that the transforming potential of this oncogene depends on AP-1 function. Expression of A-CREB displayed a weaker but significant inhibitory effect, demonstrating that both transcription factors are involved in this phenomenon.
To determine whether suppression of AP-1 function could affect cell proliferation in tumor cells arising from M-M2 expression, we performed BrdU incorporation assays in NCI-H292 mucoepidermoid carcinoma cells, which express M-M2 (21). As shown in Fig. 4F, expression of A-Fos reduced the number of proliferating NCI-H292 cells by more than 2-fold. The same effect was exerted by ectopic expression of the DN CRTC1, demonstrating that both AP-1 and CRTC1 are necessary for M-M2–dependent tumor cell proliferation. Consistent with the role observed on cellular transformation, A-CREB demonstrated a modest but significant effect on BrdU incorporation. Together, these findings demonstrate that the CRTC1 portion of the M-M2 oncoprotein is involved in cellular proliferation through AP-1 and CREB.
Discussion
The AP-1 complex comprises several transcription factors that are regulated by mitogenic stimuli and are involved in critical biological and pathological processes. The activation of AP-1 by mitogens is achieved through multiple well-characterized mechanisms, including increased synthesis of single protein components (i.e., c-Jun/c-Fos) and changes in their dimerization modules, phosphorylation levels, and DNA binding affinity (24). Currently, little is known about the immediate consequences downstream of AP-1 binding to its target gene promoters. In the present work, we have identified a novel regulatory mechanism of AP-1 transactivation. We have shown that CRTC1 mediates the AP-1–dependent transcriptional response to TPA by directly interacting with the bZip regions of c-Jun and c-Fos at the promoter level. Because the same domain of CREB interacts with the same region of CRTC1 (1), the first issue raised by our findings is whether CRTC1 can discriminate between AP-1 and CREB in response to specific stimuli. Our data with siRNAs indicate that the 2 systems likely work independently. We propose that posttranslational modifications of CRTC and/or AP-1 dictate the differential recruitment of CRTC1 toward AP-1 or CREB. Because CRTCs are highly phosphorylated proteins, one possibility is that different signals modulate the affinity of CRTCs for specific transcription factors through differential patterns of phosphorylation. Consistent with this hypothesis, we have observed that AP-1 co-precipitates with a more slowly migrating band of CRTC1, which perhaps represents a phosphorylated form. To date, phosphorylation of CRTCs has been described mainly as an inhibitory event. Indeed, CRTC2 is phosphorylated by the SIK2 kinase at Serine 171 (Ser 164 in CRTC1) (4) or by the MARK2 kinase at Ser 275 (10), which promote cytoplasmic retention through association with 14-3-3 proteins. In contrast, a recent report proposed that the mitogen-activated kinase MEKK1 phosphorylates CRTC1 at a distinct, yet uncharacterized residue that provokes nuclear translocation and transcriptional activation (13). Therefore, AP-1 may associate with an active form of CRTC1 phosphorylated at the MEKK sites.
Consistent with its role as an AP-1 enhancer, CRTC1 is involved in cellular proliferation, thus providing a novel biological function for this coactivator. Indeed, cells lacking CRTC1 fail to proliferate in response to ectopically expressed c-Jun, and CRTC1 enhances AP-1–dependent cellular growth. Because aberrant activation of cellular proliferation is a major cause of tumorigenesis, these findings have led us to investigate the role of the AP-1–CRTC1 interaction in the pathogenesis of tumors caused by the CRTC1-M2 fusion protein (21). It was previously reported that the transforming potential of this oncoprotein requires both CRTC1 and M2 domains, whereas neither of the 2 full-length proteins alone is sufficient to induce cellular transformation when overexpressed in cells (22). Gene profiling studies have shown that several CREB target genes are up-regulated by M-M2, whereas Notch targets do not seem to be affected (22, 23). Because M-M2 is known to bind CREB and recruit p300/CBP, this oncoprotein has been proposed to mimic the constitutive activation of CREB (22).
Our findings with a DN inhibitor confirm that the CRTC1 portion of M-M2 is necessary to induce cellular transformation. Because the CRTC1 region of M-M2 binds both CREB and AP-1, we analyzed the relative contribution of both transcription factors. We found that both AP-1 and, to lesser extent, CREB are needed to support the transforming effect of M-M2. Therefore, it is possible that the oncogenic potential of M-M2 is linked to its ability to constitutively activate both classes of transcription factors at the same time, due to the loss of the discriminating function toward CREB and AP-1, which likely resides in the C-terminal domain of CRTC1. In keeping with this, the spectrum of the target genes induced by the M-M2 fusion protein includes CREB (22, 23) and AP-1 targets, including MMP10 (25), PTN (26), IL-6 (27), KLF4 (28), THBS1 (29), TFF1 (30), CYR61 (31), and DUSP1 (32).
An intriguing question raised by our findings is whether aberrant regulation of CRTC1 alone may cause neoplastic transformation. In this regard, mutational inactivation of LKB1, which normally functions to promote CRTC phosphorylation and inhibition through activation of the AMPK family (33), causes the cancer predisposition syndrome Peutz-Jeghers syndrome (PJS). This suggests that constitutive dephosphorylation and activation of CRTCs may be associated with tumor formation in cells lacking Lkb1. Future studies on the PJS and related tumors will help clarify this issue.
In conclusion, our work indicates that rather than being only CREB-dedicated coactivators and sensors of metabolic signals (5–8), CRTCs also play a crucial role in AP-1–dependent cellular proliferation and transformation. CRTCs appear to regulate multiple biological outcomes through distinct signaling pathways and transcription factors, and their aberrant regulation may underlie different disease states, including diabetes and cancer.
Materials and Methods
Reagents, Antibodies, and Plasmids.
TPA, forskolin, and IBMX were purchased from Sigma. Geneticin was purchased from Invitrogen. MMP1-Luc and MMP1-Luc ΔTRE plasmids were provided by Connie Brinckerhoff. Zeo A-Fos and Zeo A-CREB were provided by Charles Vinson. CRTC1, 2, and 3 wild-type and mutant plasmids, Flag–M-M2, were described previously (4). The following antibodies were used: rabbit anti-c-Jun (Santa Cruz sc-1694, sc-45), goat anti-actin (Santa Cruz sc 1616), rabbit anti-TIMP1 (Santa Cruz sc-5538), rabbit anti-CRTC1 (Cell Signaling 2501), rabbit anti-CREB (from Marc Montminy's laboratory, Salk Institute), mouse anti-HA (Santa Cruz sc-7392), and mouse anti-Flag M2 (Sigma A8592).
Plasmids encoding HA-tagged c-Jun and c-Fos and their bZIP regions were cloned by PCR amplification of cDNA and cloned into a pcDNA3 vector (Invitrogen). To generate the 3× AP-1-Luc plasmid, an oligonucleotide containing 3 copies of the AP-1 TRE (TGACTCA) was ligated to a TK-luc promoter vector.
RT-PCR and RNA Interference.
RNA was extracted with the TRIzol reagent (Invitrogen). One microgram of total RNA was reverse-transcribed with SuperScript II reverse transcriptase and random examers (Invitrogen). PCR was performed with primers complementary to the indicated target genes. Knockdown experiments were performed using siRNA smart pools (Dharmacon). Between 50 and 100 ng of siRNA was transfected in HeLa cells with Lipofectamine 2000 (Invitrogen). After transfection, cells were grown for 72 h and treated as indicated.
Cells, Transfections, and Luciferase Assays.
HeLa and RK3E cells were grown in DMEM, 2 mM L-glutamine, 10% FBS, and antibiotics. NCI-H292 cells were grown in RPMI 1640, 10% FBS, 2 mM L-glutamine, and antibiotics. Transfections were carried out with Lipofectamine 2000. For luciferase assays, 250 ng of total DNA was transfected with Lipofectamine 2000 for 24 h in 24-well plates. For TPA treatments, 100 ng/mL of TPA was added to the culture medium for 10 h. After transfection and treatments, luciferase activity was measured using a Promega dual-luciferase assay system. Results are expressed as fold change and represent the average ± SD of at least 3 experiments, each performed in triplicate.
Coimmunoprecpitation and GST Pulldown Assays.
HeLa cells were transfected with the indicated plasmids and Lipofectamine 2000 for 24 h. After transfection, cells were lysed with RIPA buffer [0.5% sodium deoxycholate, 50 mM Tris·HCl (pH 7.6), 1% Nonidet P-40, 0.1% SDS, 140 mM NaCl, 5 mM EDTA (pH 8), 2 mM sodium pyrophosphate, and protease inhibitors], and then the cellular extracts were immunoprecipitated for 2 h. After immunoprecipitation and extensive washings with RIPA buffer, Western blot analysis was performed using standard techniques. GST pulldown assays were performed as described previously (1). GST-CRTC1 1–142 (4) and GST proteins were expressed in Escherichia coli with standard techniques.
Chromatin Immunoprecipitation.
ChIP was performed as described previously (34), with some modifications. Cells were cross-linked 10′ with 1% formaldehyde, and the reaction was stopped with 0.125 M glycine for 5′. Cells were washed and harvested, and cytoplasmatic membranes were lysed with lysis buffer (5 mM Pipes, 85 mM KCl, and 0.5% Nonidet P-40). After centrifugation, nuclei were lysed with sonication buffer [1% SDS, 10 mM EDTA, and 50 mM Tris (pH 8) supplemented with protease inhibitors] and sonicated to obtain chromatin fragments of about 400–600 nucleotides. After sonication, the lysates were precleared for 1 h, diluted with 9 volumes of dilution buffer [0.01% SDS, 1.2 mM EDTA, 16.7 mM Tris·HCl (pH 8), 1.1% Triton X-100, and 167 mM NaCl] and incubated with the specific antibodies overnight. The next day, salmon sperm–saturated protein A or G beads (Upstate) were added for 1 h, after which the lysates were washed 5 times with Buffer A [0.1% SDS, 2 mM EDTA, 20 mM Tris·HCl (pH 8), 1% Triton X-100, and 150 mM NaCl], 4 times with Buffer B [0.1% SDS, 2 mM EDTA, 20 mM Tris·HCl (pH 8), 1% Triton X-100, and 500 mM NaCl], and once with Buffer TE [10 mM Tris·HCl (pH 8) and 1 mM EDTA]. After the final washing, the immunocomplexes were eluted with elution buffer (1% SDS and 100 mM NaHCO3) 30′ at room temperature, and after the addition of 200 mM NaCl, the cross-linking was reversed with an overnight incubation at 65 °C. Subsequently, the samples were digested with proteinase K and RNase A for 2 h at 42 °C, and the DNA was purified and precipitated. Eluted DNA was PCR-amplified with primers encompassing the AP-1 site of human MMP1 promoter or the GAPDH gene as controls. The following antibodies were used: rabbit polyclonal anti-CRTC1 (Cell Signaling) and rabbit polyclonal anti–c-Jun (Santa Cruz).
Focus Formation and Proliferation Assays.
Focus formation assays were performed as described previously (21). In brief, RK3E cells were plated in 100-mm dishes and transfected with 10 μg of total DNA with the specific plasmids. After transfection, cells were grown for 3 weeks, fixed, stained with crystal violet, and assessed for foci formation.
To perform colony-formation assays, HeLa cells were transfected with the specific plasmids. After 24 h, cells were detached, diluted, and grown with G418 for 10–14 days to allow colony formation. Colonies were stained with Coomassie Blue, and colony numbers and sizes were measured. BrdU incorporation was done using a Roche BrdU-labeling assay kit as described previously (35).
Supplementary Material
Acknowledgments.
This work was supported by the Associazione Italiana per la Ricerca sul Cancro, Telethon Grant GGP07118, the Ministry of University and Research, the Ministry of Health, the Cenci-Bolognetti Foundation, the Center of Excellence for Biology and Molecular Medicine, and the Rome Oncogenomic Center. We thank Drs. Ian Clark and Connie Brinckerhoff for the MMP1 luciferase plasmids, Charles Vinson for the Zeo A-Fos and Zeo A-CREB vectors, Antonella Siena for the NCI-H292 cells, Marc Montminy for the CREB and CRTC reagents, and Michael Conkright for a critical reading of the manuscript and advice.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0808749106/DCSupplemental.
References
- 1.Conkright MD, et al. TORCs: Transducers of regulated CREB activity. Mol Cell. 2003;12:413–423. doi: 10.1016/j.molcel.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Iourgenko V, et al. Identification of a family of cAMP response element–binding protein coactivators by genome-scale functional analysis in mammalian cells. Proc Natl Acad Sci U S A. 2003;100:12147–12152. doi: 10.1073/pnas.1932773100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amelio AL, et al. A coactivator trap identifies NONO (p54nrb) as a component of the cAMP-signaling pathway. Proc Natl Acad Sci U S A. 2007;104:20314–20319. doi: 10.1073/pnas.0707999105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Screaton RA, et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Koo SH, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 6.Canettieri G, et al. Dual role of the coactivator TORC2 in modulating hepatic glucose output and insulin signaling. Cell Metab. 2005;2:331–338. doi: 10.1016/j.cmet.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Dentin R, et al. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature. 2007;449:366–369. doi: 10.1038/nature06128. [DOI] [PubMed] [Google Scholar]
- 8.Dentin R, Hedrick S, Xie J, Yates J, 3rd, Montminy M. Hepatic glucose sensing via the CREB coactivator CRTC2. Science. 2008;319:1402–1405. doi: 10.1126/science.1151363. [DOI] [PubMed] [Google Scholar]
- 9.Wu Z, et al. Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1alpha transcription and mitochondrial biogenesis in muscle cells. Proc Natl Acad Sci U S A. 2006;103:14379–14384. doi: 10.1073/pnas.0606714103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jansson D, et al. Glucose controls CREB activity in islet cells via regulated phosphorylation of TORC2. Proc Natl Acad Sci U S A. 2008;105:10161–10166. doi: 10.1073/pnas.0800796105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovacs KA, et al. TORC1 is a calcium- and cAMP-sensitive coincidence detector involved in hippocampal long-term synaptic plasticity. Proc Natl Acad Sci U S A. 2007;104:4700–4705. doi: 10.1073/pnas.0607524104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bittinger MA, et al. Activation of cAMP response element–mediated gene expression by regulated nuclear transport of TORC proteins. Curr Biol. 2004;14:2156–2161. doi: 10.1016/j.cub.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Siu YT, Ching YP, Jin DY. Activation of TORC1 transcriptional coactivator through MEKK1-induced phosphorylation. Mol Biol Cell. 2008;19:4750–4761. doi: 10.1091/mbc.E08-04-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eferl R, Wagner EF. AP-1: A double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 15.Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: Quarrel and harmony among siblings. J Cell Sci. 2004;117:5965–5973. doi: 10.1242/jcs.01589. [DOI] [PubMed] [Google Scholar]
- 16.Auble DT, Brinckerhoff CE. The AP-1 sequence is necessary but not sufficient for phorbol induction of collagenase in fibroblasts. Biochemistry. 1991;30:4629–4635. doi: 10.1021/bi00232a039. [DOI] [PubMed] [Google Scholar]
- 17.Olive M, et al. A dominant negative to activation protein-1 (AP-1) that abolishes DNA binding and inhibits oncogenesis. J Biol Chem. 1997;272:18586–18594. doi: 10.1074/jbc.272.30.18586. [DOI] [PubMed] [Google Scholar]
- 18.Conkright MD, et al. Genome-wide analysis of CREB target genes reveals a core promoter requirement for cAMP responsiveness. Mol Cell. 2003;11:1101–1108. doi: 10.1016/s1097-2765(03)00134-5. [DOI] [PubMed] [Google Scholar]
- 19.Lee W, Mitchell P, Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987;49:741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- 20.Bahr MJ, et al. Control of the tissue inhibitor of metalloproteinases-1 promoter in culture-activated rat hepatic stellate cells: Regulation by activator protein-1 DNA binding proteins. Hepatology. 1999;29:839–848. doi: 10.1002/hep.510290333. [DOI] [PubMed] [Google Scholar]
- 21.Tonon G, et al. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet. 2003;33:208–213. doi: 10.1038/ng1083. [DOI] [PubMed] [Google Scholar]
- 22.Wu L, et al. Transforming activity of MECT1-MAML2 fusion oncoprotein is mediated by constitutive CREB activation. EMBO J. 2005;24:2391–2402. doi: 10.1038/sj.emboj.7600719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coxon A, et al. Mect1-Maml2 fusion oncogene linked to the aberrant activation of cyclic AMP/CREB-regulated genes. Cancer Res. 2005;65:7137–7144. doi: 10.1158/0008-5472.CAN-05-1125. [DOI] [PubMed] [Google Scholar]
- 24.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 25.Crawford HC, Matrisian LM. Mechanisms controlling the transcription of matrix metalloproteinase genes in normal and neoplastic cells. Enzyme Protein. 1996;49:20–37. doi: 10.1159/000468614. [DOI] [PubMed] [Google Scholar]
- 26.Florin L, Maas-Szabowski N, Werner S, Szabowski A, Angel P. Increased keratinocyte proliferation by JUN-dependent expression of PTN and SDF-1 in fibroblasts. J Cell Sci. 2005;118:1981–1989. doi: 10.1242/jcs.02303. [DOI] [PubMed] [Google Scholar]
- 27.Szabo-Fresnais N, Blondeau JP, Pomerance M. Activation of the cAMP pathway synergistically increases IL-1–induced IL-6 gene expression in FRTL-5 thyroid cells: Involvement of AP-1 transcription factors. Mol Cell Endocrinol. 2008;284:28–37. doi: 10.1016/j.mce.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Mahatan CS, Kaestner KH, Geiman DE, Yang VW. Characterization of the structure and regulation of the murine gene encoding gut-enriched Kruppel-like factor (Kruppel-like factor 4) Nucleic Acids Res. 1999;27:4562–4569. doi: 10.1093/nar/27.23.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SA, Um SJ, Kang JH, Hong KJ. Expression of thrombospondin-1 in human hepatocarcinoma cell lines and its regulation by transcription factor Jun/AP-1. Mol Cell Biochem. 2001;216:21–29. doi: 10.1023/a:1011022822077. [DOI] [PubMed] [Google Scholar]
- 30.Espino PS, Li L, He S, Yu J, Davie JR. Chromatin modification of the trefoil factor 1 gene in human breast cancer cells by the Ras/mitogen-activated protein kinase pathway. Cancer Res. 2006;66:4610–4616. doi: 10.1158/0008-5472.CAN-05-4251. [DOI] [PubMed] [Google Scholar]
- 31.Tsai MS, Bogart DF, Li P, Mehmi I, Lupu R. Expression and regulation of Cyr61 in human breast cancer cell lines. Oncogene. 2002;21:964–973. doi: 10.1038/sj.onc.1205131. [DOI] [PubMed] [Google Scholar]
- 32.Furst R, et al. Atrial natriuretic peptide induces mitogen-activated protein kinase phosphatase-1 in human endothelial cells via Rac1 and NAD(P)H oxidase/Nox2-activation. Circ Res. 2005;96:43–53. doi: 10.1161/01.RES.0000151983.01148.06. [DOI] [PubMed] [Google Scholar]
- 33.Shaw RJ, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canettieri G, et al. Attenuation of a phosphorylation-dependent activator by an HDAC–PP1 complex. Nat Struct Biol. 2003;10:175–181. doi: 10.1038/nsb895. [DOI] [PubMed] [Google Scholar]
- 35.Di Marcotullio L, et al. REN(KCTD11) is a suppressor of Hedgehog signaling and is deleted in human medulloblastoma. Proc Natl Acad Sci U S A. 2004;101:10833–10838. doi: 10.1073/pnas.0400690101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.