Abstract
Gamma band modulations in neural activity have been proposed to mediate attentional processes. To support a causal link between gamma activity and attentional selection, we attempt to evoke gamma oscillations by a 50-Hz subliminal flicker. We find that a subliminal 50-Hz flicker at a target location, before target presentation, speeds up and enhances target detection and discrimination. This effect is specific to the middle of the gamma range because it is not evident at <35-Hz flicker. It requires 300 ms to build up, dissipates within 250 ms of flicker offset, and shows a tendency to invert after 500 ms. The results are discussed in relation to a role for gamma band neural synchrony in the allocation of visual attention.
Keywords: visual attention, neural synchrony, gamma, psychophysics, subliminal
The nature of the neural mechanisms underlying visual attention—the ability of humans and animals to select a limited number of stimuli from the multitude simultaneously present in the visual field for prioritized processing—remains a fundamental problem in visual neuroscience (1). A complete theory of visual attention must explain how the relative salience of selected stimuli is enhanced in neural terms, even though they are often not singled out by increased firing rates (2, 3). One recently proposed solution is the “Attention–Gamma” hypothesis, according to which synchronized gamma band (40–70 Hz) modulations in neural activity mediate attentional processes (4–10). This hypothesis is supported by a correlation, across trials, between the speed of behavioral responses in a visual detection task and the power in the gamma frequency range of V4 neurons (10–12). In these studies, Fries, Womelsdorf, and colleagues demonstrated that top-down visual attention is associated with internal gamma band synchrony in task-specific neural populations, which could be generated by top-down attentional modulations (13, 14). Thus, it is possible that selected neural representations are given a gamma band oscillatory tag by a top-down attention mechanism (15). If this is the case, it may be possible to trigger the effects of selective attention (enhanced selection and perception) by externally evoking gamma band oscillations of the relevant neural representation, thus mimicking the attentional tag.
To test this hypothesis, we examined whether external stimulus flicker at a specific location, which is expected to evoke phase-locked neural activity at the same frequency, results in attentional orientation to that location in the absence of conscious detection of the flicker; if the flicker were detectable, it could lead to an orienting of attention toward its location as a result of exogenous or endogenous processes that are not specific to the temporal modulation. To test whether subliminally evoked neural synchronization has an attentional effect, we built on recent studies demonstrating that visual flicker in the midgamma band range (40–70 Hz) entrains periodic neural responses at the same frequency in the visual cortex [refs. 16 and 17; see also supporting information (SI) Text and Fig. S1]. Because flicker within this frequency range is expected to be subliminal [the critical flicker fusion frequency is <50 Hz with luminance levels obtained on CRT monitors (18–20)], it is possible to test the Attention–Gamma hypothesis psychophysically by examining whether subliminal flicker (that should evoke neural synchronization within this frequency band) triggers attentional orientation.
We carried out a set of experiments in which three Gabor patches (arranged equidistantly on an invisible circle; Fig. 1A) were shown on a cathode ray tube (CRT) monitor, and we measured the response times (RTs) to the detection of a target— a subtle change in the spatial frequency of one of the three patches (change-target)—by using a 3-alternative forced-choice (3AFC) task. During a preview interval that preceded the target, one patch, whose location could be congruent or incongruent with the target location, was temporally modulated by either a 50-Hz or a 30-Hz flicker (the latter just below the gamma range); the “nonflickering” Gabors were presented at frequencies of 100 Hz or 120 Hz, respectively, which are too high to trigger evoked oscillatory responses (16, 17), and their contrast was set at the average of the flickering Gabor's contrast (see SI Text). Observers were also tested in a closely matched 3AFC paradigm that assessed their ability to detect the location of the flicker, with the same flicker duration and frequencies (50 and 30 Hz), but without the subsequent change-target. In a series of follow-up experiments, we extended these results with additional flicker frequencies, aperiodic temporal modulations, additional detection tasks, and we examined the time course and nature of the effect providing evidence of a dissociation between an attentional effect and the awareness of the flicker that caused it.
Fig. 1.
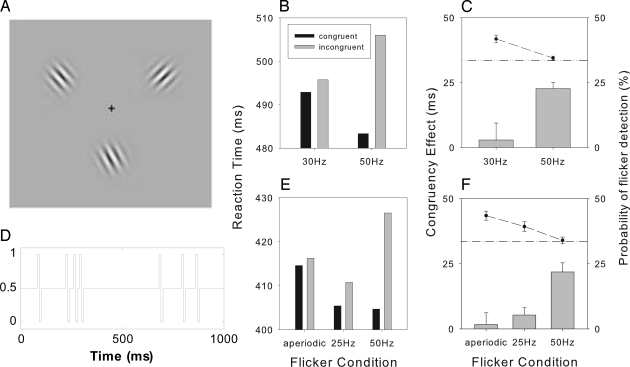
Stimuli and results of first two experiments. (A) Example of a stimulus display. Two groups of 20 observers each were tested at each of the two modulation frequencies (50/30 Hz). Each group was tested on two tasks: (i) detection of a change-target after the flicker interval, and (ii) detection of the flicker (without any additional change-target), in separate blocks (see Materials and Methods). (B) Reaction times on congruent and incongruent trials. (C) Congruency effect (Left, bar) and flicker detection rate (Right, symbols and line). The horizontal dotted/dashed line corresponds to the chance level (33%) of flicker detection. A second comparison of the 50-Hz modulation with (i) a 25-Hz flicker and (ii) an aperiodic temporal modulation was made by using a within-participant design (with trials, corresponding to 25/50-Hz or aperiodic modulations, randomized within each block). (D) Example waveform showing the contrast modulation of the cue, over a 1-s interval, in the aperiodic condition. (E) RTs. (F) Corresponding congruency effects and detection rates. Error bars (in this and subsequent figures) denote 1 SE (standard error), and where applicable the SE has been adjusted for within-subject designs (21).
Results
Attentional Effects of Subliminal Flicker and Frequency Specificity.
The localization of the 50-Hz flicker was at chance level in the detection task: 34% (SE 1%), demonstrating that the flicker was subliminal. For the 30-Hz flicker, the contrast modulation was manipulated to reduce detectability of the flicker (which could otherwise provide a conscious cue for voluntary orienting): the contrast was set to a level that resulted in low but above-chance localization accuracy, 42% (SE 1%). The slightly higher detection rate was intended to impose a conservative criterion: the evoked response at 30 Hz should be at least as strong as that at 50 Hz, to obtain a stringent test of the Attention–Gamma hypothesis.
The RTs in the change-detection task and the congruency effects (RTincongruent − RTcongruent) are given in Fig. 1. Consistent with the Attention–Gamma hypothesis, we found a robust congruency effect in the 50-Hz condition: RTs were 23 ms (SE = 4 ms) faster when the target appeared at the location preceded by the flicker cue, relative to incongruent locations (t19 = 9.34, P < 0.001); a congruency effect was evident for 19 of 20 observers, and this effect was not caused by trials on which observers perceived the flicker (Fig. S2). In contrast, there was no evidence of a reliable congruency effect in the 30-Hz condition (3 ms, SE = 7 ms; t19 = 0.44, P = 0.67), and the effect was larger with the 50-Hz than with the 30-Hz flicker (t38 = 2.83, P < 0.01; see Fig. 1B and Fig. S3).
In order to further validate this result and also to test whether the effect is caused by the periodic 50-Hz modulation rather than by any fluctuations in the firing rate of neural detectors responding to the Gabors, we carried out a second experiment in which we contrasted the gamma band (50 Hz) flicker cue with two new conditions: (i) a 25-Hz flickering cue, and (ii) a nonoscillatory (aperiodic) temporal modulation (see Fig. 1D), where “temporal events” (consisting of a 10-ms contrast increment followed by a 10-ms decrement) were presented at random times. In all conditions, the nonflickering Gabors were presented at 100 Hz. The contrast changes for the 25-Hz and aperiodic cues were modulated to prevent detection, and the allocation of trials to conditions was randomized within blocks of trials. Eight observers were tested, first on change-detection and then, separately, on flicker detection without any change-target. Once again, although the modulation was such that the flicker detection was lowest for 50 Hz (see Fig. 1F), this frequency modulation produced a reliable congruency effect (22 ms, SE = 4 ms; t7 = 3.31, P = 0.01), evident in 7 of the 8 observers, which was significantly larger than those obtained with the 25-Hz cue (t7 = 3.83, P < 0.01) and the aperiodic modulation (t7 = 2.70, P = 0.03); the congruency effects in the latter two conditions were not significantly different from zero (see Fig. 1E). As shown in Table S1, we have obtained similar congruency effects with 40-Hz but not with 35-Hz modulations. Thus, subliminal midrange gamma band, but not supraliminal, aperiodic, or below-gamma periodic, flicker results in faster detection of targets presented at the flicker location, consistent with an attentionally enhanced processing at this location.
Setting the Flicker Congruency and Validity in Opposition.
To demonstrate further the dissociation between the 50-Hz congruency effect and visual awareness of the cue (22), within the same task, and to demonstrate that the attentional congruency effects reported in the previous experiments are not contaminated by the detection of the flicker in a subset of trials, we carried out a second experiment that set the flicker, either a 50-Hz subliminal or a 25-Hz supraliminal flicker, and its cue validity in opposition (a display consisting of only two stimulus locations was used in this experiment). In this way, any perception of the 50-Hz flicker cue should result (as for the supraliminal 25-Hz cue) in a reorientation of attention to the valid location (opposite to the flicker).
Observers were informed that a flicker cue, which was either easy (25 Hz) or very difficult (50 Hz) to detect, would be presented before the change-target. The observers were told that, in 80% of the trials, the target would appear at the location opposite to the cue (valid condition), rather than at the location of the cue (invalid condition), and they were instructed that, even if they did not see a flicker, they should still do their best to respond to the change-target, as soon as they spot it. These instructions encouraged the observers to pay close attention to the presence of flicker in the preview. If observers were able to detect the flicker cue (in a fraction of trials), a positive validity effect should result (equivalent to a negative congruency effect). In contrast, if they did not detect the cue, a negative validity effect should result (positive congruency effect), if the subliminal flicker automatically oriented attention toward the cue (or gave the cued location an attentional tag), but observers were not aware of it and so could not deliberately redirect attention to the opposite site.
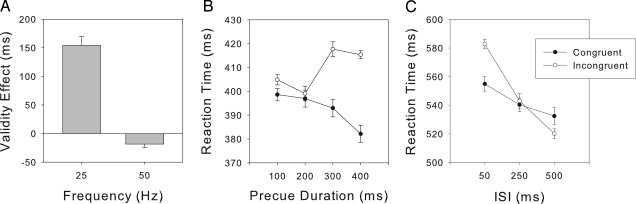
The results revealed a highly significant Frequency × Validity interaction [F (1, 5) = 113.01, P < 0.001]: with 25-Hz cues, observers were able to take advantage of their perception of the flickering cue and reorient attention to the opposite location (faster RTs on valid than on invalid trials, 405 vs. 559 ms, t5 = 9.85, P < 0.001), whereas they were not with 50-Hz cues. For the latter, RTs were slower on valid compared with invalid trials (485 vs. 466 ms, t5 = −3.31, P < 0.03): attention was exogenously oriented toward the 50-Hz flicker and, because the cue was subliminal, observers were unable to reorient their attention toward the likely target location (congruency effects depicted in Fig. 2A). The negative validity effect shows that, despite being informed of the presence of a flicker cue, it was not possible for the participants to detect the 50-Hz flicker even though it was predictive of the target location, providing observers with a strong motivation to use it (see the 25-Hz condition). Moreover, because their attention was oriented toward the subliminal 50-Hz flicker, they were faster to respond on invalid trials (where the change-targets are congruent with the flicker location). Thus, the 50-Hz priming effect was automatic and not subject to conscious strategic (top-down) control.
Fig. 2.
Results of the opposition, duration, and ISI experiments. (A) Results of the opposition experiment. A positive validity effect is obtained in the 25-Hz condition, whereas the validity effect is negative in the 50-Hz condition. (B) RTs for congruent and incongruent trials as a function of (50 Hz) flicker preview duration. The congruency effect develops only after at least 200-ms flicker duration. (C) RTs for congruent and incongruent trials with a constant-flicker preview duration (1,000 ms) but varying change-target onsets (ISI) after flicker preview offset. Although there is still a congruency effect 50 ms after flicker offset, the effect vanishes within 250 ms and produces an “inhibition of return” effect (24) after 500 ms.
Discrimination Sensitivity.
It is possible that the RT enhancement triggered by the 50-Hz flicker resulted from either shifts in response criteria for the cued location or from an enhanced perceptual sensitivity (23). To examine this, we assessed whether 50-Hz flicker influences perceptual sensitivity, separately from any shift in response criterion. To do so, we determined the thresholds of the magnitude of the spatial–frequency change (i.e., the threshold for which observers were 71% correct) for a nonspeeded discrimination of increases vs. decreases in the spatial frequency of the Gabor patches at congruent and incongruent locations. Observers' discrimination thresholds were significantly lower at congruent than at incongruent locations (t6 = −4.01, P = 0.006), as indicated by a congruent/incongruent ratio of 0.86 (SE = 0.03). Thus, the gamma-cueing produces an increase in perceptual sensitivity for the location of the flicker.
Benefits and Costs.
To examine whether the 50-Hz congruency effect was caused by an RT benefit at the cued target location, an RT cost resulting from cueing nontarget locations, or a combination of both, we repeated the 50-Hz flicker experiment (n = 10 observers) with the addition of a neutral condition in which none of the patches flickered during the preview period. The results replicated the significant congruency effect for the 50-Hz cue (30 ms, SE = 6.4; t9 = 4.67, P = 0.001). The RT for the neutral condition fell between those of the congruent and the incongruent ones [mean RT(ms): cong = 464; neut = 484; incong = 493], so that there was a significant speedup in RTs to targets after a congruent flicker (21 ms; t9 = 3.9, P = 0.004). The cost caused by incongruent flicker was smaller (9 ms) and did not reach significance.
Time Course of the Attentional Effect.
In two further experiments, we tested the dependence of the 50-Hz congruency effect on the duration of the flicker cue and on the length of the (nonflicker) interstimulus interval (ISI) between the flicker cue and the change-detection target. Fig. 2B shows that the congruency effect is not evident at short (100–200 ms) flicker durations that follow the 800- to 900-ms static preview (t6 = −1.35, P = 0.22 and t6 = −0.19, P = 0.85, respectively); rather, it emerges only at longer flicker durations of 300 and 400 ms (t6 = −4.08, P = 0.006 and t6 = −4.67, P = 0.003, respectively). Fig. 2C shows that the congruency effect persists after a very short cue–target interval (50 ms, t10 = −4.38, P < 0.01) but disappears 250 ms after offset of the flicker cue and eventually, after 500 ms, reverses such that observers exhibit a cost when the target is presented at the cued location [Congruency × ISI interaction, F(2, 20) = 11.66, P < 0.001]. That is, the effect of the flickering patch requires ≈300 ms to build up, dissipates within 250 ms after flicker offset, and shows a tendency to invert after 500 ms.
Extending the Task: Contrast Modulation and Dot Probe Experiments.
We carried out two further experiments in which two new types of targets were implemented: (i) contrast modulation and (ii) dot probe detection. Both of these targets resulted in patterns of effects identical to those in the previous experiments, ruling out the possibility that the effect is specific for targets defined by a spatial–frequency modulation. All participants (Tables S2 and S3) were faster (by 29 ms, SE = 3.42 ms, t6 = 6.86, P < 0.001) in reporting the contrast modulation of the Gabor, and all were more correct (9%, SE = 1.57%, t5 = 4.11, P < 0.01) in detecting a brief dot probe when the target location was congruent with the location of the subliminal flicker than when it was incongruent. Note that these results are not subject to speed–accuracy tradeoffs: the same conclusions are obtained when we measure the effect via RT efficiency (RT/accuracy): contrast modulation, t6 = 5.59, P < 0.001, and probe detection, t5 = 2.70, P < 0.05. Thus, the congruency effect generalizes to types of discriminations other than the spatial–frequency change.
Discussion
We have found that a subliminal, frequency-specific flicker cue (at 50 Hz) causes increased sensitivity and faster RTs to targets presented at cued locations. This finding provides evidence that attentional orienting can arise from subliminal and sustained manipulations, extending previous results with abrupt masked cues (22). The effect was caused mainly by facilitation by congruent flicker before target presentation and was found to be robust across detection tasks (spatial–frequency change, contrast change, and dot probe) and to occur only at frequencies within the midgamma band (≈50 Hz). In particular, we obtained much smaller (and nonsignificant) congruency effects with periodic modulations of 25/30 Hz and with aperiodic modulations, whose amplitude was chosen so as to permit a higher flicker detection rate (when tested in isolation, without the change-target stimulus), than that found with 50-Hz flicker (which was at chance). Furthermore, we have shown that this effect shows up even when observers have every incentive to shift their attention away from the cue (if they observe it), indicating that it takes place without awareness and is not contaminated by the perception of the cue in a subset of trials. Finally we found that the congruency effect needs >200 ms to build up and that it persists for at least 50 ms after flicker offset, but it is short-lived: it disappears within 250 ms.
The speedup in target detection caused by the presence of a 50-Hz flicker at the same location is consistent with the hypothesis that the flicker triggers modulations in neural activity in the Gamma band (16, 17) and thus mimics the attentional tag (15), normally engaged by top-down instructions or exogenous cues. The time course of the effect also indicates that it is not the outcome of a simple mechanism based on detectors sensitive to transient contrast changes at the beginning or end of the (flicker) preview period (25), but rather that it involves a continuous increase over a period (at least 200 ms). Future work should investigate further the existence of a cost resulting from 50-Hz flicker incongruent with the probe, which would suggest that, in addition to reproducing the end effect of attention, the 50-Hz flicker engages attentional mechanisms that are subject to capacity limitations or mutual inhibition (26, 27).
The psychophysical method of evoking neural synchrony has limitations in its frequency range. Because of low-pass filtering of the visual system, it is difficult to evoke synchrony in the high-gamma range (16, 17). Such neural synchrony (75–150 Hz) has recently also been associated with selective attention (28, 29). Further work, with stimulation techniques that directly target cortical circuits, is required to examine the effects of externally modulating synchrony in this frequency range. Moreover, physiological work is needed to establish clearly that (frequency-specific) evoked neural synchrony is causally related to enhanced attentional orienting. One alternative interpretation of our results is that attentional selection is associated not with the Gamma flicker, but simply with the firing rate fluctuations associated with it, which are likely to exceed those of the nonflickering Gabors (that were presented at 100 Hz). If this was the case, however, we would expect that similar congruency effects should obtain with the 30-Hz flicker (here, the nonflickering Gabors were presented at 120 Hz) or with the aperiodic cues, which are likely to benefit from stronger transients. In our experiments, however, we found null effects with such temporal modulations, when their amplitude was set such as to make detection of the flicker cue difficult, but not as difficult as for 50-Hz flicker. Still, one could argue that this is caused by lower-amplitude modulations in the relevant (V1) detectors for the 30-Hz and the aperiodic signals. Because physiological responses could not be monitored in our experiment, this possibility cannot be ruled out. However, we consider this to be implausible because the strength of neural entrainment is reduced for high frequencies (>40 Hz) caused by low-pass filtering (16, 17). A parallel reduction in response modulation of linear filters with flicker frequency is assumed in psychophysical models of flicker detection, based on cascading leaky integration (30, 31) and accounts of the decrease in flicker sensitivity with frequency (for frequencies >10 Hz). According to such models, the amplitude of the response modulation of the detectors responding to flickering Gabors is the signal used to compute their presence [say, by comparing peak with average activation (30)]. If this is the case, the higher flicker detection with 30-Hz and with aperiodic signals should correlate with stronger modulations of these detectors. Physiological monitoring of response amplitudes is needed, however, to corroborate the conjecture that flicker detection is monotonic with the amplitude of the entrained oscillations of V1 detectors and thus confirm that the attentional effects we report can be attributed solely to the frequency of evoked modulations in neural activity and not to their amplitude.
The most important result of our study is the dissociation in performance between flicker detectability and attentional enhancement: although the detection performance of the flicker (without a subsequent target) was higher in the 30-Hz compared with the 50-Hz condition, the effect of the flicker on attentional orienting (as measured by the RT for subsequent target detection) was significant for the 50-Hz condition only. As discussed above, we interpret this to indicate a dissociation of the detection of the flicker and its further attentional effects on visual processing. One possibility is that flicker detectability depends on the response amplitude (30, 31) of the corresponding temporal frequency (higher for 30 than for 50 Hz with the stimuli we used), whereas attentional enhancement depends more specifically on the frequency (higher for 40–50 Hz than 30 Hz). The results are consistent with the suggestion that gamma band neural modulations trigger attentional effects as a result of efficient summation of postsynaptic potentials (32–34), resulting in faster and more accurate responses to stimulus presentation (35).
To conclude, we suggest that, although top-down attentional orienting enhances visual processing through the generation of oscillatory neural activity, a similar enhancement can be obtained without top-down attention by an exogenous flicker cue that evokes gamma activity at the target location. As opposed to endogenous, top-down attentional orienting, the externally evoked gamma response is short-lived (dissipating shortly after the flicker) and does not engage visual awareness, possibly because of the absence of top-down feedback loops needed to sustain it.
Materials and Methods
Apparatus.
All experiments were conducted in a dimly lit room. Stimuli were presented by using a VSG 2/5 system (Cambridge Research Systems) on a Sony Trinitron multiscan E450 monitor (800 × 600 pixels). The frame rate was set at either 100 Hz (50-Hz, 25-Hz, and aperiodic conditions) or 120 Hz (30-Hz condition). Observers (all with normal or corrected-to-normal vision) maintained their viewing distance (57 cm) via a chin rest and gave their responses through a CT3 four-button response box (Cambridge Research Systems).
Attentional Effects of Subliminal Flicker and Frequency Specificity.
In the first experiment (detection of a spatial frequency change), 20 observers (18 naïve) were tested at 50 Hz, and another 20 observers (18 naïve) at 30 Hz. In both tests, observers viewed a display consisting of 3 Gabor patches (size 3°, spatial frequency 2 cpd, and deviation 0.45°), which are equally spaced on an invisible circle (radius 6°) around a central black fixation cross (always visible) on a light gray background with the same mean luminance as the Gabor patches (Fig. 1A). At the beginning of a trial, one patch flickered (30/50 Hz) for 1,000 ms (preview interval with flicker cue). After this preview interval, a change-detection target, generated by changing the spatial frequency of one of the Gabors (0.14 cpd), was presented for 600 ms. The target location was 50% congruent with the flicker cue and 50% incongruent. Observers indicated (3AFC) the location of the spatial–frequency change by pressing a spatially corresponding button as quickly as possible, and the next trial followed 1,000 ms later. Each session consisted of 5 blocks of 50 trials. For the 50-Hz test, we used flicker modulation of 10-ms on–off (monitor frequency set at 100 Hz), whereas for 30 Hz we used a flicker modulation of 16.6 ms on–off (monitor frequency set at 120 Hz). In the 30-Hz condition, the peak–trough contrast value between successive frames was determined individually for each observer, before the experiment, by using an adaptive staircase procedure that converges at ≈50% flicker detection (chance level is 33%). For all participants, error rates in the change-detection task were <10%. Mean RTs for each observer were computed for correct responses after excluding outliers (i.e., any RTs further than 2.5 SD from the mean).
After the change-detection experiment observers were tested on the detection of flicker without a subsequent change-detection. In the flicker detection task, observers viewed 180 trials of flickering cues identical to the first 1-s preview interval of the change-detection experiment, and they were instructed to indicate which patch appeared different (in flicker or any other visual property) in a 3AFC. To maintain motivation throughout this difficult task, 10% of trials contained a more detectable half-frequency flicker; such trials were excluded from analysis. Participants whose detection rate exceeded 55% were discarded, leaving a total of 20 observers that satisfied these constraints, per group.
Fifty-Hertz, 25-Hz, and Aperiodic Flicker Within-Design.
Eight observers (6 naïve) took part in the experiment; stimuli, task, and procedure were identical to experiment 1, except that three conditions where randomly intermixed: 50-Hz, 25-Hz, and aperiodic flicker. To permit random intermixing of the 50-Hz and 25-Hz conditions, the monitor frequency was set at 100 Hz. In the nonoscillatory (aperiodic) temporal modulation (see Fig. 1D), temporal events (consisting of a 10-ms contrast increment followed by a 10-ms decrement) were positioned randomly within the 1-s cue interval. The placement method was as follows. An array representing each frame of the display sequence was assigned an event with 0.06 probability, resulting in a stochastic sequence of 6 events (on average) per 100 frames (representing the 1-s cue interval). Sequential placement of events was prevented. As in experiment 1, for the 25-Hz and the aperiodic condition, the peak–trough contrast value between successive frames was set so as to permit a 50% flicker detection rate (chance level is 33%).
Discrimination Thresholds.
Seven observers (5 naïve) performed 5 interleaved staircases with congruent/incongruent-cue trials, in a task that required them to discriminate between an increase/decrease in the spatial frequency of one of the three Gabor patches. The changed patch was presented for 100 ms after the flicker cue preview interval, after which all of the stimuli disappeared. The response was nonspeeded: observers had to press the left button of the response box for a frequency decrease and the right button for an increase, independently of the target location. Frequency increases and decreases were equiprobable (P = 0.5) on a trial. The staircases started with a large spatial–frequency change value and used a 2/1 protocol that converged at a 71%-correct level. Each interleaved staircase resulted in two thresholds (one for congruent and one for incongruent locations), which were computed by averaging the value of the frequency change across the last 6 (of 8) reversals. For each observer, the 5 congruent and 5 incongruent threshold estimates were then averaged. Finally, the congruent/incongruent threshold ratio was computed to standardize the threshold differences.
Costs and Benefits.
Ten observers (8 naïve) took part in the experiment; stimuli, task, and procedure were identical to experiment 1, with the difference that the flicker cue was presented only at 50 Hz and that an additional neutral condition was included in which none of the Gabors flickered.
Time Course of the Attentional Effect: Flicker Duration and Flicker–Target Interval (ISI).
Seven (duration experiment, 5 naïve) and 11 (ISI experiment, 9 naïve) observers performed 9 blocks (450 trials) by using stimuli and procedures matched to those described above for the 50-Hz condition of the first experiment, apart from the following details: The duration experiment started with the presentation of the three nonflickering Gabor patches for a variable duration of 900, 800, 700, or 600 ms, followed by an interval during which one patch flickered, for a duration of 100, 200, 300, or 400 ms, respectively, so that the total preview interval (before the target was presented) was 1 s. The ISI experiment used a variable cue–target interstimulus interval (50, 250, or 500 ms), with nonflickering Gabor patches presented between the 50-Hz flicker cue (of 1 s) and the target.
Contrast Modulation and Dot Probe Experiments.
Seven (contrast modulation, 5 naïve) and, respectively, 6 observers (dot probe, 4 naïve) performed 5 blocks (250 trials) by using stimuli and procedures matched to those described above for the 50-Hz condition of the first experiment, apart from the following details: For the contrast modulation experiment, the target was defined as a Gabor patch changing in contrast every 100 ms. The total target duration was equal to the previous experiments (600 ms). For the dot probe experiment, the target consisted of a briefly presented (50 ms) white dot (diameter 0.2°) that appeared in the center of one Gabor patch.
Flicker Congruency and Target Location Validity in Opposition.
Six observers (4 naïve) were tested in 12 blocks (600 trials) by using a task and procedure based on the first experiment. However, in this experiment, only two Gabor patches were presented, one to the left and one to the right of the fixation cross (distance 5.91°). Observers were informed that the change-target would appear at the location opposite to that of the flicker with a probability of 0.8 and that sometimes the flicker would be easily detectable and at other times harder. If they could not detect the flicker, they should still react as fast as possible to the change-target. Thus, they knew the flicker cue indicated that the target was likely to appear at the opposite location, rather than at the location of the cue. In 20% of trials, the target appeared at the same location as the cue (invalid condition), and in 80%, it appeared at the opposite location (valid condition). Observers were tested in two (randomly intermixed) frequency conditions: 50 Hz and 25 Hz.
Supplementary Material
Acknowledgments.
We thank Yoram Bonneh, Tobias Donner, and Eddy Davelaar for helpful discussions. This work was supported by a Deutsche Forschungsgemeinschaft grant (to H.M. and M.U.) and a Deutscher Akademischer Austauschdienst postdoctoral fellowship (to F.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810496106/DCSupplemental.
References
- 1.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 2.Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- 3.Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- 4.Crick F, Koch C. Towards a neurobiological theory of consciousness. Semin Neurosci. 1990;2:263–275. [Google Scholar]
- 5.Engel AK, Fries P, Singer W. Dynamic predictions: Oscillations and synchrony in top-down processing. Nat Neurosci Rev. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 6.Fries P, Roelfsema PR, Engel AK, König P, Singer W. Neuronal synchronization as a correlate of perceptual dominance in awake squinting cats. Proc Natl Acad Sci USA. 1997;94:12699–12704. doi: 10.1073/pnas.94.23.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinmetz PN, et al. Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature. 2000;404:187–190. doi: 10.1038/35004588. [DOI] [PubMed] [Google Scholar]
- 8.Taylor K, Mandon S, Freiwald WA, Kreiter AK. Coherent oscillatory activity in monkey area V4 predicts successful allocation of attention. Cereb Cortex. 2005;15:1424–1437. doi: 10.1093/cercor/bhi023. [DOI] [PubMed] [Google Scholar]
- 9.Vidal JR, Chaumon M, O'Regan JK, Tallon-Baudry C. Visual grouping and the focusing of attention induce gamma band oscillations at different frequencies in human magnetoencephalogram signals. J Cognit Neurosci. 2006;18:1850–1862. doi: 10.1162/jocn.2006.18.11.1850. [DOI] [PubMed] [Google Scholar]
- 10.Womelsdorf T, Fries P. The role of neuronal synchronization in selective attention. Curr Opin Neurobiol. 2007;17:154–160. doi: 10.1016/j.conb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Womelsdorf T, Fries P, Mitra PP, Desimone R. Gamma band synchronization in visual cortex predicts speed of change detection. Nature. 2006;439:733–736. doi: 10.1038/nature04258. [DOI] [PubMed] [Google Scholar]
- 12.Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 13.Börgers C, Epstein S, Kopell NJ. Background gamma rhythmicity and attention in cortical local circuits: A computational study. Proc Natl Acad Sci USA. 2005;102:7002–7007. doi: 10.1073/pnas.0502366102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiesinga PH, Fellous JM, Salinas E, José JV, Sejnowski TJ. Inhibitory synchrony as a mechanism for attentional gain modulation. J Physiol (Paris) 2004;98:296–314. doi: 10.1016/j.jphysparis.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niebur E, Koch C. A model for the neuronal implementation of selective visual attention based on temporal correlation among neurons. J Comp Neurosci. 1994;1:141–158. doi: 10.1007/BF00962722. [DOI] [PubMed] [Google Scholar]
- 16.Williams PE, Mechler F, Gordon J, Shapley R, Hawken MJ. Entrainment to video displays in primary visual cortex of macaque and humans. J Neurosci. 2004;24:8278–8288. doi: 10.1523/JNEUROSCI.2716-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrmann CS. Human EEG responses to 1–100-Hz flicker: Resonance phenomena in visual cortex and their potential correlation to cognitive phenomena. Exp Brain Res. 2001;137:346–353. doi: 10.1007/s002210100682. [DOI] [PubMed] [Google Scholar]
- 18.Robson JG. Spatial and temporal contrast-sensitivity functions of visual system. J Opt Soc Am. 1966;56:1141–1142. [Google Scholar]
- 19.Kulikowski JJ, Tolhurst DJ. Psychophysical evidence for sustained and transient detectors in human vision. J Physiol. 1973;232:149–162. doi: 10.1113/jphysiol.1973.sp010261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shady S, MacLeod DI, Fisher HS. Adaptation from invisible flicker. Proc Natl Acad Sci USA. 2004;101:5170–5173. doi: 10.1073/pnas.0303452101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cousineau D. Confidence intervals in within-subject designs: A simpler solution to Loftus and Masson's method. Tutorial Quant Methods Psychol. 2005;1:42–45. [Google Scholar]
- 22.McCormick PA. Orienting attention without awareness. J Exp Psychol Hum Percept Perform. 1997;23:168–180. doi: 10.1037//0096-1523.23.1.168. [DOI] [PubMed] [Google Scholar]
- 23.Müller HJ, Humphreys GW. Luminance-increment detection: Capacity-limited or not? J Exp Psychol Hum Percept Perform. 1991;17:107–124. doi: 10.1037//0096-1523.17.1.107. [DOI] [PubMed] [Google Scholar]
- 24.Posner MI, Cohen Y, Rafal RD. Neural systems control of spatial orienting. Phil Trans R Soc London Ser B. 1982;298:187–198. doi: 10.1098/rstb.1982.0081. [DOI] [PubMed] [Google Scholar]
- 25.Dakin SC, Bex PJ. Role of synchrony in contour binding: Some transient doubts sustained. J Opt Soc Am. 2002;19:678–686. doi: 10.1364/josaa.19.000678. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg ME, Bisley JW, Powell KD, Gottlieb J. Saccades, salience, and attention: The role of the lateral intraparietal area in visual behavior. Prog Brain Res. 2006;155:157–175. doi: 10.1016/S0079-6123(06)55010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei P, Lü J, Müller HJ, Zhou X. Searching for two feature singletons in the visual scene: The localized attentional interference effect. Exp Brain Res. 2008;185:175–188. doi: 10.1007/s00221-007-1141-7. [DOI] [PubMed] [Google Scholar]
- 28.Ray S, Niebur E, Hsiao SS, Sinai A, Crone NE. High-frequency gamma activity (80–150 Hz) is increased in human cortex during selective attention. Clin Neurophysiol. 2008;119:116–133. doi: 10.1016/j.clinph.2007.09.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wyart V, Tallon-Baudry C. Neural dissociation between visual awareness and spatial attention. J Neurosci. 2008;28:2667–2679. doi: 10.1523/JNEUROSCI.4748-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sperling G, Sondhi MM. Model for visual luminance discrimination and flicker detection. J Opt Soc Am. 1968;58:1133–1145. doi: 10.1364/josa.58.001133. [DOI] [PubMed] [Google Scholar]
- 31.Watson AB. Temporal sensitivity. In: Boff K, Kaufman L, Thomas J, editors. Handbook of Perception and Human Performance. New York: Wiley; 1986. [Google Scholar]
- 32.Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Neurosci Rev. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azouz R, Gray CM. Adaptive coincidence detection and dynamic gain control in visual cortical neurons in vivo. Neuron. 2003;37:513–523. doi: 10.1016/s0896-6273(02)01186-8. [DOI] [PubMed] [Google Scholar]
- 34.MacLeod K, Bäcker A, Laurent G. Who reads temporal information contained across synchronized and oscillatory spike trains? Nature. 1998;95:693–698. doi: 10.1038/27201. [DOI] [PubMed] [Google Scholar]
- 35.Fries P, Neuenschwander S, Engel AK, Goebel R, Singer W. Rapid feature selective neuronal synchronization through correlated latency shifting. Nat Neurosci. 2001;4:194–200. doi: 10.1038/84032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.