Abstract
To identify small molecules that can induce β-cell replication, a large chemical library was screened for proliferation of growth-arrested, reversibly immortalized mouse β cells by using an automated high-throughput screening platform. A number of structurally diverse, active compounds were identified, including phorbol esters, which likely act through protein kinase C, and a group of thiophene-pyrimidines that stimulate β-cell proliferation by activating the Wnt signaling pathway. A group of dihydropyridine (DHP) derivatives was also shown to reversibly induce β-cell replication in vitro by activating L-type calcium channels (LTCCs). Our data suggest that the LTCC agonist 2a affects the expression of genes involved in cell cycle progression and cellular proliferation. Furthermore, treatment of β cells with both LTCC agonist 2a and the Glp-1 receptor agonist Exendin-4 showed an additive effect on β-cell replication. The identification of small molecules that induce β-cell proliferation suggests that it may be possible to reversibly expand other quiescent cells to overcome deficits associated with degenerative and/or autoimmune diseases.
Keywords: beta-cell proliferation, diabetes mellitus, small molecules, Wnt agonist, L-type calcium channel agonist
Type 1 diabetes mellitus (T1DM) is characterized by a marked reduction in pancreatic β-cell mass resulting in insufficient insulin secretion and, as a consequence, abnormally high blood glucose levels. Although daily insulin administration remains the most effective treatment for hyperglycemia in T1DM, it does not fully recapitulate the strict control of blood glucose that is exerted by endogenous β cells and, as a result, does not prevent diabetic patients from eventually developing major damage to the kidney, eye, vascular, and nervous systems. Transplantation of donor islet β cells has been shown to successfully normalize blood glucose levels, but is limited by the scarcity of donor islets. Increasing evidence indicates that pancreatic β cells replicate at a basal level in vivo, and can be stimulated to expand significantly to meet metabolic demand, for example, during pregnancy, obesity, or after partial pancreatectomy (1, 2). These observations suggest that it may be possible to use external stimuli to expand primary β cells ex vivo for transplantation, or even induce the regeneration of endogenous β-cell mass directly in the pancreas.
Here, we describe a high-throughput screen of a large chemical library for inducers of β-cell proliferation by using growth-arrested, reversibly immortalized mouse β cells. A number of structurally diverse molecules were identified that promote β-cell replication, including novel Wnt signaling agonists and L-type calcium channel (LTCC) agonists. We further show that the LTCC agonist 2a likely induces β-cell replication by activating Ras signaling and increased expression of cell cycle regulators, and that cotreatment of β cells with 2a and the Glp-1 receptor agonist Exendin-4 (Ex-4) exert an additive effect on β-cell proliferation.
Results and Discussion
High-Throughput Chemical Screen in Immortalized β Cells.
To identify small molecules that can induce controlled β-cell proliferation, we used the reversibly immortalized mouse β-cell line R7T1, which provides the large quantities of homogeneous, functional β cells required for large-scale cell-based screens. SV40 T antigen (TAg) oncoprotein under the control of the Tet-On system was used to immortalize mouse β cells so that these engineered β cells proliferate when TAg is induced in the presence of tetracycline (Tet), but undergo growth arrest upon withdrawal of Tet (3–5). Although not a true mimic of in vivo quiescent β cells, this system should allow us to identify small molecules that are able to induce reentry into cell cycle and proliferation of growth-arrested cells. Indeed, these engineered cells express high levels of β-cell signature markers, including insulin 1, insulin 2, and Pdx1 (Fig. S1), and are able to secrete insulin and restore and maintain euglycemia in STZ-treated diabetic mice when induced to growth arrest and transplanted (3, 5). A total of ≈850,000 compounds were screened with an automated high-throughput screening platform for their ability to induce the proliferation of growth-arrested β cells in either a 384- or 1,536-well plate format. In the primary screen, proliferation was assayed by using a luminescent assay measuring intracellular ATP content, which is directly proportional to viable cell number (see Materials and Methods). To rule out any possible Tet mimetics that induce the proliferation of β cells through the induction of SV40 TAg expression, the Tet-Off immortalized β-cell line βTC-Tet was used for a counterscreen. In this system, βTC-Tet cells proliferate in the absence of Tet, but stop dividing in its presence, hence, a Tet analog will not induce the proliferation of growth-arrested βTC-tet cells (3, 4). Approximately 80 compounds, representing 10 distinct scaffolds, passed this secondary filter, and were subsequently shown to induce the proliferation of growth-arrested immortalized mouse β cells by direct cell counting.
Identification of Novel Wnt Agonists as Inducers of β-Cell Proliferation.
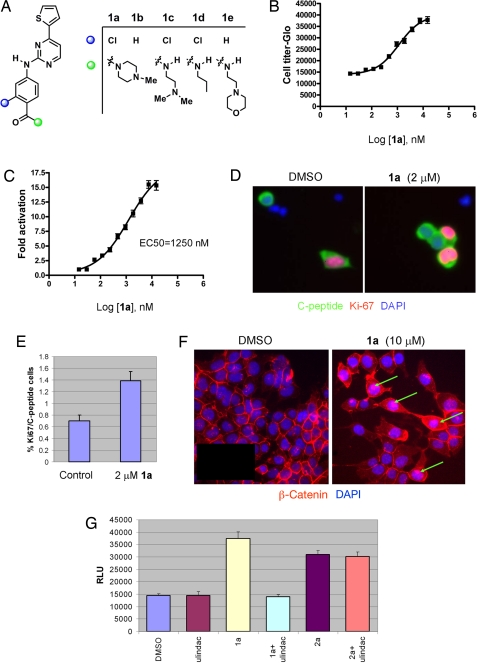
Of the structurally diverse hit compounds, a number have known mechanisms of action. For example, phorbol esters were identified that likely promote β-cell proliferation by binding and activating protein kinase C (PKC) isozymes. Another mechanism by which small molecules might promote β-cell proliferation involves activation of developmental signaling pathways, such as hedgehog and Wnt, which are critical for cellular differentiation and proliferation during embryonic development and adult homeostasis. Indeed, the Wnt pathway has recently been implicated in the regulation of β-cell proliferation based on the phenotypes of mouse models with gain and loss of function in the Wnt signaling pathway (6). Fifteen compounds, including a group of thiophene-pyrimidines, aminopyridines, and indirubin (BIO), induced the Wnt reporter Super (8X) TOPFlash (7) 5- to 50-fold (Fig. 1A, and data not shown). We further tested a representative compound in this group, the thiophene pyrimidine derivative 1a, for its effects on Wnt signaling and on β-cell proliferation. Compound 1a induced replication of growth-arrested R7T1 β cells in a dose-dependent manner with EC50s of ≈1.1 μM (Fig. 1B). Compound 1a was also able to promote the proliferation of 2 other β-cell lines, HIT-T15 and MIN6. This compound (2 μM) also increased the number of proliferating primary rat β cells staining doubly positive for Ki-67/C-peptide ≈2-fold after incubation for 72 h compared with DMSO treatment (Fig. 1 D and E). Consistent with activation of Wnt signaling, 1a induced the Super (8X) TOPFlash reporter in a dose-dependent manner with an EC50 value of ≈1.25 μM, similar to that for β-cell proliferation (Fig. 1C); at a concentration of 10 μM, 1a activated the Wnt reporter 15-fold compared with that for DMSO. In contrast, compound 1a had no activity on a Wnt reporter (7) whose β-catenin/TCF binding sites are mutated (Fig. S2A). Increased nuclear β-catenin accumulation was also observed in compound 1a-treated cells (in DMSO-treated control cells, β-catenin staining is weak and appears to concentrate at the cell surface; Fig. 1F). In addition, compound 1a-stimulated Wnt activation and β-cell proliferation were abolished by Sulindac, a Wnt signaling antagonist (Fig. S2B and Fig. 1G). A preliminary investigation of the mechanism of action of compound 1a revealed that it is a relatively selective and potent (IC50 = 64 nM) GSK3-β inhibitor, a negative regulator of Wnt signaling pathway (7 of 65 kinases profiled were inhibited by >75% at 5 μM; data not shown). Collectively, these findings suggest that compound 1a likely mediates β-cell proliferation through the Wnt signaling pathway, although we cannot exclude the possibility that other signaling pathways are also involved.
Fig. 1.
Novel Wnt agonists are inducers of β-cell proliferation. (A) Chemical structures of 5-thiophene pyrimidines. (B) Dose-dependent effects of 1a on β-cell proliferation. Relative luminescence unit (RLU) was measured by CellTiter-Glo assay after 7-day incubation of growth-arrested R7T1 β cells with 1a, which was added at day 0 and refreshed at day 4; experiments were performed in quadruplicate. All data in this paper are presented as mean ± SD, unless otherwise specified. (C) Dose-dependent effects of 1a on the activation of Super (8X) TOPFlash reporter. HEK293 cells transfected with Super (8X) TOPFlash reporter were treated with 1a at the indicated concentrations 24 h after transfection. Luciferase activity was measured 48 h after compound treatment. (D and E) The proliferative effect of 1a on rat primary β cells. (D) After incubation for 72 h with 2 μM 1a, replicating β cells were identified by double-immunofluorescence staining by using anti-C-peptide antibody to mark β cells (green) and antibody against Ki-67, a proliferation marker (red). Nuclear DNA was stained with DAPI (blue). (Scale bar, 10 μm.) (E) The percentage of Ki-67-positive cells of all C-peptide-positive cells, which corresponds to the fraction of replicating β cells. The number of proliferating primary β cells increased after incubation for 72 h in the presence of 2 μM 1a compared with DMSO control: 1a at 2 μM increases the replicating β cells ≈2-fold. (F) Compound 1a stimulates the translocation of β-catenin into the nucleus. Nuclear β-catenin accumulation (green arrows) was increased in 10 μM 1a-treated HEK293 cells; in DMSO-treated control cells, β-catenin staining is weak and appears to concentrate at the cell surface. (G) 1a-stimulated β-cell proliferation was abolished by the Wnt signaling antagonist Sulindac. R7T1 cells were treated with 10 μM 1a, 60 μM Sulindac, or 1 μM 2a as indicated in the figure. CellTiter-Glo activity was measured 7 days after compound treatment.
Agonists of L-Type Calcium Channels Promote β-Cell Proliferation.
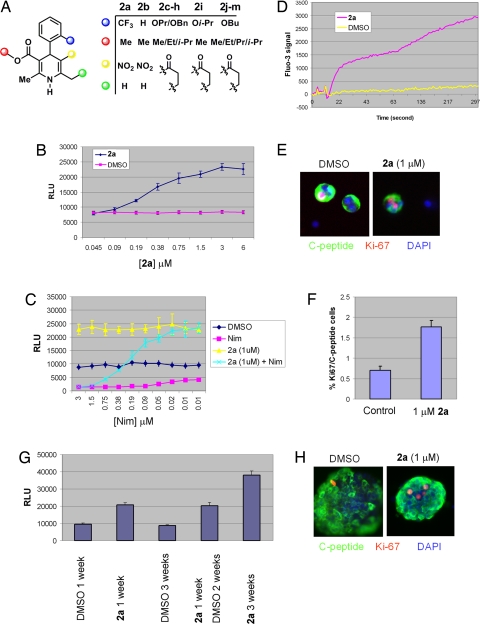
Another group of compounds identified in this screen are dihydropyridine (DHP) derivatives (Fig. 2A), known agonists and antagonists of LTCCs. That the LTCC antagonist nimodipine and agonist Bay K 8644 (2a) induced β-cell death and β-cell proliferation, respectively (Fig. 2 B and C), suggests that the calcium channel agonist activity is responsible for the proliferation of the growth-arrested β cells. Consistent with this notion, the proliferative effect of DHP derivative 2a on β cells can be blocked by nimodipine in a dose-dependent fashion (Fig. 2C), and treatment with 2a leads to a 10-fold increase in transient intracellular calcium levels (Fig. 2D). The compound also increased proliferation of 2 other β-cell lines (HIT-T15 and MIN6) in a dose-dependent manner. Moreover, compound 2a promotes the proliferation of primary rat β cells 2.5-fold as determined by the number of Ki-67 and C-peptide double-positive cells after compound incubation for 3 days (Fig. 2 E and F). We also evaluated whether the proliferative effects of 2a on growth-arrested β cells is strictly dependent on its presence. R7T β cells proliferated continuously for up to 3 weeks in the presence of 2a, but stopped dividing upon its removal (Fig. 2G), suggesting that controlled proliferation of β cells by 2a may be possible. A number of experiments indicate that the proliferative effect of 2a is because of the expansion of the β cells per se rather than a dedifferentiated cell type with higher proliferation potential from β cells. First, the expression of β-cell signature markers such as insulin 1, insulin 2, and Pdx1 is unchanged on R7T1 β cells both pretreatment and posttreatment with 2a in gene profiling analysis (Fig. S1). Second, even after being cultured with 2a for 1–2 weeks, R7T1 cells keep the same morphology and behavior—they remain small and round-shaped and form clusters (data not shown). Moreover, both in cultured primary rat and human islets treated with compound, the overwhelming majority of cells still stain for C-peptide (Figs. 2H and 4C). Finally, compound 2a also appears to increase the number of proliferating human β cells (Fig. 2H), although quantitation is difficult because of the heterogeneous nature of the human islets used.
Fig. 2.
LTCC agonists stimulate β-cell proliferation. (A) Chemical structures of dihydropyridine (DHP) derivatives. (B) Dose-dependent effect of 2a on β-cell proliferation. CellTiter-Glo assay was performed 7 days after incubation of growth-arrested R7T1 β cells with compounds; 0.1% of DMSO was used as control. Experiments were performed in quadruplicate. (C) LTCC agonists are responsible for the β-cell proliferation. Nimodipine (Nim), a known LTCC antagonist, resulted in growth-arrested R7T1 cell death. The proliferative effect of 2a (1 μM) on these β cells can be blocked by Nim in a dose-dependent fashion. At higher concentrations of Nim, β cells failed to survive even with 2a treatment; at lower concentrations of Nim, 2a-treated β cells proliferate. Each experiment was performed in quadruplicate. (D) Calcium influx is stimulated by 2a. Calcium influx was analyzed by using fluo-3 dye in MIN6 β cells. Fluo-3 dye was incubated for 1 h before compound treatment and measured by fluorescence laser imaging plate reader (FLIPR) for up to 5 min after compound treatment. (E and F) The proliferative effect of 2a on rat primary β cells. (E) After incubation for 72 h with 1 μM 2a, replicating β cells were identified by double-immunofluorescence staining by using anti-C-peptide antibody to mark β cells (green) and antibody against Ki-67, a proliferation marker (red). Nuclear DNA was stained with DAPI (blue). (Scale bar, 10 μm.) (F) The percentage of Ki-67-positive cells of all C-peptide-positive cells, which corresponds to the fraction of replicating β cells. The number of proliferating primary β cells increased after incubation for 72 h in the presence of 1 μM 2a compared with DMSO control: 2a at 1 μM increases the replicating β cells ≈2.5-fold. (G) 2a-induced β-cell proliferation is reversible. R7T1 cells were able to proliferate for up to 3 weeks in the presence of 2a, but stopped proliferation upon its removal within 1 week. (H) The proliferative effect of 2a on human primary β cells. The number of proliferating human primary β cells increased ≈1.5-fold after incubation of 1 μM 2a for 72 h compared with DMSO as control. Replicating β cells were identified by double-immunofluorescence staining by using anti-C-peptide antibody to mark β cells (green) and antibody against Ki-67, a proliferation marker (red). Nuclear DNA was stained with DAPI (blue). (Scale bar, 10 μm.)
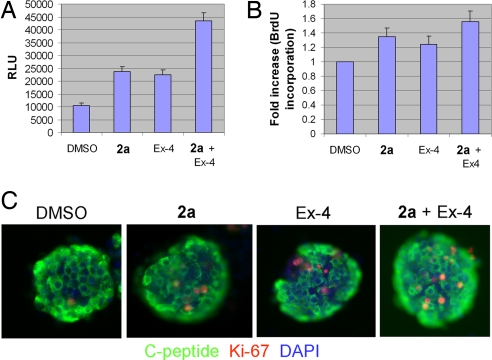
Fig. 4.
Additive effect of LTCC agonist 2a and the Glp-1 receptor agonist Ex-4. (A) Growth-arrested R7T1 β cells were incubated with DMSO, 2a (1 μM), Ex-4 (5 nM), or both for 7 days. Combined treatment with 2a and Ex-4 has an additive effect on the proliferation of growth-arrested R7T1 β cells as assessed by CellTiter-Glo (RLU). (B) HIT-T15 β cells were treated with DMSO, 2a (1 μM), Ex-4 (5 nM), or both 2a and Ex-4 for 1 day after serum-free culture for 24 h. Combined treatment with 2a and Ex-4 showed a higher proliferation rate as measured by BrdU incorporation. Values are presented as fold increase relative to DMSO-treated cells in serum-free medium; each experiment was performed in quadruplicate. (C) The proliferative effect of 2a and Ex-4 on human primary β cells. Human islets were cultured in the presence of DMSO, 2a (1 μM), Ex-4 (5 nM), or 2a and Ex-4 for 3 days. More proliferating human primary β cells were present when treated with both 2a and Ex-4. Replicating β cells were identified by double-immunofluorescence staining by using anti-C-peptide antibody to mark β cells (green) and antibody against Ki-67, a proliferation marker (red). Nuclear DNA was stained with DAPI (blue). (Scale bar, 10 μm.)
Calcium channel-mediated Ca2+ influx regulates diverse cellular processes from muscle contraction and synaptic neurotransmission to cell survival and apoptosis. As a consequence, mutations that affect calcium channel function result in clinical pathologies, referred to as calcium channelopathies. In β cells, calcium channels play a key role in controlling glucose-stimulated insulin secretion and insulin production (8). Polymorphisms in some calcium channel-encoding genes have been shown to be associated with types 1 and 2 diabetes in genome-wide association studies (9–11). However, it is also possible that these mutations lead to proliferative defects in β cells. In fact, the LTCC α1D subunit knockout displayed hypoinsulinemia and impaired glucose tolerance in adult mice along with a significant reduction of postnatal β-cell proliferation (12), suggesting that calcium channel signaling is necessary for β-cell replication. Our identification of LTCC agonists as inducers of β-cell proliferation supports the notion that calcium channel signaling modulates β-cell proliferation. Intriguingly, LTCC agonists have also been shown to induce in vivo neurogenesis (13), and more recently, isoxazole derivatives were reported to trigger neuronal differentiation in uncommitted adult neural stem cells and to increase the proliferation of committed neuroblasts by activating Ca2+ influx through both voltage-gated Ca2+ channels and N-methyl-d-aspartic acid (NMDA) receptors (14).
Mechanistic Studies of LTCC Agonists.
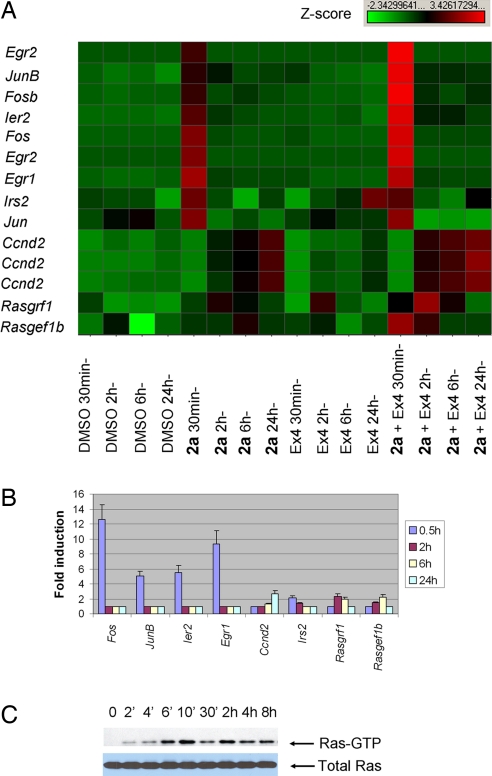
To further explore the mechanism of action of 2a, we analyzed its effects on gene expression in growth-arrested R7T1 β cells by microarray analysis (Fig. S3). Treatment of cells with 0.5 μM 2a led to a large increase in the expression of several immediate-early genes, including Fos, Jun, and Egr families, which are critical for the expression of genes important for cell survival and cell cycle progression, within 30 min (Fig. 3A). In addition, expression of Ccnd2 (cyclin D2), an important positive regulator during G1 phase, increased approximately 1.7-fold after treatment with 2a for 24 h, and IRS2, which is critical for β-cell survival and proliferation, was up-regulated 1.7-fold (Fig. 3A). The expression profiles of these genes were confirmed by quantitative real-time RT-PCR (Fig. 3B). Calcium signaling has been shown to be transduced by the activation of transcription factors such as CREB and NFAT (15, 16). In neurons, the effects of calcium signaling are mediated in part by the small GTPase Ras/MAPK pathway, based on the observation that Ras signaling is activated upon treatment with a high concentration of potassium chloride, which increases calcium influx by depolarizing the cell membrane of neurons (17, 18). We therefore investigated whether the LTCC agonist 2a is able to directly activate Ras signaling. Indeed, the level of Ras-GTP, the active Ras form, was elevated after treatment of growth-arrested R7T cells with 2a for 6 min (Fig. 3C). Ras is activated and deactivated by the recruitment of guanine nucleotide exchange factors (GEFs) and GTPase activating proteins (GAPs) to the membrane, respectively. Calcium signaling modulates Ras signaling by regulating the rapid recruitment of GEFs and GAPs to the plasma membrane (19). Strikingly, the microarray data showed that 2 GEFs (RasGRF1 and RasGEF1b) were transcriptionally up-regulated in growth-arrested R7T1 β cells upon treatment with 2a for 2–6 h (Fig. 3A). These findings were further validated by quantitative RT-PCR analysis (Fig. 3B). Consistent with these findings, Ras-GTP levels were elevated for an extended time after the peak level at 10 min of incubation (Fig. 3C). These findings suggest that LTCC agonists likely activate Ras signaling through both short- and longer-term mechanisms by the recruitment of GEFs to the plasma membrane and their enhanced expression, respectively. A sustained Ras activity is required for cell cycle initiation and progression up to the restriction point in the late G1 phase (20). How this transcriptional regulation of GEFs is mediated by LTCC-mediated calcium signaling needs further investigation (Fig. 3A).
Fig. 3.
Mechanistic studies of LTCC agonists. (A) Genes regulated by treatment with 2a (1 μM), the GLP-1 receptor agonist Exendin 4 (Ex-4, 5 nM), or both. Gene expression profiles were analyzed at 4 time points (30 min, 2 h, 6 h, and 24 h) after compound treatment of growth-arrested R7T1 β cells. Selected samples of differentially expressed genes are listed. Immediate-early response gene families, such as Fos, Jun, Egr, and Ier, were highly induced within 30 min after treatment with 2a. Irs2, a gene critical for β-cell survival and proliferation, was also up-regulated after treatment with 2a and Ex-4. Multiple entries for the same gene reflect multiple differentially expressed Affymetrix probe sets on the microarray. In addition, the expression of some RasGEFs was activated 2–6 h after treatment with 2a; this effect was enhanced by cotreating cells with Ex-4. Expression of Ccnd2 (cyclin D2) was up-regulated at 24 h after treatment with 2a; however, it too was up-regulated earlier (starting at 2 h posttreatment) and at higher levels after cotreatment with 2a and Ex-4. (B) The expression of several differentially expressed genes from microarray analysis was confirmed by real-time qRT-PCR. Growth-arrested R7T1 β cells were treated with 2a at the indicated time points; increases are relative to those found in DMSO-treated cells with each reaction performed in triplicate. (C) Activation of Ras signaling by 2a. Growth-arrested R7T1 β cells were treated with 2a at the indicated time points. Cell lysates were subjected to pull-down experiments by using the GST-Raf1 RBD fusion protein, followed by Western blot analysis to detect activated Ras (Ras-GTP, Upper); controls for Ras expression are also shown (total Ras, Lower).
Additive Effect of LTCC Agonist 2a and GLP-1 on β-Cell Replication.
Finally, we examined whether Ca2+- and cAMP-dependent pathways might function in concert to promote β-cell proliferation. The gut hormone glucagon-like peptide-1 (GLP-1) promotes β-cell proliferation and viability by increasing cAMP concentration (21), which is in part mediated by activation of the cAMP- and calcium-responsive transcription factor CREB (22). It has also been reported that cAMP and calcium pathways converge on CREB to regulate β-cell gene expression in response to glucose and incretin hormones (16). Thus, 2a and GLP-1 might be expected to exert an additive effect on β-cell proliferation. Indeed, treatment of growth-arrested R7T1 cells, HIT-T15 cells, and primary human β cells with 1 μM 2a and 5 nM of the GLP-1 analog Ex-4 led to a higher rate of proliferation than either alone (Fig. 4 A–C). These data suggest that cAMP and calcium pathways function in a concerted manner in promoting β-cell proliferation. Consistent with these findings, several early-response genes, such as Fos, FosB, JunB, Egr1, Egr2, and Ier2, were expressed at higher levels in cells treated with both 2a and Ex-4 than with either alone (Fig. 3A). Moreover, though increased Ccnd2 expression is evident by 24 h after treatment with 2a, its expression increases much earlier (at 2 h) and to a higher level at 24 h in 2a and Ex-4 cotreated cells (Fig. 3A). Similarly, combined treatment of growth-arrested R7T1 cells with 2a and Ex-4 also triggers higher expression of 2 RasGEFs, Rasgrf1, and Rasgef1b, which may contribute to the additive effect of 2a and Ex-4.
Conclusion
In summary, a large-scale high-throughput, cell-based screen identified a number of small molecules that induce pancreatic β-cell proliferation. One group of compounds induces β-cell proliferation by activating Wnt signaling, which is consistent with a recent report that GSK3β inhibitors promote β-cell survival and replication (23). Intriguingly, some known GSK3β inhibitors lack the ability to promote β-cell proliferation (23) (data not shown), possibly because of other off-target activities (i.e., they may inhibit cell cycle regulators CDKs as well) or a distinct mechanism of inhibition relative to 1a. L-type calcium channel agonists were also identified as inducers of β-cell proliferation/regeneration. Although analysis of the in vivo efficacy of 2a was hampered by unfavorable pharmacokinetic properties, this class of compounds with improved pharmacokinetics and tolerability may offer a potentially promising avenue for treatment of β-cell deficiency in diabetes, especially when combined with GLP-1 receptor agonists. In addition, other significantly more potent compounds identified in this screen whose mechanisms have yet to be established will likely shed new insights into the cellular processes that control β-cell proliferation. We are also beginning to explore whether other quiescent, or possibly even postmitotic cells, can be reversibly expanded either in vitro or in vivo to overcome deficits associated with degenerative and/or autoimmune diseases in murine models. Finally, application of a similar approach to human β cells may lead to compounds with enhanced efficacy for human β-cell proliferation, if robust strategies for immortalization can be developed that retain β-cell identity.
Materials and Methods
Cell Culture.
R7T1 β cells were expanded in growth medium (DMEM with 15% horse serum and 2.5% FBS) in the presence of 10 μg/ml doxycycline. βTC-Tet cells were expanded in growth medium (DMEM with 15% horse serum and 2.5% FBS) in the absence of 10 μg/ml doxycycline. Hamster HIT-T15 β cells were grown in F12 medium with 15% horse serum and 2.5% FBS. MIN6 cells were cultured in DMEM with 15% FBS. HEK293 cells were grown according to instructions from American Type Culture Collection (www.atcc.org/). All cells were cultivated at 37°C, with 5% CO2 in a humidified atmosphere.
High-Throughput Screen.
R7T1 β cells were expanded in large quantities in growth medium in the presence of 10 μg/ml doxycycline and then growth arrested by removal of doxycycline for 1 week. For the primary screen, growth-arrested cells were plated onto 384- or 1,536-well plates in growth medium without doxycycline at a density of 4,000 cells per well in 384-well plate or 1,000 cells per well in 1,536-well plate in an automated high-throughput screening platform (designated as day 0). A heterocycle library of 850,000 compounds (at a final concentration of 1.25 μM) was added at day 1 and refreshed at day 5, and the effects on β-cell replication were determined at day 8 by using the CellTiter-Glo assay system (a luminescent assay measuring intracellular ATP content, which is directly proportional to cell number). For data analysis, the median of a plate was calculated and the activity of each well was the signal/median of the plate. A signal in any well >1.5 of the median was regarded as a primary hit. For the reconfirmation screen, growth-arrested R7T1 cells were plated onto 384-well plates and screened against all primary hits in quadruplicate. To eliminate tetracycline mimetics among all reconfirmed primary hits, βTC-tet cells were plated onto 384-well plates at a density of 2,000 cells per well in growth medium in the presence of 10 μg/ml doxycycline, and 1.25 μM compound was added after overnight incubation and refreshed 4 days later. The effects on β-cell replication were then determined at day 8 by using the CellTiter-Glo assay system. Any compound that increased the readings >1.5-fold was regarded as a hit.
Rat or Human Islet Preparations.
Islets were isolated by the standard Liberase digestion method from the pancreata of adult Sprague–Dawley rats (200–250 g) (Liberase III; Roche Applied Science) and cultured in RPMI medium with 10% FBS. In brief, 6 ml of ice-cold Liberase solution (Roche Applied Science) was injected into the pancreas via the common bile duct. After dissection, the pancreas was incubated for 35 min at 37°C and then further dissociated by repeated pipetting by using a 10-ml pipette. Islets were purified by Histopaque 1.077 (Sigma) density gradient centrifugation and manually picked by using a stereomicroscope. Islets were allowed to recover from the isolation procedure by culture overnight in RPMI medium, 10% FBS, by using plastic dishes to which they do not attach. Human islets were obtained through the Juvenile Diabetes Research Foundation Islet Distribution Program by Islet Cell Resource Center. The use of human tissue for research was approved by our local institutional ethical committee. The purity and viability of human islets are reported to be 30%–70% and 50%–70%, respectively. Human islets were cultured in CMRL medium containing 10% FBS.
In Vitro β-Cell Proliferation Assay.
Primary β-cell proliferation assay.
Freshly isolated rat or human islets or trypsinized single-cell suspensions of rat islets were cultured in vitro in the presence or absence of compound for 72 h. Islets or single-suspension cells were fixed with 4% formalin solution (Sigma) and stained by standard immunofluorescence techniques for C-peptide (anti-human C-peptide antibody from Raybiotech, and anti-rat C-peptide antibody from Linco) and Ki-67 (anti-Ki-67 antibody from Abcam), a marker of proliferating cells. Nuclear DNA was stained with DAPI (Molecular Probes). At least 30,000 C-peptide-positive cells were counted in each experiment, performed in triplicate. The images were taken on a Nikon Eclipse TE300 microscope with 200× magnification. Proliferating C-peptide/Ki-67 double-positive β cells were counted manually.
BrdU incorporation assay.
HIT-T15 or MIN6 β-cell proliferation was assessed by 5-bromo-2-deoxyuridine (BrdU) incorporation ELISA (Roche). Cells were seeded on 96-well plates at 5 × 103 cells per well in serum-containing medium until 60%–70% confluent, and serum-starved for 24 h for HIT-T15 or 16 h for MIN6 before 24 h treatment with compounds.
Calcium Influx Assay.
Measurement of intracellular Ca2+ ([Ca2+]i) was performed by using a fluorescence laser-imaging plate reader (FLIPR; Molecular Devices). Briefly, MIN6 cells cultured in 384-well plates were incubated for 1 h with 4 μM fluo-3/AM (Molecular Probes) and 0.04% pluronic acid in physiological Locke's buffer (154 mM NaCl, 5.6 mM KCl, 1.0 mM MgCl2, 2.3 mM CaCl2, 8.6 mM Hepes, 5.6 mM glucose, and 0.1 mM glycine [pH 7.4]). After incubation, MIN6 cells were washed with Locke's buffer by using an automated cell washer to remove extracellular dye. MIN6 cells were treated with compounds at the indicated concentrations. These cells were excited at 488 nm and Ca2+-bound fluo-3 emission was recorded at 500–560 nm.
Microarray Analysis.
RNA extraction from R7T1 β cells treated with compounds at the indicated time points was performed by using RNeasy kits (Qiagen). The integrity and concentration of RNA was determined by microfluidic analysis on an Experion instrument (BioRad). Standard Affymetrix single amplification was performed by using 5 μg total RNA. Standard Affymetrix protocols were used to process Affymetrix MOE430 2 microarrays (Affymetrix). All CEL file images were processed as a single group by using gcRMA. The microarray data described have been deposited in NCBIs Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) and accessible through GEO Series accession no. GSE12802.
Real-Time RT-PCR.
Total RNA from different samples was isolated and purified by using the RNeasy Mini Kit with on-column DNase digestion (QIAGEN). Single-stranded cDNA was synthesized from 2 μg of total RNA with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). qRT-PCR was performed with the TaqMan Universal PCR Master Mix, No AmpErase UNG (Applied Biosystems) on an ABI PRISM 7700 Sequence Detection System. Assay IDs of primers and probes for each gene are described in SI Text. Mouse beta-actin was used for endogenous control. Data are represented as mean + SD of 3 independent experiments.
Ras Activation Analysis.
Ras-GTP levels were detected by using the Active Ras Pull-Down and Detection Kit, following the manufacturer's instructions (Pierce). Briefly, growth-arrested R7T1 β cells were treated with 0.5 μM compound 2a for the indicated times. Cells were then washed twice with ice-cold PBS and lysed in lysis/binding/wash buffer containing protease inhibitor mixture (Roche). Lysates (500 μg) were clarified by centrifugation, and supernatants were incubated with 80 μg of GST-Raf1-RBD fusion protein precoupled to glutathione-Sepharose beads. After incubation for 1 h at 4°C, beads were washed 3 times in lysis/binding/wash buffer, resuspended in Laemmli buffer, and boiled. Samples were analyzed by SDS-PAGE (4%–20%), followed by transfer to nitrocellulose membrane, and then were probed with anti-Ras antibody. Blots were developed by using the enhanced ECL system.
Supplementary Material
Acknowledgments.
We thank Charles Cho and Ann Herman for helpful discussions and Stephen Ho for technical assistance for microarray. This work was supported by the Novartis Research Foundation, The Skaggs Institute of Chemical Biology, and the Juvenile Diabetes Research Foundation Grant 17–2007-1040 (to P.G.S.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE12802).
This article contains supporting information online at www.pnas.org/cgi/content/full/0811848106/DCSupplemental.
References
- 1.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 2.Bouwens L, Rooman I. Regulation of pancreatic beta-cell mass. Physiol Rev. 2005;85:1255–1270. doi: 10.1152/physrev.00025.2004. [DOI] [PubMed] [Google Scholar]
- 3.Efrat S, et al. Conditional transformation of a pancreatic beta-cell line derived from transgenic mice expressing a tetracycline-regulated oncogene. Proc Natl Acad Sci USA. 1995;92:3576–3580. doi: 10.1073/pnas.92.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleischer N, et al. Functional analysis of a conditionally transformed pancreatic beta-cell line. Diabetes. 1998;47:1419–1425. doi: 10.2337/diabetes.47.9.1419. [DOI] [PubMed] [Google Scholar]
- 5.Milo-Landesman D, et al. Correction of hyperglycemia in diabetic mice transplanted with reversibly immortalized pancreatic beta cells controlled by the tet-on regulatory system. Cell Transplant. 2001;10:645–650. [PubMed] [Google Scholar]
- 6.Rulifson IC, et al. Wnt signaling regulates pancreatic beta cell proliferation. Proc Natl Acad Sci USA. 2007;104:6247–6252. doi: 10.1073/pnas.0701509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DasGupta R, Kaykas A, Moon RT, Perrimon N. Functional genomic analysis of the Wnt-wingless signaling pathway. Science. 2005;308:826–833. doi: 10.1126/science.1109374. [DOI] [PubMed] [Google Scholar]
- 8.Yang SN, Berggren PO. The role of voltage-gated calcium channels in pancreatic beta-cell physiology and pathophysiology. Endocr Rev. 2006;27:621–676. doi: 10.1210/er.2005-0888. [DOI] [PubMed] [Google Scholar]
- 9.Yamada Y, et al. Genomic variation in pancreatic ion channel genes in Japanese type 2 diabetic patients. Diabetes Metab Res Rev. 2001;17:213–216. doi: 10.1002/dmrr.193. [DOI] [PubMed] [Google Scholar]
- 10.Muller YL, et al. Variants in the Ca V 2.3 (alpha 1E) subunit of voltage-activated Ca2+ channels are associated with insulin resistance and type 2 diabetes in Pima Indians. Diabetes. 2007;56:3089–3094. doi: 10.2337/db07-0587. [DOI] [PubMed] [Google Scholar]
- 11.Sellick GS, Garrett C, Houlston RS. A novel gene for neonatal diabetes maps to chromosome 10p12.1-p13. Diabetes. 2003;52:2636–2638. doi: 10.2337/diabetes.52.10.2636. [DOI] [PubMed] [Google Scholar]
- 12.Namkung Y, et al. Requirement for the L-type Ca(2+) channel alpha(1D) subunit in postnatal pancreatic beta cell generation. J Clin Invest. 2001;108:1015–1022. doi: 10.1172/JCI13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deisseroth K, et al. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 14.Schneider JW, et al. Small-molecule activation of neuronal cell fate. Nat Chem Biol. 2008;4:408–410. doi: 10.1038/nchembio.95. [DOI] [PubMed] [Google Scholar]
- 15.Im SH, Rao A. Activation and deactivation of gene expression by Ca2+/calcineurin-NFAT-mediated signaling. Mol Cells. 2004;18:1–9. [PubMed] [Google Scholar]
- 16.Screaton RA, et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Farnsworth CL, et al. Calcium activation of Ras mediated by neuronal exchange factor Ras-GRF. Nature. 1995;376:524–527. doi: 10.1038/376524a0. [DOI] [PubMed] [Google Scholar]
- 18.Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- 19.Cook SJ, Lockyer PJ. Recent advances in Ca(2+)-dependent Ras regulation and cell proliferation. Cell Calcium. 2006;39:101–112. doi: 10.1016/j.ceca.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Coleman ML, Marshall CJ, Olson MF. RAS and RHO GTPases in G1-phase cell-cycle regulation. Nat Rev Mol Cell Biol. 2004;5:355–366. doi: 10.1038/nrm1365. [DOI] [PubMed] [Google Scholar]
- 21.Brubaker PL, Drucker DJ. Minireview: Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004;145:2653–2659. doi: 10.1210/en.2004-0015. [DOI] [PubMed] [Google Scholar]
- 22.Jhala US, et al. cAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2. Genes Dev. 2003;17:1575–1580. doi: 10.1101/gad.1097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mussmann R, et al. Inhibition of GSK3 promotes replication and survival of pancreatic beta cells. J Biol Chem. 2007;282:12030–12037. doi: 10.1074/jbc.M609637200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.