Abstract
The three cyanobacterial Kai proteins and ATP are capable of generating an autonomous rhythm of KaiC phosphorylation in a test tube. As the period is ≈24 hours and is stable in a wide temperature range, this rhythm is thought to function as the basic oscillator of the cyanobacterial circadian system. We have examined the rhythm under various temperature cycles and found that it was stably entrained by a temperature cycle of 20–28 hours. As the period length was not altered by temperature, entrainment by period change could be excluded from possible mechanisms. Instead, temperature steps between 30° and 45°C and vice versa shifted the phase of the rhythm in a phase-dependent manner. Based on the phase response curves of the step-up and step-down in temperature, phase shift by single temperature pulse was estimated using a nonparametric entrainment model (discontinuous phase jump by external stimuli). The predicted phase shift was consistent with the experimentally measured phase shift. Next, successive phase shifts caused by repeated temperature cycles were computed by two phase response curves and compared with actual entrainment of the rhythm. As the entrainment pattern observed after various combinations of temperature cycles matched the prediction, it is likely that nonparametric entrainment functions even in the simple three-protein system. We also analyzed entrainment of KaiC phosphorylation by temperature cycle in cyanobacterial cells and found both the parametric and the nonparametric models function in vivo.
Keywords: circadian clock, KaiC phosphorylation rhythm, Phase response, temperature entrainment
The circadian clock is a basic cellular system found in almost all organisms; it temporally organizes metabolism and behavior to match the alternating day/night environment (1). To function as autonomous time-keeping devices, all clocks share three prominent characteristics. First, clocks generate self-sustained oscillations under constant conditions with a ≈24-hour (circadian) period, allowing anticipation of cyclic changes in the environment, particularly in light and temperature cycles. Second, unlike typical biochemical processes, the period of circadian clocks is stable against changes in temperature and nutrient conditions, facilitating circadian timing under different external conditions. Third, it is physiologically essential that circadian clocks can be easily entrained to external time cues derived from day/night alternation.
The molecular mechanisms of circadian clocks have been studied in a wide variety of model organisms (2). Among these, studies on a prokaryotic model organism, cyanobacterium (Synechococcus elongatus PCC 7942), demonstrated that circadian oscillation of KaiC phosphorylation could be reconstituted in vitro by incubating the three Kai proteins and adenosine triphosphate (ATP) (3). Further studies have shown that an extremely weak but temperature-compensated ATPase activity of KaiC is tightly linked to the circadian period (4) and coupled to KaiC kinase/phosphatase activities to generate robust circadian oscillation (5). In cyanobacterial cells, time profiles of induction or repression of gene expression by KaiC match the circadian period (6). Furthermore, a KaiC-based transcription/translation oscillation persists in cells to generate robust circadian rhythms by coupling to the biochemical KaiC phosphorylation oscillation (7).
Daily alterations in light and temperature are the main factors that entrain the circadian oscillator (8). External light signals are processed in the cell through photoreceptor and signaling pathways to the pacemaker loop or to a photo-receiving process that is tightly linked to the time generating loop. Entrainment of circadian rhythms by temperature has also been studied from two aspects. To elucidate how the intracellular oscillators physiologically interact with the external temperature cycles, entrainment kinetics were carefully studied by applying various non–24-hour cycles (T cycles; 9, 10). On the other hand, to study molecular events that linked with external temperature signals, temperature-dependent processes such as transcription, translation, RNA processing, protein stability, and protein–protein interactions were analyzed in several model organisms (11–13).
External temperature signals might also reach the central pacemaker via nonspecific pathway such as thermal agitation of water molecules. If so, the in vitro KaiC phosphorylation rhythm might be directly stimulated by external temperature cues, whereas it is unlikely that any of the three Kai proteins sense external light signals. In this study, we examined KaiC phosphorylation rhythms under various sequences of ambient temperature and found that the in vitro oscillation of KaiC phosphorylation was stably entrained by external temperature cycles.
Two models for the entrainment of the circadian clock by external time cues (zeitgeber) have been proposed, the parametric model and the nonparametric model (8). In the parametric model, the total time of the rhythmic cycle is adjusted to the external cycle by changing times required for the external day and night periods. On the other hand, the nonparametric model depend on discontinuous phase jumps caused by day and night switchings of external conditions to adjust the effective cycle time (free-running period and phase shifts) to that of external cycle. For the circadian clock of living cells, it was generally considered that the manner of light entrainment of mammalian clock was accounted for by combination of the two models (8).
In this study, we showed that the nonparametric model can clearly account for the temperature entrainment of in vitro circadian rhythm, because entrainment of the KaiC phosphorylation rhythm was not attained by affecting the period of the oscillation but rather by phase jumps caused by step-up and step-down in the temperature cycle. These results suggest that entrainment of the in vitro rhythm by temperature can occur by direct regulation of the biochemical timing reactions of the Kai proteins.
Results
Temperature Entrainment of the KaiC Phosphorylation Rhythm In Vitro.
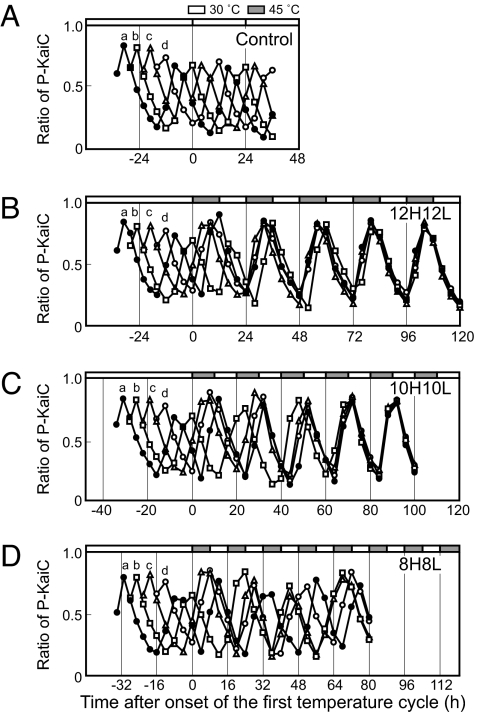
If a self-sustained rhythm is entrained by an external cycle (zeitgeber), the period of the rhythm should adjust to the external cycle so that the peak of the rhythm is stably positioned at a unique phase of the external cycle. To test whether the in vitro KaiC phosphorylation rhythm could be entrained by a temperature cycle, we prepared four mixtures of KaiA, KaiB, and KaiC at 6-hour intervals. Under the standard conditions (30°C), the rhythm of the four mixtures (curves a–d) persisted with a period of 23.1 ± 0.23 hours (n = 4), maintaining the phase angle differences determined by the time of mixing (Fig. 1A). Next, we exposed the four mixtures to temperature cycles of 12 hours at 45°C and 12 hours at 30°C (12H12L). We chose 45°C as the warm temperature because this temperature was highest one that permitted stable KaiC phosphorylation rhythm to persist in vitro. As depicted in Fig. 1B [see supporting information (SI) Fig. S1 for raw gel image], the peak of the four KaiC phosphorylation rhythms moved forward or backward depending on the original phase of the mixture. Interestingly, by the fourth temperature cycle, the traces of the mixtures overlapped, indicating that the periods of the four mixtures were extended to 24 hours (23.9 ± 0.22 h, n = 4) and the peaks coincided at ≈8 hours after the cool-to-warm (step-up) transition. This result clearly demonstrates that the in vitro KaiC phosphorylation rhythm was entrained by the 12H12L temperature cycle.
Fig. 1.
Temperature entrainment of the in vitro KaiC phosphorylation rhythm. Four mixtures of Kai proteins (a–d) were prepared at 6-hour intervals and incubated at 30°C (A). Four mixtures were incubated at 30°C for 34 hours (a, closed circle), 28 hours (b, open square), 22 hours (c, open triangle), and 16 hours (d, open circle), and then subjected to 45°C/30°C cycles of 12H12L (12 hours 45°C and 12 hours 30°C) (B), 10H10L (10 hours 45°C and 10 hours 30°C) (C), and 8H8L (8 hours 45°C and 8 hours 30°C) (D). In each experiment, aliquots of reaction mixtures were collected every 3 hours or 4 hours and subjected to SDS/PAGE and CBB staining. ImageJ software was used for densitometric analysis. The ratios of phosphorylated KaiC (P-KaiC) to total KaiC are plotted against time in the temperature cycle. Zero on the abscissa of panel A represents 34 hours after the preparation of the first mixture.
Next, we examined a 10H10L temperature cycle. As shown in Fig. 1C, the 10H10L regimen also entrained the KaiC phosphorylation rhythm, as the period approached 20 hours (19.9 ± 0.24 h, n = 4) after the fourth cycle and the peaks of the four mixtures coincided. In contrast, under an 8H8L temperature cycle, the interval between peaks changed from cycle to cycle (18.4 to 24.4 hours) and the peaks did not come together (Fig. 1D). Thus, the KaiC phosphorylation rhythm could be entrained by 12H12L and 10H10L temperature cycles, but not by an 8H8L cycle.
Precision for Temperature Compensation of the KaiC Phosphorylation Rhythm.
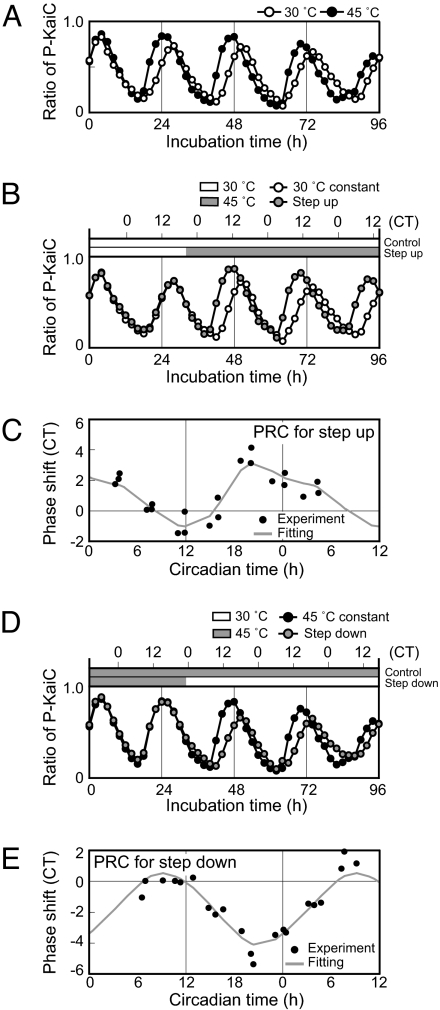
A parametric model proposed by Aschoff depends primarily on a period change generated by light flux (Aschoff's rule, 8). Although light flux did not affect the period of the KaiC phosphorylation rhythm, day/night change in temperature might entrain the rhythm by changing the period length. To test this possibility, the period of the rhythm was precisely examined at different temperatures. To exclude variations in period due to Kai protein preparation, we first mixed the Kai proteins and then divided the mixture into separate tubes to examine the period length of the same mixture at 30° and 45°C (Fig. 2A; Fig. S1). We excluded the first peak from the evaluation, as it was apparently induced by a transient process. The observed periods were 23.4 hours at 30°C and 23.3 hours at 45°C (Q10 = 1.00). We then examined additional five Q10 values using five different Kai protein preparations. The mean periods were 23.0 ± 0.56 hours (n = 6) at 30°C and 22.7 ± 0.93 hours (n = 6) at 45°C. While the absolute period length was influenced by the preparation of Kai proteins, Q10 values for five experiments were 0.98, 1.00, 1.02, 1.03, and 1.03 (average 1.01 ± 0.02, n = 6). This extraordinarily precise temperature compensation indicated that the entrainment by period change by temperature was not adopted in this case.
Fig. 2.
Phase shifts of the KaiC phosphorylation rhythm caused by temperature steps. (A) Effects of temperature on the in vitro KaiC phosphorylation rhythm. A mixture of Kai proteins was prepared and divided into two fractions, which were incubated at 30°C and 45°C. Aliquots of the mixtures were collected every 2 h, and subjected to SDS/PAGE and densitometric analyses, as in Fig. 1. The ratios of P-KaiC to total KaiC are plotted against incubation time. (B) KaiC phosphorylation rhythms after temperature step-up. The Kai protein mixture was incubated at 30°C and the temperature was stepped up to 45°C at CT 20.1 (incubation time 32 hours). Phosphorylation of KaiC was assayed and plotted as in A. CT scale was also shown along x axis with assigning the peak of the rhythm as CT 16. Aliquots were collected every 2 hours and subjected to SDS/PAGE as described in Fig. 1. (C) PRC for temperature step-up (30° to 45°C). The mixtures were exposed to temperature step-up at incubation time 16, 20, 24, 28, 32, 36, and 40 hours. Phase shifts caused by each temperature step-up were examined, normalized, and plotted against CT. Positive and negative values on the ordinate represent phase advance and delay, respectively. PRC was obtained by smoothing-spline interpolation of the data points (see text and SI Text). (D) KaiC phosphorylation rhythms following temperature step-down from 45° to 30°C. The mixture was exposed to temperature step-down at CT 23.1 (incubation time 32 hours). KaiC phosphorylation is shown as in B. (E) PRC for temperature step-down (45° to 30°C). Phase shifts of respective mixtures and PRC are shown as in C.
Phase Shifting of the KaiC Phosphorylation Rhythm by Temperature Steps.
An alternative to the parametric model is a nonparametric model originally proposed by Pittendrigh, which depends on phase shifts caused by discontinuous changes in external conditions (14, 15). By extensive studies on phase response to light step, Aschoff also supported a significance of the nonparametric entrainment in various circadian system (16). Thus, we hypothesized that the temperature entrainment (Fig. 1) of the KaiC phosphorylation rhythm might be caused by discontinuous jumps in phase caused by temperature steps. To examine this possibility, we analyzed the effects of temperature shifts from 30° to 45°C (step-up) on the KaiC phosphorylation rhythm. As shown in Fig. 2B, temperature step-up at circadian time (CT) 20.1 led to peak and trough occurrence ≈3 hours earlier than the reference rhythm (3-hour phase advance). We further examined phase shifting by step-up at various times of the incubation times (during hours 16 and 40) (Fig. S2A) and plotted the phase shifts against the phase of the temperature shift to construct a phase response curve (PRC, Fig. 2C). To reject variations caused by preparation of Kai proteins, we normalized the phase of the step-up and induced shift in CT by adjusting the period length of each rhythm to 24 hours in CT. In this plot, the peak of the rhythm was set to CT 16, because KaiC phosphorylation in cyanobacterial cells peaked at hour 16 of continuous light conditions. Next, we constructed PRC for temperature step-up by a smoothing-spline interpolation. As shown in Fig. 2C, temperature step-up at times between CT 8 and 15 delayed the phase and temperature step-up at other times caused phase advance. Maximum phase advance (≈4 hours) and delay (≈2 hours) were induced by temperature stepping at CT 20 and CT 12, respectively.
Similarly, we analyzed phase shifting after temperature step-down from 45° to 30°C (Fig. 2D; Fig. S2B). As shown in Fig. 2E, the PRC of step-down appeared to be a mirror image of that of step-up. The phase was delayed by temperature step-down at times between CT 12 and 6, whereas step-down at times between CT 6 and 12 elicited phase advance. The maximum advance was ≈2 hours by step-down at CT 8 and the maximum delay was ≈5 hours at the late dephosphorylation phase (CT 20). Note also that both temperature step-up and step-down caused larger phase shifts during the dephosphorylation phase (CT 16–4, Figs. 2C and 2E).
We also examined masking effect of temperature steps on the KaiC phosphorylation rhythm. By analyzing waveforms of the rhythms after temperature steps, we confirmed that there was little or no masking effect (1) on the rhythmic profile of the KaiC phosphorylation. For rhythms from living cells, masking effects would be inevitable because the rhythmic event was processed with many intracellular steps that should be temperature sensitive. However, it would be not the case for in vitro rhythm that we discussed here.
Phase Shifting by Temperature Pulse.
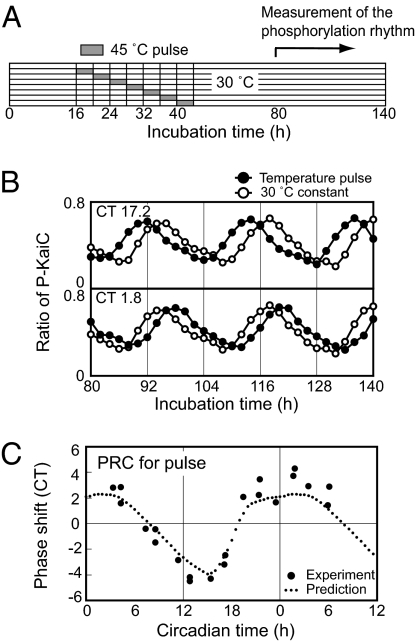
In the nonparametric entrainment model, phase shifting by temperature pulse is assumed to be the sum of phase shifts caused by step-up and step-down. In fact, phase shift of the Drosophila eclosion rhythm by high-temperature pulse can be deduced from a sum of step-up and step-down phase shifts (17). If a phase shift by temperature step-up is completed before step-down, the phase shift h(φ) of the high-temperature pulse at phase φ is calculated by the following equation:
where f(φ) and g(φ) respectively represent phase shift by step-up and step-down (in CT) and A and τ represent duration of pulse and free-running period (in hours), respectively.
To examine the nonparametric model, we examined actual phase shifting by administering 4-hour pulses at various times of incubation (hours 16–44, Fig. 3A). Figure 3B depicts temperature pulses starting at hours 28 and 36. The phase was advanced by the pulse starting at CT 17.2 (hour 28) but delayed by the pulse starting at CT 1.8 (hour 36). We next plotted phase shifts caused by 21 different temperature pulses ranging across the circadian cycle (Fig. 3C). On the other hand, we calculated phase shifting by 4-hour high-temperature pulses using Eq. 1 and overlaid the results with the observed phase shifts. As shown in Fig. 3C, the predicted PRC correlated well with the experimental results, suggesting that nonparametric entrainment occurs in this biochemical oscillator that consists of only three proteins.
Fig. 3.
Phase shift of KaiC phosphorylation rhythm by high temperature pulse. (A) Protocol to assay phase shifts by high temperature pulses. After various durations of incubation at 30°C (incubation time 16–40 hours), samples were subjected to 45°C pulses for 4 hours and then returned to 30°C. After the pulses, KaiC phosphorylation rhythms were monitored for 60 hours (incubation time 80–140 hours). (B) KaiC phosphorylation rhythms following 4-h pulses at 45°C administered at CT 17.2 (incubation time 28 h) (upper panel) and CT 1.8 (incubation time 36 hours) (lower panel). KaiC phosphorylation rhythms with (closed) or without (open) the pulse were plotted against incubation time. SDS/PAGE and densitometric analyses were carried out as in Fig. 1. (C) PRC for 4-hour pulses at 45°C. The mixtures were exposed to temperature pulses, and the induced phase shifts (in CT) were plotted against onset of the temperature pulse (on abscissa in CT). A PRC deduced from the nonparametric model based on the PRCs of temperature step (Fig. 2) is shown by the dashed line (see Text and SI Text).
Range of Entrainment of the KaiC Phosphorylation Rhythm by External Temperature Cycle.
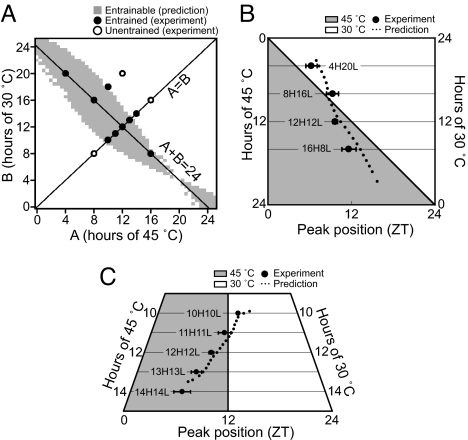
To determine whether the nonparametric model holds for entrainment by temperature cycles, we analyzed the rhythm of KaiC phosphorylation under cycles of various combinations of high- and low-temperature periods. When peaks repeated with cycle length of temperature and were positioned at a specific phase of the cycle, we judged that stable entrainment was established (Fig. S3). Results of entrainments by various temperature cycles are shown with closed or open circles in diagrams of incubation period of 30°C versus that of 45°C. As shown in Fig. 4A, when cycle length was 24 hours, the rhythm was entrained with cycles that had 8–20 hours of high temperature. When the cycle was symmetric, rhythm was entrained by temperature cycles of 20–28 hours but not by cycles of 16 or 32 hours.
Fig. 4.
Entrainment of the KaiC phosphorylation rhythm by various temperature cycles. (A) Mixtures were incubated under various temperature regimens consisting of 30 and 45°C for at least four cycles, and entrainment was evaluated based on period length and peak positions. The temperature cycles that entrained the rhythm were plotted with closed circles on a diagram of hours at high and low temperature. Temperature cycles that failed to entrain were plotted with open circles. Based on the phase shifts caused by temperature steps (Fig. 2), the predicted range of entrainment was shown in gray (see SI Text). (B) Peak position in zeitgeber (ZT) scale of the rhythms entrained by temperature cycles of 24-hour period (A+B = 24). The peaks of the entrained rhythms were plotted on ZT (closed circle). Attached bars represent standard deviation for four experiments. A solid line depicts peak position predicted by the nonparametric model (see SI Text). (C) Peak position in ZT scale of the rhythms entrained by symmetrical temperature cycles (A = B). Results and prediction are shown as in (B).
We then used the nonparametric model to predict a range of entrainment by cycles of various fractions of high and low temperature (Fig. 4A). When stable entrainment is attained under a cycle of A hours at high temperature and B hours at low temperature, Eq. 2 should be satisfied with a specific phase of f(φs) (phase of onset of high temperature in entrained status in CT) because, under entrained conditions, the difference in free-running period of the rhythm and external cycle length should be compensated by two phase shifts accompanied by a temperature cycle (see SI Text for details of the prediction).
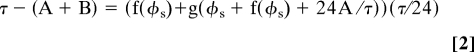 |
where factor τ/24 converts time in CT to real hours. As shown in Fig. 4A, most of the temperature cycles that entrained the rhythm (closed circle) fell within the predicted range of entrainment. On the other hand, protocols that failed to entrain the rhythm (open circle) fell outside the range.
Figures 4B and 4C depict the peak position of the entrained rhythm in the temperature cycle in a zeitgeber time (ZT) scale, a time scale for an external cycle in 24 hours. As shown in Fig. 4C, the longer the period of T-cycle was applied, the earlier the peak of the phosphorylation rhythm occurred. This relationship was commonly observed when the circadian clock was entrained by various T-cycles (9, 10). Based on φs that satisfied Eq. 2, the predicted peak positions were also plotted in Figs. 4B and 4C (see SI Text for details). The observed peak positions of the entrained rhythms corresponded well with those predicted by the nonparametric model. These results also support that entrainment of the KaiC phosphorylation rhythm in vitro follows the nonparametric entrainment model. In addition, we confirmed that a fitting of PRC of temperature step to cosine curve also well predicted the experimental results (Fig. S4).
In sum, our predictions for phase shifting by 4-hour, single temperature pulse (Fig. 3C) and for repeated temperature cycles of various lengths (Fig. 4) are consistent with our experimental results. Thus, our assumption that phase shifting by a temperature step is complete before onset of the next stimulus seems to be valid, as it is unlikely we would have observed a fit otherwise.
KaiC Phosphorylation Rhythms in Cyanobacterial Cells Under Temperature Cycling.
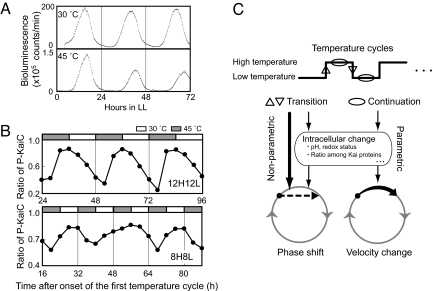
We next examined the KaiC phosphorylation rhythm in cyanobacterial cells under two temperatures, as Synechococcus cells can proliferate even at 45°C in LL conditions. Although bacterial luciferase loses most of its activity at this temperature, we were able to detect a weak but reliable bioluminescence with a robust rhythm of a 26.3-hour period (Fig. 5A). As the period from the turbidostat culture at 30°C was 24.6 hours, the period of the cellular oscillator is slightly affected by temperature (Q10 = 0.96).
Fig. 5.
Temperature entrainment of the KaiC phosphorylation rhythm in Synechococcus cells. (A) Circadian rhythms monitored by bioluminescence under LL (continuous light) at 30°C and 45°C. A wild-type strain carrying a luciferase reporter construct was grown under LL at 30°C or 45°C at a constant optical density (at 730 nm) of 0.3. After two cycles of 12-hours light/12-hours dark to synchronize their clocks, bioluminescence was monitored under LL. Bioluminescence from the liquid culture was plotted against hours in LL. (B) KaiC phosphorylation rhythm in cyanobacterial cells exposed to temperature cycles of 45°C/30°C. Cyanobacterial cells were grown in LL at 30°C and then subjected to temperature cycles of 12 hours at 45°C and 12 hours at 30°C (12H12L) or 8 hours at 45°C and 8 hours at 30°C (8H8L). Cells were collected every 4 hours after the second temperature step-up. A 1-μg quantity of total cellular protein was used for Western blot analysis. KaiC phosphorylation ratio was plotted against the time after onset of the first temperature cycle. (C) A model for entrainment of the cyanobacterial circadian clock by temperature cycle. In the upper part, a temperature cycle and its effective factors for entrainment are shown. Ovals indicate continuation of temperature and up and down arrowheads indicate discontinuous jumps in temperature. The lower part illustrates possible effects of factors to a limit-cycle of the circadian pacemaker. See text for more explanation.
Cells were next cultured under temperature cycles of 12H12L. As shown in Fig. 5B, KaiC phosphorylation in these cells showed a rhythm of 24-hour period and appeared to be entrained by the 12H12L cycle because the peak of the rhythm was located at specific points of the temperature cycle. In contrast, the KaiC phosphorylation state under the 16-hour temperature cycle (8H8L) did not oscillate with a 16-hour period; rather, it weakly oscillated with an ≈24-hour period and an unstable peak position. These results indicate that the 8H8L temperature cycle failed to entrain the KaiC phosphorylation rhythm in the cell. For more information see Figs. S5 and S6.
Discussion
By repeating external temperature cycles, we demonstrated that the in vitro oscillation persisted and established a solid phase relationship with the external temperature cycle after 4–5 transient cycles (18) (Fig. 1). We also confirmed that the KaiC phosphorylation rhythm shared a common relationship between position of peak of the entrained rhythms and length of entraining T-cycle with other circadian systems (9, 10) (Fig. 4). As the KaiC phosphorylation rhythms was already reported to satisfy self-sustainability of rhythm and temperature compensation of the period, the result here implies that the Kai oscillator satisfies all three criteria of circadian rhythm. That is, we confirmed entrainability of the in vitro rhythm in this study, whereas phase shifting of the rhythm by temperature pulse itself was previously reported (19).
Temperature entrainment of circadian rhythms has been studied in many organisms at various levels. An extensive study of the eclosion rhythm of Drosophila pseudoobscura (17) demonstrated that entrainment by temperature can be ascribed to phase shifting by discontinuous temperature changes (both step-up and step-down). The fact that the rhythm could be predicted by accumulation of phase shifting at a given step strongly supports nonparametric or discontinuous entrainment in Drosophila. Interestingly, we have found similar entrainment kinetics in the simplest circadian oscillation in vitro. As shown in Fig. 4A, the entrainment of the KaiC phosphorylation rhythm to temperature cycles was predicted by phase shifting with temperature transitions between 30° and 45°C. Moreover, the range of entrainment and the peak position of the entrained rhythm precisely matched the prediction (Figs. 4B and 4C). Thus, nonparametric entrainment by temperature may be a common principle for entrainment both in fly brain and in the simple biochemical system of Kai proteins, because temperature can shift the phase of the rhythm but fails to alter period length (temperature compensation of period).
The nonparametric entrainment of self-sustained oscillation by a stimulus implies that the stimulus alters a state variable of the oscillation because this type of entrainment is based on discontinuous phase shifting of the oscillation. How, then, does temperature change induce the phase shift? It is important to note that the phosphorylation state of KaiC would not be the primary target of the temperature step, because the phosphorylation state of KaiC did not change acutely but gradually changed after the temperature step (Fig. S2). Other processes that may cause the phase shifting include changes of the KaiC intramolecular structure that can be associated with the timebase ATPase activity (4), changes in the state of the KaiC hexamer, or changes in complex formation between KaiC and KaiB or KaiA (19, 20). At present, we have not yet found such changes, which may be too subtle to detect directly.
KaiC phosphorylation rhythm in vivo was entrained by a 12H12L cycle. If a period of warm temperature is shorter than 24 hours and that of cold temperature is longer than 24 hours, or vice versa, the total cycle time for one turn of the temperature cycle can be adjusted to 24 hours. However, the period of the in vivo bioluminescence rhythm was longer than 24 hours at both warm and cold temperatures (Fig. 5). Thus, it is difficult to adjust the total time for the temperature cycle to 24 hours by period adjustment only (parametric entrainment). Therefore, nonparametric temperature entrainment mechanism, as we observed in vitro, could contribute to the entrainment of the cyanobacterial cellular clock.
In some aspects, entrainment in vivo is different from that observed in vitro. Note that both in vivo waveform and peak position of the KaiC phosphorylation rhythm entrained by the 12H12L regimen differed from those in vitro (Figs. 1B, 5B). At the molecular level, various intracellular processes are likely responsible for these differences. As shown in Fig. 5C, possible factors that contribute to entrainment in vivo include 1) change in intracellular environment (pH or redox state), 2) change in concentration of intracellular Kai proteins caused by effects on synthesis and degradation, and 3) factors that might affect timebase ATPase activity. Some of these factors derived from the alteration in temperature might alter the velocity of the KaiC oscillator to facilitate the entrainment that we observed in this study. In fact, we found that the period of in vivo rhythm was changed by temperature (Fig. 5A). Other factors might enhance nonparametric entrainment by discontinuously altering the status of the pacemaking reaction in KaiC. In addition, the KaiC transcription/translation loop that functions independently of the KaiC phosphorylation rhythm (7) might also enhance entrainment.
Materials and Methods
Reconstitution of KaiC Phosphorylation Rhythms In Vitro and Regulation of Temperature.
Purification of recombinant KaiA, KaiB, and KaiC proteins and reconstitution of the KaiC phosphorylation rhythm in vitro were performed as described elsewhere (5), except that 3 μg/mL vancomycin (final concentration) was added to repress bacterial contamination. For temperature regulation, mixtures of Kai proteins and ATP were incubated with a thermal-block incubator (BI-515, ASTEC). Incubation temperatures were changed by quickly moving the mixtures to different incubators that were equilibrated at 30° or 45°C. Effective temperature step-up and step-down were completed within 5 minutes. To estimate period length and phase of the peak of the rhythm, time courses of KaiC phosphorylation ratio for each experiment were fitted to the cosine function by a nonlinear least-square mean procedure (see SI Text for details).
Cyanobacterial Strains and Culture for In Vivo Analysis.
Synechococcus elongatus PCC 7942 carrying a PkaiBC-luxAB bioluminescence reporter (NUC42) was used in this study. Cells were cultured in modified BG-11 liquid medium under continuous light conditions (LL) of 46 μmol m−2 s−1 in a continuous culture system to maintain an optical density (at 730 nm) of 0.3. Culture bioluminescence was monitored as described previously (6). To reduce thermal noise, the temperature of the photomultiplier tube of the monitoring system was maintained at 30°C. A small portion of the culture was introduced into a flow cell placed at the top of the detector within 5 minutes to monitor bioluminescence. The continuous culture system was maintained at the designated temperature by flow of temperature-regulated water through stainless tubing immersed in the liquid culture. The fluctuation of temperature of the liquid culture was 0.1°C. Temperature step-up to 45°C and step-down to 30°C were completed within 20 minutes. To assay KaiC, Western blotting analysis was performed as previously described (6).
Supplementary Material
Acknowledgments.
We thank Drs. S. Daan (University of Groningen) and T. Oyama for their helpful discussions, and H. Kondo, T. Nishikawa, and M. Tamura for their technical support. This research was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (15GS0308 to T. K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806741106/DCSupplemental.
References
- 1.DeCoursey PJ. In: Chronobiology: Biological timekeeping. Dunlap JC, Loros JJ, DeCoursey PJ, editors. Sunderland, MA: Sinauer; 2004. pp. 3–24. [Google Scholar]
- 2.Stillman B, Stewart D. In: Clocks and Rhythms. Stillman B, Stewart D, editors. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2008. [Google Scholar]
- 3.Nakajima M, et al. Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science. 2005;308:414–415. doi: 10.1126/science.1108451. [DOI] [PubMed] [Google Scholar]
- 4.Terauchi K, et al. The ATPase activity of KaiC determines the basic timing for circadian clock of cyanobacteria. Proc Natl Acad Sci USA. 2007;104:16377–16381. doi: 10.1073/pnas.0706292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishiwaki T, et al. A sequential program of dual phosphorylation of KaiC as a basis for circadian rhythm in cyanobacteria. EMBO J. 2007;26:4029–4037. doi: 10.1038/sj.emboj.7601832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murayama Y, Oyama T, Kondo T. Regulation of circadian clock gene expression by phosphorylation states of KaiC in cyanobacteria. J Bacteriol. 2008;190:1691–1698. doi: 10.1128/JB.01693-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitayama Y, Nishiwaki T, Terauchi K, Kondo T. Dual KaiC-based oscillations constitute the circadian system of cyanobacteria. Genes Dev. 2008;22:1513–1521. doi: 10.1101/gad.1661808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson CH, et al. In: Chronobiology: Biological timekeeping. Dunlap JC, Loros JJ, DeCoursey PJ, editors. Sunderland, MA: Sinauer; 2004. pp. 67–105. [Google Scholar]
- 9.Hoffmann K. Zur Beziehung zwischen Phasenlage und Spontanfrequenz bei der endogenen Tagesperiodik. Z Naturforschg. 1971;18:154–157. [Google Scholar]
- 10.Merrow M, Brunner M, Roenneberg T. Assignment of circadian function for the Neurospora clock gene frequency. Nature. 1999;399:584–586. doi: 10.1038/21190. [DOI] [PubMed] [Google Scholar]
- 11.Glaser FT, Stanewsky R. Temperature synchronization of the Drosophila circadian clock. Curr Biol. 2005;15:1352–1363. doi: 10.1016/j.cub.2005.06.056. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Merrow M, Loros JJ, Dunlap JC. How temperature changes reset a circadian oscillator. Science. 1998;281:825–829. doi: 10.1126/science.281.5378.825. [DOI] [PubMed] [Google Scholar]
- 13.Lahiri K, et al. Temperature regulates transcription in the zebrafish circadian clock. PLoS Biol. 2005;3:e351. doi: 10.1371/journal.pbio.0030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pittendrigh CS. The circadian oscillation in Drosophila pseudoobscura pupae: A model for the photoperiodic clock. Z Pflanzenphysiol. 1966;54:275–307. [Google Scholar]
- 15.Pittendrigh CS. Temporal organization: Reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993;55:17–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- 16.Aschoff J. Masking and parametric effects of high-frequency light-dark cycles. Jpn J physiol. 1999;49:11–18. doi: 10.2170/jjphysiol.49.11. [DOI] [PubMed] [Google Scholar]
- 17.Zimmerman WF, Pittendrigh CS, Pavlidis T. Temperature compensation of the circadian oscillation in Drosophila pseudoobscura and its entrainment by temperature cycles. Insect Physiol. 1968;14:669–684. doi: 10.1016/0022-1910(68)90226-6. [DOI] [PubMed] [Google Scholar]
- 18.Yoshii T, et al. Temperature cycles drive Drosophila circadian oscillation in constant light that otherwise induces behavioural arrhythmicity. Eur J Neurosci. 2005;22:1176–1184. doi: 10.1111/j.1460-9568.2005.04295.x. [DOI] [PubMed] [Google Scholar]
- 19.Mori T, et al. Elucidating the ticking of an in vitro circadian clockwork. PLoS Biol. 2007;5:e93. doi: 10.1371/journal.pbio.0050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kageyama T, et al. Cyanobacterial circadian pacemaker: Kai protein complex dynamics in the KaiC phosphorylation cycle in vitro. Mol Cell. 2006;23:161–171. doi: 10.1016/j.molcel.2006.05.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.