Abstract
Molecular and cellular interactions coordinating the origin and fate of neural stem cells (NSCs) in the adult brain are far from being understood. We present a protein complex that controls proliferation and migration of adult NSCs destined for the mouse olfactory bulb (OB). Combinatorial selection based on phage display technology revealed a previously unrecognized complex between the soluble protein netrin-4 and laminin γ1 subunit that in turn activates an α6β1 integrin-mediated signaling pathway in NSCs. Differentiation of NSCs is accompanied by a decrease in netrin-4 receptors, indicating that netrin-4 participates in the continual propagation of this stem cell population. Notably, the stem cells themselves do not synthesize netrin-4. Further, we show that netrin-4 is produced by selected GFAP-positive astrocytes positioned close to newborn neurons migrating in the anterior part of the rostral migratory stream (RMS) and within the OB. Our findings present a unique molecular mechanism mediating astrocytic/neuronal crosstalk that regulates ongoing neurogenesis in the adult olfactory system.
Keywords: olfactory system, phage display, merogenesis, rostral migratory stream, astrocytes
Netrins are a family of secreted proteins initially described as developmental axon attractants (1). Yet, with critical roles in tissue morphogenesis (2–4), vascular patterning (5), and angiogenesis (6–8), netrins have become recognized to mediate functions far beyond axonal guidance. Netrin-4 is expressed in both neural and nonneural tissues (8–11). In the nervous system, it promotes neurite extension from olfactory bulb (OB) explants but the molecular mechanisms underlying this phenomenon remain unknown. In the vasculature of the kidney, heart, and ovary, the protein is located in the basement membrane. Integrins and netrins, along with the established netrin-1 and netrin-4 receptors DCC and UNC5, appear to cooperate to regulate multiple aspects of development (3, 12, 13). However, certain netrin-4 functions are mediated by neogenin (8) and likely by other, as yet unidentified receptors (14, 15).
Here, we provide evidence for a direct relationship between netrin-4, laminin γ1 chain, and α6β1 integrin in the context of neural stem cells (NSCs). By exploiting the power of the unbiased phage display combinatorial approach (16–18), followed by peptide affinity chromatography and functional validation, we found that a netrin-4/laminin γ1 chain complex binds to α6β1 integrin, with subsequent activation of the mitogen-activated protein (MAP) kinase signaling pathway, resulting in NSC migration and proliferation. We also show that in the adult mouse brain, netrin-4 is produced by a subset of astrocytes on the edge of the rostral migratory system (RMS) close to the site of neuron entrance into the OB. Our findings support the conclusion that netrin-4 is a regulatory factor in NSC biology and uncover a previously unrecognized mechanism in which a multimeric complex functions as an integrated supramolecular switch that regulates NSC fate.
Results and Discussion
Isolation of Peptides Binding to NSCs and Receptor Identification.
We profiled (16–18) the surface of a representative prototypical clone of murine NSCs (C17.2) that can self-renew, differentiate into all neural lineages, and populate developing or degenerating regions of the central nervous system (19, 20). Cells proliferating in vitro were exposed to a random phage display peptide library (CX7C: C, cysteine; X, any residue) (16–18); marked enrichment was obtained after 3 rounds of selection [supporting information (SI) Fig. S1A]. Similarity BLAST search analysis of peptides displayed by the enriched phage population showed that the sequence CGLPYSSVC contained a motif embedded within domain IV of the netrin-4 protein (Leu-404–Val-410) (9, 10). Detailed sequence alignment revealed that the region mimicked by the peptide motif is not similar to any other known member of the netrin and laminin families of proteins (Fig. S1B). Three-dimensional models of the Cys-391–Cys-410 motif of netrin-4 and the peptide CGLPYSSVC revealed similarities in primary sequence and structure (Fig. S1C).
We evaluated the binding of selected phage to C17.2 NSCs and found that phage displaying CGLPYSSVC specifically bound to NSCs in contrast to negative controls (insertless phage and phage displaying an unrelated peptide sequence) (Fig. S1D). In addition, given that netrin-4 can form dimers (9), we evaluated the binding of the netrin-4-like phage to netrin-4 and observed increased phage binding to immobilized netrin-4 (Fig. S1E). Inhibition of phage binding by an anti-netrin-4 antibody confirmed specificity (Fig. S1F).
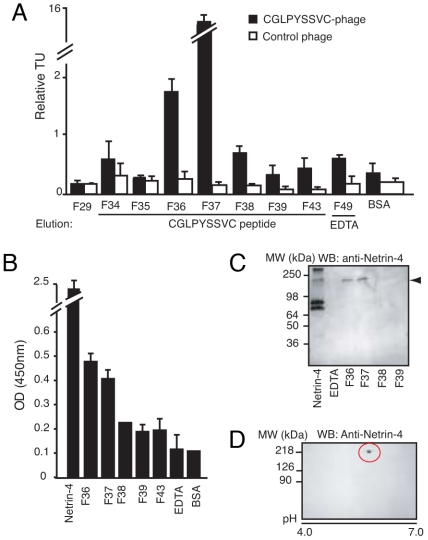
We used peptide column affinity chromatography to identify binding partners of CGLPYSSVC. Total cell extracts of the C17.2 NSCs were loaded onto a CGLPYSSVC peptide column, and specific proteins were eluted with a solution of the synthetic corresponding peptide. The eluate(s) potentially containing the receptor(s) were determined by phage binding to purified proteins of 8 fractions covering different points of the targeted elution. The CGLPYSSVC-phage bound preferentially to F36 and F37, in comparison to the flanking fractions or controls. Control phage showed only background binding to the tested fractions (Fig. 1A).
Fig. 1.
Purification of binding partner(s) of the CGLPYSSVC-phage. (A) Identification of fractions containing the targeted receptor by phage binding assay. (B) ELISA showing that a protein recognized by the anti-netrin-4 antibody was eluted in F36 and F37. (C) Immunoblotting analysis revealed that the anti-netrin-4 antibody recognizes a protein of 220 kDa (arrowhead) in F36 and F37. (D) Two-dimensional electrophoresis gel of crude NSC lysate. A single protein with ≈220-kDa molecular weight and isoelectric point ≈5.2 is recognized by the anti-netrin-4 antibody.
Given that the CGLPYSSVC-phage specifically binds to immobilized netrin-4 (Fig. S1 E and F), we predicted that netrin-4 itself might serve as a binding partner. To test this possibility, we used ELISA to examine the targeted fractions for netrin-4. Enhanced reactivity was observed in F36 and F37 relative to the flanking fractions or to control proteins (Fig. 1B). Next, we used immunoblotting in attempts to confirm the identity of the targeted protein. Surprisingly, the protein recognized by the anti-netrin-4 antibody exhibited a molecular mass of ≈220 kDa (Fig. 1C), whereas netrin-4 is described as a secreted protein of ≈80 kDa (9, 10). Immunoprecipitation with anti-netrin-4 antibody revealed the presence of the high-molecular-weight protein in total NSCs lysate (Fig. S1G). Furthermore, we observed that NSCs themselves appear not to produce netrin-4, as it was not detected in either total cell extracts (Fig. 1D and Fig. S1G) or cell culture media (data not shown). Two-dimensional electrophoresis confirmed that the anti-netrin-4 antibody cross-reacts with a single protein of ≈220 kDa (Fig. 1D).
We evaluated whether netrin-4 would bind to DCC and UNC5, well-known netrin-1 receptors (21) that are also capable of binding netrin-4 (14), although not necessarily in all types of cells (15). We observed binding of netrin-4 to DCC, UNC5H1, and UNC5H2 (Fig. S2 A–E). We show that the CGLPYSSVC-phage specifically and consistently binds to DCC, UNC5H1, and UNC5H2 (Fig. S2F). However, we did not detect expression of these receptors on C17.2 NSCs by either RT-PCR or immunoblotting (data not shown). This result indicates that these ligand-receptor systems are unlikely to be relevant in the context of NSCs.
Netrin-4, Laminin γ1 Chain, and the α6β1 Integrin Interact on the Surface of NSCs.
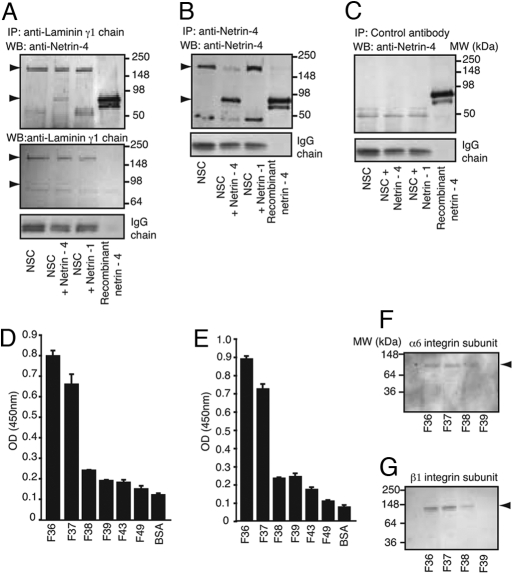
Mass spectroscopy and database analysis identified the laminin γ1 chain as the candidate partner for the netrin-4-like peptide. Consistent with this, interaction of netrin-4 and the short arms of laminin has been recently reported (22). Reciprocal co-immunoprecipitation experiments demonstrated that such interaction is also effective in the context of NSCs (Fig. 2). Cells were treated either with netrin-4 or netrin-1. Total cell extracts of treated and untreated cells were immunoprecipitated either with anti-laminin γ1 chain or with anti-netrin-4 antibodies (Fig. 2 A and B). Anti-laminin γ1 chain coprecipitates both the γ1 chain and netrin-4 (Fig. 2A) only when netrin-4 was added to the wells. Immunoprecipitation with control antibody (Fig. 2C) revealed no detectable binding. Moreover, no specific interaction was observed when co-immunoprecipitation experiments were performed with anti-netrin-1 antibody (Fig. S3A). Another antibody specific to netrin-4 (Fig. S3B) was used to confirm the binding of netrin-4 to laminin γ1 chain (Fig. S3C).
Fig. 2.
Netrin-4 binds to laminin γ1 chain. (A and B) Ligand-dependent co-immunoprecipitation. As indicated by the arrowheads, netrin-4 (≈80 kDa) and laminin γ1 chain (≈220 kDa) form a precipitable complex when netrin-4 is added. (C) In similar experiments, no coprecipitation was observed when the cell lysate was immunoprecipitated by control antibodies. (D and E) ELISA revealed that α6 (D) and β1 (E) integrin subunits were present in F36 and F37. (F and G) Immunoblotting confirming the identity of the netrin-4 receptor candidates.
Structural similarities among members of the laminin and netrin families (10, 23) explain why an antibody raised against netrin-4 might also recognize an equivalent epitope in the γ1 chain. In contrast to the other members of the family, netrin-4 most closely resembles laminin β chain in primary amino acid sequence (10). Yet, we observed binding of netrin-4 to the γ1 chain. This result invites the speculation that such a relationship might establish stable interactions of laminin and netrins in the extracellular matrix. Indeed, its importance in basement membrane assembly has been recently shown and a putative role in epithelial branching morphogenesis of developing organs suggested (22).
We next attempted to identify the molecule(s) responsible for the anchoring of the netrin-4/laminin γ1 chain complex on the surface of NSCs. Cell interaction with laminin is primarily mediated by integrins, which are central molecules in cell adhesion and proliferation. To date, 24 integrin heterodimers have been identified in vertebrates (24). Among these, α6β1, α3β1, α7β1, α2β1, and α6β4 (25) are the major known laminin-binding integrins.
We analyzed the targeted peptide-eluted fractions for these integrins. ELISA-based experiments revealed that both the α6 and β1 integrin subunits were present in high concentrations in F36 and F37 (Fig. 2 D and E). Immunoblotting with specific antibodies confirmed the identity of these subunits (Fig. 2 F and G). Subunits α1, α2, α3, α7, and β4 were not found in any of the analyzed fractions (data not shown). Furthermore, molecular imaging showed colocalization of α6β1 integrin and laminin γ1 chain on cells activated with netrin-4 but not with netrin-1 (Fig. S4). Collectively, these results establish that netrin-4 binds to the laminin γ1 chain and that this molecular complex further interacts with α6β1 integrin on the surface of NSCs.
The Ternary Protein Complex Netrin-4/Laminin γ1 Chain/α6β1 Integrin Is Functional in NSCs.
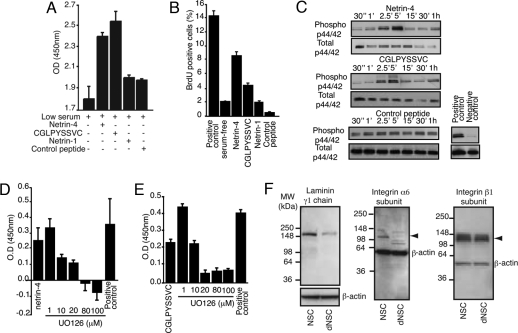
Integrins are major regulatory molecules in signal induction and modulation. Integrin-mediated cell activation affects cell adhesion, spreading, migration, and proliferation (24). We used adhesion, migration, and cell proliferation assays to access mechanisms by which α6β1 integrin cooperates with netrin-4 and its partner laminin γ1 chain to affect NSC behavior. We found an enhanced proliferative response by the C17.2 NSC (Fig. 3 A and B) and by primary neural progenitors isolated from the adult mouse brain (Fig. S5 A and B) when either netrin-4 or CGLPYSSVC peptide were added to the culture medium. As ERK1/2 phosphorylation has been linked to integrin-mediated cell proliferation (26), we investigated the effect of netrin-4 and the CGLPYSSVC peptide in the activation of the MAP kinase pathway. C17.2 NSCs were treated with either netrin-4 or synthetic peptide and protein phosphorylation was analyzed by (i) immunoblotting (Fig. 3C) and (ii) immunofluorescence (Fig. S5C). We show in Fig. 3C specific phosphorylation of ERK1/2 at 2.5 and 5 min after cell activation with both netrin-4 and CGLPYSSVC peptide. In contrast, an unrelated control peptide did not induce ERK1/2 phosphorylation. Moreover, treatment of NSCs with UO126 (a specific inhibitor of the MAP kinase pathway) before addition of netrin-4 (Fig. 3D) or peptide (Fig. 3E) prevented cell proliferation.
Fig. 3.
Netrin-4 affects proliferation, adhesion, and migration of NSCs in vitro. (A) Both netrin-4 and the peptide CLPGYSSVC induce NSC proliferation. (B) Incorporation of bromodeoxyuridine (BrdU) by proliferating NSCs. Experiments were performed in serum-free medium containing recombinant proteins and peptides as indicated. We used medium containing 10% FCS as a positive control. (C) Phosphorylation of ERK1/2 was investigated in different time points after cell activation. Total p44/42 was used as loading control. (D) Increasing concentrations of UO126 specifically inhibit the proliferative effect of netrin-4 (D) and CLPGYSSVC peptide (E). (F) The laminin γ1 chain and α6 integrin subunit are downregulated after NSC differentiation. Immunoblots were developed with anti-laminin γ1 chain (Left), anti-α6 integrin subunit (Middle), and anti-β1 integrin subunit antibodies (Right). Anti-β-actin antibody was used as loading control.
Because stem cell proliferation and differentiation are tightly regulated events, we sought to determine whether expression and activity of the netrin-4/laminin γ1 chain/α6β1 integrin complex would be modified during NSC differentiation. A mixed population of cells composed of neurons, oligodendrocytes, astrocytes, and progenitor cells was obtained by acutely arresting proliferation of the C17.2 NSCs with mitomycin-C, while maintaining a culture medium supportive of all differentiated and undifferentiated neural cell types (20, 27) (Fig. S6).
We next asked whether netrin-4 would differentially affect the adhesion and migration of proliferating and differentiated C17.2 NSCs. Netrin-4 induced adhesion and spreading of NSCs (Fig. S7A) and primary progenitors (Fig. S7B). Moreover, the presence of soluble netrin-4 in the culture media increased the adhesion of NSCs to laminin by 60%, indicating a synergistic effect of laminin and netrin-4. However, when a mixed population of differentiated C17.2 NSCs was assayed for its capacity to adhere to netrin-4, we observed a complete abrogation of adhesion of differentiated NSCs to netrin-4 and a marked increase in cell adhesion to netrin-1 (≈60%). Accordingly, addition of specific antibodies against netrin-4, α6 integrin subunit, or laminin γ1 chain reduced cell adhesion by ≈70, 50, and 40%, respectively (Fig. S7C), confirming that the attachment of NSCs to netrin-4 is mediated by the ternary complex netrin-4/laminin γ1 subunit/α6β1 integrin.
In addition, we examined the promigratory activity of netrin-4. Netrin-4 and all control proteins induced migration of C17.2 NSCs (Fig. S7D) and primary progenitor cells (Fig. S7E). Again, the presence of netrin-4 in the shared media increased the migratory activity of undifferentiated NSCs toward laminin, a result not observed with differentiated NSCs. The latter migrated toward neither netrin-4 nor netrin-1, but responded to fibronectin as a chemoattractant (Fig. S7D).
We reasoned that alterations in receptor expression after NSC differentiation would likely be responsible for the decrease in adhesion of differentiated NSCs to netrin-4. This is indeed the case; immunoblotting revealed a marked reduction in expression of the laminin γ1 chain (Fig. 3F Left) and the α6 integrin subunit (Fig. 3F Middle) in differentiated NSCs, in comparison to the undifferentiated cell population. As expected, we did not detect alterations in the expression levels of the β1 integrin subunit (Fig. 3E Right). Consistently, we also observed a decrease in phage binding and internalization in differentiated NSCs (Fig. S7F). These results, while not quantitative, confirm the downregulation of targeted receptors and the specificity of phage binding.
Taken together, we have shown that the protein complex netrin-4/laminin γ1 chain/α6β1 integrin promotes NSC proliferation, adhesion, and migration in vitro and that downregulation of the netrin-4 receptors occurs after NSC differentiation.
NSCs Produce a Variant Form of Laminin.
Laminin is thought to be involved in the development of the brain (28) and in the regulation of NSC migration, expansion, and differentiation into neurons and astrocytes (29). Glial cells of mammalian brain produce a variant form of laminin composed only of β and γ chains (30). In addition, 4- to 8-cell embryos synthesize only β and γ chains and fertilized eggs appear to produce only the β chain (31). Evidently, the function of laminin in cell–cell interaction at early stages of embryogenesis may depend upon the expression of the β and γ chains only.
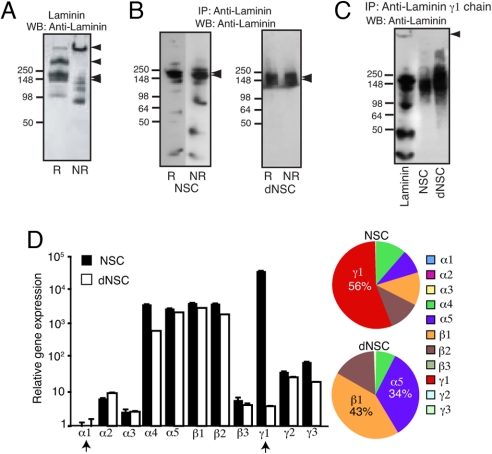
Therefore, we sought to determine the molecular composition of the laminin produced by NSCs. Laminin-1 and laminin γ1 chain were immunoprecipitated from NSCs and differentiated NSC lysates (Fig. 4). Purified laminin-1 solubilized in the same lysis buffer served as a reference (Fig. 4A). Under nonreducing conditions, laminin migrated essentially as a high-molecular-weight band after SDS/PAGE (Fig. 4A). When reduced, two major bands were observed: the β and γ chains, each of ≈220 kDa molecular weight, and the α chain (≈440 kDa) (Fig. 4A). Analysis of proteins immunoprecipitated by anti-laminin-1 antibody showed that both proliferating and differentiated NSCs produced only the 220-kDa major band (Fig. 4B) whereas the 440-kDa subunit and the full laminin-1 molecule were absent. Immunoprecipitation with anti-laminin γ1 chain antibody produced similar results (Fig. 4C). Next, we used quantitative real-time PCR to study the gene expression profile of all of the laminin chains in NSCs before and after differentiation (Fig. 4D). We confirmed that neither proliferating nor differentiated NSCs produced the α1 chain, the laminin subunit required for the assembly of laminin-1 and -3 (32). Moreover, we observed a drastic reduction in mRNA levels of the γ1 chain after cell differentiation, as previously demonstrated at the protein level (Fig. 3F). Analysis of the relative number of gene copies for each of the tested genes revealed that the γ1 chain represented 56% of all of the chains produced by NSCs. After cell differentiation, α5 and β1 chains were the major chains produced.
Fig. 4.
NSC and differentiated NSC produce different laminin chains. (A) Solubilized laminin-1 was resolved on 4–20% SDS/PAGE under reducing (R) and nonreducing (NR) conditions. (Left) Arrowheads point to the intact laminin-1 trimer (≈900 kDa), the α chain (400 kDa), and the γ and β chains (≈200 kDa). (B) Immunoprecipitation of laminin produced by NSCs (Left) and differentiated NSCs (Right). Only chains of 220 kDa were observed, indicated by the arrowheads. (C) An anti-laminin γ1 chain antibody precipitates laminin and its individual chains from a purified laminin solution, whereas only the γ and β chains were precipitated from NSCs and differentiated NSC lysates. (D) Quantitative real-time PCR measurement of laminin subunits. Expression was normalized such that the average expression of the less abundant gene copies (α chains) was set to 1.
Together, these results show that a chain switch accompanies NSC differentiation and reinforce the importance of the γ1 chain in NSC biology. The consequences of such alterations in gene expression are to be determined, but some aspects merit further consideration. First, it is not clear whether NSCs produce and secrete the laminin γ1 chain as a single protein, for it is generally accepted that α chains are the only laminin subunits secreted in the absence of the other chains (32). Second, one could speculate that other laminins carrying the γ1 chain (such as laminin-4, -6, -7, -8, -9, -10, and -11) may undergo degradation upon secretion and thereby release the γ1 chain for association with other proteins. Third, one cannot rule out the possibility that the γ1 chain may associate with netrin-4 as part of an intact laminin. Further experiments will be required to gain a deeper understanding of the roles of laminins in the behavior of NSCs.
Netrin-4 Expression in the OB of the Adult Mouse Brain.
In our in vitro studies, we demonstrated that netrin-4 acts together with laminin γ1 chain and α6β1 integrin to coordinate NSC behavior. Moreover, the fact that netrin-4 receptors are diminished after NSC differentiation indicates that such a mechanism might be relevant in neurogenesis. As the generation of new progenitor cells in the normal adult brain occurs only in 3 germinal regions—the widely recognized subventricular zone of the lateral ventricle (SVZ), the less widely recognized anterior part of the RMS approaching the OB, and the subgranular zone of the dentate gyrus of the hippocampal formation (SGZ)—we used histological approaches to investigate netrin-4 mRNA and protein expression in these specific areas of the mouse brain (Fig. S8 and Fig. 5).
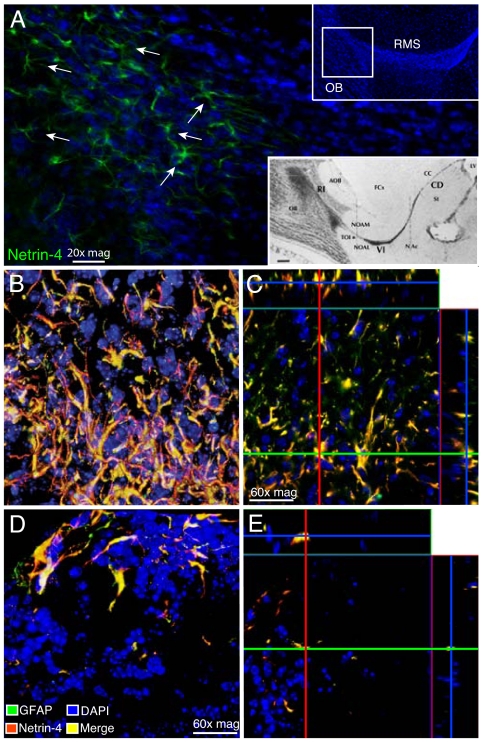
Fig. 5.
Expression of netrin-4 in the OB of adult mouse brain. (A) Netrin-4 is expressed most strongly by astrocytes at the base of the OB (location indicated in the square within the Inset at the Upper Right). Arrows point to astrocyte-like cells (green). The location of the netrin-4-expressing astrocytes is identified with respect to the regional anatomy in the Inset at the Lower Right, slightly modified from Fig. 1 of Jankovski and Sotelo (41). Labels of the 3 zones, from caudal to rostral: CD, caudodorsal; VI, ventral intermediate; and RI, rostral intrabulbar. Labels around the RMS: AOB, accessory olfactory bulb; CC, corpus callosum; FCx, frontal cortex; NAc, nucleus accumbens septi; NOAL, nucleus olfactorius anterior pars lateralis; NOAM, nucleus olfactorius anterior pars medialis; St, nucleus caudatum/putamen (striatum); TOI, tractus olfactorius intermedius; and (*), position of netrin-4-expressing astrocytes. (B and C) Double-label immunofluorescence of netrin-4-expressing astrocytes viewed by confocal microscopy. Yellow represents points of colocalization of netrin-4 (red) and GFAP (green). DAPI (blue) was used for staining of cell nuclei. Red and green lines (C) indicate points in the orthogonal planes, demonstrating that netrin-4 is present in the cytoplasm and at the surface of GFAP-positive astrocytes. (D and E) We also detected netrin-4-positive astrocytes in the glomerular layer of the OB.
We searched the Allen Brain Atlas (33), a digital atlas of gene expression, for sites of netrin-4 expression in the adult C57BL/6 mouse brain and extracted data indicating high levels of netrin-4 mRNA in the OB (Fig. S8A). Other areas where netrin-4 mRNA is detectable include the medulla, cerebellum, zona incerta, cerebral cortex, and hippocampal formation, including subiculum (data not shown). The Allen Brain Atlas also shows netrin-4 mRNA in mitral cell bodies and small cells in the inner granular layer of the OB, in cells within the adult stem cell niche in the anterior wall of the lateral ventricle, and in the granular layer of the cerebellar cortex. Further analysis demonstrated colocalization of the α6 integrin subunit and netrin-4 mRNAs in the OB (Fig. S8B), suggesting that α6 integrin subunit/netrin-4 protein interactions likely occur in this region.
Our analysis of serial sagittal sections of adult mouse brain revealed that netrin-4 is produced by astrocytes located mainly along the border of the RMS at and near its entrance into the OB, close to the (arbitrary) boundary between the Jankovski/Sotelo ventral intermediate (VI) and rostral intrabulbar (RI) segments (Fig. 5A). Colocalization of netrin-4 and GFAP (Fig. 5B) and delineating their characteristic size and “star-like” cell shape identified astrocytes as the netrin-4-producing cells. Confocal imaging of double-stained sections showed colocalization of GFAP and netrin-4 in the cell body and cytoplasmic processes of the astrocytes and additional netrin-4 at or close to the cell surface (Fig. 5C). These netrin-4-positive astrocytes lie close to the neuron precursors and young neurons in the anterior part of the RMS, destined mainly for the glomerular layer of the OB (34, 35), where they continuously increase the proportion of newly generated glomerular layer interneurons throughout adult life. Netrin-4-positive astrocytes were also found among the granule cell neurons within the glomerular layer of the OB (Fig. 5 D and E). We observed that not every GFAP-positive astrocyte was stained for netrin-4 (Fig. S9 A–C) and that the location of double-stained astrocytes varied within the OB from mouse to mouse. The reasons for this variability are yet to be determined but might include waves of astrocytic activation across successive glomeruli, among other nonmutually exclusive possibilities.
Of note, we have also analyzed the expression of netrin-4 in the SVZ of the adult mouse brain (Fig. S9 D–G), a recognized source of new neurons destined to migrate in the RMS to become predominantly GABAergic granule cell neurons of the OB. Immunofluorescence and confocal microscopy studies demonstrated that netrin-4 is not expressed in GFAP+ or PDGFRα+/GFAP+ type B cells of the adult brainstem cell niche in the wall of the lateral ventricle (LV) (Fig. S9 D–G), a result that strongly supports our original hypothesis that netrin-4 production is restricted to astrocytes located in the OB and along the border of the anterior part of the RMS in adult mice. It remains to be established whether the difference in netrin-4 distribution between the area immediately ventral to the anterior part of the RMS plus the glomerular layer of the OB, on the one hand, and the SVZ bordering the LV, on the other hand, is a determinant of the different terminal sites in the OB for neurons arising from these 2 germinal sources and of their very different rates of turnover within the bulb. Another possibility is that the netrin-4-expressing astrocytes have a role in restricting young neurons to the migration path into the OB.
In conclusion, our data suggest netrin-4 as a new regulator of neurogenesis in the adult brain and define the astrocyte as one cell type that synthesizes netrin-4 and possibly releases it to closely related NSCs or their progeny. Astrocytes in germinal layers of the adult brain, classified as type B cells, can directly function as neurogenic stem cells (36). In the olfactory system, astrocytic function may include tissue remodeling associated with mitosis and migration of other cells. Astrocyte production of laminin and other extracellular matrix components such as tenascin-C and chondroitin sulfate has been demonstrated under both normal and pathological conditions (29, 37). To add further levels of complexity and diversity, there appear to be multiple subsets of GFAP-expressing astrocytes in the olfactory system, distinguished by whether or not they express surface markers such as vimentin and nestin (38, 39). The subset of astrocytes that we have shown to contain netrin-4 may be involved in multiplication and migration of those neuron precursors, reviewed by Ihrie and Alvarez-Buylla (39), destined for the glomerular layer of the OB and arising not in the SVZ of the lateral ventricle, but in the less widely recognized prebulbar sector of the RMS.
All one can state securely at present is that the presence of netrin-4 in the OB, along with the functional description of the netrin-4/laminin γ1 chain/α6β1 integrin complex at the protein and cellular levels, establishes a unique molecular relationship capable of affecting neural cell behavior. While it remains to be determined whether these observations will translate also to the human brain (40), this work provides a working model framework for further studies.
Materials and Methods
Refer to SI Text for details.
NSCs.
The clone C17.2 was originally generated by enhanced expression of the self-renewal-promoting stemness gene myc in proliferating NSCs isolated from neonatal mouse cerebellum (19). Myc was enhanced to preserve multipotency, self-renewal, and the undifferentiated state in vitro but is nevertheless constitutively self-regulated upon contact, engraftment, and/or differentiation. These engraftable cells remain stable through multiple passages without losing multipotency, self-renewal capacity, engraftability, or the ability to differentiate appropriately and nontumorigenically in response to temporal and spatial developmental cues. They also can be serially transplanted (27).
Culture Method.
The NSCs from clone C17.2 were cultured as monolayers in medium containing 83% DMEM and 0.11 g/L pyruvate with pyridoxine (Invitrogen), 10% FCS (Sigma), 5% horse serum (Invitrogen), 1% L-glutamine (200 mM) (Invitrogen), and 1% penicillin G/streptomycin SO4/fucidin (Invitrogen). Cells were passaged by trypsin treatment once per week at a maximun dilution of 1:10.
Supplementary Material
Acknowledgments.
We thank Drs. Ricardo R. Brentani and E. Helene Sage for critical reading of the manuscript, Dr. Glauco R. Souza for assistance with peptide modeling, and Michael G. Ozawa for help with confocal microscopy. This work was supported by grants from the National Institutes of Health and the Department of Defense and by awards from the Gillson–Longenbaugh Foundation (to R.P. and W.A.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813286106/DCSupplemental.
References
- 1.Serafini T, et al. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 2.Srinivasan K, Strickland P, Valdes A, Shin GC, Hinck L. Netrin-1/neogenin interaction stabilizes multipotent progenitor cap cells during mammary gland morphogenesis. Dev Cell. 2003;4:371–382. doi: 10.1016/s1534-5807(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 3.Yebra M, et al. Recognition of the neural chemoattractant netrin-1 by integrins alpha6beta4 and alpha3beta1 regulates epithelial cell adhesion and migration. Dev Cell. 2003;5:695–707. doi: 10.1016/s1534-5807(03)00330-7. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, et al. Novel role for netrins in regulating epithelial behavior during lung branching morphogenesis. Curr Biol. 2004;14:897–905. doi: 10.1016/j.cub.2004.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eichmann A, Le Noble F, Autiero M, Carmeliet P. Guidance of vascular and neural network formation. Curr Opin Neurobiol. 2005;15:108–115. doi: 10.1016/j.conb.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Park KW, et al. The axonal attractant netrin-1 is an angiogenic factor. Proc Natl Acad Sci USA. 2004;101:16210–16215. doi: 10.1073/pnas.0405984101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu X, et al. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432:179–186. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- 8.Lemji E, et al. Netrin-4 inhibits angiogenesis via binding to neogenin and recruitment of Unc5B. Proc Natl Acad Sci USA. 2008;105:12491–12496. doi: 10.1073/pnas.0804008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koch M, et al. A novel member of the netrin family, beta-netrin, shares homology with the beta chain of laminin: identification, expression, and functional characterization. J Cell Biol. 2000;151:221–234. doi: 10.1083/jcb.151.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin Y, Sanes JR, Miner JH. Identification and expression of mouse netrin-4. Mech Dev. 2000;96:115–119. doi: 10.1016/s0925-4773(00)00369-5. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez-Bolado G, Eichele G. Analysing the developing brain transcriptome with the GenePaint platform. J Physiol. 2006;575:347–352. doi: 10.1113/jphysiol.2006.112763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikolopoulos SN, Giancotti FG. Netrin-integrin signaling in epithelial morphogenesis, axon guidance and vascular patterning. Cell Cycle. 2005;4:131–135. [PubMed] [Google Scholar]
- 13.Liu G, et al. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat Neurosci. 2004;7:1222–1232. doi: 10.1038/nn1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cirulli V, Yebra M. Netrins: beyond the brain. Nat Rev Mol Cell Biol. 2007;8:296–306. doi: 10.1038/nrm2142. [DOI] [PubMed] [Google Scholar]
- 15.Wilson BD, et al. Netrins promote developmental and therapeutic angiogenesis. Science. 2006;313:640–644. doi: 10.1126/science.1124704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolonin MG, et al. Ligand-directed surface profiling of human cancer cells with combinatorial peptide libraries. Cancer Res. 2006;66:34–40. doi: 10.1158/0008-5472.CAN-05-2748. [DOI] [PubMed] [Google Scholar]
- 17.Arap W, et al. Steps toward mapping the human vasculature by phage display. Nat Med. 2002;8:121–127. doi: 10.1038/nm0202-121. [DOI] [PubMed] [Google Scholar]
- 18.Giordano RJ, Cardó-Vila M, Lahdenranta J, Pasqualini R, Arap W. Biopanning and rapid analysis of selective interactive ligands. Nat Med. 2001;7:1249–1253. doi: 10.1038/nm1101-1249. [DOI] [PubMed] [Google Scholar]
- 19.Snyder EY, et al. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell. 1992;68:33–51. doi: 10.1016/0092-8674(92)90204-p. [DOI] [PubMed] [Google Scholar]
- 20.Parker MA, et al. Expression profile of an operationally-defined neural stem cell clone. Exp Neurol. 2005;194:320–332. doi: 10.1016/j.expneurol.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Arakawa H. Netrin-1 and its receptors in tumorigenesis. Nat Rev Cancer. 2004;4:978–987. doi: 10.1038/nrc1504. [DOI] [PubMed] [Google Scholar]
- 22.Schneiders FI, et al. Binding of netrin-4 to laminin short arms regulates basement membrane assembly. J Biol Chem. 2007;282:23750–23758. doi: 10.1074/jbc.M703137200. [DOI] [PubMed] [Google Scholar]
- 23.Yurchenco PD, Wadsworth WG. Assembly and tissue functions of early embryonic laminins and netrins. Curr Opin Cell Biol. 2004;16:572–579. doi: 10.1016/j.ceb.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 24.ffrench-Constant C, Colognato H. Integrins: versatile integrators of extracellular signals. Trends Cell Biol. 2004;14:678–686. doi: 10.1016/j.tcb.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Belkin AM, Stepp MA. Integrins as receptors for laminins. Microsc Res Tech. 2000;51:280–301. doi: 10.1002/1097-0029(20001101)51:3<280::AID-JEMT7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 26.Hunter T, Eckhart W. The discovery of tyrosine phosphorylation: it's all in the buffer! Cell. 2004;116:35–39. doi: 10.1016/s0092-8674(04)00049-2. [DOI] [PubMed] [Google Scholar]
- 27.Snyder EY, Yoon C, Flax JD, Macklis JD. Multipotent neural precursors can differentiate toward replacement of neurons undergoing targeted apoptotic degeneration in adult mouse neocortex. Proc Natl Acad Sci USA. 1997;94:11663–11668. doi: 10.1073/pnas.94.21.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- 29.Liesi P, Dahl D, Vaheri A. Laminin is produced by early rat astrocytes in primary culture. J Cell Biol. 1983;96:920–924. doi: 10.1083/jcb.96.3.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liesi P, Risteli L. Glial cells of mammalian brain produce a variant form of laminin. Exp Neurol. 1989;105:86–92. doi: 10.1016/0014-4886(89)90175-1. [DOI] [PubMed] [Google Scholar]
- 31.Cooper AR, MacQueen HA. Subunits of laminin are differentially synthesized in mouse eggs and early embryos. Dev Biol. 1983;96:467–471. doi: 10.1016/0012-1606(83)90183-5. [DOI] [PubMed] [Google Scholar]
- 32.Yurchenco PD, et al. The alpha chain of laminin-1 is independently secreted and drives secretion of its beta- and gamma-chain partners. Proc Natl Acad Sci USA. 1997;94:10189–10194. doi: 10.1073/pnas.94.19.10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 34.Hack MA, et al. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 2005;8:865–872. doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- 35.Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- 36.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 37.Brodkey JA, et al. Focal brain injury and upregulation of a developmentally regulated extracellular matrix protein. J Neurosurg. 1995;82:106–112. doi: 10.3171/jns.1995.82.1.0106. [DOI] [PubMed] [Google Scholar]
- 38.Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 39.Ihrie RA, Alvarez-Buylla A. Cells in the astroglial lineage are neural stem cells. Cell Tissue Res. 2008;331:179–191. doi: 10.1007/s00441-007-0461-z. [DOI] [PubMed] [Google Scholar]
- 40.Curtis MA, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 41.Jankovski A, Sotelo C. Subventricular zone-olfactory bulb migratory pathway in the adult mouse: cellular composition and specificity as determined by heterochronic and heterotopic transplantation. J Comp Neurol. 1996;371:376–396. doi: 10.1002/(SICI)1096-9861(19960729)371:3<376::AID-CNE3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.