Abstract
D-serine is a physiologic coagonist with glutamate at NMDA-subtype glutamate receptors. As D-serine is localized in glia, synaptically released glutamate presumably stimulates the glia to form and release D-serine, enabling glutamate/D-serine cotransmission. We show that serine racemase (SR), which generates D-serine from L-serine, is physiologically inhibited by phosphatidylinositol (4,5)-bisphosphate (PIP2) presence in membranes where SR is localized. Activation of metabotropic glutamate receptors (mGluR5) on glia leads to phospholipase C-mediated degradation of PIP2, relieving SR inhibition. Thus mutants of SR that cannot bind PIP2 lose their membrane localizations and display a 4-fold enhancement of catalytic activity. Moreover, mGluR5 activation of SR activity is abolished by inhibiting phospholipase C.
Keywords: D-serine; metabotropic glutamate receptor; NMDA transmission; phosphatidylinositol (4,5); bisphosphate
D-serine is an endogenous physiologic agonist of glutamate-NMDA receptors acting at a site that is also targeted by glycine (1–4). Thus, depletion of D-serine but not glycine by treatment with D-amino acid oxidase (5) or D-serine deaminase (6) selectively abolishes NMDA neurotransmission. Hormonally altered astrocytic coverage of hypothalamic neurons regulates NMDA transmission in a D-serine-dependent fashion (7). Mice with targeted deletion of serine racemase (SR), the enzyme that physiologically generates D-serine from L-serine, display altered NMDA transmission (8). SR and D-serine were first identified in glia (9) but also occur in neurons (10). SR is regulated by multiple factors. It requires pyridoxal phosphate and also has an absolute requirement for ATP that is not hydrolyzed during SR activation (11). SR binds to glutamate receptor interacting protein (GRIP), which also binds to AMPA-glutamate receptors (12). GRIP activates SR, enabling AMPA neurotransmission to impact D-serine formation and release. SR is also regulated by nitric oxide (NO) (13). Specifically, SR is physiologically S-nitrosylated, which inhibits catalytic activity via competition with ATP. NMDA transmission stimulates S-nitrosylation of SR, providing inhibitory feedback on presynaptic formation of D-serine.
Because D-serine functions as a coagonist with glutamate, we have conceptualized that released glutamate activates D-serine formation and release, leading to coordinate action of the 2 agonists at postsynaptic NMDA receptors. AMPA neurotransmission, via GRIP, presumably activates SR and D-serine release in neurons in which GRIP and AMPA receptors are primarily localized. Although AMPA receptors have been detected in cultured type II astrocytes (14), mammalian glia typically lack functional AMPA receptors (15). However, glia do possess metabotropic glutamate receptors that signal by activating phospholipase C (PLC) (15, 16). In the present study we show that glial SR, associated with cell membranes in presumed proximity to phospholipids such as phosphatidylinositol (4, 5)-bisphosphate (PIP2), binds selectively to PIP2, which physiologically inhibits the enzyme by competing for ATP. Metabotropic glutamate transmission markedly stimulates SR activity by relieving the PIP2 inhibition.
Results
SR Binds to Phospholipids.
Dumin and associates (17) reported localizations of SR to membranous structures in intact rat brain. We have examined the intracellular localization of SR in intact glia from primary cultures of mouse cerebral cortex (Fig. 1A and SI Materials and Methods). SR is prominently associated with the plasma membrane but also is detected in granular structures that may be vesicles associated with the plasma membrane or other cellular components. Membrane associations of SR might reflect interactions with phospholipids.
Fig. 1.
SR binds to phospholipids. (A) Immunocytochemistry of SR in mouse primary culture glia is consistent with localization to the plasma membrane and small granular structures that may be vesicles. SR is highlighted in green while the glial marker, glial fibrillary acidic protein (GFAP), is shown in red. (B) PIP strip assays with purified His-SR reveal prominent binding to a variety of lipids. Singly phosphorylated PIPs provide the most prominent binding with somewhat lesser binding for various forms of PIP2 and least for PIP3. PtdIns, phosphatidylinositol; PE, phosphatidylethanolamine; PC, phosphatidylcholine; PA, phosphatidic acid; and PS, phosphatidylserine. (C) Liposomal assays in vitro with His-SR give similar findings as in B. PI, phosphatidylinositol. (D) PolyPIPosome assays in HEK293 cells transfected with myc-tagged SR show greatest binding to PI(4,5)P2. (E) Similar results as in D are seen in intact mouse brain. Akt, a known interactor of lipids, is used as a positive control. (F) Fluorescence anisotropy assays with pure SR and BODIPY-TMR-labeled PIP2 reveal specific interactions between SR and PIP2 with a Kd of ≈1 μM. Boiled SR does not interact with PIP2. (G) Fluorescence anisotropy of the PH domain of PLCδ1 provides a Kd of ≈0.25 μM, similar to values for SR. Unlabeled PIP2 potently displaces binding with a Ki of 0.1 μM. Bars represent the mean ± SEM of 3 independent experiments each performed in triplicate.
We examined binding of SR to a variety of lipids in vitro on PIP strips (Fig. 1B). Binding is evident only for phosphorylated PIPs. The most prominent binding occurs for singly phosphorylated PIPs with somewhat lesser binding for various forms of PIP2 and least for PI(3,4,5)P3 (PIP3). We obtain similar results in liposomes in vitro with PI(4)P displaying the greatest binding to SR followed by PI(4,5)P2 and lesser binding for phosphatidic acid (PA) and PI (Fig. 1C).
Lipid binding is also detectable in cell lysates. In HEK293 cell lysates the binding of PI(4,5)P2, the principal physiologic form of PIP2, exceeds that of PIP3 (Fig. 1D). Similarly, in lysates of mouse brain PIP2 binds to SR more robustly than PIP3 (Fig. 1E).
We used real-time fluorescence anisotropy to quantitate the affinity of SR-PIP2 binding (Fig. 1 F and G). We observe saturable binding of SR to PIP2 with a dissociation constant (Kd) of ≈1 μM, a much smaller value than the estimated physiologic cellular PIP2 concentration of 10 μM (18). To evaluate the significance of this binding pattern, we examined binding to PIP2 of the Pleckstrin Homology (PH) domain of PLCδ1 (Fig. 1G), the most robust known interactor of PIP2 with a reported Kd of ≈1 μM (19). Our fluorescence anisotropy measurements reveal a comparable binding affinity with a Kd of ≈0.25 μM, similar to values for SR binding. Unlabeled PIP2 potently displaces binding with a Ki value of 0.1 μM.
PIP2 Inhibits SR.
We examined the influence of a variety of phospholipids upon the catalytic activity of purified SR, assaying the generation of both D-serine and pyruvate (13). In comparing mixtures of phosphorylated PIPs with other phospholipids, we observe the greatest inhibition with the PIP mixture (Fig. 2A). Phosphatidic acid provides modest inhibition, while the other lipids are ineffective. Among the various PIPs we observe the greatest inhibition with the two forms of PIP2, PI(4,5)P2 and PI(3,5)P2 (Fig. 2B). The head groups of the lipids do not appear to be sufficient for SR inhibition, as various inositol phosphates fail to influence SR activity (Fig. 2C). Inhibition of SR by PIP2 is concentration dependent with an IC50 of ≈13 μM (Fig. 2D).
Fig. 2.
PIP2 inhibits SR. (A) Assays of SR in the presence of 50-μM lipids reveal specific inhibition by a mixture of PIPs, while PA moderately inhibits SR. 1-stearoyl-2-arachidonoyl-sn-glycerol (SAG) and 1-oleoyl-2-acetyl-sn-glycerol (OAG) are diacylglycerol derivatives. (B) SR is markedly inhibited by 50 μM PIP2 and PIP3. The singly phosphorylated PIs do not significantly impact activity. (C) Inositol phosphate head groups lacking the hydrophobic fatty acid component do not inhibit SR. (D) PI(4,5)P2 concentration dependently inhibits SR with an IC50 of 13 μM. Bars represent the mean ± SEM of 3 independent experiments each performed in triplicate.
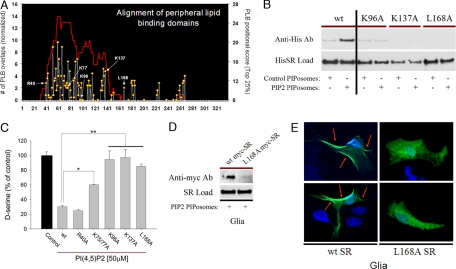
To ascertain regions of SR that might be responsible for PIP2 binding, we used the gestalt domain detection algorithm–basic local alignment tool (GDDA-BLAST) (20–23). We detect a robust signal for peripheral lipid-binding domains within SR (Fig. 3A). Specific surface residues that appear to be important include K137 and L168. We examined the binding to PIP2 of SR with selective mutations (Fig. 3B). PIP2 binding is almost completely lost with SR-K96A, SR-K137A, and SR-L168A. We explored the influence of these mutations on PIP2 inhibition of SR activity (Fig. 3C). PIP2 (50 μM) inhibits catalytic activity of wild-type SR ≈70%, while similar inhibition occurs for SR-R40A. SR-K96A, SR-K137A, and SR-L168A are almost totally resistant to inhibition by PIP2 while SR-K75/77A is partially resistant.
Fig. 3.
Mutation of specific SR residues prevents its inhibition via PIP2 binding. (A) GDDA-BLAST analysis reveals a hidden peripheral lipid-binding (PLB) domain within the SR sequence. Specific potential lipid interacting surface residues are also identified. (B) PIP2 PolyPIPosome assay with pure wild-type vs. mutant SR proteins reveals K96, K137, and L168 as potential lipid binding residues, because their mutation to alanine hampers interaction with PIP2. (C) SR activity assays with or without PIP2 show that SR-K96A, K137A, and L168A are resistant to PIP2 inhibition. All 3 mutants are basally as active as the wild-type protein. (D) Mouse primary culture glia transfected with myc-tagged wild-type SR or the L168A mutant demonstrate a requirement of L168 for interaction with PIP2 in glia, as L168A does not coprecipitate with PIP2 PolyPIPosomes. (E) In intact primary glia the nonlipid interacting myc-tagged L168A mutant SR has a diffuse cytosolic distribution, whereas the wild-type protein is highly concentrated at lipid membranes, particularly the plasma membrane (arrows). SR is represented in green, while the nuclear stain, DAPI, is shown in blue. Bars represent the mean ± SEM of 3 independent experiments each performed in triplicate.
We examined the influence of the L168A mutation of SR on binding to PIP2 and localization of SR in primary cultures of glia. In these glial cell lysates SR-L168A fails to bind to PIP2 (Fig. 3D). Immunocytochemical analysis reveals SR-L168A diffusely distributed within the cytosol, contrasting with wild-type SR, which is highly concentrated at lipid membranes, particularly the plasma membrane (Fig. 3E). Thus, binding of SR to PIP2 in the membranes is critical for its physiologic localization.
PIP2 Inhibits SR by Competing with ATP.
Structural modeling of SR reveals that those amino acids whose mutation abolishes PIP2 inhibition are located in the vicinity of the ATP binding site of SR (Fig. 4A). Accordingly, we examined interactions between PIP2, ATP, and SR.
Fig. 4.
PIP2 inhibits SR by competing with ATP. (A) Model of mammalian SR showing ATP in yellow, magnesium ion in black, leucine 168 in pink, and lysines 75, 77, 96, and 137 in green. The PIP2 interacting residues are adjacent to the ATP binding site on SR, presumably interfering with the activation of SR by ATP. (B) Fluorescence anisotropy reveals that the binding of SR to PIP2 is competitively reversed by ATP in a concentration-dependent fashion. (C) Liposomal assays also show that ATP reverses the SR–PIP2 interaction in a concentration-dependent manner. (D) Pure His-SR enzyme activity was measured in the presence or absence of PIP2 and increasing concentrations of ATP. PIP2 inhibits SR activity by interfering with ATP, as PIP2 causes a 100-fold increase in the EC50 for ATP with no change in maximal activation. (E) SR enzyme activity measured in the presence or absence of PIP2 and increasing L-serine concentration displays a substantial reduction in Vmax with no alteration in Km. Hence, PIP2 inhibits SR noncompetitively. Bars represent the mean ± SEM of 3 independent experiments each performed in triplicate.
In fluorescence anisotropy experiments, binding of SR to PIP2 is competitively relieved by ATP in a concentration-dependent fashion (Fig. 4B). Similarly, in liposomal assays ATP inhibits PIP2 binding to SR in a concentration-dependent manner (Fig. 4C).
We monitored the influence of PIP2 on activation of SR by ATP (Fig. 4D). In untreated preparations ATP stimulates SR activity with an EC50 of ≈9 μM. PIP2 elicits a rightward parallel shift of the concentration-response curve consistent with competitive inhibition. By contrast, concentration-response experiments with L-serine reveal noncompetitive interactions between PIP2 and L-serine (Fig. 4E).
Metabotropic Glutamate Activation Stimulates SR by Reversing PIP2 Inhibition.
Our characterization of PIP2 disposition indicates that it is a physiologic inhibitor of SR. Metabotropic glutamate transmission via the metabotropic glutamate receptor (mGluR5) activates PLC. We wondered whether mGluR5 stimulation influences SR activity by activating PLC to degrade the PIP2 that normally inhibits the enzyme. In HEK293 cells with overexpressed SR and mGluR5, the mGluR agonist dihydroxyphenylglycine (DHPG) augments SR activity 4-fold [Fig. 5A and supporting information (SI) Fig. S1]. This effect reflects stimulation by DHPG of mGluR5, as HEK293 cells without overexpressed mGluR5 do not respond to DHPG (data not shown). The activation of SR is attributable to degradation of PIP2, as it is prevented by treatment with the PLC inhibitor U73122.
Fig. 5.
Metabotropic glutamate receptor activation stimulates SR by reversing PIP2 inhibition. (A) HEK293 cells transfected with wild-type SR and mGluR5 display a 4-fold increase in SR activity upon treatment with 50 μM of the mGluR agonist DHPG for 30 min. The PLC inhibitor U73122 (10 μM) reverses mGluR5-mediated activation of SR. The non-PIP2 binding mutant L168A is 4 times more active basally than the wild-type enzyme. Additionally, L168A activity does not appear to be altered by either DHPG or U73122, reflecting an absence of mGluR5-mediated regulation. (B) Glial cells endogenously expressing mGluR5 and SR show increased D-serine production with 50 μM DHPG treatment for 30 min. U73122 (10 μM) reverses this effect, demonstrating that glutamate transmission activates SR by degrading phospholipids. Bars represent the mean ± SEM of 3 independent experiments, each performed in triplicate.
To ascertain whether the relatively low basal activity of SR in the HEK293 cells stems from physiologic inhibition by endogenous PIP2, we examined SR activity in cells overexpressing SR-L168A, which does not bind PIP2 (Fig. 5A). Catalytic activity of SR-L168A is quadruple the levels of wild-type SR, indicating that endogenous PIP2 normally represses SR. If mGluR5 stimulation activates SR by reversing inhibition by PIP2, then it should be unable to stimulate SR that cannot bind PIP2. DHPG fails to augment the activity of SR-L168A, providing further evidence that metabotropic glutamate transmission stimulates SR by relieving PIP2 inhibition.
We wondered whether metabotropic glutamate transmission in intact glia can influence SR activity. In glial cultures DHPG increases SR activity 4-fold (Fig. 5B). This stimulation is reversed by treatment with the PLC inhibitor U73122, demonstrating that the glutamate transmission activates SR by degrading phospholipids. These observations are consistent with previous findings wherein stimulation of mGluR increased D-serine release from glial cultures, an effect blocked by antagonists of the receptor (24).
Discussion
In the present study we demonstrate a unique mode for the physiologic regulation of SR by glutamatergic neurotransmission. In glia SR is localized to membranes, particularly the plasma membrane, where it has access to PIP2. PIP2 binds SR and physiologically inhibits its catalytic activity by competing with the activating effects of ATP. Metabotropic glutamate transmission, acting via mGluR5, leads to stimulation of phospholipase C, which degrades PIP2, relieving the inhibition of SR. The pronounced stimulation of SR by metabotropic glutamate transmission appears to elicit a major increase in D-serine production and release. These findings provide a model that facilitates the coordinated interactions of glutamate and D-serine (Fig. 6): depolarization of nerve terminals releases glutamate, which can act directly upon NMDA receptors while stimulating closely apposed glia to generate D-serine, which joins with glutamate to coactivate postsynaptic NMDA receptors.
Fig. 6.
Model for D-serine signaling in the brain. D-serine is synthesized from L-serine by SR and stored primarily within astrocytes ensheathing neuronal synapses containing NMDA receptors. SR and D-serine may also occur in neurons (10). When the presynaptic neuron releases glutamate, it acts not only on the postsynaptic neuron, but also on the ensheathing astrocyte, resulting in the activation of metabotropic glutamate receptors and consequent degradation of PIP2 by PLC; subsequent activation of SR occurs through ATP binding. In the synaptic cleft, D-serine binds to the glycine/D-serine binding site on the NMDA receptor and, in conjunction with L-glutamate, elicits opening of the receptor channel. This model affords a potential activation mechanism of the D-serine synthesis needed for NMDA neurotransmission.
PIP2 has been implicated in the regulation of numerous ion channels and transporters, both as an activator and as an inhibitor (18, 19, 25). Signaling via receptor activation of PLC and depletion of PIP2 has been reported for the vanilloid receptor (VR)1, which mediates pain perception (25). Painful stimuli such as bradykinin and nerve growth factor activate PLC to deplete PIP2 and abrogate its inhibition of the VR1 channel. Muscarinic cholinergic receptor activation inhibits K(ATP) channels via PLC-mediated PIP2 depletion (25), and similar effects have been reported for α-adrenergic linked GIRK potassium channels (25), including desensitization of these channels in response to prolonged muscarinic cholinergic activation (25). PIP2 regulates channels by competing with ATP similar to their competition for SR.
Regulation of D-serine formation by metabotropic glutamate transmission may have pathophysiologic and physiologic significance because of links to NMDA transmission. For instance, D-serine may participate in the overactivation of NMDA receptors that underlies much of the brain damage associated with vascular stroke (26). Drugs that block the D-serine–glycine site of the NMDA receptor relieve stroke damage (26). A direct involvement of SR in stroke arises from our observations of markedly decreased stroke damage in SR gene knockout mice (our unpublished data). Selective inhibitors of mGluR5 also protect against stroke (27), which may reflect facilitation of NMDA transmission by mGluR activation. Thus, the mGluR activation of SR may underlie the involvement of metabotropic glutamate transmission in vascular stroke.
Materials and Methods
PIP Strip Assays.
PIP strip assays were done as described previously (20).
Liposomal Assays.
Liposomal assays either were carried out using premade biotin-tagged PolyPIPosomes containing PIP2 (Echelon Biosciences) or were generated as a lipid mixture with a ratio of 3:2:2:3 phosphatidylcholine:phosphatidylethanolamine:phosphatidylserine:specific lipid-of-interest, all from Avanti Polar Lipids. Liposomes were made by sonicating the mixture for 10 min 3 times at 25 °C with liquid N2 freezing in between each session in buffer containing 0.2 M sucrose, 20 mM KCl, 20 mM Hepes (pH 7.4), and 0.01% Na Azide. The protein(s) of interest (0.3 μg) were diluted in 900 μl of reaction buffer containing 0.12 M NaCl, 1 mM EGTA, 0.2 mM CaCl2, 1 mM MgCl2, 5 mM KCl, 20 mM Hepes (pH 7.4), and 1 mg/ml BSA and mixed with 100 μl of the sonicated lipid solution. The samples were immediately incubated for 15 min at 37 °C and then centrifuged for 40 min at 100,000 × g in a Beckman Ti 70.1 rotor. The samples were immediately placed on ice and the supernatant was vacuum suctioned carefully so as not to disturb the pellet. Lithium dodecyl sulfate (LDS) loading buffer was added and Western blots were carried out. For PolyPIPosome experiments, 2 μl of PolyPIPosomes were incubated overnight with purified proteins or 100 μg of cell or organ extracts at 4 °C. The next day, 30 μl of Neutravidin beads were added to pull down the lipid–protein mixtures for 1 h at 25 °C. Following 5 washes with 1 ml reaction buffer as detailed above, the beads were mixed with LDS loading buffer and processed for Western blotting.
Fluorescence Anisotropy Experiments.
Fluorescence anisotropy experiments used purified His-SR or PLCδ1 PH domain as follows. The pure proteins were mixed with 100 nM BODIPY-TMR-labeled PIP2 (Echelon Biosciences) in 20 mM Tris (pH 7.7) with or without ATP or unlabeled PIP2. Changes in the fluorescence anisotropy of the lipid in the presence of the proteins were measured in real time, using the T-format Spex Fluorolog-3 (J. Y. Horiba) set at an absorption wavelength of 542 nm and an emission wavelength of 574 nm. An increase in anisotropy indicates greater lipid–protein interaction.
Immunocytochemistry Analysis.
Primary glial cultures were grown until ≈50% confluency at 37 °C. The cells were quickly rinsed with PBS and fixed with fresh 4% paraformaldehyde in PBS for 15 min at 25 °C. The cells were then washed with PBS, permeabilized, blocked with 0.1% Triton in 5% goat serum for 1 h at 25 °C, and then washed again with PBS. Primary antibodies to SR, GFAP, and myc were added at 1:200 dilution in 1% goat serum in PBS overnight at 4 °C. Following washes with PBS, secondary antibodies conjugated with specific fluorophores were added per manufacturer's instructions for 1 h at 25 °C. The cells were finally washed, put under Anti-Fade DAPI Fluorophore mounting media from SouthernBiotech, and subjected to confocal imaging using a Zeiss 510 Meta confocal microscope.
GDDA-BLAST Analysis.
Peripheral lipid-binding (PLB) domain boundaries of SR were analyzed with GDDA-BLAST as previously described (21). Using the PLB boundaries derived using GDDA-BLAST, the positional scores were calculated using the Smith–Waterman algorithm with BLOSUM 45 and 62 to generate an alignment in the query sequence with profiles that were positive by GDDA-BLAST. Raw scores for each residue are calculated by scoring a value of 2 for identities and value of 1 for positive substitutions. These positions were tallied and the cumulative score was annotated vs. the amino acid position. Subsequently, positional scores for BLOSUM 45 and 62 were averaged, rank ordered, and thresholded to those residues in the top 25%.
Supplementary Material
Acknowledgments.
We thank Dr. Adam Resnick for his valuable suggestions, Dr. Jon Lorsch for help with the fluorescence anisotropy experiments, and Dr. Paul Worley for providing the mGluR5 construct and antibody. This work was supported by a National Institutes of Health National Research Service Award (1 F30 MH074191–01A2) (to A.K.M.) and by Grant MH18501 and Research Scientist Award DA00074 (to S.H.S.). This project was also supported by a grant from the Pennsylvania Department of Health, using Tobacco Settlement Funds (D.v.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813105106/DCSupplemental.
References
- 1.Mustafa AK, Kim PM, Snyder SH. D-serine as a putative glial neurotransmitter. Neuron Glia Biol. 2004;1(3):275–281. doi: 10.1017/S1740925X05000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolosker H, Dumin E, Balan L, Foltyn VN. D-amino acids in the brain: D-serine in neurotransmission and neurodegeneration. FEBS J. 2008;275(14):3514–3526. doi: 10.1111/j.1742-4658.2008.06515.x. [DOI] [PubMed] [Google Scholar]
- 3.Oliet SH, Mothet JP. Molecular determinants of D-serine-mediated gliotransmission: from release to function. Glia. 2006;54(7):726–737. doi: 10.1002/glia.20356. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto A, Nishikawa T, Oka T, Takahashi K. Endogenous D-serine in rat brain: N-methyl-d-aspartate receptor-related distribution and aging. J Neurochem. 1993;60(2):783–786. doi: 10.1111/j.1471-4159.1993.tb03219.x. [DOI] [PubMed] [Google Scholar]
- 5.Mothet JP, et al. D-serine is an endogenous ligand for the glycine site of the N-methyl-d-aspartate receptor. Proc Natl Acad Sci USA. 2000;97:4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shleper M, Kartvelishvily E, Wolosker H. D-serine is the dominant endogenous coagonist for NMDA receptor neurotoxicity in organotypic hippocampal slices. J Neurosci. 2005;25(41):9413–9417. doi: 10.1523/JNEUROSCI.3190-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panatier A, et al. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125(4):775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 8.Basu AC, et al. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.130. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolosker H, Blackshaw S, Snyder SH. Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-d-aspartate neurotransmission. Proc Natl Acad Sci USA. 1999;96:13409–13414. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kartvelishvily E, Shleper M, Balan L, Dumin E, Wolosker H. Neuron-derived D-serine release provides a novel means to activate N-methyl-d-aspartate receptors. J Biol Chem. 2006;281(20):14151–14162. doi: 10.1074/jbc.M512927200. [DOI] [PubMed] [Google Scholar]
- 11.De Miranda J, Panizzutti R, Foltyn VN, Wolosker H. Cofactors of serine racemase that physiologically stimulate the synthesis of the N-methyl-d-aspartate (NMDA) receptor coagonist D-serine. Proc Natl Acad Sci USA. 2002;99:14542–14547. doi: 10.1073/pnas.222421299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim PM, et al. Serine racemase: activation by glutamate neurotransmission via glutamate receptor interacting protein and mediation of neuronal migration. Proc Natl Acad Sci USA. 2005;102:2105–2110. doi: 10.1073/pnas.0409723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mustafa AK, et al. Nitric oxide S-nitrosylates serine racemase, mediating feedback inhibition of D-serine formation. Proc Natl Acad Sci USA. 2007;104(8):2950–2955. doi: 10.1073/pnas.0611620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci USA. 1995;92(9):3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tritsch NX, Bergles DE. Defining the role of astrocytes in neuromodulation. Neuron. 2007;54(4):497–500. doi: 10.1016/j.neuron.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Fellin T, D'Ascenzo M, Haydon PG. Astrocytes control neuronal excitability in the nucleus accumbens. Scientific World Journal. 2007;7:89–97. doi: 10.1100/tsw.2007.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dumin E, et al. Modulation of D-serine levels via ubiquitin-dependent proteasomal degradation of serine racemase. J Biol Chem. 2006;281(29):20291–20302. doi: 10.1074/jbc.M601971200. [DOI] [PubMed] [Google Scholar]
- 18.McLaughlin S, Wang J, Gambhir A, Murray D. PIP(2) and proteins: interactions, organization, and information flow. Annu Rev Biophys Biomol Struct. 2002;31:151–175. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438(7068):605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- 20.van Rossum DB, et al. Phospholipase Cgamma1 controls surface expression of TRPC3 through an intermolecular PH domain. Nature. 2005;434(7029):99–104. doi: 10.1038/nature03340. [DOI] [PubMed] [Google Scholar]
- 21.Ko KD, et al. Phylogenetic profiles as a unified framework for measuring protein structure, function, and evolution. Phys Arch. 2008 arXiv:0806.239, q-bio.Q. [Google Scholar]
- 22.Chang GS, et al. Phylogenetic profiles reveal evolutionary relationships within the “twilight zone” of sequence similarity. Proc Natl Acad Sci USA. 2008;105(36):13474–13479. doi: 10.1073/pnas.0803860105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Rossum DB, et al. TRP_2, a lipid/trafficking domain that mediates diacylglycerol-induced vesicle fusion. J Biol Chem. 2008;283(49):34384–34392. doi: 10.1074/jbc.M804707200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mothet JP, et al. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc Natl Acad Sci USA. 2005;102(15):5606–5611. doi: 10.1073/pnas.0408483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE. 2001;2001(111):RE19. doi: 10.1126/stke.2001.111.re19. [DOI] [PubMed] [Google Scholar]
- 26.Kemp JA, McKernan RM. NMDA receptor pathways as drug targets. Nat Neurosci. 2002;5:1039–1042. doi: 10.1038/nn936. [DOI] [PubMed] [Google Scholar]
- 27.Szydlowska K, Kaminska B, Baude A, Parsons CG, Danysz W. Neuroprotective activity of selective mGlu1 and mGlu5 antagonists in vitro and in vivo. Eur J Pharmacol. 2007;554(1):18–29. doi: 10.1016/j.ejphar.2006.09.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.