The chromophore-binding domain of cyanobacteriochrome AnPixJ was overproduced in E. coli, purified and crystallized in its red-absorbing form. Diffraction data were then collected to a resolution of 1.8 Å.
Keywords: cyanobacteriochromes, AnPixJ, chromophore-binding domains
Abstract
Cyanobacteriochromes form a recently defined superfamily of tetrapyrrole-based photoreceptors that are distantly related to conventional red/far-red photoreceptor phytochromes. Among these molecules, AnPixJ from Anabaena sp. PCC 7120 is a novel photoreceptor that shows reversible photoconversion between green-absorbing and red-absorbing forms, which is in contrast to the properties of conventional phytochromes. In order to better understand the structural basis of this unique photoconversion mechanism, the chromophore-binding domain of AnPixJ (AnPixJ-GAF2) was heterologously overproduced and purified, and crystallization of both forms was attempted. Blue crystals of the red-absorbing form of AnPixJ-GAF2 were successfully obtained; they belonged to space group P43212 and contained one monomer per asymmetric unit. Diffraction data were collected to a resolution of 1.8 Å using synchrotron-radiation beamline BL-5A at the Photon Factory.
1. Introduction
The phytochromes in plants, fungi, algae, cyanobacteria and some bacteria are photoreceptors that bind linear tetrapyrroles and mostly exhibit reversible photoconversion between red-absorbing and far-red-absorbing forms (Rockwell et al., 2006 ▶). This conversion has been ascribed to isomerization between the C15-Z and C15-E isomers of the phytochromobilin, phycocyanobilin or biliverdin chromophores. The photosensory module of phytochromes is divided into the three domains PAS (Per/ARNT/Sim), GAF (cGMP-phosphodiesterase/adenylate cyclase/FhlA) and PHY (phytochrome-specific) (Montgomery & Lagarias, 2002 ▶). A Cys residue which covalently ligates the chromophore via a thioether bond is located either within the GAF domain in plant and cyanobacterial phytochromes or in the upstream knotted region prior to the PAS domain in bacterial and fungal phytochromes (Lamparter et al., 2002 ▶). The recently determined crystal structures of the red-absorbing form of PHY-truncated bacterial phytochromes (Wagner et al., 2005 ▶, 2007 ▶; Yang et al., 2007 ▶) suggest that the GAF domain plays a crucial role in the functioning of the chromophore.
The GAF domain has been defined as a superfamily with diverse sequences and that contains a specific three-dimensional structural fold (Aravind & Ponting, 1997 ▶). The domain is widely distributed throughout the three domains of life (Okamoto & Ohmori, 2003 ▶; Galperin et al., 2001 ▶). Recent genome projects have revealed that freshwater and terrestrial cyanobacteria possess an extremely large number of GAF-domain proteins (Ohmori et al., 2001 ▶; Ashby & Houmard, 2006 ▶). Phylogenetic analysis has further shown that a number of GAF domains found in cyanobacterial signalling proteins are homologous to but clearly distinct from those contained in the phytochromes. Moreover, they are not accompanied by PAS or PHY domains. Recently, some of these GAF domains have been demonstrated to bind to tetrapyrroles and form photoactive holoproteins known as ‘cyanobacteriochromes’ (Yoshihara & Ikeuchi, 2004 ▶; Ikeuchi & Ishizuka, 2008 ▶).
GAF domains in the phototaxis regulators SyPixJ1 from Synechocystis sp. PCC 6803 and TePixJ from Thermosynechococcus elongatus BP-1 bind linear tetrapyrroles and show reversible photoconversion between blue-absorbing and green-absorbing forms (Yoshihara et al., 2004 ▶, 2006 ▶; Ishizuka et al., 2006 ▶). Notably, the chromophore of TePixJ is not phycocyanobilin but phycoviolobilin, although phycocyanobilin is initially incorporated (Ishizuka et al., 2007 ▶). The GAF domain in the SyCikA photoreceptor from Synechocystis sp. PCC 6803 shows unidirectional photoconversion from a violet-absorbing to a yellow-absorbing form and partial dark reversion from the yellow-absorbing to the violet-absorbing form, suggesting that it potentially acts as a violet-light sensor (Narikawa, Kohchi et al., 2008 ▶). SyCcaS is a novel green–red reversible photoreceptor that activates autophosphorylation of its histidine kinase domain and promotes phosphotransfer to the cognate response regulator CcaR, leading to the induction of a phycobilisome linker gene cpcG2 under green–orange light (Katayama & Ikeuchi, 2006 ▶; Hirose et al., 2008 ▶).
AnPixJ from the heterocyst-forming cyanobacterium Anabaena sp. PCC 7120 is a signalling protein consisting of four cyanobacteriochrome-type GAF domains and a methyl-accepting chemotaxis regulator domain (Narikawa, Fukushima et al., 2008 ▶). Thus, the overall sequence of AnPixJ is very similar to those of SyPixJ1 and TePixJ. However, the GAF domains of AnPixJ belong to a new subgroup that is distantly related to those of SyPixJ1 and TePixJ. Consistently, the second GAF domain of AnPixJ (AnPixJ-GAF2), when prepared from cyanobacterial cells or phycocyanobilin-producing Escherichia coli, exhibits a novel reversible photoconversion between the green-absorbing and red-absorbing forms. Denaturation analysis further revealed that the green-absorbing and red-absorbing forms harbour the phycocyanobilin with an E-configuration and a Z-configuration at the C15=C16 double bond, respectively. This means that the red-absorbing form of AnPixJ-GAF2 corresponds to that of phytochromes and that photoconversion from the red-absorbing form to the green-absorbing form involves a Z-to-E isomerization in the same manner as in phytochromes. Time-resolved spectral analysis has further demonstrated that the initial intermediates from the red-absorbing form also resemble those of the phytochromes, but that the final product, the more unusual green-absorbing form, is distinctly different.
In our present communication, we wished to gain further molecular insights into this unique photoconversion mechanism. We thus attempted the structural determination of both the red-absorbing and the green-absorbing forms of AnPixJ-GAF2, which was co-produced with phycocyanobilin in E. coli. We report the successful purification, crystallization and preliminary X-ray crystallographic analysis of the red-absorbing form of AnPixJ-GAF2.
2. Materials and methods
2.1. Protein overproduction and purification
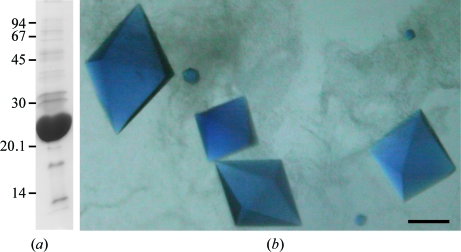
The plasmids (pKT271 and pAnPixJ-GAF2) used in this study have been described previously (Narikawa, Fukushima et al., 2008 ▶). pKT271 encodes haemoxygenase and PcyA (phycocyanobilin:ferredoxin oxidoreductase) to generate phycocyanobilin from haem via biliverdin (Mukougawa et al., 2006 ▶). pAnPixJ-GAF2 is an expression vector for the second GAF domain (residues 221–397) of AnPixJ. To prepare AnPixJ-GAF2 protein, BL21 (DE3) cells transformed with pKT271 and pAnPixJ-GAF2 were precultured at 310 K for 25 h in 2.5 l Luria–Bertani medium containing 2% glucose, 20 µg ml−1 kanamycin and 20 µg ml−1 chloramphenicol. Isopropyl β-d-1-thiogalactopyranoside was then added to a final concentration of 1 mM and the cells were cultured at 300 K overnight and harvested by centrifugation. The resulting cell pellet was frozen at 193 K, thawed at 277 K and then resuspended in 50 ml buffer A consisting of 20 mM Na HEPES pH 7.5, 100 mM NaCl and 10%(w/v) glycerol. The cell suspension was passed through a French Press (three passages at 147 MPa) and the obtained cell extracts were centrifuged at 109 200g for 30 min at 277 K. The supernatant containing the His-tagged protein was applied onto a nickel-affinity column (His-Trap Chelating HP, GE Healthcare). After washing the column with ten volumes of buffer A containing 30 mM imidazole, proteins were eluted using a step gradient of 50, 100 and 200 mM imidazole in buffer A. Most His-tagged proteins were recovered in the 200 mM imidazole fraction, which was dialyzed three times against 30 volumes of buffer A to remove imidazole. The homogeneity of the purified proteins was confirmed by SDS–PAGE (Fig. 1 ▶ a). The protein concentrations were determined using the Bradford method as described by the manufacturer (Bio-Rad Laboratories, Hercules, California, USA). The molecular masses of the purified proteins were determined using size-exclusion column chromatography (Superdex 200 PC 3.2/30, SMART system, GE Healthcare). Purified proteins were concentrated to approximately 10 mg ml−1 in buffer A and the sample was used at this concentration in all subsequent crystallization experiments.
Figure 1.
(a) SDS–PAGE gel showing purified recombinant AnPixJ-GAF2. Molecular-mass markers (kDa) are indicated on the left. (b) Blue crystals of the red-absorbing form of AnPixJ-GAF2 with dimensions of 0.4 × 0.2 × 0.2 mm. Scale bar, 0.1 mm.
2.2. Crystallization
Crystallization screening of the red-absorbing and the green-absorbing forms of AnPixJ-GAF2 was carried out using Crystal Screens I and II (Hampton Research, Riverside, California, USA) and Ozma PEG 8K 48-salt (Emerald Biosystems, Bainbridge Island, Washington, USA) with the hanging-drop vapour-diffusion method at 277 K. Crystallization droplets were prepared on siliconized cover slips by mixing 1 µl protein solution with 1 µl reservoir solution and were equilibrated against 180 µl of the same reservoir solution. The red-absorbing form of AnPixJ-GAF2 was crystallized using a reservoir solution consisting of 20%(w/v) PEG 8K containing 200 mM potassium iodide as a crystallizing agent. All crystallization experiments were performed under green safety light.
2.3. Data collection
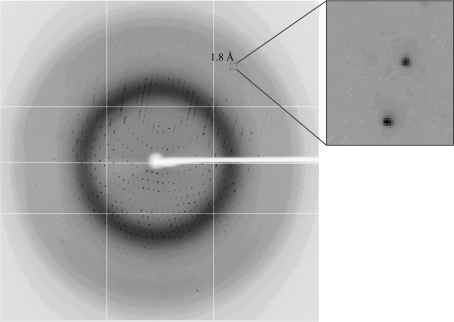
For data collection under cryogenic conditions, crystals were soaked for a few minutes in reservoir solution containing 20%(v/v) glycerol, mounted in a nylon loop and flash-frozen in a liquid-nitrogen bath. After freezing the crystals, white light was used to prepare the X-ray experiments. X-ray diffraction data were collected on beamline BL5A of the Photon Factory, Tsukuba, Japan, which was equipped with an ADSC Quantum-315 CCD detector and a Rigaku GN2 cryosystem. Since the crystals were obtained from an iodide-containing solution, iodide may be introduced into specific sites in the crystals to serve as a heavy atom for phase determination. We collected a native data set and an iodide single-wavelength anomalous dispersion (SAD) data set; the wavelengths used were 1.0000 and 1.6000 Å, respectively. Diffraction images were collected with an oscillation angle of 1° and an exposure time of 1 s per image in a nitrogen-gas stream at 100 K. All diffraction data were processed and scaled with the HKL-2000 program package (Otwinowski & Minor, 1997 ▶). The data-collection statistics are summarized in Table 1 ▶.
Table 1. Diffraction data statistics for crystals of the red-absorbing form of AnPixJ-GAF2.
Values in parentheses are for the highest resolution shell.
| Native data | Iodide SAD data | |
|---|---|---|
| Data processing | ||
| X-ray source | PF BL-5A | PF BL-5A |
| Wavelength (Å) | 1.0000 | 1.6000 |
| Temperature (K) | 100 | 100 |
| Resolution (Å) | 50–1.80 (1.86–1.80) | 50–2.20 (2.28–2.20) |
| Total reflections | 389475 | 792364 |
| Unique reflections | 28763 | 16094 |
| Redundancy | 13.5 (13.3) | 49.3 (26.5) |
| Completeness (%) | 99.7 (98.9) | 100 (99.9) |
| Rmerge(I)† (%) | 5.7 (39.9) | 7.4 (49.7) |
| I/σ(I) | 15.9 (5.0) | 7.5 (3.5) |
| Crystallographic data | ||
| Crystal system | Tetragonal | |
| Space group | P43212 | |
| Unit-cell parameters (Å, °) | a = b = 69.1, c = 124.1, α = β = γ = 90 | |
| VM (Å3 Da−1) | 3.22 | |
| No. of molecules in ASU | 1 |
R
merge(I) = 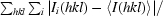
 , where Ii(hkl) is the value of the ith measurement of the intensity of a reflection and 〈I(hkl)〉 is the mean value of the intensity of that reflection.
, where Ii(hkl) is the value of the ith measurement of the intensity of a reflection and 〈I(hkl)〉 is the mean value of the intensity of that reflection.
3. Results and discussion
Based on SDS–PAGE analysis, the purity of the expressed recombinant proteins was deemed to be sufficient for crystallization (Fig. 1 ▶ a). The molecular mass of AnPixJ-GAF2 was estimated to be 23 kDa by SDS–PAGE, which is in good agreement with the theoretical value (22.8 kDa) for this protein. The purified protein was eluted from a gel-filtration column at a position corresponding to the monomer. Thus, we assumed that the native protein was monomeric in solution. Crystals of the red-absorbing form of AnPixJ-GAF2 appeared within 1–3 d and reached final dimensions of 0.4 × 0.2 × 0.2 mm in four weeks (Fig. 1 ▶ b). Since it is known that the red-absorbing form is the dark-stable form (Narikawa, Fukushima et al., 2008 ▶), it should remain stable during long-term incubation in the dark. X-ray diffraction data were collected to a resolution of 1.80 Å (Fig. 2 ▶). The crystals belong to the tetragonal space group P43212 or P41212, with unit-cell parameters a = b = 69.1, c = 124.1 Å. Assuming the presence of one protein molecule in the asymmetric unit, the Matthews coefficient (V M) was determined to be 3.22 Å3 Da−1, corresponding to a solvent content of 61.8%.
Figure 2.
X-ray diffraction image from a crystal of AnPixJ-GAF2.
To obtain the phase information, we first attempted molecular replacement using MOLREP from the CCP4 program suite. The search models showed approximately 20% identity to AnPixJ-GAF2 and included DrBphP-CBD (PDB codes 1ztu and 2o9c; Wagner et al., 2005 ▶, 2007 ▶) and RpBphP3-CBD (PDB code 2ool; Yang et al., 2007 ▶). However, we could not obtain good solutions. Instead, difference Patterson and anomalous difference Patterson maps were calculated to check iodide binding using both the iodide SAD and the native data sets (Collaborative Computational Project, Number 4, 1994 ▶). Both maps indicated that the space group was P43212 and clearly showed four unique Harker peaks, (u = 0.5000, v = 0.5000, w = 0.4572), (u = 0.1332, v = 0.0000, w = 0.5000), (u = 0.4337, v = 0.4337, w = 0.2496) and (u = 0.3659, v = 0.5000, w = 0.0423), corresponding to four iodide-binding sites. Efforts towards structure determination using the iodide SAD method are currently in progress.
Thus far, we have not obtained crystals of the green-absorbing form of AnPixJ-GAF2 and work is ongoing in our laboratory to achieve this.
Acknowledgments
We thank Professor Takayuki Kohchi for his kind gift of pKT271. We also thank the staff at beamline BL-5A, KEK, Japan for their support during data collection. This work was supported in part by Grants-in-Aid for Scientific Research (to RN, MI and GK) from the Ministry of Education and Science, Japan.
References
- Aravind, L. & Ponting, C. P. (1997). Trends Biochem. Sci.22, 458–459. [DOI] [PubMed]
- Ashby, M. K. & Houmard, J. (2006). Microbiol. Mol. Biol. Rev.70, 472–509. [DOI] [PMC free article] [PubMed]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763.
- Galperin, M. Y., Nikolskaya, A. N. & Koonin, E. V. (2001). FEMS Microbiol. Lett.203, 11–21. [DOI] [PubMed]
- Hirose, Y., Shimada, T., Narikawa, R., Katayama, M. & Ikeuchi, M. (2008). Proc. Natl Acad. Sci. USA, 105, 9528–9533. [DOI] [PMC free article] [PubMed]
- Ikeuchi, M. & Ishizuka, T. (2008). Photochem. Photobiol. Sci.7, 1159–1167. [DOI] [PubMed]
- Ishizuka, T., Narikawa, R., Kohchi, T., Katayama, M. & Ikeuchi, M. (2007). Plant Cell Physiol.48, 1385–1390. [DOI] [PubMed]
- Ishizuka, T., Shimada, T., Okajima, K., Yoshihara, S., Ochiai, Y., Katayama, M. & Ikeuchi, M. (2006). Plant Cell Physiol.47, 1251–1261. [DOI] [PubMed]
- Katayama, M. & Ikeuchi, M. (2006). Frontiers in Life Sciences, edited by M. Fujiwara, S. Ishiura & N. Sato, pp. 65–90. Kerala, India: Research Signpost.
- Lamparter, T., Michael, N., Mittmann, F. & Esteban, B. (2002). Proc. Natl Acad. Sci. USA, 99, 11628–11633. [DOI] [PMC free article] [PubMed]
- Montgomery, B. L. & Lagarias, J. C. (2002). Trends Plant Sci.7, 357–366. [DOI] [PubMed]
- Mukougawa, K., Kanamoto, H., Kobayashi, T., Yokota, A. & Kohchi, T. (2006). FEBS Lett.580, 1333–1338. [DOI] [PubMed]
- Narikawa, R., Fukushima, Y., Ishizuka, T., Itoh, S. & Ikeuchi, M. (2008). J. Mol. Biol.380, 844–855. [DOI] [PubMed]
- Narikawa, R., Kohchi, T. & Ikeuchi, M. (2008). Photochem. Photobiol. Sci.7, 1253–1259. [DOI] [PubMed]
- Ohmori, M. et al. (2001). DNA Res.8, 271–284. [DOI] [PubMed]
- Okamoto, S. & Ohmori, M. (2003). Genome Inform.14, 442–443.
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Rockwell, N. C., Su, Y. S. & Lagarias, J. C. (2006). Annu. Rev. Plant Biol.57, 837–858. [DOI] [PMC free article] [PubMed]
- Wagner, J. R., Brunzelle, J. S., Forest, K. T. & Vierstra, R. D. (2005). Nature (London), 438, 325–331. [DOI] [PubMed]
- Wagner, J. R., Zhang, J., Brunzelle, J. S., Vierstra, R. D. & Forest, K. T. (2007). J. Biol. Chem.282, 12298–12309. [DOI] [PubMed]
- Yang, X., Stojkovic, E. A., Kuk, J. & Moffat, K. (2007). Proc. Natl Acad. Sci. USA, 104, 12571–12576. [DOI] [PMC free article] [PubMed]
- Yoshihara, S. & Ikeuchi, M. (2004). Photochem. Photobiol. Sci.3, 512–518. [DOI] [PubMed]
- Yoshihara, S., Katayama, M., Geng, X. & Ikeuchi, M. (2004). Plant Cell Physiol.45, 1729–1737. [DOI] [PubMed]
- Yoshihara, S., Shimada, T., Matsuoka, D., Zikihara, K., Kohchi, T. & Tokutomi, S. (2006). Biochemistry, 45, 3775–3784. [DOI] [PubMed]