Abstract
The newly-discovered tectal longitudinal column (TLC) spans the paramedian region of the mammalian tectum. It has connections with several nuclei of the auditory system. In this report, we provide the first detailed description of the responses of TLC neurons to auditory stimuli, including monaural and binaural tones and amplitude modulated tones. For comparison, responses in the inferior colliculus (IC) were also recorded. Neurons in the TLC were sensitive to similar ranges of frequency as IC neurons, could have comparably low thresholds, and showed primarily excitatory responses to stimulation of the contralateral ear with either phasic or sustained response patterns. Differences of TLC compared to IC neurons included broader frequency tuning, higher average threshold, longer response latencies, little synchronization or rate tuning to amplitude modulation frequency and a smaller degree of inhibition evoked by stimulation of the ipsilateral ear. These features of TLC neurons suggest a role for the TLC in descending auditory pathways.
Keywords: midbrain tectum, inferior colliculus, superior colliculus, periaqueductal gray
INTRODUCTION
The tectal longitudinal column (TLC) is a recently-discovered, large nucleus of the mammalian brain (Saldaña et al., 2007). The TLC is located in the paramedian region of the midbrain tectum near the midline and immediately dorsal to the dorsomedial column of the periaqueductal gray matter (PAG). It spans the tectum longitudinally and occupies a territory that has traditionally been considered the most medial part of the deep layers of the superior colliculus (SC) and the most medial part of the inferior colliculus (IC). Its caudal third is crossed by the commissure of the IC and its rostral third by the enlarged, rostral half of the commissure of the SC (see Figure 1 of Saldaña et al. 2007). In the rat, it is ~3.5 mm long and 350 µm wide and contains approximately 11,500 neurons, a number comparable to that of most auditory nuclei of the lower brainstem (Kulesza et al., 2002). Similarity in cytoarchitectural features has led to identification of the TLC in rodents, lagomorphs, carnivores, non-human primates, and humans, indicating that the nucleus is conserved across mammals (Saldaña et al. 2007).
Figure 1. Coronal sections of rat midbrain.
A. Nissl stain (cresyl violet). Cell bodies of the TLC are near the midline, medial and ventral to the superior colliculus and dorsal to the periaqueductal gray. B. Retrograde labeling in the TLC following an injection of FluoroGold into the superior olivary complex. The FluoroGold was rendered permanently visible by immunocytochemistry using a rabbit anti-FluoroGold primary antiserum (see Saldaña et al., 2007 for details). C. Nissl stain showing an electrode track from a TLC recording (between arrows) and a lesion placed in the DC as the electrode was withdrawn (arrowhead). Scale in C = 0.5 mm.
The TLC receives direct projections from major auditory centers such as the IC and the primary auditory cortex (Morest and Oliver, 1984; Saldaña and Merchán, 1992; Saldaña et al., 1996; Saldaña and Merchán, 2005). Moreover, most TLC neurons are labeled by retrograde neuroanatomical tracers injected into the ipsilateral superior olivary complex (Faye-Lund, 1986; Saldaña et al., 2007). Because of these connections with auditory centers, we hypothesized that the TLC was part of the auditory system; indeed, our initial neural recordings from the TLC identified responses to sound that were of relatively long-latency with a range of tuning to sound frequency (Saldaña et al., 2007). For the present report, we used rats to provide the first detailed information on the auditory responses of TLC neurons, including responses to binaurally presented tones and amplitude modulated tones. For comparison, we also recorded responses from neurons in the IC of the same rats. In addition, some penetrations encountered distinctive auditory responses in the dorsolateral PAG, which lies ventrolateral to the TLC.
MATERIALS and METHODS
Animal
The experiments were in rats of the Sprague-Dawley (n=4) and Long Evans strains (n=18) showing no signs of outer or middle ear pathology. All animal procedures were approved by the Institute of Animal Care and Use Committee of The University of North Carolina at Chapel Hill and conformed to the National Institutes of Health guidelines and protocols.
Surgery
Rats were anesthetized with an intraperitoneal urethane (25% in saline, 1.5 g/kg). The head of each rat was immobilized using a custom designed head bite bar and head clamp. The animal’s temperature was maintained at a constant 37°C using a heating pad and rectal probe. One ml of 1% lidocaine with 1:100,000 epinephrine was infiltrated into the skin and subcutaneous tissue over the skull to provide additional anesthesia and hemostasis. After removing the scalp by sharp dissection, the periostium was removed exposing bregma, lambda, and the sagittal sutures. Using binocular microscopy and a high speed drill, craniotomies were created over the TLC and IC. The drilling locations were determined stereotaxically based on coordinates in a rat brain atlas (Paxinos and Watson, 1998)
Recording techniques
The electrode (glass coated Pt-W wire, tip size 1–2 µm, impedances of 10–20 MOhms) was zeroed at bregma using micromanipulators and binocular microscopy. It was advanced into the brain from outside the chamber using a piezoelectric microdrive (Burleigh Instruments Inc, Fishers, NY). Extracellular neural activity was amplified 5,000–25,000 times and monitored acoustically and on an oscilloscope. Action potentials were isolated with a time/level window discriminator and spike times recorded to an accuracy of 1 µs. Our recordings consisted of well-isolated single neurons and small clusters of neurons. All of the illustrated examples are from single neurons while the population distributions include single neurons and small clusters. No differences in the distributions were seen between the single neurons and small cluster responses. A typical recording session lasted about 8 hours.
Acoustic stimulation
Sound delivery tubes were positioned and fixed bilaterally at the rat’s external acoustic meatus. The amplitudes and phases of the sounds were calibrated across frequency (60 Hz – 50 kHz) using band-limited noise and a probe tube that extended to the end of the sound delivery tube. The probe tube was previously calibrated so that the calibration reflected the delivery of sound near the eardrum. The speakers (Beyer DT-8) could provide output at 80 dB or higher for frequencies up to about 30 kHz, but the maximum output declined at higher frequencies. Isolation of the ears was achieved using foam pads at the end of the sound delivery tubes resulting in cross-talk attenuation of at least 40 dB. Auditory stimuli consisted of tones, sinusoidally amplitude modulated (SAM) tones, clicks, and noise delivered to one or both ears. Acoustic stimuli were generated using System II components from Tucker-Davis Technologies. These included an array processor, digital-to-analog converter, 50 kHz anti-aliasing filter, and programmable attenuators. Custom software created in MATLAB allowed for the independent control of the frequencies, intensities, and timing of signals to the two ears.
Data collection and analysis
Low-pass noise bursts with cut-off frequencies of 8–20 kHz and levels up to 80 dB were used as the auditory search stimulus. After obtaining and isolating an auditory response, tones over a range of frequencies and intensities (0.25 and 0.5 octave and 5–10 dB step sizes) were presented to determine the neuron’s best frequency (BF), or frequency of maximal response at the lowest intensity tested. The threshold at BF was then determined for most neurons by varying the intensity at the BF. In a subset of neurons, the characteristic frequency (CF), or frequency of minimum threshold, was determined from an automatic tuning curve algorithm that tracked threshold responses according to a user-defined spike criterion. The sharpness of frequency tuning was evaluated using the Q10-dB (CF/bandwidth at 10 dB above threshold).
The response latency was measured as the median time from the onset of the tone to the first action potential. Median latencies were used to avoid a disproportionate influence of outliers that can occur when the mean latency is calculated. The window used was 3–53 ms. For a neuron to be included there had to be first spikes within this window for at least 50% of the repetitions, and their timing had to be significantly tighter than would be expected by chance spikes based on the spontaneous activity. Response types were determined from post-stimulus time (PST) histograms. Neurons were classified as either phasic or sustained, depending on the duration of the response throughout the stimulus.
To examine the ability of TLC and IC neurons to follow envelope modulations, SAM tones with a carrier at the CF were tested over a range of modulation frequencies (1.5 –1000 Hz). Synchronization indices were determined from cycle histograms (Goldberg and Brown, 1969), and the ability to phase-lock to the envelope modulations was determined using the Rayleigh test for uniformity (Mardia and Jupp, 1999). The phase-locking to the modulation frequency was considered significant for p-values less than 0.001.
Binaural responses were determined with tones at CF or with noise. Each ear was first tested with monaural stimulation at different levels to determine if the response was excitatory (E), inhibitory (I) or had no effect (O). As excitation can be weak to monaural stimulation and inhibition difficult to detect in the absence of spontaneous activity, the binaural interaction was also tested with tones at the most excitatory ear (usually the contralateral) at a constant level while the intensity at the other ear was varied. The criterion for classifying a neuron as EE (excited by the contralateral and ipsilateral ears, respectively) or EI or IE (excited by stimulation to one ear and inhibition to the other) was a 20% increase or reduction in the firing compared to the stimulation of the excitatory ear alone. Neurons for which binaural stimulation had less of an effect were classed as EO or OE. In some neurons that showed a binaural response, the responses to interaural phase differences in the envelope were tested with binaural beats of SAM tones, created by a difference of 1 Hz in modulation frequencies between the ears. The difference in modulation frequencies creates a dynamically fluctuating interaural phase difference of the envelope. Synchronization to the 1 Hz binaural beat frequency was evaluated using the Rayleigh test for uniformity (Mardia and Jupp, 1999), with synchronization considered significant at p<0.001.
Histology
Electrolytic lesions were made at the end of most experiments to histologically identify recording sites within the TLC. The brain was fixed by cardiac perfusion of phosphate-buffered formalin. Serial 50 µm frozen sections were cut in the coronal plane and stained with cresyl violet. The TLC itself was typically not lesioned as a single lesion could obliterate most or all of the TLC in the coronal sections and make reconstruction difficult. Instead, most lesions were made after the electrode passed through the TLC or as it was withdrawn. In a few cases, histology was not available because the animal died prior to lesioning. In these cases, the recordings were stereotaxically defined as within the TLC (see Fig. 3).
Figure 3. No evidence of tonotopy in the TLC.
A and B. Examples from two individual cases where neurons with widely different frequency tuning were found in close proximity. In A, neuron a was 120 µm deep to neuron b in the same penetration, and neuron c was from a different penetration 250 µm anterior. In B, all three neurons were from the same penetration, with neuron b located 130 µm deep to neuron a, and neuron c 250 µm deep to neuron b. C. Distribution of penetrations. Filled circles – penetrations localized to the TLC. Open circles – penetrations localized to the dorsolateral PAG. Boxes – location of the TLC determined anatomically (Saldaña et al., 2007). D. Distribution of BFs in the TLC. There was no trend for BFs to be distributed along the rostrocaudal axis of the TLC.
RESULTS
We recorded from 87 units in the TLC and 109 in the IC. Experiments were originally performed in Sprague-Dawley rats (7 TLC and 11 IC units) to remain consistent with anatomical studies where the TLC was initially identified. Once it was determined that auditory responses were present in this strain, further experiments were conducted in the Long-Evans strain, because non-pigmented animals are known to sometimes have unusual physiology (Bock and Steel, 1984). After completing the study the neurons from the Spraque-Dawley strain were not outliers in any of the distributions and the data from the two strains was pooled. Recordings made in the IC allowed for a characterization of responses to auditory stimuli in neurons from a well-described nucleus of the same animals used to record from the TLC. In addition to providing an important baseline for evaluating the responses to auditory stimulation in a novel nucleus, these recordings also provided an internal control for the quality of the physiological recording in terms of thresholds and the ability to stimulate both ears.
Localization of the TLC
In coronal sections the TLC appears located medial and ventral to the SC and dorsal to the PAG (Fig. 1A). The TLC can be seen most easily when its cells are labeled following an injection of FluroGold into the superior olivary complex (Fig. 1B). An example of a penetration into the TLC is shown in Fig. 1C. The lesion in this case was made after the electrode was withdrawn from the region with auditory responsiveness corresponding to the TLC, in a location defined in a previous study as the dorsal column (DC, Saldaña et al., 2007).
The TLC could be consistently identified through physiological properties. After traversing the medial cortex, a typical penetration would encounter light-sensitive responses in the most medial portion of the superficial layers of the SC as determined using a flashlight or a laser pointer in the darkened sound-attenuated chamber. Locations in this medial part of the SC did not respond to noise used as a search stimulus. A distance of 200–300 µm with no neural responses to sound or light, corresponding to DC, would follow the light-sensitive region. Entrance into the TLC was marked by a background of auditory responsiveness and no sensitivity to light. The TLC is limited in dorsal-to-ventral extent (Figs. 1A and B), and auditory responses were obtained for less than 500 µm before entering the dorsomedial PAG, where neurons did not respond to sound.
Frequency tuning
Penetrations through the IC had a clear tonotopy; neurons with low CFs and BFs were encountered dorsally and these frequencies increased with depth. Neurons typically had “V” shaped tuning curves although examples of neurons with broad frequency tuning covering most of the responsive range were also seen. In Fig. 2A, tuning curves encountered in a dorsal to ventral series (a–e) during a single penetration are shown. The dorsal-to-ventral distance was 1.8 mm, and the tonotopy is readily apparent.
Figure 2.
Representative threshold tuning curves to contralateral tones for neurons located in the IC (A) and the TLC (B). The neurons in the IC were from a single penetration and show a clear tonotopy as the electrode moved dorsally from neurons a–e. The neurons from the TLC are from different cases.
In the TLC, tuning curves showed a mixed population of neurons with “V” shaped responses (e.g., Fig. 2B, a–d) and more broadly tuned neurons (e.g, Fig. 2B, 1–4). The tuning curves in this figure were all obtained from different penetrations. The TLC is thin in dorsoventral extent so that in many penetrations only one neuron was recorded, and three was the maximum recorded in a single penetration.
Unlike the IC, tonotopy in the TLC was not readily apparent. Figures 3A and B show examples of tuning curves recorded from neurons in close proximity to each other that show a wide range of tuning. In Fig. 3C the distribution of all penetrations containing neurons localized to the TLC is shown (filled circles). The boxes (dotted lines) indicate the location of the TLC determined from the previous study (Saldaña et al., 2007). Most penetrations were within this region, and the remaining penetrations were with 0.25 mm of the expected coordinates, which is a reasonable error in stereotaxic positioning. A few penetrations (open symbols) encountered neurons that were histologically localized to the dorsolateral PAG. The coordinates of these penetrations were lateral to the TLC or overlapped with the lateral part of the TLC. The BFs of neurons relative to the rostrocaudal axis are shown in Fig. 3D. There was no correlation of BF with rostrocaudal position (linear regression, r=0.09, p=0.49).
The distributions of BFs and CFs and minimum thresholds for both nuclei spanned a range (Figs. 4A and B). The lowest neural thresholds for both the IC and TLC across frequencies approximated the behavioral thresholds in rats (solid line, from Kelly and Masterton, 1977). Overall, the thresholds of TLC neurons (47.2 ± 2.66 s.e.m., n=50) were higher than those of IC neurons (mean = 39.3 ± 2.36 s.e.m., n=54). The difference in the mean thresholds between the two nuclei was significant (t-test, df = 102, t=2.24, p<0.05).
Figure 4. Comparison of thresholds and frequency tuning widths between the IC and TLC.
A. Thresholds at CF for neurons in the IC. The solid line is the behavioral audiogram for rats (Kelly and Masterton, 1977). B. Thresholds at CF for neurons in the TLC. C. A comparison of Q10-dB values for the IC and TLC.
Tuning sharpness was measured at 10 dB above threshold (Q10-dB) in a subset of neurons (Fig. 4C). There was overlap in Q10-dB between the two nuclei, but on average the tuning of TLC neurons (mean = 1.8 ± 0.29 s.e.m., n=28) was broader than that of IC neurons (2.8 ± 0.39 s.e.m.). The difference in the tuning sharpness between the two nuclei was significant (t-test, t=2.082 df=45, p<0.05).
PST response types in the TLC and IC
Both nuclei showed a mixture of phasic and sustained responses. Examples of PST histograms to tones at BF from each nucleus are shown in Fig. 5. Phasic neurons (Fig. 5A for the IC and Fig. 5F for the TLC) gave only one or a few spikes at the beginning of the stimulus. Sustained neurons fired throughout the duration of the stimulus. The variety of sustained neurons was greater in the IC than in the TLC. In the IC, sustained neurons could show weak (Fig. 5B) or strong (Fig. 5C) adaptation or a chopping pattern (Fig. 5D). Some sustained neurons had a relatively long latency (Fig. 5E). In the TLC, sustained neurons were in general weakly adapting (Fig. 5G) and showed a sustained response even to very long stimuli (Fig. 5H, 5100 ms duration, same neuron as Fig. 5G). Phasic neurons were less common in the TLC than the IC (14% vs. 33%, Fig. 5I).
Figure 5. Responses in both the IC and TLC could be categorized as phasic or sustained to a contralateral tone burst at BF.
The intensity in each case was 70 dB SPL, and the duration was 75 ms for A–F and 5100 ms for G. PSTs were constructed from 30 repetitions and a 1 ms bin width, except for G which had 2 repetitions and a 30 ms bin width. A. A phasic response from the IC. BF = 2.3 kHz. B. A sustained response from the IC showing little adaptation. BF = 11.3 kHz. C. A sustained response from the IC showing a greater degree of adaptation BF=14.3 kHz. D. A sustained response from an IC neuron that showed chopping near the onset of the stimulus BF=6.7 kHz‥ E. A sustained response from the IC with a long-latency. BF = 6.7 kHz. F. A phasic response from the TLC. BF = 2.4 kHz. G. A sustained response from the TLC. BF = 24 .5 kHz. H. The same neuron as in G, showing a sustained response to a long stimulus duration (5100 ms). I. Distribution of sustained and onset type neurons in the IC and TLC. A higher proportion of TLC neurons had a sustained response.
Latencies
A major difference between the TLC and IC was the distribution of first spike latencies. Using high intensity tones (typically 70 dB SPL) at BF, the median latency in the TLC (18.6 ± 7.33 ms, n=33) was longer than in the IC (12.8 ± 5.12 ms, n=66), and this difference was significant (t-test, df=97, t=4.56,p<0.0001). Despite this difference, there was overlap between the distributions from the two nuclei (Fig. 6) and some TLC neurons had short latencies (<10 ms).
Figure 6. Distribution of response latencies for IC and TLC neurons.
Responses to SAM tones
Another difference between the TLC and the IC was a relative inability of TLC neurons to follow the envelope modulations of SAM tones. Sensitivity to the SAM tones was measured as functions of rate and synchronization index. A synchronization index of 1.0 means that the neuron consistently fired at the same point in the phase of the modulation cycle, while an index of zero means that the firing was random with regard to modulation phase. Figure 7 shows examples of typical responses to the SAM tones in each nucleus. Four neurons from the IC (Fig. 7A) each showed a strong effect of the modulation frequency on the firing rate (left panel) and a high degree of synchronization over a wide range of modulation frequencies (right panel). In four neurons from the TLC (Fig. 7B), there were only small effects of the modulation on the firing rate and significant synchronization was seen in only two of the neurons. In the two neurons with significant synchrony the maximum synchronization was low compared to that in the IC neurons and occurred for a smaller number of modulation frequencies. Of all neurons tested, 72% of IC neurons (23 of 32) showed significant synchronization to any of the modulation frequencies tested (typically 3.125–800 Hz in 0.5 octave steps) compared to only 38% of TLC neurons (11 of 29). The maximum synchronization values for the TLC were generally lower than for the IC (Fig. 7C). The mean synchronization for the TLC was 0.51 ± 0.186 (s.d) and for the IC was 0.76 ± 0.187, and the difference was significant (t-test, df = 32, t=3.69, p<.0001). In addition, the average frequency range over which significant synchronization occurred was reduced in the TLC compared to the IC (1.4 compared to 3.8 octaves) and the mean maximum frequency for synchronization was lower as well (94 compared to 220 Hz).
Figure 7. Examples of rate and synchronization modulation transfer functions to SAM tones.
The stimulus was 3 repetitions of an 1100 ms-long SAM tone to the contralateral ear at 70 dB SPL with carrier at BF and 100% modulation. A. IC neurons. These neurons were tuned to AM in discharge rate and showed a high degree of synchronization to the modulation frequency. B. TLC neurons. These neurons showed little tuning to AM in rate and had a low degree of synchronization to the modulation frequency. C. The distributions of maximum synchronization for neurons in the IC and TLC. The synchronization values in the TLC were in general lower than in the IC.
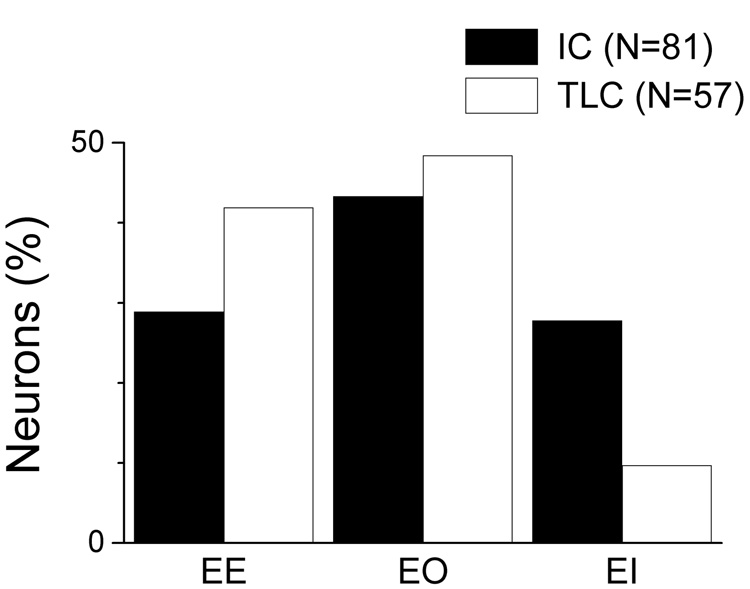
Binaural responses
A further difference between the TLC and IC was the distribution of binaural responses. Most TLC neurons were best driven contralaterally and ipsilateral effects were usually either absent or excitatory, with inhibition seen more rarely. In contrast, ipsilateral inhibition was common in the IC. The distribution of the three binaural response types in the two nuclei is shown in Fig. 8. Neurons with an EO binaural type (contralateral excitation, no ipsilateral response) were common in both nuclei, but in the TLC the EE type was common and the EI type was rare while in the IC the EE and EI binaural types were about equally distributed. Although tested in relatively few neurons, sensitivity to interaural phase was more common in IC neurons than in the TLC. In the IC, 6 of 10 neurons that showed a binaural interaction showed significant synchronization to interaural phase of the envelope (see Methods), while in the TLC only 3 of 13 neurons did so. The maximum synchronization in the IC was 0.55 and exceeded 0.25 in all six neurons, while in the TLC the maximum synchronization was less than 0.25 in all three neurons.
Figure 8. Binaural response types in the IC and TLC.
Distributions of EI, EO and EE neurons in both nuclei.
Dorsolateral periaqueductal gray
In addition to recording from the IC and TLC, we encountered neurons with distinctive auditory responsiveness on penetrations lateral to the TLC (see Fig. 3C). Histological verification showed that these neurons lay in the dorsolateral PAG. All of the responses (n=8) were similar; they showed an onset response to tones at CF or to noise, had a high threshold (>60 dB SPL), and a relatively short latency (6–12 ms). They also required a long interval between stimuli, as shown for one example in Fig. 9. The stimulus was low-pass noise (cut-off of 8000 Hz, 75 ms duration) at 80 dB SPL, to which the neuron gave an onset response with latency of ~7 ms. The neuron responded only occasionally at interstimulus intervals of 250 or 500 ms, responded more often for interstimulus intervals of 750 and 1000 ms, and responded to nearly every stimulus at interstimulus intervals of 1500 and 2000 ms.
Figure 9. Raster plots of responses from a neuron in the dorsolateral PAG as the interstimulus interval was changed.
Neurons in the dorsolateral PAG typically required long intervals before they would respond and then yielded a well-time spike at stimulus onset. The stimulus was low-pass noise (cut-off frequency of 16000 Hz) at 80 dB SPL in both ears. BF = 13.5 kHz.
DISCUSSION
This report provides the first detailed description of the response properties of neurons in the TLC to auditory stimuli. We observed distinct differences between the TLC and the IC, a well-studied auditory nucleus from which the TLC receives at least some of its input (Saldaña and Merchán, 1992; Saldaña and Merchán, 2005). Differences include a lack of tonotopy, higher thresholds, broader frequency tuning, increased prevalence of sustained over phasic firing, increased latencies, very limited sensitivity to amplitude modulation, and primarily excitation from both ears. These responses properties suggest a role for the TLC in modulation of descending auditory information.
Comparison of TLC and IC responses
The IC was studied along with the TLC for two reasons. First, the IC is a major nucleus in the ascending auditory pathway, with major properties in common with other auditory structures such as the auditory cortex. In addition, collaterals of commissural IC axons and axons projecting to the thalamus branch to innervate the TLC (Saldaña, unpublished observations). Thus, recording from the TLC and the IC in the same preparation allows for a comparison with one of the structures providing auditory input to the TLC. Second, the IC is well-studied in the rat and other species, so recordings in the IC provide an internal control for the quality of the recordings. Without this direct comparison, differences observed in a novel nucleus such as the TLC could be attributed to recording conditions. Because in general the responses in IC neurons were comparable to those in previous studies in the rat (Kelly et al., 1991; Palombi and Caspary, 1996), we have confidence that our recordings in the TLC are representative.
Measurements in the IC identified a variety of properties that have been previously described in rats. A dorsal-to-ventral tonotopy was clear, with the range of CFs and thresholds consistent with the behavioral audiogram (Kelly and Masterton, 1977). The frequency tuning of many IC neurons was “V” shaped but some were more broadly tuned (Kelly et al., 1991; Hernández et al., 2005). Responses could be phasic or sustained, and there was a variety of sustained types that could be identified in PST histograms (Palombi and Caspary, 1996). Latencies were often short, less than 10 ms, although the range extended to 30 ms. Sounds delivered to the contralateral ear were typically excitatory, while ipsilateral sounds could increase the contralateral response, decrease it, or have no effect, as in previous studies (Kelly et al., 1991; Kelly and Li, 1997). And finally, IC neurons showed a high degree of phase-locking to amplitude modulated stimuli, as previously seen (Rees and Møller, 1983; Rees and Møller, 1987; Palombi et al., 2001).
Unlike the IC, there was no obvious tonotopy in the TLC. We observed neurons in close proximity to have a wide range of frequency tuning, and across all penetrations there was no tonotopy observed in the rostrocaudal dimension. The IC contributes to the innervation of the TLC, and single fibers from the IC can extend for long distances in the TLC giving off putative terminals at a wide range of rostrocaudal locations (Saldaña, unpublished observations). This observation is consistent with our finding of no tonotopic order in the rostrocaudal axis. However, a more complex tonotopy remains possible, as for example in the radial dimension as observed in the dorsal nucleus of the lateral lemniscus in rats (Merchán et al., 1994), or the mosaic-like pattern seen the ventral nucleus of the lateral lemniscus of cats (Malmierca et al, 1998).
On average, neurons in the TLC had higher thresholds and were less sharply tuned than IC neurons (Fig. 4). The differences were small, and neurons with relatively sharp, “V” shaped tuning curves with low thresholds were also seen, so frequency tuning and a high degree of sensitivity remain important properties of TLC neurons. Still, the relatively high thresholds and broad tuning of many TLC neurons, as well as the overall greater degree of sustained firing even to very long stimulus durations (5 s), indicates that TLC neurons may be sensitive to high levels of background sound levels rather than identification of foreground sources. Sensitivity to background sound levels is necessary for adjusting the sensitivity of the auditory system and improving signal detection in noisy environments, an important role of the descending auditory system.
A role in descending rather than ascending auditory information is further suggested by the long latencies in the TLC compared to the IC. The median latency in the TLC (18.6 ms) is longer than that of neurons in the rat’s primary auditory cortex (Doron et al., 2002). However, the distribution of latencies in the TLC was quite broad, and some of the neurons had short latencies (< 10 ms). These neurons may show the influence of the direct projections from the IC or other auditory nuclei of the brainstem.
The poor ability of neurons in the TLC to phase-lock to the envelopes of SAM tones is also indicative of a level quite removed from the periphery. The upper end of AM frequencies to which neurons can synchronize declines from the auditory nerve to the IC and then declines further at the level of the auditory cortex (reviewed by Langner, 1992; Joris et al., 2004). The level of synchronization to frequencies below the upper cut-off does not decline, however, and sensitivity to higher modulation frequencies may be replaced to some extent by a rate rather than a temporal code (Langner, 1992; Lu et al., 2001; Liang et al., 2002). The poor ability of TLC neurons to synchronize to even low SAM frequencies or to show rate changes with SAM is unusual in the auditory system.
Finally, the responses to binaural stimulation in the TLC were unlike those in the IC. In most TLC neurons, input from the ipsilateral side either had no effect or was excitatory. In contrast, ipsilateral inhibition was common in IC neurons. Ipsilateral inhibition is important for encoding the interaural level differences (Irvine, 1986), a relevant cue for sound localization in the azimuthal dimension. In addition, neurons in the TLC were in general insensitive to interaural phase differences, unlike those in the IC. These binaural properties suggest that the TLC does not have a specific role in auditory spatial processing.
Auditory response properties of TLC neurons differ from those of SC neurons
The responses of TLC neurons to auditory stimuli also differed from those previously reported from the deep and intermediate layers of the superior colliculus (SC), and this difference strengthens the identity of the TLC as a separate nucleus. The characteristics of auditory SC neurons differ substantially from those of TLC neurons in several ways. First, in the rat, up to 21% of neurons in the SC respond to both visual and auditory stimuli (Gaese and Johnen, 2000). In contrast, the TLC neurons in our sample only responded to auditory stimuli. Second, in the intermediate and deep layers of the cat SC, the distribution of CFs is biased toward the higher frequency end of the animal’s audiogram (Wise and Irvine, 1983; Hirsch et al., 1985). No such bias was seen in the TLC. Third, only about 20% of SC neurons have a sustained response to acoustic stimuli (Hirsch et al., 1985). In contrast, more than 80% of TLC neurons showed a sustained response. To our knowledge, no previous studies have recorded in the medialmost part of the deep SC of the rat, so differences between TLC and SC neurons have previously gone unreported.
In addition to the lack of visual responses in the TLC, there was a 200–300 µm-deep zone between the end of visual responses in the SC and the beginning of auditory responses in the TLC that was unresponsive to either stimulus. The histology of this “dorsal column” also differs both from the TLC and from the deep SC (Saldaña et al., 2007).
Functional considerations
The physiological and anatomical evidence both indicate a role for the TLC in the descending auditory system. The descending auditory system is diffuse, consisting of many independent connections beginning in the auditory cortex and including each level down to projections from the brainstem to the cochlea itself (Huffman and Henson, 1990; Saldaña et al., 1996; Feng and Ratnam, 2000). It is unlikely that this system has a single functional role. At the level of the SOC and olivocochlear neurons, roles for the descending system include improving hearing in noise, assisting in discrimination between two signals in noise, and helping to block out irrelevant acoustic input while attending to visual stimuli (Feng and Ratnam, 2000). In general, the properties of TLC neurons suggest a role in reducing the effect of background noise rather than in the identification or localization or specific acoustic signals. In particular, the lack of sensitivity to SAM or binaural cues, as commonly seen in neurons of the ascending auditory system, makes a role in identification or localization for TLC neurons unlikely. In contrast, the long latencies, broad tuning and sustained responses of TLC neurons, even to very long stimuli, suggests that the neurons could be sensitive to ongoing signals that represent “noise” in that they do not convey specific information. Although not thoroughly quantified in most neurons, TLC neurons were in general responsive to our noise search stimulus.
The position of the TLC in the tectum suggests a role in multisensory integration. Although our crude visual stimulation did not result in responses of TLC neurons, it is not known if the responses to sound might be modulated by visual stimulation or eye position. The TLC is well placed to receive such information, as the nucleus is crossed by fibers in the commissure of the superior colliculus, a complex tract that contains axons from over forty tectal and non-tectal sources (Huerta and Harting, 1984). Although the contribution of each of these crossed projections to the innervation of the TLC remains unknown, the TLC probably receives direct input from commissural SC neurons, whose axons give off collaterals in the vicinity of the midline in both the rat (Yamasaki et al., 1984) and the hamster (Rhoades et al., 1986; Chebat et al., 2006).
In terms of more specific effects, targets of TLC projections include the superior paraolivary nucleus (SPON) and ventral nucleus of the (VNTB) of the superior olivary complex. The SPON is a major source of GABAergic inhibition to the IC (Kulesza and Berrebi, 2000). The responses of SPON neurons in the rat are primarily to the offset of contralateral stimuli and represent rebound responses from the strong inhibition provided by the medial nucleus of the trapezoid body (MNTB) in response to sounds in the contralateral ear. Because the responses to the offsets are well-timed, SPON neurons show a high degree of phase-locking to amplitude modulated stimuli (Kulesza et al., 2003). Similar responses were reported from the superior olivary complex of rabbits (Kuwada and Batra, 1999). The only tectal projections to the SPON originate in the TLC (Saldaña, unpublished observations), and previous data using staining for glutamic acid decarboxylase, the synthetic enzyme for GABA production, suggest that most TLC neurons are GABAergic (Mugnaini and Oertel, 1985). If so, the TLC inputs could contribute as late arriving sustained inhibition. By arriving later than the inputs from the MNTB, the inhibition may be able to limit or modulate the rebound response provided by the SPON neurons. Such an effect of GABA on SPON neuron has been seen, and although tentatively attributed to intrinsic collaterals of SPON neurons, the neurons of the TLC could also contribute to this effect (Kulesza et al., 2007).
In addition to projections from the TLC, the VNTB receives direct projections from the IC (Faye-Lund, 1986; Vetter et al., 1993) and the auditory cortex (Feliciano et al., 1995; Mulders and Robertson, 2000). Some of its neurons send projections to the cochlea as part of the medial olivocochlear system (Vetter and Mugnaini, 1992; Warr and Boche, 2003). Responses of VNTB neurons are little known, but in both the rat (Warr and Beck, 1996) and the guinea pig (Schofield and Cant, 1992) this nucleus has heterogeneous neural populations that, in addition to the cochlea, also innervate the IC and the cochlear nucleus. In considering a functional role for the TLC to VNTB projection it would be of interest to determine which population of VNTB neurons receives projection from the TLC.
Auditory responsive neurons of the dorsolateral PAG
In penetrations made just lateral to the TLC we encountered distinct auditory responses in the dorsolateral PAG. These neurons had short-latency onset responses, high thresholds, and required long intervals between stimuli. These responses are consistent with a role in behaviors related to the acoustic startle reflex. Interestingly, López et al. (1999) have reported a minor projection to the periaqueductal gray from the cochlear root neurons, a neuron type that constitutes the first step in the acoustic startle reflex (Merchán et al., 1988; López et al., 1993; Lee et al., 1996).
The sources of auditory input to the dorsolateral PAG are currently unknown. While the dorsolateral PAG lacks the conspicuous spinal and medullary inputs that target other PAG columns, it is innervated by projections from the prefrontal cortex and, to a lesser extent, the hypothalamus (reviewed by Keay and Bandler, 2004). In addition to auditory input from cochlear root neurons, other auditory input may also arrive via the IC, as indicated in a recent preliminary report (Larue et al., 2005). Clearly, considerable research on the connections of the dorsolateral PAG and the morphofunctional features of its auditory responsive neurons needs to be done before our observations can be placed in a meaningful biological context.
Summary
The auditory responses of TLC neurons differ greatly from those typical of neurons in nuclei that form the core of the ascending auditory pathway, such as the central nucleus of the IC. The responses characteristics of TLC neurons suggest a role in processing background rather than foreground features of the auditory scene and its position in the mammalian tectum suggest a role in sensorimoter integration of auditory information with other modalities.
Acknowledgments
Support: Research supported (in part) by NIH Short Term Research Training Grant, the UNC Medical Alumni Association Endowment Fund, and by NIH grant DC-03948 to DCF, and by grants MCyT (BFI2000-1358 and BFU2004-05909) and JCyL (SA079/01 and SA007C05) to E.S.
List of abbreviations
- BF
Best frequency
- CF
Characteristic frequency
- DC
dorsal column
- GABA
Gamma-aminobutyric acid
- E
Excitatory response
- I
Inhibitory response
- IC
Inferior colliculus
- MNTB
medial nucleus of the trapezoid body
- O
No response
- PAG
Periaqueductal grey
- PST
Post-stimulus time histogram
- SAM
Sinusoidal amplitude modulation
- SC
Superior colliculus
- SPL
Sound pressure level
- SPON
Superior paraolivary nucleus
- TLC
Tectal longitudinal column
- VNTB
Ventral nucleus of the trapezoid body
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bock GR, Steel KP. Use of albino animals for auditory research. Hear Res. 1984;13:201–202. doi: 10.1016/0378-5955(84)90109-6. [DOI] [PubMed] [Google Scholar]
- Chebat DR, Boire D, Ptito M. Development of the commissure of the superior colliculus in the hamster. J Comp Neurol. 2006;494:887–902. doi: 10.1002/cne.20856. [DOI] [PubMed] [Google Scholar]
- Doron NN, Ledoux JE, Semple MN. Redefining the tonotopic core of rat auditory cortex: physiological evidence for a posterior field. J Comp Neurol. 2002;453:345–360. doi: 10.1002/cne.10412. [DOI] [PubMed] [Google Scholar]
- Faye-Lund H. Projection from the inferior colliculus to the superior olivary complex in the albino rat. Anat Embryol (Berl) 1986;175:35–52. doi: 10.1007/BF00315454. [DOI] [PubMed] [Google Scholar]
- Feliciano M, Saldaña E, Mugnaini A. Direct projections from the rat primary auditory neocortex to nucleus sagulum, paralemniscal regions, superior olivary complex and cochlea nuclei. Auditory Neurosci. 1995;1:287–308. [Google Scholar]
- Feng AS, Ratnam R. Neural basis of hearing in real-world situations. Annu Rev Psychol. 2000;51:699–725. doi: 10.1146/annurev.psych.51.1.699. [DOI] [PubMed] [Google Scholar]
- Gaese BH, Johnen A. Coding for auditory space in the superior colliculus of the rat. Eur J Neurosci. 2000;12:1739–1752. doi: 10.1046/j.1460-9568.2000.00054.x. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Brown PB. Response of binaural neurons of dog superior olivary complex to dichotic tonal stimuli: some physiological mechanisms of sound localization. J. Neurophysiol. 1969;32:613–636. doi: 10.1152/jn.1969.32.4.613. [DOI] [PubMed] [Google Scholar]
- Hernández O, Espinosa N, Pérez-González D, Malmierca MS. The inferior colliculus of the rat: a quantitative analysis of monaural frequency response areas. Neuroscience. 2005;132:203–217. doi: 10.1016/j.neuroscience.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Hirsch JA, Chan JC, Yin TC. Responses of neurons in the cat's superior colliculus to acoustic stimuli. I. Monaural and binaural response properties. J Neurophysiol. 1985;53:726–745. doi: 10.1152/jn.1985.53.3.726. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Harting JK. The mammalian superior colliculus: studies of its morphology and connections. In: Vanegas H, editor. Comparative neurology of the optic tectum. New York: Plenum Press; 1984. pp. 687–773. [Google Scholar]
- Huffman RF, Henson OW., Jr The descending auditory pathway and acousticomotor systems: connections with the inferior colliculus. Brain Res Rev. 1990;15:295–323. doi: 10.1016/0165-0173(90)90005-9. [DOI] [PubMed] [Google Scholar]
- Irvine DRF. The Auditory Brainstem. New York: Progress in Sensory Physiology Springer-Verlag; 1986. [Google Scholar]
- Joris PX, Schreiner CE, Rees A. Neural processing of amplitude-modulated sounds. Physiol Rev. 2004;84:541–577. doi: 10.1152/physrev.00029.2003. [DOI] [PubMed] [Google Scholar]
- Kelly JB, Masterton B. Auditory sensitivity of the albino rat. J Comp Physiol Psychol. 1977;91:930–936. doi: 10.1037/h0077356. [DOI] [PubMed] [Google Scholar]
- Kelly JB, Li L. Two sources of inhibition affecting binaural evoked responses in the rat's inferior colliculus: the dorsal nucleus of the lateral lemniscus and the superior olivary complex. Hear Res. 1997;104:112–126. doi: 10.1016/s0378-5955(96)00182-7. [DOI] [PubMed] [Google Scholar]
- Kelly JB, Glenn SL, Beaver CJ. Sound frequency and binaural response properties of single neurons in rat inferior colliculus. Hear. Res. 1991;56:273–280. doi: 10.1016/0378-5955(91)90177-b. [DOI] [PubMed] [Google Scholar]
- Kulesza RJ, Viñuela A, Saldaña E, Berrebi AS. Unbiased stereological estimates of neuron number in subcortical auditory nuclei of the rat. Hear Res. 2002;168:12–24. doi: 10.1016/s0378-5955(02)00374-x. [DOI] [PubMed] [Google Scholar]
- Kulesza RJ, Jr, Berrebi AS. Superior paraolivary nucleus of the rat is a GABAergic nucleus. J Assoc Res Otolaryngol. 2000;1:255–269. doi: 10.1007/s101620010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesza RJ, Jr, Spirou GA, Berrebi AS. Physiological response properties of neurons in the superior paraolivary nucleus of the rat. J Neurophysiol. 2003;89:2299–2312. doi: 10.1152/jn.00547.2002. [DOI] [PubMed] [Google Scholar]
- Kulesza RJ, Jr, Kadner A, Berrebi AS. Distinct roles for glycine and GABA in shaping the response properties of neurons in the superior paraolivary nucleus of the rat. J Neurophysiol. 2007;97:1610–1620. doi: 10.1152/jn.00613.2006. [DOI] [PubMed] [Google Scholar]
- Kuwada S, Batra R. Coding of sound envelopes by inhibitory rebound in neurons of the superior olivary complex in the unanesthetized rabbit. J Neurosci. 1999;19:2273–2287. doi: 10.1523/JNEUROSCI.19-06-02273.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner G. Periodicity coding in the auditory system. Hear Res. 1992;60:115–142. doi: 10.1016/0378-5955(92)90015-f. [DOI] [PubMed] [Google Scholar]
- Larue DT, Prieto JJ, Winer JA. The central gray and the inferior colliculus: An auditory-limbic interface. Assoc. for Res. Otolaryngol. Abs. 2005;28:242. [Google Scholar]
- Lee Y, López DE, Meloni EG, Davis M. A primary acoustic startle pathway: obligatory role of cochlear root neurons and the nucleus reticularis pontis caudalis. J Neurosci. 1996;16:3775–3789. doi: 10.1523/JNEUROSCI.16-11-03775.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Lu T, Wang X. Neural representations of sinusoidal amplitude and frequency modulations in the primary auditory cortex of awake primates. J Neurophysiol. 2002;87:2237–2261. doi: 10.1152/jn.2002.87.5.2237. [DOI] [PubMed] [Google Scholar]
- López DE, Merchán MA, Bajo VM, Saldaña E. The cochlear root neurons in the rat, mouse and gerbil. In: Merchán MA, Juiz JM, Godfrey DA, Mugnaini E, editors. The Mammalian Cochlear Nuclei: Organization and Function, NATO ASI Series. New York: Plenum Publishing Co.; 1993. pp. 291–301. [Google Scholar]
- López DE, Saldaña E, Nodal FR, Merchán MA, Warr WB. Projections of cochlear root neurons, sentinels of the rat auditory pathway. J Comp Neurol. 1999;415:160–174. [PubMed] [Google Scholar]
- Lu T, Liang L, Wang X. Temporal and rate representations of time-varying signals in the auditory cortex of awake primates. Nat Neurosci. 2001;4:1131–1138. doi: 10.1038/nn737. [DOI] [PubMed] [Google Scholar]
- Mardia KV, Jupp PE. Directional Statistics. New York: John Wiley and Sons, Inc; 1999. [Google Scholar]
- Merchán MA, Saldaña E, Plaza I. Dorsal nucleus of the lateral lemniscus in the rat: concentric organization and tonotopic projection to the inferior colliculus. J. Comp. Neurol. 1994;342:259–278. doi: 10.1002/cne.903420209. [DOI] [PubMed] [Google Scholar]
- Merchán MA, Collia F, López DE, Saldaña E. Morphology of cochlear root neurons in the rat. J Neurocytol. 1988;17:711–725. doi: 10.1007/BF01260998. [DOI] [PubMed] [Google Scholar]
- Morest DK, Oliver DL. The neuronal architecture of the inferior colliculus in the cat: defining the functional anatomy of the auditory midbrain. J. Comp. Neurol. 1984;222:209–236. doi: 10.1002/cne.902220206. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Oertel WH. An atlas of the distribution of GABAergic neurons and terminals in the rat CNS as revealed by GAD immunocytochemistry. In: Björlund A, Hökfelt T, editors. Handbook of chemical neuroanatomy, Vol. 4: GABA and Neuropeptides in the CNS, Part I. Amsterdam: Elsevier; 1985. pp. 436–608. [Google Scholar]
- Mulders WH, Robertson D. Evidence for direct cortical innervation of medial olivocochlear neurones in rats. Hear Res. 2000;144:65–72. doi: 10.1016/s0378-5955(00)00046-0. [DOI] [PubMed] [Google Scholar]
- Palombi P, Backoff PM, Caspary DM. Responses of young and aged rat inferior colliculus neurons to sinusoidally amplitude modulated stimuli. Hear Res. 2001;153:174–180. doi: 10.1016/s0378-5955(00)00264-1. [DOI] [PubMed] [Google Scholar]
- Palombi PS, Caspary DM. Physiology of the young adult Fischer 344 rat inferior colliculus: responses to contralateral monaural stimuli. Hear Res. 1996;100:41–58. doi: 10.1016/0378-5955(96)00115-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. San Diego: Academic Press; 1998. [Google Scholar]
- Rees A, Møller AR. Responses of neurons in the inferior colliculus of the rat to AM and FM tones. Hearing Res. 1983;10:301–330. doi: 10.1016/0378-5955(83)90095-3. [DOI] [PubMed] [Google Scholar]
- Rees A, Møller AR. Stimulus properties influencing the responses of inferior colliculus neurons to amplitude-modulated sounds. Hear Res. 1987;27:129–143. doi: 10.1016/0378-5955(87)90014-1. [DOI] [PubMed] [Google Scholar]
- Rhoades RW, Mooney RD, Szczepanik AM, Klein BG. Structural and functional characteristics of commissural neurons in the superior colliculus of the hamster. J Comp Neurol. 1986;253:197–215. doi: 10.1002/cne.902530207. [DOI] [PubMed] [Google Scholar]
- Saldaña E, Merchán MA. Intrinsic and commissural connections of the rat inferior colliculus. J Comp Neurol. 1992;319:417–437. doi: 10.1002/cne.903190308. [DOI] [PubMed] [Google Scholar]
- Saldaña E, Merchán MA. Intrinsic and commissural connections of the inferior colliculus. In: Winer JA, Schreiner CE, editors. The Inferior Colliculus. New York: Springer; 2005. pp. 155–181. [Google Scholar]
- Saldaña E, Feliciano M, Mugnaini E. Distribution of descending projections from primary auditory neocortex to inferior colliculus mimics the topography of intracollicular projections. J Comp Neurol. 1996;371:15–40. doi: 10.1002/(SICI)1096-9861(19960715)371:1<15::AID-CNE2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Saldaña E, Viñuela A, Marshall AF, Fitzpatrick DC, Aparicio MA. The TLC: a novel auditory nucleus of the mammalian brain. J Neurosci. 2007;27:13108–13116. doi: 10.1523/JNEUROSCI.1892-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield BR, Cant NB. Organization of the superior olivary complex in the guinea pig: II. Patterns of projection from the periolivary nuclei to the inferior colliculus. J. Comp. Neurol. 1992;317:438–455. doi: 10.1002/cne.903170409. [DOI] [PubMed] [Google Scholar]
- Vetter DE, Mugnaini E. Distribution and dendritic features of three groups of rat olivocochlear neurons. A study with two retrograde cholera toxin tracers. Anat Embryol (Berl) 1992;185:1–16. doi: 10.1007/BF00213596. [DOI] [PubMed] [Google Scholar]
- Vetter DE, Saldaña E, Mugnaini E. Input from the inferior colliculus to medial olivocochlear neurons in the rat: a double label study with PHA-L and cholera toxin. Hear Res. 1993;70:173–186. doi: 10.1016/0378-5955(93)90156-u. [DOI] [PubMed] [Google Scholar]
- Warr WB, Beck JE. Multiple projections from the ventral nucleus of the trapezoid body in the rat. Hear Res. 1996;93:83–101. doi: 10.1016/0378-5955(95)00198-0. [DOI] [PubMed] [Google Scholar]
- Warr WB, Boche JE. Diversity of axonal ramifications belonging to single lateral and medial olivocochlear neurons. Exp Brain Res. 2003;153:499–513. doi: 10.1007/s00221-003-1682-3. [DOI] [PubMed] [Google Scholar]
- Wise LZ, Irvine DR. Auditory response properties of neurons in deep layers of cat superior colliculus. J Neurophysiol. 1983;49:674–685. doi: 10.1152/jn.1983.49.3.674. [DOI] [PubMed] [Google Scholar]
- Yamasaki DS, Krauthamer G, Rhoades RW. Organization of the intercollicular pathway in rat. Brain Res. 1984;300:368–371. doi: 10.1016/0006-8993(84)90848-5. [DOI] [PubMed] [Google Scholar]