Abstract
The ventilatory response to hypoxia depends on the carotid body function and sleep-wake states. Therefore, the response must be measured in a consistent sleep-wake state. In mice, EMG with behavioral indices (coordinated movements, CMs; myoclonic twitches, MTs) has been used to assess sleep-wake states. However, in neonatal mice EMG instrumentation could induce stress, altering their behavior and ventilation. Accordingly, we examined: (1) if EMG can be eliminated for assessing sleep-wake states; (2) behavioural characteristics and carotid body-mediated respiratory control during sleep with EMG (EMG+) or without EMG (EMG−). Seven-day-old DBA/2J and A/J mice were divided into EMG+ and EMG− groups. In both strains, CMs occurred when EMG was high; MTs were present during silent/low EMG activity. The durations of high EMG activity and of CMs were statistically indifferent. Thus, CMs can be used to indicate wake state without EMG. The stress caused by EMG-instrumentation may be distinctively manifested based on genetic background. Prolonged agitation was observed in some EMG+ DBA/2J (5 of 13), but not in A/J mice. The sleep time and MT counts were indifferent between the groups in DBA/2J mice. The EMG+ A/J group showed longer sleep time and less MT counts than the EMG− A/J group. Mean respiratory variables (baseline, hyperoxic/hypoxic responses) were not severely influenced by EMG+ in either strain. Individual values were more variable in EMG+ mice. Carotid body-mediated respiratory responses (decreased ventilation upon hyperoxia and increased ventilation upon mild hypoxia) during sleep were clearly observed in these neonatal mice with or without EMG instrumentation.
Keywords: Carotid body, control of breathing, electromyogram, hyperoxia, hypoxia
Introduction
The carotid body is the primary mammalian oxygen sensor responsible for initiating reflex responses in many organs (Fitzgerald and Shirahata, 1997). Prominent responses to hypoxia and hyperoxia are the increase and the decrease in ventilation, respectively. Thus, ventilatory responses to changing inspired oxygen tension have been used for assessing the function of the carotid body with precautions, because these responses are also dependent on the central respiratory control system. Sleep-wake state influences the central respiratory control system (Reviewed by Krimsky and Leiter, 2005;Shea, 1996;Phillipson and Bowes, 1986): the ventilatory response to hypoxia is attenuated during sleep compared to awake. Therefore, the ventilatory responses to hypoxia must be measured under a known sleep-wake state.
Traditional sleep-wake classification in large mammals has been performed by assessing the electroencephalogram (EEG), electromyogram (EMG) and eye movement (electrooculogram; EOG) (McGinty and Beahm, 1984). In the adult mouse, however, sleep-wake states have been classified based on EEG and EMG recordings exclusively without EOG (Schaub et al., 1998;Tagaito et al., 2001). EOG has been omitted due to the apparent technical difficulties. These methods have been widely accepted, but EEG instrumentation in neonatal rodents is challenging. Recently, two groups of investigators have suggested that sleep can be objectively defined in neonatal rodents based on nuchal EMG and behavioral analysis without EEG (Karlsson and Blumberg, 2002;Karlsson et al., 2004;Durand et al., 2005). Although these studies used EMG instrumentation to provide important information regarding sleep and state-dependent breathing in neonates, some issues remain unresolved. For example, EMG instrumentation may be injurious to neonates, cause stress to the animals, and possibly change sleep-wake architecture. EMG instrumentation in neonates also requires some restraint during measurement. This may influence baseline respiration and the response to hypoxia (Dauger et al., 1998). Stress related to EMG instrumentation and restrain can be avoided if sleep-wake states can be determined by behavioral indices exclusively. Accordingly, the purposes of the current study were: (1) to test if EMG can be eliminated to assess sleep-wake states; (2) to examine behavioral characteristics and carotid body-mediated respiratory control during sleep with EMG (EMG+) or without EMG (EMG−). We assessed our objectives using two inbred strains of mice: DBA/2J and A/J. These strains of mice have been our models for studying the carotid body function, morphology, genetics and development (Balbir et al., 2006;Balbir et al., 2007;Yamaguchi et al., 2003). Further, adult mice of these two strains have demonstrated disparate behavioral responses under certain stress conditions (Bouwknecht and Paylor, 2002).
Results
Animals
Table 1 shows the basic animal characteristics for the mice. The body weight and temperature of the pups were not significantly different among the four groups (one-way ANOVA, p> 0.05). EMG− animals settled in the chamber within 15 minutes. Some EMG+ animals showed continual attempts to dislodge the EMG electrodes by sudden movements or scratching. When these behaviors continued more than 30 minutes, we considered the animals were under prolonged stress. Five DBA/2J mice that were instrumented with EMG showed prolonged stress (see below) and were excluded from Table 1 and further analysis.
Table 1.
Basic physical characteristics of the animals
| Strain | DBA/2J | A/J | ||
|---|---|---|---|---|
| Group | EMG+ | EMG+ | EMG+ | EMG− |
| n | 8 | 11 | 7 | 7 |
| Weight (g) | 4.09 ± 0.30 | 4.17 ± 0.20 | 3.84 ± 0.37 | 4.04 ± 0.06 |
| Temperature (°C) | 34.5 ± 0.1 | 34.2 ± 0.3 | 34.3 ± 0.2 | 34.4 ± 0.4 |
Behavior and breathing during the initial 15-min observation phase
An example of typical recordings for EMG, behavior (movement of animals), and breathing is shown in Figure 1A. Myoclonic twitch (MT) was defined as phasic, rapid, and independent movement of a limb or a tail. It was recorded as a short pulse. MTs were observed while basal EMG activity was silent to low. Most MTs (88 ± 3% in the DBA/2J strain and 77 ± 7% in the A/J strain) corresponded to an EMG spike burst which lasted less than one second. Some MTs, such as tail movement, were not accompanied by EMG spikes. During silent/low EMG activity, respiratory traces had little noise and we were able to measure the respiratory frequency (fR) and the tidal volume (VT) without major difficulties. Coordinated movement (CM) was defined as the prolonged movement of multiple limbs and the head. It was recorded as a square wave trace in Fig 1A. CMs were observed only with high EMG activity which was clearly distinguished from silent/low EMG activity. During high EMG activity, respiratory traces were often distorted due to movement artifact. The total duration of high EMG activity and that of CMs (seconds) were similar within an individual mouse during the observation phase (15 minutes), although variations were observed between different mice. No significant difference was found between the total duration of high EMG activity and the duration of CM within the strain (Figure 1B). DBA/2J mice spent significantly more time in the high EMG/CM state than A/J mice.
Figure 1.
Sleep-wake assessment in 7-day old mice. (A) Sample tracing of nuchal EMG activity, behavior, and breathing in EMG-instrumented pups during the sleep observation phase. Sleep was defined as silent to low EMG activity with or without the presence of myclonic twitches (MTs). Arrows show EMG spikes (< 1 sec burst) that correspond to MTs. During sleep the respiratory trace was stable. Awake was defined as high EMG activity and the presence of coordinated movement (CM) for an extended time period. During the awake period, the respiratory trace was unstable due to movement artifact. (B) High EMG activity and CM are closely associated. The total duration of high EMG activity and that of CMs (seconds) were similar within an individual mouse during the observation phase (15 minutes), although variations were observed between different mice. The duration of high EMG events and the duration of CMs did not differ significantly in either strain (p>0.05; unpaired t-test). Values are means ± SEM.
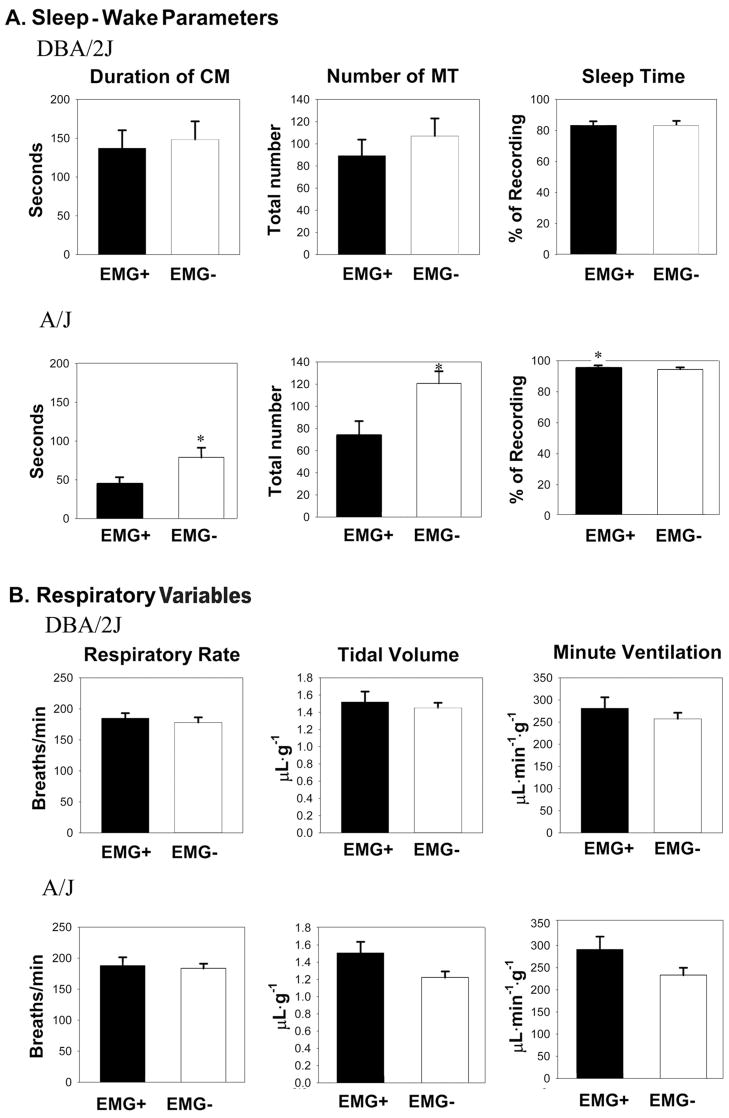
The effects of the EMG-instrumentation on behaviour were strain dependent. In the DBA/2J mice, 13 pups were instrumented with EMG electrodes and showed agitated behaviour: They attempted to dislodge the electrodes by continual scratching and sudden movements of the whole body. Five pups did not stop these behaviors for more than 30 minutes and were excluded from the further experiments. The EMG− pups adapted to the chamber within 15 minutes. On the other hand, EMG+ A/J pups did not show any attempts to dislodge the electrode. Further, all A/J pups settled in the chamber within 15 minutes. Other behavior parameters are shown in Figure 2A. In the DBA/2J strain, EMG-instrumentation did not significantly affect the CM duration or the total number of MTs. However, in the A/J strain, the duration of CM and the number of MTs was significantly greater in the pups without EMG compared to the pups with EMG. In the EMG+ pups, sleep was defined as silent to low nuchal EMG activity with the presence of MTs and the absence of CM. In the EMG− pups, sleep was defined as periods of no CMs with the presence of MTs. No differences were observed in the sleep time between the EMG+ and EMG− in DBA/2J mice. However, longer sleep time was observed in EMG+ A/J mice compared to EMG− A/J mice.
Figure 2.
Comparison of sleep-wake parameters and respiratory variables between the pups with (EMG+) and without EMG instrumention (EMG−). (A) Total CM durations during the 15 minute observation period did not differ significantly between the EMG+ and EMG− DBA/2J pups. CM duration was greater in the EMG−A/J pups versus the EMG+ A/J pups. The total number of MTs in both groups was not significantly different in the DBA/2J strain (middle panel). The total number of MTs was greater in the EMG−A/J pups versus the EMG+ A/J pups. Sleep time between the two groups was not significantly different in the DBA/2J strain. However, sleep time was greater in the EMG+ A/J pups compared to its EMG− counterpart (right panel). (B) Respiratory variables (fR, VT, V̇E) were measured during the periods of sleep. All respiratory variables during the sleep observation phase did not differ significantly between the two groups of pups in either strain (unpaired t-test). Values are means ± SEM and significance is denoted by a * p< 0.05, unpaired t-test.
As mentioned above, CMs distorted the respiratory trace, and we measured respiratory variables (fR, VT) during the periods without CM. The EMG instrumentation did not influence baseline fR, VT, and therefore, calculated minute ventilation (V̇E; μL · min−1 · g−1; = fR × VT) in both strains of pups. However, baseline fR varied more in EMG+ A/J mice compared to EMG− A/J mice, as seen by a larger SEM (Table 2) and a larger coefficient of variation (CV; more variable) in EMG+ A/J mice (Fig. 3).
Table 2.
Ventilatory responses to hyperoxia and hypoxia in 7-day old mice (mean ± SEM)
| Strain | DBA/2J | A/J | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | EMG+ (n=8) | EMG− (n=11) | EMG+ (n=7) | EMG− (n=7) | ||||||||||||
| Gas (%O2) | 21% | 100% | 15% | 21% | 21% | 100% | 15% | 21% | 21% | 100% | 15% | 21% | 21% | 100% | 15% | 21% |
| fR breath/min | 170 ± 5 | 128 ± 7 * | 218 ± 8 * | 178 ± 9 | 170 ± 7 | 125 ± 5 * | 205 ± 7 * | 173 ± 4 | 184 ± 17 | 147 ± 12 * | 221 ± 22 * | 197 ± 17 | 173 ± 6 | 125 ± 5 * | 243 ± 10 * | 176 ± 6 |
| VT μL/g | 1.46 ± 0.10 | 1.41± 0.14 | 1.57 ± 0.08 | 1.41 ± 0.11 | 1.46 ± 0.09 | 1.42 ± 0.14 | 1.46 ± 0.07 | 1.41 ± 0.11 | 1.49 ± 0.12 | 1.23 ± 0.09* | 1.36 ± 0.09 | 1.30 ± 0.08 | 1.30 ± 0.06 | 1.36 ± 0.15 | 1.42 ± 0.07 | 1.38 ± 0.08 |
| V̇E μL/min/g | 251.9 ± 25.5 | 165.2 ± 19.6* | 360.1 ± 30.7 * | 240.0 ± 16.3 | 244.9 ± 13.5 | 160.5 ± 18.5 * | 305.8 ± 24.7 * | 234.5 ± 18.5 | 271.3 ± 29.2 | 176.9 ± 12.2 * | 274.9 ± 38.4 | 253.8 ± 22.1 | 224.8 ± 9.9 | 170.5 ± 19.8 | 344.2 ± 23.9 * | 241.4 ± 12.1 |
, significantly different from the control (21%) (ANOVA, repeated measure; <0.05).
Figure 3.
Scatter plots of respiratory frequency changes in response to different oxygen tensions. Pups were either EMG-instrumented (closed symbols; EMG+) or without EMG (open symbols; EMG−). Each symbol represents one animal. In general, values were more variable in EMG+ animals. In particular, EMG+ A/J mice showed a larger coefficient of variation (CV) at every phase of gas challenges.
Ventilatory responses in the gas challenge phase
Gas challenges (hyperoxia and hypoxia) were introduced during periods without high EMG and/or CMs. In general, both strains of mice with or without EMG responded to hyperoxia and hypoxia (Table 2). Changes in fR were predominant, and hyperoxia decreased fR and V̇E, and hypoxia increased fR and V̇E. All respiratory variables returned to control levels during recovery. Although mean values were similar between the EMG+ and EMG− animals, the scatter plots of the fR indicate more variations in the animals with EMG (Fig. 3).
Discussion
Ventilatory responses to hyperoxia and/or hypoxia have been often used to assess the carotid body function. These measurements are particularly useful in clinical settings or in small animals, where the direct measurements of the carotid body output are practically impossible. However, ventilatory responses are also dependent on sleep-wake states, and therefore, ventilatory responses must be measured in a known sleep-wake condition. The standard recordings of the EEG, EMG and EOG for determining sleep-wake states cannot always be applied to small and developing animals. In the neonatal mouse, EEG instrumentation and EOG recording is not feasible. However, Karlsson & Blumberg (2002) and Karlsson et al. (2004) established a two-state model (sleep and wake) for neonatal rats using EMG and behavioral criteria. Our question was whether we can determine sleep-wake states using exclusively behavioral indices. We found that CMs were useful indices. They were observed only during periods of high EMG activity (Figure 1). The duration of CMs was not significantly different from the duration of high EMG in both strains of mice. Durand et al. (2005), in the neonatal mouse, appeared to demonstrate a similar phenotype (i.e. correlation of CMs and high EMG activity). Previous work in adult mice have also reported similar observations where high EMG activity is associated with the awake state and this activity can be clearly differentiated from EMG activity associated with the sleep state (Schaub et al., 1998;Tagaito et al., 2001). Further, non-invasive sleep-wake characterization has been reported in the adult mouse (Pack et al., 2007) and the study suggested that periods of no gross body movement do indeed correspond to sleep. Thus, if we take a position of the two-state (sleep and wake) model, sleep-wake states in neonatal mice can be characterized successfully based on CMs without the use of EMG electrodes.
The behavioral indices do not differentiate REM and non-REM sleep. Durand et al. (2005), using mice, sub-classified sleep into active sleep and quiet sleep based on silent and low EMG amplitude, respectively. In our study, however, a clear distinction between silent and low levels of EMG amplitude could not be consistently delineated (Figure 1A). In young animals, active sleep and quiet sleep are considered to be precursors to REM and non-REM sleep, respectively (Frank and Heller, 1997). Both REM and non-REM sleep originate from active sleep (Frank and Heller, 1997), and active sleep is predominant (~80% of total sleep time) in five-day old mice (Durand et al., 2005). Thus, these facts could preclude the need to differentiate states within sleep at this age in mice.
The instrumentation of the EMG appears to cause stress in the neonatal mice. Without EMG instrumentation, the pups settled in the recording chamber within 15 minutes after the transfer. However, in the DBA/2J mice, 5 animals expressed prolonged (>30 minutes) agitation and were excluded. In the pups that did not show the prolonged agitation, their behavioral indices were indifferent between EMG+ and EMG− (DBA/2J). Although no A/J pups showed prolonged agitation, they may have expressed their stress in different manners: less CM duration, less number of MTs, and more sleep time in EMG+ mice compared to EMG− mice. Thus, A/J mice’s sleep architecture may have changed due to EMG instrumentation.
A concern was if EMG instrumentation influences the ventilatory responses to gas challenges. Overall responses were not severely affected by the EMG instrumentation. However, the baseline fR and the responses to the gas challenges among individual mice were more variable in the EMG+ A/J group (Fig. 3). The manifestation of stress clearly differed between the two strains, suggesting genetics plays a role. Hence, EMG instrumentation may influence respiratory variables and responses in some strains of mice. Other non-invasive methods such as wrist movement (Mullaney et al., 1980), heart rate variability (Lewicke et al., 2004) and respiratory characteristics (Haddad et al., 1987) have been used to classify sleep-wake states in neonates. For smaller animals, such as rodents, heart rate analysis would require instrumentation thus introducing some levels of stress and restraint. Because we could reasonably assess sleep-wake state by monitoring movement behaviour, and because EMG instrumentation induces stress and behavioral changes including ventilation, we have concluded that a non-invasive method for sleep-wake assessment by monitoring the behavioral indices would be a more suitable approach to measure ventilatory responses to gas challenges in neonatal mice.
The carotid body is the primary oxygen sensor for mammals and its role in initiating ventilatory responses is very well known (Bowes et al., 1981;Fitzgerald and Shirahata, 1997;Gonzalez et al., 1994). The sensitivity of the carotid body to hypoxia, and subsequent hypoxic ventilatory response, is low at birth but increases with age. Studies in different mammals suggest that hypoxic sensitivity of the carotid body reaches a mature level during the first few weeks of life (1–4 wks depending on the species) (Bamford et al., 1999;Wasicko et al., 1999;Carroll et al., 1993;Donnelly and Doyle, 1994). In this study we used a transient hyperoxia, as well as a short and very mild hypoxia (15% O2). Although blood gas data were not available for this study, Tagaito et al. (2001) have shown in mice that 15% inspired O2 yields an oxygen tension of 61 mmHg, which is sufficient to stimulate the carotid body. This level of hypoxia did not arouse mice from sleep (Rubin et al., 2003). Prolonged hypoxia induces respiratory depression due to brain hypoxia (Neubauer et al., 1990). Also, severe hypoxia, often used for studies in mice, decreases body temperature (Wood, 1991), thereby confounding ventilatory results. Our protocols minimized the potential influences of the central chemoreceptors and of temperature on the ventilatory responses. Taken together, we postulate that the ventilatory changes we observed mostly reflect the carotid body function.
In conclusion, we confirmed that sleep-wake states of 7-day old mice can be assessed based on the behavioral indices without EMG instrumentation. Our results contribute to the growing body of research that utilizes non-invasive methods to examine sleep in animal models, especially neonates. Further, we found that the carotid body appears to play a role in respiratory control during sleep in 7-day old DBA/2J and A/J mice.
Experimental Procedures
Animals
Seven-day old, male DBA/2J (n = 24) and A/J mice (n =14) were used. Breeding pairs (obtained from Jackson Laboratories; Bar Harbor, ME) were housed in the animal facility of the Johns Hopkins Bloomberg School of Public Health with proper temperature (~22°C) and regulated light cycle (12:12-h light/dark cycle). Animals used in this study were bred at this facility. Food (Agway Pro-lab RMH 1000) and water were available ad libitum before experimentation. All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health. Protocols for animal experimentation were approved by The Johns Hopkins University Animal Care and Use Committee.
EMG Instrumentation
In one set of animals per strain, nuchal EMG electrodes were implanted. After a small amount of lidocaine (Astrazenica; Wilmington, DE) was applied, custom designed bipolar EMG electrodes (either stainless steel or platinum-iridium; 0.25mm in diameter) were quickly inserted through the skin bilaterally into the nuchal muscle. The mouse was placed in a plexiglass crib, and the EMG electrodes were secured to the crib with a small narrow strip of adhesive tape. A copper plate placed at the bottom of the crib served as the ground electrode. The EMG signal was amplified × 1,000 (Grass Instruments, Model 79E; West Warwick, RI) with filter settings at 10 kHz high-frequency cutoff and 300 Hz low-frequency cutoff, and digitally acquired using the Biopac Acquisition System (Model MP150; Santa Barbara, CA). The EMG signal was collected at a sample rate of 2500 samples/sec. In a second set of animals in each strain, EMG electrodes were not inserted and the animals were free of any restraint.
Animal Behavior
Animal behavior was continuously monitored by the experimenter for the length of the protocol based on criteria previously described (Karlsson and Blumberg, 2002). The movements of the animals were recorded as electrical signals in the acquisition system. Briefly, a myoclonic twitch was defined as phasic, rapid, and independent movement of a limb or tail and recorded as a short square pulse. Coordinated movement was defined as the prolonged movement of multiple limbs and/or head. Sleep-wake classification in the EMG-instrumented animals was adapted from previous reports (Karlsson and Blumberg, 2002;Durand et al., 2005). EMG activity was classified in two states: silent/low or high activity. Sleep was defined as silent to low nuchal EMG activity with or without the presence of MTs and in the absence of CM. Wakefulness was defined as high nuchal EMG activity and the presence of CM. In the non-EMG-instrumented pups, we used the following criteria for sleep-wake classification: sleep was defined as periods of no gross movement accompanied by the presence of MTs. Wakefulness was defined as the presence of CM. Consideration of the appropriateness of these criteria is presented in the Discussion section.
Whole Body-Plethysmography
Respiratory frequency (fR; breaths · min−1), tidal volume (VT; μL · g−1), minute ventilation (V̇E; μL · min−1 · g−1; = fR × VT) were measured via continuous-flow whole-body plethysmography as described by Drorbaugh and Fenn (1955). The system consisted of a custom designed 50 mL chamber with multiple ports. A reference chamber was not used, because in pilot experiments (Balbir et al., 2006) the use of a reference chamber did not significantly affect the results. A piezoresistive microphone pressure transducer (Endevco; San Juan Capistrano, CA) was instrumented to a port in the chamber. The signal was amplified and filtered (30 mV balance voltage; 0.5 kHz high frequency cutoff, 60 Hz notch filter), and digitally acquired similar to the EMG. The respiratory signal was digitally bandpass filtered (0.75–10 Hz) following acquisition. Normoxic, dry air (21% O2) was continuously passed through the chamber (100 mL/minute) to prevent CO2 and water accumulation. The chamber was also equipped with an OM-4 O2 sensor (Microelectrodes Inc.; Bedford, NH) in order to observe real-time changes in O2 tension during gas challenges. A heating lamp placed above and a circulating warm water pad placed below the chamber were used to maintain chamber temperature in a thermoneutral range (32.8–33.4 °C). Chamber temperature was measured before and after the experiment. Animal temperature was measured by placing a TS67–170 thermistor probe (Oven Industries INC.; Mechanicsburg, PA) on the interscapular skin region (Blumberg and Sokoloff, 1998;Durand et al., 2005) only after the experiment in order to prevent excessive handling before the experiment.
Protocol
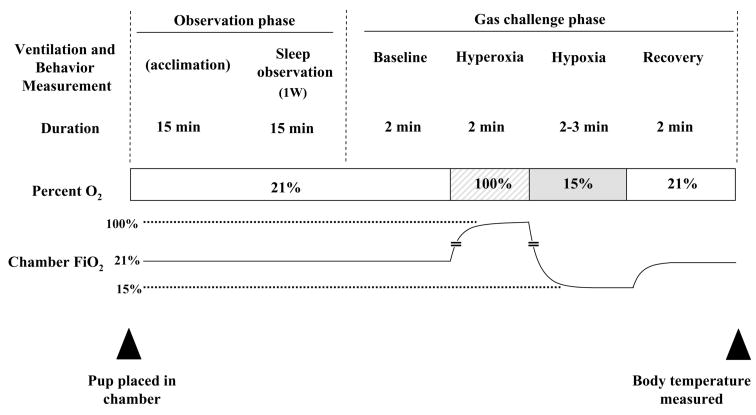
Figure 4 represents the experimental protocol and timeline. Pups in a plexiglass crib were placed inside the plethysmograph chamber for an initial 15-minute acclimation. Animals manifesting stress were allowed longer acclimation times. Stress included attempts to dislodge the EMGs by sudden movements or scratching, and hyperactivity in the chamber. Animals that showed continual stress for more than 30 minutes were excluded. Breathing, EMG activity, and behavior were recorded for a 15-minute period (sleep observation phase) under the normoxic condition. Immediately following this phase was the gas challenge phase which proceeded as follows: 2-minute baseline/normoxic period, followed by a 2-minute hyperoxia period (100% O2), a 3-minute hypoxia period (15%O2) and a 2-minute recovery/normoxic period. Gas challenges were given to the mice while they were judged to be asleep. Breathing, EMG activity, and behavior were recorded during this phase as well.
Figure 4. Protocol for sleep-wake assessment and the measurement of respiratory variables.
Animals were placed inside the plethysmograph chamber. After an initial 15 minutes of acclimation, breathing, EMG activity, and behavior were recorded for the length of the experiment. After 15 minutes of sleep observation the gas challenge phase proceeded as follows: 2-minute baseline/normoxic period, followed by a 2-minute hyperoxia period (100% O2), a 3-minutes hypoxia period (15%O2) and a 2-minute recovery/normoxic period. Gas challenges were given to the mice while they were judged to be asleep.
Analysis
Ventilatory data during sleep were collected and analyzed. VT calibration was done before each experiment by injecting 5 μL, 10 μL, and 20 μL of air into the chamber using a micro-syringe (Hamilton; Reno, NV) for a 3-point calibration. In the sleep observation phase, a set of ten tidal breaths was sampled while the animal was judged to be in the sleep state for at least six consecutive seconds. Ten sets of these tidal breaths were randomly collected and analyzed over the 15-minute period. In the gas challenge phase, three sets of ten tidal breaths during sleep were measured during the baseline (normoxia), hyperoxia (at saturation), hypoxia (at nadir) and recovery periods (normoxia). All data sets were expressed as mean ± SEM. Student’s unpaired t-test or ANOVA repeated measures with Tukey test was used to evaluate statistical significance. Differences were considered to be statistically significant when p<0.05.
Acknowledgments
The authors thank Mr. John Howell for the development of the gas delivery system as well as all other electronic devices used for this study. This work was supported by HL72293, HL81345 and AHA 0255358N. A. Balbir and R.S. Fitzgerald were supported by T32-HL07534 and HL50712, respectively.
Abbreviations
- CM
Coordinated movement
- EEG
electroencephalogram
- EMG
electromyogram
- EOG
electrooculogram
- MT
myoclonic twitch
- fR
respiratory frequency
- V̇E
minute ventilation
- VT
tidal volume
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- Balbir A, Okumura M, Schofield B, Coram J, Tankersley C, Fitzgerald RS, O’donnell CP, Shirahata M. Genetic regulation of chemoreceptor development in DBA/2J and A/J strains of mice. Adv Exp Med Biol. 2006;580:99–104. doi: 10.1007/0-387-31311-7_15. [DOI] [PubMed] [Google Scholar]
- Balbir A, Lee H, Okumura M, Biswal S, Fitzgerald RS, Shirahata M. A search for genes that may confer divergent morphology and function in the carotid body between two strains of mice. Am J Physiol Lung Cell Mol Physiol. 2007;292:L704–L715. doi: 10.1152/ajplung.00383.2006. [DOI] [PubMed] [Google Scholar]
- Bamford OS, Sterni LM, Wasicko MJ, Montrose MH, Carroll JL. Postnatal maturation of carotid body and type I cell chemoreception in the rat. Am J Physiol. 1999;276:L875–L884. doi: 10.1152/ajplung.1999.276.5.L875. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Sokoloff G. Thermoregulatory competence and behavioral expression in the young of altricial species--revisited. Dev Psychobiol. 1998;33:107–123. doi: 10.1002/(sici)1098-2302(199809)33:2<107::aid-dev2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Paylor R. Behavioral and physiological mouse assays for anxiety: a survey in nine mouse strains. Behav Brain Res. 2002;136:489–501. doi: 10.1016/s0166-4328(02)00200-0. [DOI] [PubMed] [Google Scholar]
- Bowes G, Townsend ER, Kozar LF, Bromley SM, Phillipson EA. Effect of carotid body denervation on arousal response to hypoxia in sleeping dogs. J Appl Physiology. 1981;51:40–45. doi: 10.1152/jappl.1981.51.1.40. [DOI] [PubMed] [Google Scholar]
- Carroll JL, Bamford OS, Fitzgerald RS. Postnatal maturation of carotid chemoreceptor responses to O2 and CO2 in the cat. J Appl Physiol. 1993;75:2383–2391. doi: 10.1152/jappl.1993.75.6.2383. [DOI] [PubMed] [Google Scholar]
- Dauger S, Nsegbe E, Vardon G, Gaultier C, Gallego J. The effects of restraint on ventilatory responses to hypercapnia and hypoxia in adult mice. Respir Physiol. 1998;112:215–225. doi: 10.1016/s0034-5687(98)00027-9. [DOI] [PubMed] [Google Scholar]
- Donnelly DF, Doyle TP. Developmental changes in hypoxia-induced catecholamine release from rat carotid body, in vitro. J Physiol. 1994;475:267–275. doi: 10.1113/jphysiol.1994.sp020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics. 1955;16:81–87. [PubMed] [Google Scholar]
- Durand E, Dauger S, Pattyn A, Gaultier C, Goridis C, Gallego J. Sleep-disordered breathing in newborn mice heterozygous for the transcription factor Phox2b. Am J Respir Crit Care Med. 2005;172:238–243. doi: 10.1164/rccm.200411-1528OC. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RS, Shirahata M. Systemic responses elicited by stimulating the carotid body: primary and secondary mechamisms. In: Gonzalez C, editor. Carotid Body Chemoreceptors. Springer-Verlag; 1997. pp. 171–202. [Google Scholar]
- Frank MG, Heller HC. Development of REM and slow wave sleep in the rat. Am J Physiol. 1997;272:R1792–R1799. doi: 10.1152/ajpregu.1997.272.6.R1792. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Haddad GG, Jeng HJ, Lai TL, Mellins RB. Determination of sleep state in infants using respiratory variability. Pediatr Res. 1987;21:556–562. doi: 10.1203/00006450-198706000-00010. [DOI] [PubMed] [Google Scholar]
- Karlsson KA, Blumberg MS. The union of the state: myoclonic twitching is coupled with nuchal muscle atonia in infant rats. Behav Neurosci. 2002;116:912–917. doi: 10.1037//0735-7044.116.5.912. [DOI] [PubMed] [Google Scholar]
- Karlsson KA, Kreider JC, Blumberg MS. Hypothalamic contribution to sleep-wake cycle development. Neurosci. 2004;123:575–582. doi: 10.1016/j.neuroscience.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Krimsky WR, Leiter JC. Physiology of breathing and respiratory control during sleep. Semin Respir Crit Care Med. 2005;26:5–12. doi: 10.1055/s-2005-864197. [DOI] [PubMed] [Google Scholar]
- Lewicke A, Sazonov E, Schuckers SA. Sleep-wake identification in infants: heart rate variability compared to actigraphy. Conf Proc IEEE Eng Med Biol Soc. 2004;1:442–445. doi: 10.1109/IEMBS.2004.1403189. [DOI] [PubMed] [Google Scholar]
- McGinty DJ, Beahm EL. Neurobiology of Sleep. In: Saunders NA, Sullivan CE, editors. Sleep and Breathing. New York, NY: Marcel Dekker; 1984. pp. 1–89. [Google Scholar]
- Mullaney DJ, Kripke DF, Messin S. Wrist-actigraphic estimation of sleep time. Sleep. 1980;3:83–92. doi: 10.1093/sleep/3.1.83. [DOI] [PubMed] [Google Scholar]
- Neubauer JA, Melton JE, Edelman NH. Modulation of respiration during brain hypoxia. J Appl Physiol. 1990;68:441–451. doi: 10.1152/jappl.1990.68.2.441. [DOI] [PubMed] [Google Scholar]
- Pack AI, Galante RJ, Maislin G, Cater J, Metaxas D, Lu S, Zhang L, Smith RV, Kay T, Lian J, Svenson K, Peters LL. Novel method for high-throughput phenotyping of sleep in mice. Physiol Genomics. 2007;28:232–238. doi: 10.1152/physiolgenomics.00139.2006. [DOI] [PubMed] [Google Scholar]
- Phillipson EA, Bowes G. Control of breathing during sleep. In: Fishman AP, Cherniack NS, Widdicombe JG, Geiger SR, editors. Handbook of Physiology, Section 3: The Respiratory System. Bethesda, MD: American Physiological Society; 1986. pp. 649–689. [Google Scholar]
- Rubin AE, Polotsky VY, Balbir A, Krishnan JA, Schwartz AR, Smith PL, Fitzgerald RS, Tankersley CG, Shirahata M, O’donnell CP. Differences in sleep-induced hypoxia between A/J and DBA/2J mouse strains. Am J Respir Crit Care Med. 2003;168:1520–1527. doi: 10.1164/rccm.200304-462OC. [DOI] [PubMed] [Google Scholar]
- Schaub CD, Tankersley C, Schwartz AR, Smith PL, Robotham JL, O’donnell CP. Effect of sleep/wake state on arterial blood pressure in genetically identical mice. J Appl Physiol. 1998;85:366–371. doi: 10.1152/jappl.1998.85.1.366. [DOI] [PubMed] [Google Scholar]
- Shea SA. Behavioural and arousal-related influences on breathing in humans. Exp Physiol. 1996;81:1–26. doi: 10.1113/expphysiol.1996.sp003911. [DOI] [PubMed] [Google Scholar]
- Tagaito Y, Polotsky VY, Campen MJ, Wilson JA, Balbir A, Smith PL, Schwartz AR, O’donnell CP. A model of sleep-disordered breathing in the C57BL/6J mouse. J Appl Physiol. 2001;91:2758–2766. doi: 10.1152/jappl.2001.91.6.2758. [DOI] [PubMed] [Google Scholar]
- Wasicko MJ, Sterni LM, Bamford OS, Montrose MH, Carroll JL. Resetting and postnatal maturation of oxygen chemosensitivity in rat carotid chemoreceptor cells. J Physiol. 1999;514(Pt 2):493–503. doi: 10.1111/j.1469-7793.1999.493ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SC. Interactions between hypoxia and hypothermia. Annu Rev Physiol. 1991;53:71–85. doi: 10.1146/annurev.ph.53.030191.000443. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Balbir A, Schofield B, Coram J, Tankersley CG, Fitzgerald RS, O’donnell CP, Shirahata M. Structural and functional differences of the carotid body between DBA/2J and A/J strains of mice. J Appl Physiol. 2003;94:1536–1542. doi: 10.1152/japplphysiol.00739.2002. [DOI] [PubMed] [Google Scholar]