Abstract
The third meeting of the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) focused on selecting promising measures for each of the cognitive constructs selected in the first CNTRICS meeting. In the domain of perception, the 2 constructs of interest were gain control and visual integration. CNTRICS received 5 task nominations for gain control and three task nominations for visual integration. The breakout group for perception evaluated the degree to which each of these tasks met prespecified criteria. For gain control, the breakout group for perception believed that 2 of the tasks (prepulse inhibition of startle and mismatch negativity) were already mature and in the process of being incorporated into multisite clinical trials. However, the breakout group recommended that steady-state visual-evoked potentials be combined with contrast sensitivity to magnocellular vs parvocellular biased stimuli and that this combined task and the contrast-contrast effect task be recommended for translation for use in clinical trial contexts in schizophrenia research. For visual integration, the breakout group recommended the Contour Integration and Coherent Motion tasks for translation for use in clinical trials. This manuscript describes the ways in which each of these tasks met the criteria used by the breakout group to evaluate and recommend tasks for further development.
Keywords: perception, CNTRICS, sensory processes
Perceptual processes are viewed as being among the key domains for development of measures that can be used in clinical trials in schizophrenia. This topic was discussed at the first consensus meeting of Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) and summarized in Biological Psychiatry vol. 64(1).1 At this initial meeting, the high priority constructs within perception were identified as (1) gain control and (2) integration.2 Gain control was defined as processes that allow sensory systems to adapt and optimize their responses to stimuli within a surrounding context. Integration was defined as processes linking the output of neurons that individually code local (typically small) attributes of a scene into global (typically larger) complex structure for guiding behavior. Both of these domains have features that make them attractive and valuable for clinical trials in schizophrenia.2 Specifically, (1) both can be readily measured in humans, (2) both show evidence of impairment in schizophrenia, (3) both have moderately strong links to neural circuits, (4) both are partially understood in terms of the mechanisms, (5) animal models exist for gain control, but not for integration, (6) links to neuropsychopharmacology are present for both, but are stronger for gain control, (7) both can be applied to human neuroimaging, and (8) both have moderate links to functional outcome.
The goal of the third CNTRICS meeting was to identify promising paradigms within these 2 perceptual domains. The task was to take paradigms from these domains that were nominated by a broad group of experts and to evaluate the paradigms according to a list of criteria to determine which ones are highly promising for immediate development. The criteria for evaluating paradigms include the following: (1) construct validity, (2) clarity of a link to neural circuit, (3) clarity of a link to cognitive mechanism, (4) availability of an animal model, (5) link to a neural system through neuropsychopharmacology, (6) amenable for use in human neuroimaging studies, (7) evidence of impairment in schizophrenia, (8) link to functional outcome in schizophrenia, and (9) good psychometric properties.
A breakout group was devoted to evaluating the nominated paradigms, which are shown in the Table 1. The purpose of this article is to briefly summarize some of the key decisions from this breakout group and then to provide more detailed descriptions of each of the recommended paradigms.
Table 1.
Perception in Schizophrenia
| Gain control: Neurons adapting their response levels to take into account their immediate context to make best use of a limited dynamic signaling range |
| Tasks recommended for immediate development |
| Contrast-contrast effect task |
| Steady-state visual-evoked potentials to magnocellular- vs parvocellular-biased stimuli (with contrast sensitivity task) |
| Already mature tasks |
| Prepulse inhibition of startle |
| Mismatch negativity |
| Integration: the processes linking the output of neurons that code local attributes of a scene into global complex structure |
| Tasks recommended for immediate development |
| Contour integration task |
| Coherent motion detection task |
For the measures of gain control (see table 1), it was decided that there were two distinctly different types of measures: those that are already well-established in the schizophrenia literature and used in multisite trials vs those that are still largely unexplored. The breakout group thought that it would not be fair to evaluate both types of measures in the same exercise, so the 2 more mature measures (ie, prepulse inhibition of startle [PPI] and mismatch negativity [MMN]) were separated from the less established measures (ie, contrast-contrast sensitivity and steady-state evoked potentials to magnocellular- [M] vs parvocellular [P]-biased stimuli). The 2 mature tasks were considered to be reasonable measures of gain control because they involve neural adaptation to immediate context, despite the fact that neither was viewed as a prototypical measure of gain control. It was noted that an assessment of contrast sensitivity to M- vs P-biased stimuli could be easily added to the steady-state evoked potential assessment with little additional time or burden, and so the description below includes both elements.
The 2 measures of gain control that were recommended for immediate development (contrast-contrast sensitivity and steady-state evoked potentials/contrast sensitivity to M- vs P-biased stimuli) scored strongly across the key criteria. Two areas of limitations were noted, namely that there were not strong animal models, and there were few data on links to functional status in schizophrenia. The limitation for animal models was not an absence of models, but that these models typically involved cats or nonhuman primates, as opposed to rodents. Rodent models have clear advantages for preclinical drug development activities because they are much faster and cheaper to implement than cat or nonhuman primate models. Hence, these models are more feasible for screening a large number of compounds for procognitive effects. It was also noted that the steady-state evoked potentials involved electrophysiological methods that would present a substantial practical challenge for multisite clinical trials. Nonetheless, practicality was not a criterion at this stage of paradigm evaluation.
For the construct of integration (see table 1), 2 measures were listed as recommended for immediate development: the contour integration task and the coherent motion detection task. A third task, the babble task, was nominated as well. In this task, participants are presented with a dense array of voices in which it is difficult to detect coherent words or phrases. The experimenter then measures the degree to which individuals report hearing “spurious” words or phrases. Hoffman et al.3 have found that individuals who go on to develop schizophrenia are more likely to report hearing such spurious words or phrases. Although the breakout group felt that this task was highly interesting, the group believed that more work was needed to establish its basic construct validity and neural basis before it was ready for translation for use in clinical trials contexts. The construct validity for the contour integration task was considered strong. Some ambiguity in construct validity was noted for the coherent motion detection task regarding whether the task is mainly conducted by global vs local processing. Also when evaluated by the criteria, both measures recommended for immediate translation had similar limitations, including questions about applications to rodent models, few data on links to outcome in schizophrenia, and few psychometric data such as test-retest reliability. In the section below, we provide descriptions of each of these tasks for each of the 2 constructs to provide guidance for future research that will facilitate the translation of these paradigms into use in clinical trials contexts in schizophrenia.
Gain Control
Task Recommended for Immediate Development
Contrast-Contrast Effect Task.
Description
Contrast-contrast effect (CCE) Task is based on a well-characterized visual illusion in which contrast sensitivity is strongly modulated by the visual properties of adjacent or surrounding stimuli4–6 and follows closely the paradigm in a study by Dakin et al.5 In healthy subjects, the presence of a high contrast surround results in decreased contrast sensitivity, ie, the same level of contrast is perceived as being lower when it is surrounded by a high-contrast stimulus compared with when it is not surrounded by this stimulus. This illusion demonstrates contrast gain control, which is necessary for optimization of visual processing. Subjects view a circular patch (1.3° diameter) presented in the center of the field of view, which consists of blob-like shapes (8 c/degree bandpass-filtered noise) with a contrast of 40%. This central patch is presented either with or without a high contrast (95%) surround for 1000 ms. Subjects then view a reference contrast patch and indicate which patch had a higher contrast. The contrast of the reference patch is varied to provide a psychometric function for contrast perception. An adaptive method of constant stimuli can be used.7 This procedure has the advantage that one can simultaneously estimate accuracy and precision. The data from each subject can be fit with a cumulative Gaussian function to estimate the accuracy (bias/intercept) and precision (slope) of the subject's contrast perception.
Construct Validity
The large number of consistent and convergent behavioral and functional studies based on this and related tasks in humans and animals strongly support the construct validity of the CCE task as a measure of contrast gain control. By definition, gain control is a process in which the relative magnitude of input and output signals is dynamically modulated in an adaptive manner. From an ecological perspective, there is an obvious benefit to dynamically adjust contrast sensitivity in accordance to the visual qualities of a given visual scene. The varying need for contrast gain is effectively operationalized in this task by the presence or absence of a high-contrast surround. Numerous electrophysiologic studies8–15 and a recent human functional magnetic resonance imaging (fMRI) study16 attest to the modulation of neural activity as a function of the presence of a high-contrast surround. For example, in single-unit studies, the response rate of neurons to a visual stimulus within its classic receptive field is strongly diminished in the presence of a high-contrast surround, which is outside of the neuron's receptive field. Likewise, a human study by Zenger-Landholt and Heeger16 demonstrated strong correspondence between behavioral performance and V1 activity as a function of the presence of a high-contrast surround.
Neural Systems
There have been a very large number of studies examining the neural basis of contextual modulation of contrast processing. While the specific neural mechanisms have yet to be clarified, there has been substantial progress in identifying candidate neural systems and pathways. Converging evidence from psychophysics and fMRI indicates that the CCE is linked to visual processing within the primary visual cortex (V1). The study by Zenger-Landolt found robust contextual modulation of the fMRI signal in V1, V2, and V3, but the best correlation with behavioral responses and, consequently, perceptual changes induced by contextual modulation, was with V1 signal changes.16 Some investigators have hypothesized localized lateral inhibitory mechanisms via horizontal fibers within V117 while others have proposed feedback mechanisms ultimately acting on inhibitory neurons in V1.8 The suppressive effects of both of these models are hypothesized to act through γ-aminobutyric acid (GABA) neurotransmission of inhibitory interneurons. Alternatively, feedforward mechanisms, in which contextual modulation arises in the lateral geniculate nucleus (LGN) of the thalamus, have also been proposed.12,13 In addition to these models, others have postulated that multiple mechanisms may account for the CCE.18,19
Pharmacological or Behavioral Manipulation of Task Performance
The effects of pharmacological or nonpharmacological treatment on contrast gain control are unknown at present. However, prior studies have outlined the involvement of dopamine, GABA, and acetylcholine systems in processes related to CCE task performance. Consequently, these neurotransmitter systems are potential targets of pharmacological agents seeking to address deficits in contrast gain control. Dopamine modulation of visual contrast detection has been documented in animals and humans. It is thought that dopamine's primary locus of modulation is mediated by D2 receptors in the retina, but some studies have also shown extra-retinal sites of action (for review, see20,21). A recent study by Chen et al.22 suggests that the atypical neuroleptics, with relatively less dopamine activity compared with typical neuroleptics, are not associated with alterations in contrast sensitivity in subjects with schizophrenia. One of the most commonly cited mechanisms for the contextual modulation of contrast sensitivity is through surround suppression. In turn, the predominant neurobiological mechanism thought to mediate surround suppression is lateral inhibition through GABA neurotransmission.17 Ozeki et al.13 found a modest effect of application of GABA A receptor inhibition on the magnitude of contextual effects on contrast sensitivity. A recent study in monkeys demonstrated anatomic and functional evidence of nicotinic receptors being a mediator of visual gain control. Nicotinic receptors were found to be expressed abundantly presynaptically on thalamocortical fibers, specifically in layer 4c of V1. Furthermore, the application of nicotine at these synapses resulted in response gain to contrast stimuli that prior to treatment were subthreshold.23 In addition to pharmacological interventions, some lines of evidence suggest a potential role for cognitive training in treating deficits in gain control. There is now compelling evidence that top-down modulation acts on early stages of visual processing, even in V124 and for the role of attentional modulation of contrast gain.25
Animal Models
The CCE, alternatively referred to as surround suppression in the literature, has been one of the most replicated findings in visual neuroscience, having been studied abundantly in animals, mostly cats10,12–14 and macaques.8,9,11,15 Although, as alluded to above, the specific mechanism of the CCE has yet to be clarified, the large body of neuroanatomical and neurochemical knowledge in the visual system of these animals provide unparalleled opportunities for translational research. The vast majority of animal models, however, are based on electrophysiologic and not perceptual dependent measures. Therefore, the direct translation of animal results to human clinical studies must be done with this limitation in mind.
Performance in Schizophrenia
There is one published study that has applied the CCE task to subjects with schizophrenia.5 This study demonstrated notable robustness in detecting a group difference in performance and interpretive specificity. Healthy and psychiatric control subjects experienced significantly greater biasing effect of the high contrast surround on contrast perception, compared with patients with schizophrenia. The robustness of this difference was such that there was nearly complete separation between patients with schizophrenia and controls on this measure. One of the most compelling aspects of the CCE task for schizophrenia research is that patients are predicted to actually perform better than control subjects. This result strongly argues against generalized deficits accounting for the results. In addition to the study by Dakin et al., there have been several other recent studies in schizophrenia that have either employed similar tasks or have examined contextual modulation of other visual processes.26–28 Among these studies, there is consensus that subjects with schizophrenia demonstrate altered contextual modulation of visual processing. The convergence of results across multiple paradigms and laboratories strongly argue for the reliability and robustness of gain control deficits in schizophrenia.
Psychometric Data
An important limitation of this task is that there has not been extensive testing of its psychometric properties. Test-retest reliability has not been assessed. As a related matter, the impact of practice or training also requires investigation. An attractive feature of this task is that psychometric functions can be obtained for subjects. From these functions, separate indicators of precision (the minimum size of contrast differences that are detectable, which is indicated by the slope of the function) and bias (reflecting the amount of offset that is needed between the target and the surround to produce a perceptual match) can be obtained, allowing us to examine discrimination accuracy independent of response bias (as with other signal-detection analyses). This flexibility suggests the absence of floor/ceiling effects.
Future Directions
While the availability of well-validated animal models of the CCE task and the accrued knowledge about the basic neuroanatomical and neurochemical components of the neural systems and circuits related to this task presents an excellent opportunity to identify the specific neural mechanisms giving rise to deficits in contrast gain control in schizophrenia, several challenges remain. First, some basic psychometric qualities of this task, such as test-retest reliability, practice effects, and ceiling/floor effects, must be assessed. Although the deficit in schizophrenia appears very robust, the CCE task must be applied to independent samples by additional investigators in order to assess its reliability. Second, there is at present a paucity of knowledge on the efficacy of any interventions in schizophrenia. There are several potential pathways that may serve as targets for nonpharmacological and pharmacological treatments and present future opportunities of study. Finally, there is a great need, on a conceptual and empirical level, to incorporate contrast gain control deficits within a larger theoretical framework for the behavioral and higher order cognitive deficits in schizophrenia. This effort may take the form of uncovering correlations between CCE task deficits and clinical or cognitive features.
Steady-State Visual-Evoked Potentials and Contrast Sensitivity to M- and P-Biased Stimuli
Description
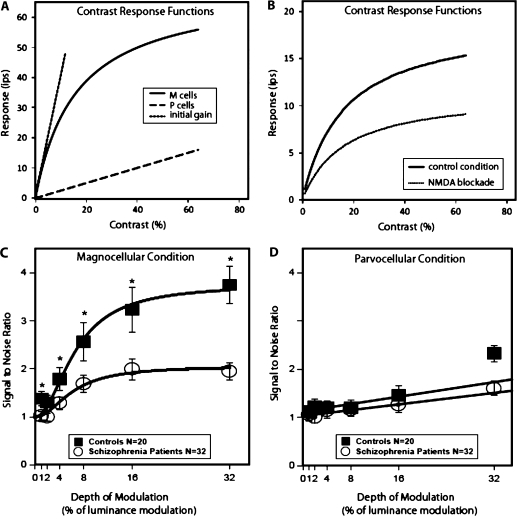
This particular steady-state visual-evoked potential (ssVEP) task using M- or P-biased stimuli was developed by Zemon and Gordon.29 This task is based on the differential response to contrast of the M and P pathways, which begin in the retina and project via the LGN to primary visual cortex. The M pathway shows a steeply rising increase in response to increases in low contrast and then nearly saturates at about 16–32% contrast30,31 (figure 1A). The P pathway does not respond until about 10% contrast or greater and has a linear increase in response throughout the entire contrast range.30,32 The slope of the linear portion of the contrast response curve is referred to as contrast gain, and it is about 10 times greater for the M than P pathway.
Fig. 1.
(A) Contrast Response Functions Recorded From Macaque Monkey Retinal Ganglion Cells (Adapted With Permission From Kaplan and Shapley30). M cells show much higher gain (slope) than P cells at low contrasts. The initial gain of the M cells is shown in the dashed straight line derived from the M-cell curve. The gain of the P cells is the dashed line fitted to the P-cell function; (B) N-methyl-D-aspartate (NMDA) antagonists produce shallower gain at low contrast and a much lower plateau in visual-evoked potential responses, indicating decreased signal amplification (adapted with permission from Kwon et al.48); (C and D) Visual-evoked potential responses (adapted with permission from Butler et al.33) using the M- and P-biased steady-state visual-evoked potential technique described in this article. The patient visual-evoked potential contrast response curve in the M-biased condition shows similar decreased gain at low luminance contrast and decreased plateau as seen with NMDA antagonist administration, indicating decreased signal amplification.
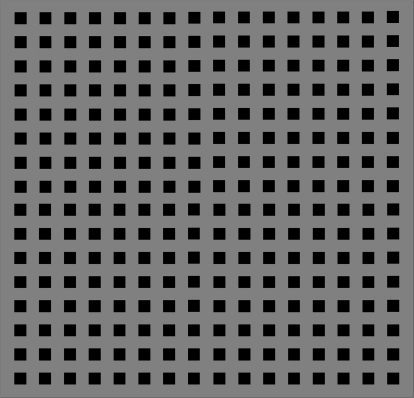
To emphasize contributions from the M pathway to the ssVEP, isolated check stimuli (figure 2) were kept within the low-contrast region.29 To emphasize contributions from the P pathway to the ssVEP, checks were modulated around a high static contrast (pedestal) to avoid the low-contrast regions where magnitudes of M-pathway responses rise steeply with increase in contrast.
Fig. 2.
Example of isolated check stimuli used in the steady-state visual-evoked potential task.
A key aspect of the procedure is that the same depths of luminance modulation (DOM) (0, 1, 2, 4, 8, 16, and 32%) are used for both the M- and P-biased conditions. To produce the M-biased conditions in which stimuli appear and disappear, the DOM of the checks is equal to the mean contrast (pedestal). Thus, the checks reach a peak contrast double that of the mean value. For example, if the mean of the checks is set at 4%, then the DOM is also 4% and the pattern reaches a peak contrast of 8% at one point in the cycle and 0% contrast a half cycle later. Under the P-biased condition, a high mean contrast (pedestal) of 48% is often used.
A second important feature is that data can be collected quickly. Responses are typically recorded from an electrode at a midline occipital site (Oz) referenced to a second electrode on the vertex of the head (Cz). Patterns are modulated quickly (eg, at 12 Hz) in a contrast sweep such that DOM is increased in each second of a 7-s run. Thus, in a single run, all 7 DOMs are presented and there are 12 stimulus presentations per second. The M- and P-biased conditions are presented separately, and there are 10 runs of each condition. Fourier analysis is used to obtain the response at the stimulus frequency for each 1-s electroencephalography epoch in each run, and mean amplitude and phase values are computed over the 10 runs. Signal-to-noise ratios (SNRs) are also obtained33,35 as are estimates of contrast gain and contrast gain control.33,34
A second task that may be used to assess M- and P-pathway responses involves psychophysical contrast sensitivity. This is a classic behavioral task that has been used for over 50 years.36–38 Sine-wave gratings presented at various spatial frequencies from low to high are the typical stimuli. Spatial frequency refers to the number of pairs of light and dark bars in a degree of visual angle such that lower spatial frequencies indicate wider bars and higher spatial frequencies indicate thinner bars. Different psychophysical methods have been used to measure contrast sensitivity functions (CSFs).37 A preferred method is 2-alternative forced-choice tracking in which, for instance, gratings are presented randomly to one half of a visual display, while the other half has a uniform field.33 Participants are asked to state which side of the display contains the grating. Contrast is varied across trials using an up-down transform rule to determine the contrast threshold for each spatial frequency condition (eg, the contrast at which the presence of the grating is correctly detected 70.7% of the time). Contrast sensitivity is the reciprocal of this threshold. High-contrast sensitivity indicates good performance. CSFs are inverted U-shaped functions with a peak in the mid-range of spatial frequencies (4–6 cycles per degree) and fall off at lower and higher spatial frequencies.
Construct Validity
Gain control, as defined in the CNTRICS initiative, refers to processes that allow sensory systems to adapt and optimize their responses to stimuli within a particular context.2 The nonlinear response to contrast with steep gain at low contrast and amplitude compression and phase advance with increases in contrast was first described in cat retinal ganglion cells and termed “contrast gain control.”39,40 Contrast gain control refers to the change in slope of the amplitude function as contrast increases. Contrast gain control is present in M, but not P, neurons41 and divisive contrast gain control is operating at higher contrasts to limit the responses of the M pathway. The divisive contrast gain control in the M pathway is thought to arise from shunting inhibition, which appears to be GABAA mediated.29,42
Thus, unlike the P response to contrast which shows low gain and is linear over the entire range of contrast, the M response to contrast is an example of adapting and optimizing responses by showing high gain (slope) at low contrast where it is needed in order to respond to low contrast and, given that responses cannot continue to rise at that rate, compression of responses at higher contrast so that the M pathway can still respond to high contrast, albeit with lower gain.
The psychophysical contrast sensitivity task generally produces thresholds that are in the low-contrast range (ie, produces responses with high-contrast sensitivity). High-contrast sensitivity in the CSF indicates high-contrast gain. In addition, the M pathway uses contrast gain control and CSFs reflect M-pathway responses under a variety of conditions.
Neural Systems
The contrast response functions obtained in healthy humans under M- and P-biased pedestal ssVEP conditions29,33,43,44 (figures 1c and 1d) are very similar to those recorded from M and P neurons in macaque retina (figure 1A)30,41,45,46 and LGN30,41,45,46, supporting the concept that neural M and P responses are being examined. In psychophysical studies of contrast sensitivity, transient and sustained mechanisms have been posited to explain the resulting CSF. These psychophysical mechanisms appear to correspond to M and P activity, respectively. Changes in the shape of the CSF with manipulation of spatiotemporal stimulus conditions have led researchers to conclude that brief presentations of gratings over a wide range of spatial frequencies yield M-dominated responses, and long duration presentation of spatial frequency gratings yield P-dominated responses47.
Pharmacological or Behavioral Manipulation of Task Performance
For the contrast response curve, microinfusion of N-methyl-D-aspartate (NMDA) antagonists into cat LGN and primary visual cortex reduce contrast gain as well as the maximum response (figure 1B)48,49. NMDA may also be involved in the high-contrast sensitivity seen in the psychophysical contrast sensitivity task. Dopamine20,50 and nicotinic cholinergic receptors23 appear to play a role in contrast sensitivity measurements.
Animal Models
Like humans, monkeys have M and P pathways and produce contrast response curves in electrophysiological studies that are very similar to the ssVEP functions described above for humans. Although cats do not have M and P divisions, they produce contrast response curves similar to those recorded from M cells in primates.48,49 In addition, similar results were seen for monkeys, in which recordings were obtained from the dura mater above the brain, and humans using the same apparatus and ssVEP stimuli described above.51
CSFs can be obtained psychophysically as well as electrophysiologically. CSFs have been obtained in a number of species including goldfish, cat, and falcon and can be obtained behaviorally in macaques.52,53
Performance in Schizophrenia
To date, two studies in which the ssVEP task was used demonstrated preferential deficits in the M pathway in schizophrenia patients including decreased contrast gain.33,43 A subset of patients were also found to have decreased shunting inhibition.54 A number of studies 33,55–59 though not all60 show decreased contrast sensitivity in patients with schizophrenia. The high-contrast sensitivity suggests M-pathway deficits, although others have argued for a P-pathway role in this deficit within the high spatial frequency region.
Psychometric Data
An advantage of the ssVEP task is that behavioral responses are not required and participants only have to fixate on the screen, which reduces the burden. The 95% confidence intervals for the 10 runs per person demonstrate consistent data and good reliability within an individual.29 In addition, SNRs are typically high, indicating signals that are considerably larger than the variability within a condition.29,33,43 Also, the large between-group effects observed using SNR indicate good reliability within a group.33,43 A number of individuals were retested on another day and results show good reproducibility (Pamela D Butler, Vance Zemon, Daniel C Javitt). CSFs do not have a ceiling effect, but could have a floor effect because threshold can never be greater than 100% contrast (ie, contrast sensitivity of 1 is a lower limit in performance). Practice effects are negligible with the 2-alternative forced choice tracking method.37 In addition, there is good agreement in observers in the shape of the function and absolute values of contrast sensitivity.
Future Directions
For the ssVEP, a full study of reproducibility across days needs to be done. While a body of single-cell data exist to support the role of M and P pathways in the generation of both ssVEP and CSFs, additional single-cell investigations may serve to support the use of macaques as an animal model and the conclusion that there are distinct contributions from M and P cells to these response measures. This work would include examination of the specific pedestal conditions for ssVEP and a variety of spatiotemporal conditions for CSFs in single-cell studies of M and P neurons. For both tasks, further patient, prodromal, and pharmacological studies are needed. In particular, the amenability of ssVEP and CSFs to modulation by pharmacological or behavioral interventions needs to be examined.
Gain Control
Already Mature Tasks
Prepulse Inhibition of the Eyeblink Component of the Startle.
Description
The startle response is a set of reflexive responses to strong, sudden acoustic or tactile stimuli that can be studied in all mammals. PPI is considered a measure of “sensorimotor gating” because it involves both sensory stimuli and motor responses. In PPI, the startle response elicited by a startling stimulus is measured in the presence or absence of a weak prepulse stimulus, which can be in the same or a different modality. The weak prepulse strongly inhibits the response to the subsequent startling stimulus. PPI is not a form of habituation and is not correlated with auditory gating measured as event-related potentials (ERPs) elicited by pairs of identical clicks presented at 500 ms intervals.61 In humans, startle is assessed in most cases via the eyeblink component of the startle reflex, using electromyographic recordings. In animals, the whole-body flinch aspect of the startle response is quantified using an accelerometer that is sensitive to dynamic movements.
Most studies use brief (20–40 ms) startle-eliciting acoustic stimuli, with intensities varying from 105 to 115 dB and presented for 20–50 ms. On some trials in a test session, the startle pulse is preceded at 30–500 ms by a prepulse, which is not thought to elicit a startle response by itself. PPI is usually expressed as the percentage of inhibition of the startle amplitude on prepulse trials relative to the amplitude during startle pulse trials, although difference scores and raw values should be assessed especially when group differences in startle reactivity are evident.62
An extensive literature in both humans and animals has demonstrated the parametric responsiveness of PPI to many factors, including stimulus intensities, rise times, durations, and modalities; controlled vs noncontrolled background noise63; pure tone stimuli vs white noise stimuli64 instructions given to the participants regarding attending to the stimuli65–67; sex differences68; strain differences;69,70 and, in humans, differences in which eye is monitored as well as smoking behavior and personality factors.
Construct Validity
Gating functions are believed to be impaired in schizophrenia, which theoretically can lead to increased distractibility, cognitive fragmentation, and thought disorder.71–74 PPI is thought to reflect a largely automatic, preattentional, and unlearned sensorimotor gating mechanism. Indeed, deficient PPI in schizophrenia has been observed with intervals between the prepulse and startle pulse being too short (eg, 30 or 60 ms) to be influenced by the conscious allocation of attentional resources.75 In some descriptions, PPI is seen as a mechanism to protect the processing of the first of successive stimuli. In other contexts, PPI has been considered as an operational measure of gain control impacting perception.
Neural Systems
The similarity of PPI across species supports the suggestion that the neurobiological mechanisms underlying the PPI in humans can be examined productively in animals.76–78 Whereas structures at the level of the brainstem control the startle response per se79,80 forebrain structures modulate the inhibitory functions of the prepulse via cortico-striato-pallido-pontine circuitry.77,80,81 This modulatory system includes the limbic cortex (medial prefrontal cortex, amygdala, and ventral hippocampus), the thalamus, the ventral striatum (nucleus accumbens), the ventral pallidum, and the pontine tegmentum.77,78,80,82 Most if not all of these structures have also been implicated in the pathophysiology of schizophrenia.77 Furthermore, fMRI studies have shown that most of these regions are altered by startle and/or PPI in humans, some being affected differentially in schizophrenia patients relative to control subjects.66,83
Pharmacological or Behavioral Manipulation of Task Performance
An extremely extensive literature describes the effects of pharmacological manipulations on startle and PPI in rats (summarized in Geyer et al.84 and Jones et al.85). In mice, the literature on pharmacological effects is also substantial and is growing.86 In addition, many of the genes implicated in schizophrenia have been examined in mice by studying the effects of relevant genetic manipulations on PPI.86,87 In both rats and mice, strain differences and gene expression studies have also added to our understanding of the neurobiological substrates influencing PPI.69,70 In humans, more limited pharmacological data are available (reviewed in75,88,89). It should be noted that some disparities between pharmacological effects on PPI have been noted when comparing mice with rats or rats with humans.77,78,90–92 Recent work has begun to explore the ability of some atypical antipsychotics, and potentially other treatments, to increase PPI specifically in healthy volunteers who exhibit low baseline levels of PPI.93 Such studies may enable the development of proof-of-concept studies for either antipsychotic or procognitive agents.
Animal Models
The cross-species nature of startle and PPI enables the use of animal models of induced deficits that are extremely similar to the gating deficits seen in schizophrenia. Beginning with the initial demonstrations of the ability of dopamine agonists and glutamatergic antagonists to disrupt PPI in rats,94 the rodent PPI models have evolved into at least 4 distinct models.77,84,95 These models have PPI measures in common but are differentiated by the manipulations used to disrupt PPI: (1) dopamine agonists, (2) serotonin agonists, (3) NMDA receptor antagonists, and (4) developmental manipulations such as isolation rearing or neonatal lesions of the ventral hippocampus.84 In contrast to the first 3 models, which are based on changes induced by acutely administered psychotomimetic drugs, the fourth PPI model may help to assess environmental or developmental contributions to PPI deficits.96–98 In addition to rodents, some work has begun to establish nonhuman primate models of PPI and pharmacological manipulations relevant to schizophrenia.99
Performance in Schizophrenia
As first reported in 1978100 and confirmed subsequently in many laboratories (reviewed in 64,75), PPI is reduced in schizophrenia patients. PPI deficits in schizophrenia patients are seen in patients treated with first generation antipsychotic drugs as well as in first-break patients who had never been treated with any antipsychotics.101 Although schizophrenia was the original focus of psychiatric PPI studies, subsequent research has demonstrated that PPI is reduced in patients suffering from a variety of neuropsychiatric disorders.64,75 For example, PPI deficits have also been found in obsessive compulsive disorder, Tourette's syndrome, Huntington's disorder, panic disorder, bipolar disorder, Asperger's syndrome, and others.102 These disorders are all characterized by PPI deficits and by abnormalities of gating in sensory, motor, or cognitive domains.
Psychometric Data
The several reviews cited above include extensive information regarding the psychometrics of PPI. In addition, it should be noted that PPI is quite stable over time in rodents and in both healthy human volunteers and clinically stable patients with schizophrenia.103,104
The MMN
Description
MMN is an ERP response that is elicited when a series of standard stimuli is interrupted periodically by deviant, or “oddball,” stimuli. MMN can be elicited using auditory and visual deviant stimuli that differ in one type of physical property (eg, pitch, duration, intensity) from the standard stimuli. Although MMN can be elicited with visual stimuli, it is most commonly recorded using auditory stimuli and there is more information on the neural subsystems and psychopharmacological effects on MMN with auditory stimuli. Thus, this brief review will focus largely on the auditory-elicited MMN. For a more in-depth review, including discussion on heritability and genes associated with MMN, see105.
In the typical auditory MMN paradigm, a standard auditory stimulus (eg, a 1000-Hz, 50-ms tone) is presented repeatedly with a brief interstimulus interval (eg, 500 ms). On approximately 10% of the trials, a deviant auditory stimulus that differs in one physical property (eg, a 1000-Hz, 100-ms tone) is presented. Subjects are usually instructed to ignore the tones, are shown a silent movie, or perform a secondary visual processing task. MMN is calculated as the difference between the ERP elicited by the deviant stimuli and the ERP elicited by the standard stimuli. Response onset can occur as early as 50 ms after the onset of the deviant stimuli and peaks at approximately 200 ms postonset. MMN elicited by auditory stimuli has its maximum response at frontocentral sites. In humans, MMN reflects a largely preattentive and automatic measure of change detection and is thought to represent an echoic memory process.106,107 MMN is not under the voluntary control of the subject and does not require any overt response. Therefore, MMN is seen as an effective means to measure preattentional auditory mechanisms in neuropsychiatric populations in which there may be questions about whether subjects are fully able and motivated to perform active cognitive tasks.
Neural Systems
Several different studies, using varying methodologies, have been conducted to determine the neural source of the auditory MMN response. Source localization of the MMN ERP,108 magnetoencephalography,109 and functional MRI110 studies have localized the auditory MMN to the primary and secondary auditory cortices. Specifically, this includes the superior temporal gyrus, while others find additional contributions from bilateral dorsolateral prefrontal cortices.111
Pharmacological or Behavioral Manipulation of Task Performance
NMDA receptor–mediated glutamate dysfunction is thought to underlie MMN deficits in certain neuropsychiatric diseases, including schizophrenia.112 NMDA antagonists have been shown to diminish MMN amplitude in primate models112 as well as selectively diminish MMN amplitude in healthy control subjects while sparing other auditory-related ERP activity.113–115
Animal Models
Animal models of MMN are very valuable in the neuroanatomical and psychopharmacological examination of normal and dysfunctional MMN. Reliable MMN data that closely match MMNs recorded in humans have been recorded from rats,116 cats,117 chimpanzees,118 as well as monkeys.112 The results of these studies imply that animal models can be used to test newly developed pharmacological treatments to improve MMN in schizophrenia patients.
Performance in Schizophrenia
Deficits in MMN amplitude and latency have repeatedly been shown in schizophrenia patients using varying deviant stimuli, including duration deviants, frequency deviants, and intensity deviants.119–122 A meta-analysis of MMN studies in schizophrenia patients123 showed a mean effect size of approximately 1.0. Moreover, it appears duration deviant stimuli tend to result in a larger MMN deficit compared with frequency deviant stimuli, though the difference was not statistically significant. MMN also appears to be insensitive to antipsychotic medication in schizophrenia, as it is not affected by first-generation antipsychotic medications or risperidone, olanzapine, or clozapine.124–126
MMN deficits in schizophrenia have recently been shown to be correlated with measures of functional outcome. In a sample of chronic schizophrenia patients, Light and Braff121 found that MMN was significantly negatively correlated with the Global Assessment of Functioning (GAF) scale127 as well as a measure of independent living,128 with MMN accounting for up to 42% of the variance in the functional status of the patients. Moreover, the relationship between MMN and functional status in schizophrenia patients has been shown to be stable over time.122 These connections to functioning have been replicated. Kiang et al.129 found that MMN elicited by duration-deviant tones was associated with GAF scores in a sample of 18 schizophrenia patients. Kawakubo and Kasai130 found that poorer duration MMN, elicited using phonemes rather than pure tones, was correlated with lower GAF scores. This group131 also found that better phoneme-deviant MMNs were associated with better scores on a social skills acquisition program after 3 months in a sample of 13 schizophrenia patients. Using a visual MMN task, Urban et al.132 found that schizophrenia patients below the median on the GAF had significantly smaller MMN amplitudes than normal controls and patients who were above the median on the GAF.
Psychometric Data
In healthy controls, MMN has high test-retest reliability coefficients in the range of 0.60–0.80, with higher reliability seen using duration deviant stimuli.133 In schizophrenia patients, MMN also appears to be stable over time, with one study finding no differences in MMN amplitudes recorded at least 1 year apart.122 MMN amplitude in frontal sties also appears to be stable in schizophrenia patients during acute and postacute phases,134 suggesting that MMN amplitude deficits are a relatively stable trait characteristic of the disease.
Visual Integration
Contour Integration Test
Description.
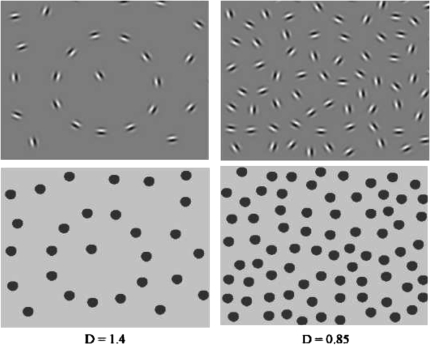
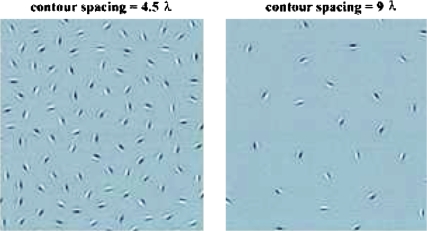
In this task, the visual integrative mechanisms responsible for linking contour segments together are probed by employing stimuli with a continuous path of Gabor signals embedded in noise (see figure 3). Participants are typically asked either to identify the location of the contour (eg, a line or a circular shape, depending on the task) within the larger stimulus field or to determine in which direction an egg-shaped contour is pointing (left or right) in a 2-alternative forced-choice task. Gabor elements are Gaussian-modulated sinusoidal luminance distributions that closely model the known spatial frequency processing properties of cells in area V1. Use of Gabor elements provides superior measurement of orientation sensitivity, and grouping of orientation cues, compared to stimuli with unknown effects on V1 neurons (eg, arbitrarily constructed lines and dots). The embedded contours in stimuli employing Gabor elements cannot be detected by purely local filters or by the known types of orientation tuned neurons with large receptive fields.135 The long-range orientation correlations along the path of the contour can only be found by the integration of local orientation measurements into an emergent shape representation (see figure 3).
Fig. 3.
Examples of Gabor-defined contours with different D values (top left: D = 1.4, top right: D = 0.85.). In the bottom panels, Gabor elements were replaced by disks to demonstrate the importance of correlated orientation cues in visual integration. Without orientation cues in these bottom panels, the contour remains invisible at D < 1, and this is the range where visual integration depends on long-range spatial interactions. Note that these panels focus on the stimulus region containing the contour only. In the actual test stimuli, the areas represented by the panels below would comprise about one-eighth of the total stimulus display.
Construct Validity
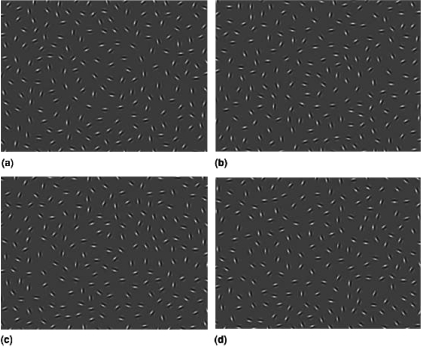
Numerous studies using such tasks have explored the conditions under which human observers perceive or do not perceive contours (reviewed in2). These findings support concept of Field et al.136 of the “association field,” in which neurons whose orientations are correlated in a manner that suggests the presence of a contour have facilitatory effects on each other, whereas neurons that encode elements whose orientation varies randomly with surrounding elements have an inhibitory effect on each other. Moreover, findings from psychophysical studies are consistent with computational models derived from information theory, in which receptive and contextual fields interact to enhance the salience of phenomena that can be grouped based on statistical regularities.137,138 Validation of the concept of visual integration from psychophysical studies has come from tasks that manipulate one or more of the following 3 parameters: (1) signal-noise ratio (delta or Δ) which refers to ratio of the average spacing between adjacent background elements to the average spacing between adjacent contour elements; contours are more difficult to detect as the ratio decreases (see figure 3); (2) the orientation of contour elements; contours are more difficult to detect as the elements are jittered and the correlation between the angles of adjacent elements decreases (see figure 4); (3) the spacing between contour elements; for children, but not healthy adults, contours are more difficult to detect as contour elements become further apart, even when Δ is kept constant by removing background elements as contour element spacing increases (see figure 5).
Fig. 4.
Samples of images from the 2-alternative forced choice version of the task. Top left: 0 degree jitter, top right: 7–8 degree jitter, bottom left: 15–16 degree jitter, and bottom right: 27–28 degree jitter. These panels show only the region of the display containing the contour. The actual stimuli contain approximately 75% additional space that contains only noise elements.
Fig. 5.
Performance in the contour-integration task is determined by the relative noise density (D), and it might also be determined by the absolute (cortical) spatial range of interactions (ie, the distance across which elements can be integrated into a single object). Left panel—contour spacing is small: 4.5 λ (λ = wavelength of Gabor signal or the width of the dark section of the stimulus). Right panel—contour spacing is large: 9 λ. These are partial presentations of the cards showing only the contour area. D = 0.85 in both cases. Adult performance as defined by D at threshold does not vary significantly in the tested contour-spacing range, which means that adults are limited only by relative noise density. However, children integrate large-spaced contours with a greater difficulty, which indicates the possibility of shorter interaction ranges in their case, and also in cases of neuropsychiatric disorders with compromised contour integration.
Neural Systems
The behavioral findings using contour integration tasks are supported by microelectrode studies in animals that indicate facilitatory effects of flanker elements with orientations similar to, or strongly correlated with, a target element, and inhibitory effects of random orientation surrounds.139 Moreover, behavioral findings fit closely with the predictions of the cortical processing model of visual integration proposed by Yen and Finkel140 in which horizontal connections mediate context-dependent facilitatory and inhibitory interactions among neurons coding stimulus orientation. The contour integration task has demonstrated sensitivity to visual integration deficits in both anisometropic and strabismic ambyopia, disorders where integration deficits are limited to the early visual cortex regions subserving the disordered eye, showing clear differences between amblyopic and fellow eyes.141,142 fMRI data in humans143 and monkeys144 indicate a visual cortex basis for contour integration, and a recent study in schizophrenia patients and healthy controls indicates that these same regions (eg, V2–V4) are underactivated during contour perception in schizophrenia (S. Silverstein, S. Berten, B. Essex, I. Kovacs, T. Susmaras, D. Little, unpublished data). In addition, recent work suggests that contour integration mechanisms may be mediated by NMDA-mediated glutamatergic effects.145
Pharmacological or Behavioral Manipulation of Task Performance
We are unaware of studies with these specific contour integration tasks that have examined whether performance can be modified through either specific pharmacological or behavioral interventions. However, as noted below, performance can improve during treatment in individuals with schizophrenia,146,147 suggesting that such deficits are amenable to modification. Given hypotheses about the mechanisms supporting contour integration described above, promising avenues for pharmacological manipulation would be agents that modulate NMDA receptor function.145
Performance in Schizophrenia
The contour integration task has shown evidence of impairment in schizophrenia in all studies in which it has been used (S. Silverstein, S. Berten, B. Essex, I. Kovacs, T. Susmaras, D. Little, unpublished data).148–153 Performance on the contour integration test is related to level of disorganization, but not positive or negative symptoms in schizophrenia.146–148,154 Moreover, among schizophrenia patients who demonstrate impairments on admission to acute-care-level treatment, test scores improve significantly over time, and the degree of change is related to improvement in disorganized symptoms.146,147
Psychometric Data
Past studies have demonstrated adequate reliability and minimal practice effects. Silverstein et al152 tested 87 people (including schizophrenia patients, other psychotic patients, and nonpatient controls) over 2 consecutive days using a signal-noise ratio (Δ) task variant. For controls only, across the first 2 days (collapsed across both conditions), the single measures ICC was 0.77, P < .001. For the entire sample, the single measures ICC was 0.66, P = .005 (These reliability estimates were calculated for the current article and are not found in the original article cited.). In a study using a version of the task also employing a signal-noise ratio (Δ) manipulation, there were no significant differences in test-retest performance across 4 same-day administrations in children, or across 6 same-day administrations in adults.155 In addition, studies of practice effects in nonclinical and amblyopic samples indicate virtually no change in performance across repeated administrations in a single day or 2 consecutive administrations on the same day152,155 using a version of the test that varies Δ only. When multiple administrations are used across multiple days, however, allowing for sleep-dependent perceptual learning, some minimal practice effects were observed.152,153 For example, using the orientation manipulation variant of the test with 12 healthy controls for 5 consecutive days, day 5 was the only day where scores differed significantly (P = .015) from day 1.153 Kovacs et al.156 reported that, when tested over three consecutive days, practice effects were not evident until the third day, and these were greater in children than in adults. Silverstein et al.,152 using a version that varied Δ only, demonstrated the largest practice effects. However, in this study, the test was given twice a day for 4 consecutive days. To date, studies have not specifically assessed practice effects or test-retest reliability over periods greater than 5 days. However, in Uhlhaas et al.,147 nondisorganized schizophrenia patients, psychotic patients with disorders other than schizophrenia, and psychiatric controls did not perform differently when tested on admission and discharge to a psychiatric unit (mean length of stay was 23 [SD = 22.2] days). Only the disorganized schizophrenia group demonstrated significant improvement, and this was significantly correlated with reduction in disorganized symptoms.
Future Directions
Future research will need to determine which of the three task versions described above are the most sensitive to visual integration deficits in individuals with schizophrenia. In addition, although each version of the task is relatively brief (20 min or shorter), work is needed to determine the minimum number of trials necessary both to discriminate patients from controls and to be sensitive to treatment effects. In addition, work is needed to determine whether visual integration deficits as measured by contour integration are amenable to modulation by pharmacological or behavioral interventions.
Coherent Motion Detection
Description
Detection of coherent motion is a perceptual task used widely for assessing visual motion processing. Like many perceptual tasks, development of the coherent motion detection task 157,158 is based on the premise that, by systematically adjusting the signal strength of visual motion, one can quantitatively determine properties of the visual motion processing system. As one such property, perceptual performance during motion detection can be measured as a function of signal strength of the visual stimulus.
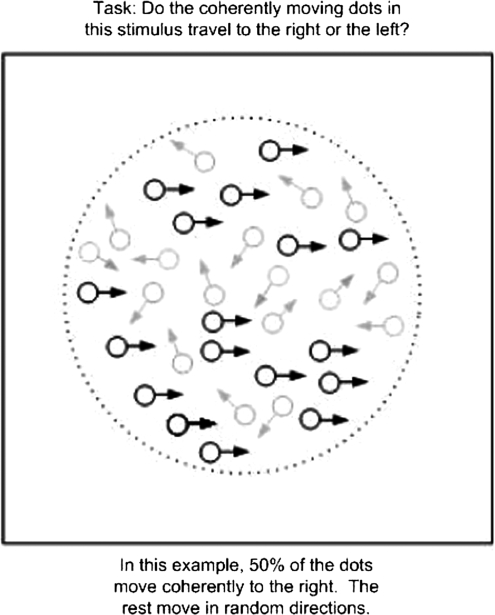
Coherent motion detection tasks utilize a specific motion stimulus called a random dot pattern (RDP) that consists of signal and noise components (figure 6). The signal is an array of dots moving coherently in one direction (eg, rightward) whereas the noise is another array of dots moving in random directions. These 2 arrays of moving dots interleave spatially within a certain region as well as temporally within a certain display time. The proportion of the signal dots in a RDP determines its motion signal strength—the greater the percentage of the signal dots, the stronger motion signal strength a RDP possesses. The task, when applied in a behavioral domain, is to identify the direction of motion of the signal dots in the presence of the noise dots.
Fig. 6.
Examples of stimuli from a coherent motion task.
Construct Validity
Given the special spatial and temporal configurations in a RDP, the ability to detect the direction of motion provides an effective measure of visual integration. To perceive the direction of motion of the stimulus, one must integrate visual information about the signal dots that are distributed spatially in random locations and temporally at random times. Focus on one particular spatial location or one particular time would not lead to success in detecting the globally defined direction of motion. The requirement of combining visual signals across space and time makes coherent motion detection a useful task for assessing integration and therefore lends the task a high degree of construct validity. It has been used in studying a variety of populations—human and animal, young and elderly, and healthy and diseased.
Neural Systems
Perceptual performance in coherent motion detection is mediated by neural computation in the visual motion systems. The primary neural computation includes direction selectivity and spatial integration—the former refers to a property that is characterized as the selective responsiveness of a neural unit to one specific direction of motion (but not to the opposite direction of motion), whereas the latter refers to a summation process that combines visual signals across space. Neurophysiological studies in monkeys have identified neural units that are responsive to coherent motion.159 Robust neuronal responses to coherent motion in motion-sensitive brain areas such as the middle temporal (MT) area have been directly linked to correct perceptual responses.160 Neuroimaging studies in humans have shown that the same cortical regions (eg, MT) are significantly activated in the presence of coherent motion,161 confirming the evidence found from neurophysiological studies. One important feature in these findings is that the magnitude of both neuronal responses and cortical activations at MT is proportional to the signal strength of coherent motion, mimicking the established relationship between accuracy of perceptual performance and signal strength of coherent motion.
Pharmacological or Behavioral Manipulation of Task Performance
Several types of neurotransmission appear to modulate detection of coherent motion. GABAergic activity plays an important role in forming and shaping direction selectivity, a neural property essential for motion detection.162 Agonistic action of serotonin (via Psilocybin) can lead to a selective impairment in detection of coherent motion.163 The impact of pharmacological modulations on detection of coherent motion is still an area that awaits further investigation.
Performance in Schizophrenia
Compared with healthy individuals, schizophrenia patients perform the task with significantly lower accuracies and require greater levels of signal strength in order to achieve similar levels of accuracies.164–167 This result indicates that processing of coherent motion signals is deficient in this psychiatric disorder. On the other hand, a preliminary study showed that the relatives of schizophrenia patients and patients with bipolar disorder had normal performance on this task, suggesting specificity of coherent motion detection deficit in clinical schizophrenia.168 This result, however, needs to be confirmed in larger and independent populations. The deficient performance in detecting coherent motion has been associated with smooth pursuit eye movement dysfunction in schizophrenia patients.164,169
Cortical mechanisms underlying coherent motion detection have also been investigated in schizophrenia. Although normal detection of coherent motion primarily involves the occipital cortex, decreased neural activation in occipital areas such as MT (as expected), as well as increased neural activation in the frontal cortex, were found while schizophrenia patients performed this task.170 This result suggests that coherent motion detection in schizophrenia involves not only sensory but also cognitive processing in the cortex.
Psychometric Data
Many kinds of physiological (as well as behavioral) data are available to attest to the validity of the coherent motion detection task in measuring visual integration. Yet, the reliability of performance measurements in individuals, particularly in patients, still needs to be evaluated.
Future Directions
Thus far, this task has been used primarily within research laboratories. How to adapt this laboratory-based task in clinical settings is a future direction that merits effort. Additionally, the interaction between visual and cognitive processing remains an elusive topic in schizophrenia research. Detection of coherent motion may provide a probe into both visual and visual cognitive processes, in which the bottom-up and top-down mechanisms pertaining to visual integration can be concurrently and comprehensively evaluated in schizophrenia patients. Another prospective direction may be to evaluate the efficacy of antipsychotic agents on modulating behavioral performance during coherent motion detection tasks.
Funding
National Institute of Mental Health (NIMH) and the Department of Veterans Affairs (VA) to M.F.G. MH43292 and J.K.W.; NIMH to P.D.B., MH66374 Y.C., MH61824 and J.H.Y.; consulting compensation from Abbott, Acadia, Addex, Amgen, AstraZeneca, Bristol-Myers Squibb, Jazz, Organon, Nura, San Diego Instruments, Serono, and Wyeth-Ayerst to M.A.G.; MH52885 research grant support from National Institute of Drug Abuse (NIDA DA02925, NIMH, and the US Veteran's Administration VISN 22 Mental Illness Research, Education, and Clinical Center to M.A.G.; NIMH, NARSAD, Stanley Medical Research Institute, Janssen, and AstraZeneca to S.S.
M.F.G. serves as a consultant to Amgen, Acadia, Astellas, Bristol-Myers Squibb, Dainippon Sumitomo Pharma, Eli Lilly, Lundbeck, Memory Pharmaceuticals, Otsuka Pharmaceutical, Sanofi-Aventis Pharmaceuticals, and Solvay.
Acknowledgments
We would also like to thank Jody Conrad, Carol Cox, and Deb Tussing whose efforts have been invaluable in the CNTRICS process. Ryan McBain provided helpful comments on the coherent motion detection task section. In addition, we would like to thank the other members of the perception breakout group, including Jeffrey Baker, Deanna Barch, Paul Blum, Robert Buchanan, Sam Chan, Steve Dakin, John Evenden, Melissa Fisher, Judy Ford, Magali Haas, Ron Mangun, Steve Marder, Daniel Mathalon, Michael Palfreyman, Daniel Ragland, Milton Strauss, Carol Tamminga, Steve Taylor, Jared Young, Jessica Turner, and Curtis Tatsuoka.
References
- 1.CNTRICS. Cognitive Neuroscience Approaches to Treatment Development of Impaired Cognition in Schizophrenia: proceedings of the First Meeting of the CNTRICS Initiative. Biol Psychiatry. 2008;64:1–78. [Google Scholar]
- 2.Butler PD, Silverstein SM, Dakin SC. Visual perception and its impairment in schizophrenia. Biol Psychiatry. 2008;64:40–47. doi: 10.1016/j.biopsych.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman RE, Woods SW, Hawkins KA, et al. Extracting spurious messages from noise and risk of schizophrenia-spectrum disorders in a prodromal population. Br J Psychiatry. 2007;191:355–356. doi: 10.1192/bjp.bp.106.031195. [DOI] [PubMed] [Google Scholar]
- 4.Chubb C, Sperling G, Solomon JA. Texture interactions determine perceived contrast. Proc Natl Acad Sci U S A. 1989;86:9631–9635. doi: 10.1073/pnas.86.23.9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dakin S, Carlin P, Hemsley D. Weak suppression of visual context in chronic schizophrenia. Curr Biol. 2005;15:R822–R82. doi: 10.1016/j.cub.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Xing J, Heeger DJ. Measurement and modeling of center-surround suppression and enhancement. Vision Res. 2001;41:571–583. doi: 10.1016/s0042-6989(00)00270-4. [DOI] [PubMed] [Google Scholar]
- 7.Watt RJ, Andrews DP. Adaptive probit estimation of psycometric functions. Curr Psychol Rev. 1981;1:205–214. [Google Scholar]
- 8.Bair W, Cavanaugh JR, Movshon JA. Time course and time-distance relationships for surround suppression in macaque V1 neurons. J Neurosci. 2003;23:7690–7701. doi: 10.1523/JNEUROSCI.23-20-07690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavanaugh JR, Bair W, Movshon JA. Nature and interaction of signals from the receptive field center and surround in macaque V1 neurons. J Neurophysiol. 2002;88:2530–2546. doi: 10.1152/jn.00692.2001. [DOI] [PubMed] [Google Scholar]
- 10.DeAngelis GC, Robson JG, Ohzawa I, Freeman RD. Organization of suppression in receptive fields of neurons in cat visual cortex. J Neurophysiol. 1992;68:144–163. doi: 10.1152/jn.1992.68.1.144. [DOI] [PubMed] [Google Scholar]
- 11.Jones HE, Wang W, Sillito AM. Spatial organization and magnitude of orientation contrast interactions in primate V1. J Neurophysiol. 2002;88:2796–2808. doi: 10.1152/jn.00403.2001. [DOI] [PubMed] [Google Scholar]
- 12.Naito T, Sadakane O, Okamoto M, Sato H. Orientation tuning of surround suppression in lateral geniculate nucleus and primary visual cortex of cat. Neuroscience. 2007;149:962–975. doi: 10.1016/j.neuroscience.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Ozeki H, Sadakane O, Akasaki T, Naito T, Shimegi S, Sato H. Relationship between excitation and inhibition underlying size tuning and contextual response modulation in the cat primary visual cortex. J Neurosci. 2004;24:1428–1438. doi: 10.1523/JNEUROSCI.3852-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sceniak MP, Ringach DL, Hawken MJ, Shapley R. Contrast’s effect on spatial summation by macaque V1 neurons. Nat Neurosci. 1999;2:733–739. doi: 10.1038/11197. [DOI] [PubMed] [Google Scholar]
- 15.Zipser K, Lamme VA, Schiller PH. Contextual modulation in primary visual cortex. J Neurosci. 1996;16:7376–7389. doi: 10.1523/JNEUROSCI.16-22-07376.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zenger-Landolt B, Heeger DJ. Response suppression in v1 agrees with psychophysics of surround masking. J Neurosci. 2003;23:6884–6893. doi: 10.1523/JNEUROSCI.23-17-06884.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert CD, Wiesel TN. Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. J Neurosci. 1989;9:2432–2442. doi: 10.1523/JNEUROSCI.09-07-02432.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angelucci A, Bullier J. Reaching beyond the classical receptive field of V1 neurons: horizontal or feedback axons? J Physiol Paris. 2003;97:141–154. doi: 10.1016/j.jphysparis.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Webb BS, Dhruv NT, Solomon SG, Tailby C, Lennie P. Early and late mechanisms of surround suppression in striate cortex of macaque. J Neurosci. 2005;25:11666–11675. doi: 10.1523/JNEUROSCI.3414-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bodis-Wollner I. Visual deficits related to dopamine deficiency in experimental animals and Parkinson’s disease patients. Trends Neurosci. 1990;13:296–302. doi: 10.1016/0166-2236(90)90113-o. [DOI] [PubMed] [Google Scholar]
- 21.Djamgoz MB, Hankins MW, Hirano J, Archer SN. Neurobiology of retinal dopamine in relation to degenerative states of the tissue. Vision Res. 1997;37:3509–3529. doi: 10.1016/S0042-6989(97)00129-6. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Levy DL, Sheremata S, Nakayama K, Matthysse S, Holzman PS. Effects of typical, atypical, and no antipsychotic drugs on visual contrast detection in schizophrenia. Am J Psychiatry. 2003;160:1795–1801. doi: 10.1176/appi.ajp.160.10.1795. [DOI] [PubMed] [Google Scholar]
- 23.Disney AA, Aoki C, Hawken MJ. Gain modulation by nicotine in macaque v1. Neuron. 2007;56:701–713. doi: 10.1016/j.neuron.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silver MA, Ress D, Heeger DJ. Neural correlates of sustained spatial attention in human early visual cortex. J Neurophysiol. 2007;97:229–237. doi: 10.1152/jn.00677.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrone MC, Denti V, Spinelli D. Different attentional resources modulate the gain mechanisms for color and luminance contrast. Vision Res. 2004;44:1389–1401. doi: 10.1016/j.visres.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Norton D, Ongur D. Altered center-surround motion inhibition in schizophrenia. Biol Psychiatry. 2008;64:74–77. doi: 10.1016/j.biopsych.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Must A, Janka Z, Benedek G, Keri S. Reduced facilitation effect of collinear flankers on contrast detection reveals impaired lateral connectivity in the visual cortex of schizophrenia patients. Neurosci Lett. 2004;357:131–134. doi: 10.1016/j.neulet.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 28.Tadin D, Kim J, Doop ML, et al. Weakened center-surround interactions in visual motion processing in schizophrenia. J Neurosci. 2006;26:11403–11412. doi: 10.1523/JNEUROSCI.2592-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zemon V, Gordon J. Luminance-contrast mechanisms in humans: visual evoked potentials and a nonlinear model. Vision Res. 2006;46:4163–4180. doi: 10.1016/j.visres.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan E, Shapley R. The primate retina contains two types of ganglion cells, with high and low contrast sensitivity. Proc Natl Acad Sci U S A. 1986;83:2755–2757. doi: 10.1073/pnas.83.8.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplan E, Shapley RM. X and Y cells in the lateral geniculate nucleus of macaque monkeys. J Physiol (Lond) 1982;330:125–143. doi: 10.1113/jphysiol.1982.sp014333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tootell RB, Hamilton SL, Switkes E. Functional anatomy of macaque striate cortex. IV. Contrast and magno-parvo streams. J Neurosci. 1988;8:1594–1609. doi: 10.1523/JNEUROSCI.08-05-01594.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butler PD, Zemon V, Schechter I, et al. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen Psychiatry. 2005;62:495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zemon V, Hartmann EE, Gordon J, Prunte-Glowazki A. An electrophysiological technique for assessment of the development of spatial vision. Optom Vis Sci. 1997;74:708–716. doi: 10.1097/00006324-199709000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Victor JD, Mast J. A new statistic for steady-state evoked potentials. Electroencephalogr Clin Neurophysiol. 1991;78:378–388. doi: 10.1016/0013-4694(91)90099-p. [DOI] [PubMed] [Google Scholar]
- 36.Kelly DH. Visual contrast sensitivity. Optica Acta. 1977;24:107–129. [Google Scholar]
- 37.Kelly DH, Savoie RE. A study of sine-wave contrast sensitivity by two psychophysical methods. 1973;14 [Google Scholar]
- 38.Robson JG. Spatial and temporal contrast-sensitivity functions of the visual system. J Opt Soc Am. 1966;56:1141–1142. [Google Scholar]
- 39.Shapley RM, Victor JD. The effect of contrast on the transfer properties of cat retinal ganglion cells. J Physiol. 1978;285:275–298. doi: 10.1113/jphysiol.1978.sp012571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shapley RM, Victor JD. How the contrast gain control modifies the frequency responses of cat retinal ganglion cells. J Physiol. 1981;318:161–179. doi: 10.1113/jphysiol.1981.sp013856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benardete EA, Kaplan E, Knight BW. Contrast gain control in the primate retina: P cells are not X-like, some M cells are. Vis Neurosci. 1992;8:483–486. doi: 10.1017/s0952523800004995. [DOI] [PubMed] [Google Scholar]
- 42.Borg-Graham LJ, Monier C, Fregnac Y. Visual input evokes transient and strong shunting inhibition in visual cortical neurons. Nature. 1998;393:369–373. doi: 10.1038/30735. [DOI] [PubMed] [Google Scholar]
- 43.Butler PD, Schechter I, Zemon V, et al. Dysfunction of early stage visual processing in schizophrenia. Am J Psychiatry. 2001;158:1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- 44.Greenstein VC, Seliger S, Zemon V, Ritch R. Visual evoked potential assessment of the effects of glaucoma on visual subsystems. Vision Res. 1998;38:1901–1911. doi: 10.1016/s0042-6989(97)00348-9. [DOI] [PubMed] [Google Scholar]
- 45.Derrington AM, Lennie P. Spatial and temporal contrast sensitivities of neurones in lateral geniculate nucleus of macaque. J Physiol. 1984;357:219–240. doi: 10.1113/jphysiol.1984.sp015498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaplan E, Purpura K, Shapley RM. Contrast affects the transmission of visual information through the mammalian lateral geniculate nucleus. J Physiol. 1987;391:267–288. doi: 10.1113/jphysiol.1987.sp016737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Legge G. Sustained and transient mechanisms in human vision: temporal and spatial properties. Vision Res. 1978;18:69–82. doi: 10.1016/0042-6989(78)90079-2. [DOI] [PubMed] [Google Scholar]
- 48.Fox K, Sato H, Daw N. The effect of varying stimulus intensity on NMDA-receptor activity in cat visual cortex. J Neurophysiol. 1990;64:1413–1428. doi: 10.1152/jn.1990.64.5.1413. [DOI] [PubMed] [Google Scholar]
- 49.Kwon YH, Nelson SB, Toth LJ, Sur M. Effect of stimulus contrast and size on NMDA receptor activity in cat lateral geniculate nucleus. J Neurophysiol. 1992;68:182–196. doi: 10.1152/jn.1992.68.1.182. [DOI] [PubMed] [Google Scholar]
- 50.Bodis-Wollner I, Tzelepi A. The push-pull action of dopamine on spatial tuning of the monkey retina: the effects of dopaminergic deficiency and selective D1 and D2 receptor ligands on the pattern electroretinogram. Vision Res. 1998;38:1479–1487. doi: 10.1016/s0042-6989(98)00028-5. [DOI] [PubMed] [Google Scholar]
- 51.Zemon V, Mehta AD, Schroeder CE, Gordon J. Parallel pathways in humans and monkeys: a VEP study. Invest Ophthalmol Vis Sci. 1995;36:690. [Google Scholar]
- 52.DeValois RL, DeValois KK. Spatial Vision. New York, NY: Oxford University Press; 1990. [Google Scholar]
- 53.Stavros KA, Kiorpes L. Behavioral measurement of temporal contrast sensitivity development in macaque monkeys (Macaca nemestrina) Vision Res. 2008;48:1335–1344. doi: 10.1016/j.visres.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zemon V, Butler PD, Gordon J, et al. Neural dysfunction in schizophrenia: contrast-response functions and a nonlinear model. Abstr Soc Neurosci. 2004;347:12. [Google Scholar]
- 55.Butler PD, Tambini A, Yovel G, et al. What’s in a face? Effects of stimulus duration and inversion on face processing in schizophrenia. Schizophr Res. 2008;103:283–292. doi: 10.1016/j.schres.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keri S, Antal A, Szekeres G, Benedek G, Janka Z. Spatiotemporal visual processing in schizophrenia. J Neuropsychiatry Clin Neurosci. 2002;14:190–196. doi: 10.1176/jnp.14.2.190. [DOI] [PubMed] [Google Scholar]
- 57.Slaghuis WL. Contrast sensitivity for stationary and drifting spatial frequency gratings in positive- and negative-symptom schizophrenia. J Abnorm Psychol. 1998;107:49–62. doi: 10.1037//0021-843x.107.1.49. [DOI] [PubMed] [Google Scholar]
- 58.Slaghuis WL. Spatio-temporal luminance contrast sensitivity and visual backward masking in schizophrenia. Exp Brain Res. 2004;156:196–211. doi: 10.1007/s00221-003-1771-3. [DOI] [PubMed] [Google Scholar]
- 59.Chen Y, Levy DL, Sheremata S, Holzman PS. Compromised late-stage motion processing in schizophrenia. Biol Psychiatry. 2004;55:834–841. doi: 10.1016/j.biopsych.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 60.Chen Y, Palafox GP, Nakayama K, Levy DL, Matthysse S, Holzman PS. Motion perception in schizophrenia. Arch Gen Psychiatry. 1999;56:149–154. doi: 10.1001/archpsyc.56.2.149. [DOI] [PubMed] [Google Scholar]
- 61.Oranje B, Geyer MA, Bocker KB, Leon Kenemans J, Verbaten MN. Prepulse inhibition and P50 suppression: commonalities and dissociations. Psychiatry Res. 2006;143:147–158. doi: 10.1016/j.psychres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 62.Swerdlow NR, Martinez ZA, Hanlon FM, et al. Toward understanding the biology of a complex phenotype: rat strain and substrain differences in the sensorimotor gating-disruptive effects of dopamine agonists. J Neurosci. 2000;20:4325–4336. doi: 10.1523/JNEUROSCI.20-11-04325.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davis M, Mansbach RS, Swerdlow NR, Campeau S, Braff DL, Geyer MA. Apomorphine disrupts the inhibition of acoustic startle induced by weak prepulses in rats. Psychopharmacology (Berl) 1990;102:1–4. doi: 10.1007/BF02245735. [DOI] [PubMed] [Google Scholar]
- 64.Braff DL, Geyer MA, Light GA, et al. Impact of prepulse characteristics on the detection of sensorimotor gating deficits in schizophrenia. Schizophr Res. 2001;49:171–178. doi: 10.1016/s0920-9964(00)00139-0. [DOI] [PubMed] [Google Scholar]
- 65.Dawson ME, Schell AM, Hazlett EA, Nuechterlein KH, Filion DL. On the clinical and cognitive meaning of impaired sensorimotor gating in schizophrenia. Psychiatry Res. 2000;96:187–197. doi: 10.1016/s0165-1781(00)00208-0. [DOI] [PubMed] [Google Scholar]
- 66.Hazlett EA, Buchsbaum MS, Tang CY, et al. Thalamic activation during an attention-to-prepulse startle modification paradigm: a functional MRI study. Biol Psychiatry. 2001;50:281–291. doi: 10.1016/s0006-3223(01)01094-0. [DOI] [PubMed] [Google Scholar]
- 67.Heekeren K, Meincke U, Geyer MA, Gouzoulis-Mayfrank E. Attentional modulation of prepulse inhibition: a new startle paradigm. Neuropsychobiology. 2004;49:88–93. doi: 10.1159/000076416. [DOI] [PubMed] [Google Scholar]
- 68.Swerdlow NR, Geyer MA, Hartman PL, et al. Sex differences in sensorimotor gating of the human startle reflex: all smoke? Psychopharmacology (Berl) 1999;146:228–232. doi: 10.1007/s002130051111. [DOI] [PubMed] [Google Scholar]
- 69.Ellenbroek BA, Geyer MA, Cools AR. The behavior of APO-SUS rats in animal models with construct validity for schizophrenia. J Neurosci. 1995;15:7604–7611. doi: 10.1523/JNEUROSCI.15-11-07604.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paylor R, Crawley JN. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology (Berl) 1997;132:169–180. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- 71.Braff DL, Geyer MA. Sensorimotor gating and schizophrenia. Human and animal model studies. Arch Gen Psychiatry. 1990;47:181–188. doi: 10.1001/archpsyc.1990.01810140081011. [DOI] [PubMed] [Google Scholar]
- 72.Geyer MA, Braff DL. Startle habituation and sensorimotor gating in schizophrenia and related animal models. Schizophr Bull. 1987;13:643–668. doi: 10.1093/schbul/13.4.643. [DOI] [PubMed] [Google Scholar]
- 73.McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. Br J Med Psychol. 1961;34:103–116. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- 74.Venables PH. Input dysfunction in schizophrenia. Prog Exp Pers Res. 1964;72:1–47. [PubMed] [Google Scholar]
- 75.Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- 76.Swerdlow NR, Braff DL, Taaid N, Geyer MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiatry. 1994;51:139–154. doi: 10.1001/archpsyc.1994.03950020063007. [DOI] [PubMed] [Google Scholar]
- 77.Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- 78.Swerdlow NR, Platten A, Shoemaker J, Pitcher L, Auerbach P. Effects of pergolide on sensorimotor gating of the startle reflex in rats. Psychopharmacology (Berl) 2001;158:230–240. doi: 10.1007/s002130100856. [DOI] [PubMed] [Google Scholar]
- 79.Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- 81.Fendt M, Li L, Yeomans JS. Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology (Berl) 2001;156:216–224. doi: 10.1007/s002130100794. [DOI] [PubMed] [Google Scholar]
- 82.Vollenweider FX, Geyer MA. A systems model of altered consciousness: integrating natural and drug-induced psychoses. Brain Res Bull. 2001;56:495–507. doi: 10.1016/s0361-9230(01)00646-3. [DOI] [PubMed] [Google Scholar]
- 83.Kumari V, Gray JA, Geyer MA, et al. Neural correlates of tactile prepulse inhibition: a functional MRI study in normal and schizophrenic subjects. Psychiatry Res. 2003;122:99–113. doi: 10.1016/s0925-4927(02)00123-3. [DOI] [PubMed] [Google Scholar]
- 84.Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- 85.Jones DNC, Gartlon JE, Minassian A, Perry W, Geyer MA. Developing new drugs for schizophrenia: from animals to the clinic. In: McArthur R, Borsini F, editors. Animal and Translational Models for CNS Drug Discovery: Psychiatric Disorders. New York, NY: Elsevier Inc.; 2008;Vol. 1:199–262. [Google Scholar]
- 86.Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Mol Psychiatry. 2002;7:1039–1053. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- 87.Ralph RJ, Varty GB, Kelly MA, et al. The dopamine D2, but not D3 or D4, receptor subtype is essential for the disruption of prepulse inhibition produced by amphetamine in mice. J Neurosci. 1999;19:4627–4633. doi: 10.1523/JNEUROSCI.19-11-04627.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hamm AO, Weike AI, Schupp HT. The effect of neuroleptic medication on prepulse inhibition in schizophrenia patients: current status and future issues. Psychopharmacology (Berl) 2001;156:259–265. doi: 10.1007/s002130100827. [DOI] [PubMed] [Google Scholar]
- 89.Kumari V, Sharma T. Effects of typical and atypical antipsychotics on prepulse inhibition in schizophrenia: a critical evaluation of current evidence and directions for future research. Psychopharmacology (Berl) 2002;162:97–101. doi: 10.1007/s00213-002-1099-x. [DOI] [PubMed] [Google Scholar]
- 90.Abel KM, Allin MP, Hemsley DR, Geyer MA. Low dose ketamine increases prepulse inhibition in healthy men. Neuropharmacology. 2003;44:729–737. doi: 10.1016/s0028-3908(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 91.Bitsios P, Giakoumaki SG, Frangou S. The effects of dopamine agonists on prepulse inhibition in healthy men depend on baseline PPI values. Psychopharmacology (Berl) 2005;182:144–152. doi: 10.1007/s00213-005-0056-x. [DOI] [PubMed] [Google Scholar]
- 92.Vollenweider FX, Remensberger S, Hell D, Geyer MA. Opposite effects of 3,4-methylenedioxymethamphetamine (MDMA) on sensorimotor gating in rats versus healthy humans. Psychopharmacology (Berl) 1999;143:365–372. doi: 10.1007/s002130050960. [DOI] [PubMed] [Google Scholar]
- 93.Csomor PA, Stadler RR, Feldon J, Yee BK, Geyer MA, Vollenweider FX. Haloperidol differentially modulates prepulse inhibition and p50 suppression in healthy humans stratified for low and high gating levels. Neuropsychopharmacology. 2008;33:497–512. doi: 10.1038/sj.npp.1301421. [DOI] [PubMed] [Google Scholar]
- 94.Geyer MA, Swerdlow NR, Mansbach RS, Braff DL. Startle response models of sensorimotor gating and habituation deficits in schizophrenia. Brain Res Bull. 1990;25:485–498. doi: 10.1016/0361-9230(90)90241-q. [DOI] [PubMed] [Google Scholar]
- 95.Ellenbroek BA. Pre-attentive processing and schizophrenia: animal studies. Psychopharmacology (Berl) 2004;174:65–74. doi: 10.1007/s00213-003-1684-7. [DOI] [PubMed] [Google Scholar]
- 96.Le Pen G, Moreau JL. Disruption of prepulse inhibition of startle reflex in a neurodevelopmental model of schizophrenia: reversal by clozapine, olanzapine and risperidone but not by haloperidol. Neuropsychopharmacology. 2002;27:1–11. doi: 10.1016/S0893-133X(01)00383-9. [DOI] [PubMed] [Google Scholar]
- 97.Lipska BK, Swerdlow NR, Geyer MA, Jaskiw GE, Braff DL, Weinberger DR. Neonatal excitotoxic hippocampal damage in rats causes post-pubertal changes in prepulse inhibition of startle and its disruption by apomorphine. Psychopharmacology (Berl) 1995;122:35–43. doi: 10.1007/BF02246439. [DOI] [PubMed] [Google Scholar]
- 98.Powell SB, Geyer MA. Developmental markers of psychiatric disorders as identified by sensorimotor gating. Neurotox Res. 2002;4:489–502. doi: 10.1080/10298420290030578. [DOI] [PubMed] [Google Scholar]
- 99.Linn GS, Negi SS, Gerum SV, Javitt DC. Reversal of phencyclidine-induced prepulse inhibition deficits by clozapine in monkeys. Psychopharmacology (Berl) 2003;169:234–239. doi: 10.1007/s00213-003-1533-8. [DOI] [PubMed] [Google Scholar]
- 100.Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- 101.Ludewig K, Geyer MA, Vollenweider FX. Deficits in prepulse inhibition and habituation in never-medicated, first-episode schizophrenia. Biol Psychiatry. 2003;54:121–128. doi: 10.1016/s0006-3223(02)01925-x. [DOI] [PubMed] [Google Scholar]
- 102.Geyer MA. The family of sensorimotor gating disorders: comorbidities or diagnostic overlaps? Neurotox Res. 2006;10:211–220. doi: 10.1007/BF03033358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ludewig K, Geyer MA, Etzensberger M, Vollenweider FX. Stability of the acoustic startle reflex, prepulse inhibition, and habituation in schizophrenia. Schizophr Res. 2002;55:129–137. doi: 10.1016/s0920-9964(01)00198-0. [DOI] [PubMed] [Google Scholar]
- 104.Ludewig S, Ludewig K, Geyer MA, Hell D, Vollenweider FX. Prepulse inhibition deficits in patients with panic disorder. Depress Anxiety. 2002;15:55–60. doi: 10.1002/da.10026. [DOI] [PubMed] [Google Scholar]
- 105.Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Naatanen R, Paavilainen P, Alho K, Reinikainen K, Sams M. Do event-related potentials reveal the mechanism of the auditory sensory memory in the human brain? Neurosci Lett. 1989;98:217–221. doi: 10.1016/0304-3940(89)90513-2. [DOI] [PubMed] [Google Scholar]
- 107.Näätänen R. Attention and Brain Function. Hillsdale, NJ: Lawrence A. Erlbaum Associates; 1992. [Google Scholar]
- 108.Park HJ, Kwon JS, Youn T, et al. Statistical parametric mapping of LORETA using high density EEG and individual MRI: application to mismatch negativities in schizophrenia. Hum Brain Mapp. 2002;17:168–178. doi: 10.1002/hbm.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alho K, Winkler I, Escera C, et al. Processing of novel sounds and frequency changes in the human auditory cortex: magnetoencephalographic recordings. Psychophysiology. 1998;35:211–224. [PubMed] [Google Scholar]
- 110.Schall U, Johnston P, Todd J, Ward PB, Michie PT. Functional neuroanatomy of auditory mismatch processing: an event-related fMRI study of duration-deviant oddballs. Neuroimage. 2003;20:729–736. doi: 10.1016/S1053-8119(03)00398-7. [DOI] [PubMed] [Google Scholar]
- 111.Alho K. Cerebral generators of mismatch negativity (MMN) and its magnetic counterpart (MMNm) elicited by sound changes. Ear Hear. 1995;16:38–51. doi: 10.1097/00003446-199502000-00004. [DOI] [PubMed] [Google Scholar]
- 112.Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci U S A. 1996;93:11962–11967. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Umbricht D, Koller R, Vollenweider FX, Schmid L. Mismatch negativity predicts psychotic experiences induced by NMDA receptor antagonist in healthy volunteers. Biol Psychiatry. 2002;51:400–406. doi: 10.1016/s0006-3223(01)01242-2. [DOI] [PubMed] [Google Scholar]
- 114.Heekeren K, Daumann J, Neukirch A, et al. Mismatch negativity generation in the human 5HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology (Berl) 2008;199:77–88. doi: 10.1007/s00213-008-1129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kreitschmann-Andermahr I, Rosburg T, Demme U, Gaser E, Nowak H, Sauer H. Effect of ketamine on the neuromagnetic mismatch field in healthy humans. Brain Res Cogn Brain Res. 2001;12:109–116. doi: 10.1016/s0926-6410(01)00043-x. [DOI] [PubMed] [Google Scholar]
- 116.Umbricht D, Vyssotki D, Latanov A, Nitsch R, Lipp HP. Deviance-related electrophysiological activity in mice: is there mismatch negativity in mice? Clin Neurophysiol. 2005;116:353–363. doi: 10.1016/j.clinph.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 117.Csepe V, Karmos G, Molnar M. Evoked potential correlates of stimulus deviance during wakefulness and sleep in cat–animal model of mismatch negativity. Electroencephalogr Clin Neurophysiol. 1987;66:571–578. doi: 10.1016/0013-4694(87)90103-9. [DOI] [PubMed] [Google Scholar]
- 118.Ueno A, Hirata S, Fuwa K, et al. Auditory ERPs to stimulus deviance in an awake chimpanzee (Pan troglodytes): towards hominid cognitive neurosciences. PLoS ONE. 2008;3:e1442. doi: 10.1371/journal.pone.0001442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Javitt DC, Shelley AM, Silipo G, Lieberman JA. Deficits in auditory and visual context-dependent processing in schizophrenia: defining the pattern. Arch Gen Psychiatry. 2000;57:1131–1137. doi: 10.1001/archpsyc.57.12.1131. [DOI] [PubMed] [Google Scholar]
- 120.Salisbury DF, Shenton ME, Griggs CB, Bonner-Jackson A, McCarley RW. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Arch Gen Psychiatry. 2002;59:686–694. doi: 10.1001/archpsyc.59.8.686. [DOI] [PubMed] [Google Scholar]
- 121.Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch Gen Psychiatry. 2005;62:127–136. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- 122.Light GA, Braff DL. Stability of mismatch negativity deficits and their relationship to functional impairments in chronic schizophrenia. Am J Psychiatry. 2005;162:1741–1743. doi: 10.1176/appi.ajp.162.9.1741. [DOI] [PubMed] [Google Scholar]
- 123.Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 124.Umbricht D, Javitt D, Novak G, et al. Effects of clozapine on auditory event-related potentials in schizophrenia. Biol Psychiatry. 1998;44:716–725. doi: 10.1016/s0006-3223(97)00524-6. [DOI] [PubMed] [Google Scholar]
- 125.Umbricht D, Javitt D, Novak G, et al. Effects of risperidone on auditory event-related potentials in schizophrenia. Int J Neuropsychopharmacol. 1999;2:299–304. doi: 10.1017/S1461145799001595. [DOI] [PubMed] [Google Scholar]
- 126.Korostenskaja M, Dapsys K, Siurkute A, Maciulis V, Ruksenas O, Kahkonen S. Effects of olanzapine on auditory P300 and mismatch negativity (MMN) in schizophrenia spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:543–548. doi: 10.1016/j.pnpbp.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 127.APA APAToD-I. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. Washington D.C.: American Psychiatric Association; 2000. [Google Scholar]
- 128.Rapaport M, Bazzetta J, McAdams LA, Patterson T, Jeste DV. Validation of the scale of functioning in older outpatients with schizophrenia. Am J Geriatr Psychiatry. 1996;4:218–228. doi: 10.1097/00019442-199622430-00005. [DOI] [PubMed] [Google Scholar]
- 129.Kiang M, Light GA, Prugh J, Coulson S, Braff D, Kutas M. Cognitive, neurophysiological and functional correlates of proverb interpretation abnormalities in schizophrenia. J Int Neuropsychol Soc. 2007;13:653–663. doi: 10.1017/S1355617707070816. [DOI] [PubMed] [Google Scholar]
- 130.Kawakubo Y, Kasai K. Support for an association between mismatch negativity and social functioning in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1367–1368. doi: 10.1016/j.pnpbp.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 131.Kawakubo Y, Kamio T, Iwanami A, et al. Phonetic mismatch negativity predicts social skills acquisition in schizophrenia. Psychiatry Res. 2007;152:261–265. doi: 10.1016/j.psychres.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 132.Urban A, Kremlåcek J, Masopust J, Libiger J. Visual mismatch negativity among patients with schizophrenia. Schizophr Res. 2008;102:320–328. doi: 10.1016/j.schres.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 133.Kathmann N, Frodl-Bauch T, Hegerl U. Stability of the mismatch negativity under different stimulus and attention conditions. Clin Neurophysiol. 1999;110:317–323. doi: 10.1016/s1388-2457(98)00011-x. [DOI] [PubMed] [Google Scholar]
- 134.Shinozaki N, Yabe H, Sato Y, et al. The difference in mismatch negativity between the acute and post-acute phase of schizophrenia. Biol Psychol. 2002;59:105–119. doi: 10.1016/s0301-0511(01)00129-6. [DOI] [PubMed] [Google Scholar]
- 135.Dakin SC, Hess RF. Spatial-frequency tuning of visual contour integration. J Opt Soc Am A Opt Image Sci Vis. 1998;15:1486–1499. doi: 10.1364/josaa.15.001486. [DOI] [PubMed] [Google Scholar]
- 136.Field DJ, Hayes A, Hess RF. Contour integration by the human visual system: evidence for a local “association field. Vision Res. 1993;33:173–193. doi: 10.1016/0042-6989(93)90156-q. [DOI] [PubMed] [Google Scholar]
- 137.Watt RJ, Phillips WA. The function of dynamic grouping in vision. Trends Cogn Sci. 2000;4:447–454. doi: 10.1016/s1364-6613(00)01553-9. [DOI] [PubMed] [Google Scholar]
- 138.Phillips WA, Singer W. In search of common foundations for cortical computation. Behav Brain Sci. 1997;20:657–683. doi: 10.1017/s0140525x9700160x. discussion 683–722. [DOI] [PubMed] [Google Scholar]
- 139.Kapadia MK, Ito M, Gilbert CD, Westheimer G. Improvement in visual sensitivity by changes in local context: parallel studies in human observers and in V1 of alert monkeys. Neuron. 1995;15:843–856. doi: 10.1016/0896-6273(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 140.Yen SC, Finkel LH. Extraction of perceptually salient contours by striate cortical networks. Vision Res. 1998;38:719–741. doi: 10.1016/s0042-6989(97)00197-1. [DOI] [PubMed] [Google Scholar]
- 141.Chandna A, Pennefather PM, Kovacs I, Norcia AM. Contour integration deficits in anisometropic amblyopia. Invest Ophthalmol Vis Sci. 2001;42:875–878. [PubMed] [Google Scholar]
- 142.Kovacs I, Polat U, Pennefather PM, Chandna A, Norcia AM. A new test of contour integration deficits in patients with a history of disrupted binocular experience during visual development. Vision Res. 2000;40:1775–1783. doi: 10.1016/s0042-6989(00)00008-0. [DOI] [PubMed] [Google Scholar]
- 143.Altmann CF, Bulthoff HH, Kourtzi Z. Perceptual organization of local elements into global shapes in the human visual cortex. Curr Biol. 2003;13:342–349. doi: 10.1016/s0960-9822(03)00052-6. [DOI] [PubMed] [Google Scholar]
- 144.Kourtzi Z, Tolias AS, Altmann CF, Augath M, Logothetis NK. Integration of local features into global shapes: monkey and human FMRI studies. Neuron. 2003;37:333–346. doi: 10.1016/s0896-6273(02)01174-1. [DOI] [PubMed] [Google Scholar]
- 145.Phillips WA, Silverstein SM. Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behav Brain Sci. 2003;26:65–82. doi: 10.1017/s0140525x03000025. discussion 82–137. [DOI] [PubMed] [Google Scholar]
- 146.Uhlhaas PJ, Silverstein SM. Perceptual organization in schizophrenia spectrum disorders: empirical research and theoretical implications. Psychol Bull. 2005;131:618–632. doi: 10.1037/0033-2909.131.4.618. [DOI] [PubMed] [Google Scholar]
- 147.Uhlhaas PJ, Phillips WA, Silverstein SM. The course and clinical correlates of dysfunctions in visual perceptual organization in schizophrenia during the remission of psychotic symptoms. Schizophr Res. 2005;75:183–192. doi: 10.1016/j.schres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 148.Silverstein SM, Kovacs I, Corry R, Valone C. Perceptual organization, the disorganization syndrome, and context processing in chronic schizophrenia. Schizophr Res. 2000;43:11–20. doi: 10.1016/s0920-9964(99)00180-2. [DOI] [PubMed] [Google Scholar]
- 149.Uhlhaas PJ, Phillips WA, Schenkel LS, Silverstein SM. Theory of mind and perceptual context-processing in schizophrenia. Cognit Neuropsychiatry. 2006;11:416–436. doi: 10.1080/13546800444000272. [DOI] [PubMed] [Google Scholar]
- 150.Schenkel LS, Spaulding WD, DiLillo D, Silverstein SM. Histories of childhood maltreatment in schizophrenia: relationships with premorbid functioning, symptomatology, and cognitive deficits. Schizophr Res. 2005;76:273–286. doi: 10.1016/j.schres.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 151.Schenkel LS, Spaulding WD, Silverstein SM. Poor premorbid social functioning and theory of mind deficit in schizophrenia: evidence of reduced context processing? J Psychiatr Res. 2005;39:499–508. doi: 10.1016/j.jpsychires.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 152.Silverstein SM, Hatashita-Wong M, Schenkel LS, et al. Reduced top-down influences in contour detection in schizophrenia. Cognit Neuropsychiatry. 2006;11:112–132. doi: 10.1080/13546800444000209. [DOI] [PubMed] [Google Scholar]
- 153.Kozma-Wiebe P, Silverstein SM, Feher A, Kovacs I, Ulhaas P, Wilkniss SM. Development of a world-wide web based contour integration test. Comput Human Behav. 2006;22:971–980. [Google Scholar]
- 154.Uhlhaas PJ, Phillips WA, Mitchell G, Silverstein SM. Perceptual grouping in disorganized schizophrenia. Psychiatry Res. 2006;145:105–117. doi: 10.1016/j.psychres.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 155.Pennefather PM, Chandna A, Kovacs I, Polat U, Norcia AM. Contour detection threshold: repeatability and learning with ‘contour cards’. Spat Vis. 1999;12:257–266. doi: 10.1163/156856899x00157. [DOI] [PubMed] [Google Scholar]
- 156.Kovacs I, Kozma P, Feher A, Benedek G. Late maturation of visual spatial integration in humans. Proc Natl Acad Sci U S A. 1999;96:12204–12209. doi: 10.1073/pnas.96.21.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Julesz B. Foundation of Cylopean Perception. Chicago, IL: University of Chicago Press; 1971. [Google Scholar]
- 158.Newsome WT, Wurtz RH. Probing visual cortical function with discrete chemical lesions. Trends Neurosci. 1988;11:394–400. doi: 10.1016/0166-2236(88)90076-8. [DOI] [PubMed] [Google Scholar]
- 159.Newsome W, Pare EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) J Neurosci. 1988;8:2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Britten K, Shadlen M, Newsome W, Movshon J. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci. 1992;12:4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rees G, Friston K, Koch C. A direct quantitative relationship between the functional properties of human and macaque V5. Nat Neurosci. 2000;3:716–723. doi: 10.1038/76673. [DOI] [PubMed] [Google Scholar]
- 162.Egelhaaf M, Borst A, Pilz B. The role of GABA in detecting visual motion. Brain Res. 1990;509:156–160. doi: 10.1016/0006-8993(90)90325-6. [DOI] [PubMed] [Google Scholar]
- 163.Carter OL, Pettigrew JD, Burr DC, Alais D, Hasler F, Vollenweider FX. Psilocybin impairs high-level but not low-level motion perception. Neuroreport. 2004;15:1947–1951. doi: 10.1097/00001756-200408260-00023. [DOI] [PubMed] [Google Scholar]
- 164.Stuve T, Friedman L, Jesberger JA, Gilmore G, Strauss ME, Meltzer H. The relationship between smooth pursuit performance, motion perception and sustained visual attention in patients with schizophrenia and normal controls. Psychol Med. 1997;27:143–152. doi: 10.1017/s0033291796004230. [DOI] [PubMed] [Google Scholar]
- 165.Chen Y, Nakayama K, Levy DL, Matthysse S, Holzman PS. Processing of global, but not local, motion direction is deficient in schizophrenia. Schizophr Res. 2003;61:215–227. doi: 10.1016/s0920-9964(02)00222-0. [DOI] [PubMed] [Google Scholar]
- 166.Li CS. Impaired detection of visual motion in schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:929–934. doi: 10.1016/s0278-5846(02)00207-5. [DOI] [PubMed] [Google Scholar]
- 167.Kim D, Wylie G, Pasternak R, Butler PD, Javitt DC. Magnocellular contributions to impaired motion processing in schizophrenia. Schizophr Res. 2006;82:1–8. doi: 10.1016/j.schres.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chen Y, Bidwell LC, Holzman PS. Visual motion integration in schizophrenia patients, their first-degree relatives, and patients with bipolar disorder. Schizophr Res. 2005;74:271–281. doi: 10.1016/j.schres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 169.Slaghuis WL, Holthouse T, Hawkes A, Bruno R. Eye movement and visual motion perception in schizophrenia II: global coherent motion as a function of target velocity and stimulus density. Exp Brain Res. 2007;182:415–426. doi: 10.1007/s00221-007-1003-3. [DOI] [PubMed] [Google Scholar]
- 170.Chen Y, Grossman E, Bidwell LC. Differential patterns of occipital and prefrontal cortical activations during motion processing: evidence from normal and schizophrenic brains. Cogn Affect Behav Neurosci. 2008;8:293–303. doi: 10.3758/cabn.8.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]