Abstract
Compelling evidence from epidemiological studies suggest beneficial roles of dietary phytochemicals in protecting against chronic disorders such as cancer, and inflammatory and cardiovascular diseases. Emerging findings suggest that several dietary phytochemicals also benefit the nervous system and, when consumed regularly, may reduce the risk of disorders such as Alzheimer’s and Parkinson’s diseases. The evidence supporting health benefits of vegetables and fruits provide a rationale for identification of the specific phytochemicals responsible, and for investigation of their molecular and cellular mechanisms of action. One general mechanism of action of phytochemicals that is emerging from recent studies is that they activate adaptive cellular stress response pathways. From an evolutionary perspective, the noxious properties of such phytochemicals play an important role in dissuading insects and other pests from eating the plants. However at the relatively small doses ingested by humans that consume the plants, the phytochemicals are not toxic and instead induce mild cellular stress responses. This phenomenon has been widely observed in biology and medicine, and has been described as ‘preconditioning’ or ‘hormesis’. Hormetic pathways activated by phytochemicals may involve kinases and transcription factors that induce the expression of genes that encode antioxidant enzymes, protein chaperones, phase-2 enzymes, neurotrophic factors and other cytoprotective proteins. Specific examples of such pathways include the sirtuin – FOXO pathway, the NF-κB pathway and the Nrf-2 –ARE pathway. In this article we describe the hormesis hypothesis of phytochemical actions with a focus on the Nrf2/ARE signaling pathway as a prototypical example of a neuroprotective mechanism of action of specific dietary phytochemicals.
Index entries: Nrf2, antioxidant response element, hormesis, sirtuin, stress, sulforaphane, resveratrol
Introduction
Phytochemicals serve numerous functions in plants and contribute to their color, flavor, smell and texture. Increasing data suggest associations between the type of food people eat, their health and their life expectancy; the consumption of vegetables and fruits may protect against cancers, cardiovascular disease and neurodegenerative disorders (Heber, 2004). Phytochemicals include compounds with various biological properties (i.e. antioxidant, antiproliferative, DNA repair) which have presumably evolved, in part, to allow plants to cope with environmental challenges including exposure to radiation and toxins, and defense against pests and infectious agents (Tuteja et al., 2001; Huffman, 2003). Chemicals that are concentrated in the skin of fruits and the growing buds of vegetables include those that function as natural pesticides and, indeed, identification and large-scale production of such “biopesticides” has received much attention from both basic science and commercialization perspectives (Isman, 2006). These chemicals may be produced by the plants themselves or by endophytes (symbiotic bacteria or fungi) that live in the plants (Sudakin, 2003).
The term hormesis has long been used to describe the phenomenon where a specific chemical is able to induce biologically opposite effects at different doses; most commonly there is a stimulatory or beneficial effect at low doses and an inhibitory or toxic effect at high doses (Calabrese et al., 2007). In the case of natural compounds an example of hormesis is vitamin A which in relatively low amounts is essential for normal development and eye function, but in high amounts can cause anorexia, headaches, drowsiness, altered mental states and other symptoms (Penniston and Tanumihardjo, 2006). In the present article, we describe evidence supporting a major role for hormesis as a mechanism of action of phytochemicals on cells and organisms, with a focus on the health-promoting and neuroprotective actions of phytochemicals.
The Concept and Features of Hormesis
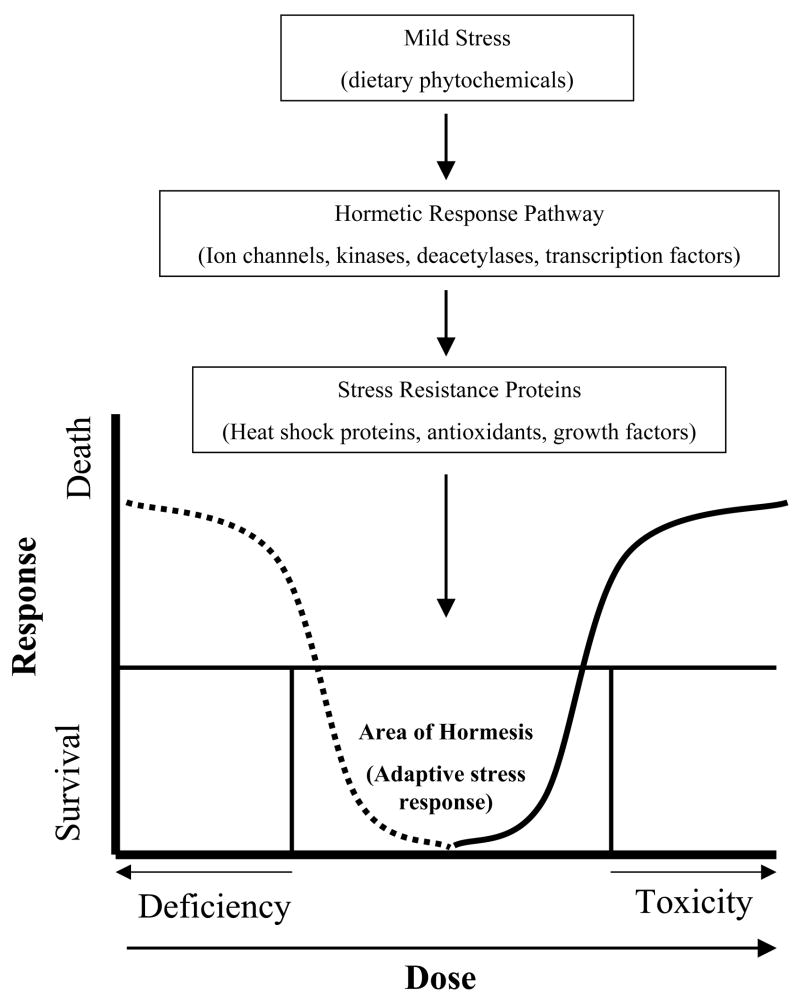
The term hormesis is commonly used by toxicologists to describe biphasic dose response curve such that a chemical has a stimulatory effect at low doses, but is toxic at high doses (Fig. 1). Recently the concept of hormesis has been adopted in the fields of biology and medicine to portray the adaptive response of cells and organisms to moderate stress (Mattson, 2008). In other words, a mild stress induces the activation of signaling pathways, leading to intrinsic changes conferring resistance to a more severe stress (Fig. 1). Typically, the stress-inducing agent elicits molecular responses that not only protect the cell against higher doses of the same agent, but also against other agents or even less specific stressors including oxidative, metabolic and thermal stress. Major components of the hormetic response include various stress resistance proteins such as heat-shock proteins, antioxidants, and growth factors (Mattson and Cheng, 2006; Mattson 2008). Classical examples of hormetic stress are exercise and calorie restriction (CR). Epidemiological studies have consistently demonstrated that moderate levels exercise and CR promote good health, whereas excessive levels are harmful (Fontana and Klein, 2007; Radak et al., 2008). As mentioned above, the need to protect themselves against bacteria, fungi, viruses, and hazardous environmental changes, has lead plants to concentrate defensive chemicals in their most vulnerable parts (i.e. leaves, flowers and roots). Like moderate exercise or CR, many of these ‘poisons’ also exhibit hormetic properties, being harmful at high doses yet beneficial at relevantly low doses.
Figure 1.
The concept and biphasic dose-response characteristic of hormesis. Hormesis can be initiated by exposure to various environmental stressors including ingestion of phytochemicals. Such exposures typically result in mild cellular stress involving free radical production, ion fluxes and increased energy demand. As a result, adaptive stress response pathways are activated leading to the synthesis of proteins that protect cells against more severe stress. Examples of stress resistance proteins include antioxidants, protein chaperones, growth factors, and proteins involved in the regulation of energy metabolism and cellular calcium homeostasis.
We are of the opinion that the amounts of cell stress-inducing phytochemicals in the varieties of fruits and vegetable normally consumed by humans fall within the low dose stimulatory range of concentrations. However, it is well-known that some plants and fungi produce and concentrate toxins in amounts sufficient to cause sickness or death in humans (Schwarting, 1963; Berger and Guss, 2005). It is also clear that consumption of phytochemicals in form of concentrated supplements has the potential for adverse health consequences if the doses consumed exceed the toxic threshold. It is therefore imperative that each individual phytochemical is extensively evaluated, including detailed dose-response studies, for its safety and effectiveness in regard to disease prevention. Meta-analyses performed by Calabrese and others have provided valuable information regarding dose-response effects of a range of natural and synthetic chemicals, as well as endogenous signaling molecules (for example, nitric oxide, adenosine, opioids, adrenergic agent, prostaglandins, estrogens, androgens, 5-hydroxytryptamine and dopamine) (Cook and Calabrese, 2007; Hayes, 2007).
Examples of Hormetic Dietary Phytochemicals
There has been considerable public and scientific interest in the use of dietary components to prevent or treat human diseases such as cancer, diabetes, cardiovascular and neurodegenerative diseases (Liu, 2004; Mattson and Cheng, 2006). Due to the increasing number of phytochemicals being identified and characterized, and the space limitations of this article, we would like to highlight the biological properties of several compounds that are of particular interest from the perspective of hormesis, but have not yet received widespread attention. Many of the phytochemicals that have recently been reported to exert neuroprotective effects in various experimental models of neurological disorders, were previously shown to have cytostatic or cytotoxic effects on cancer cells. These kinds of historical data emphasize the hormetic nature of the neurobiological activities of these phytochemicals.
Chalcone, an α, β-unsaturated aromatic ketone is present in Angelica keiskei Koidzumi, a plant traditionally used in Japanese’s cuisine (Akihisa et al., 2003). Chalcone and its derivatives allegedly possess antibacterial, anti-fungal, anti-tumor, and anti-inflammatory activities. Some of the mechanisms underlying chalcone properties are only now being discovered. For example, the anti-inflammatory effects of chalcones rely on their ability to regulate nitric oxide (NO) and cytokine production in macrophages (Alcaraz et al., 2004; Ban et al., 2004), as well as to prevent tumor necrosis factor-α (TNF-α) and lipopolysaccharide (LPS)-induced neutrophil adhesion (Madan et al., 2000). In addition, it has also been shown that chalcone suppresses the activity of cycloxygenase-2 and 5-lipoxygenase (Araico et al., 2006). In experimental models chalcone administration inhibits chemically-induced pulmonary and mammary carcinogenesis (Wattenberg et al., 1994). Chalcone derivatives show anti-tumor activity in vitro (Ye et al., 2004; Ye et al., 2005). Nishimura et al., (2007) reported that two chalcone derivatives, isobavachalcone and xanthoangelol H, exhibit high cytotoxicity against neuroblastoma cell lines IMR-32 and NB-39 by activating a pathway involving caspases 9 and 3. However, neither compound had a detrimental effect on normal cerebellar granule cells at the same concentrations tested, thus offering the possibility to use this natural compound as an efficacious and safe potential treatment against neuroblastoma.
Ferulic acid (FA) is a phytochemical commonly found in fruits and vegetables such as tomatoes, sweet corn and rice (Srinivasan et al., 2007). FA is a phenolic compound with three distinctive structural motifs that can possibly contribute to its free radical scavenging capability (Srinivasan et al., 2007). It has been reported that FA decreases the levels of inflammatory mediators (prostaglandin E2 and TNF-α; Ou et al., 2003), and nitric oxide synthase (iNOS) expression and function (Tetsuka et al., 1996). In addition, hydrophobic ester derivatives of FA seem to have enhanced inhibitory activity on iNOS protein expression in LPS/interferon- γ (IFN-γ) activated RAW264.7 cells (Murakami et al., 2002). These findings suggest that FA and its esters might be potential anti-inflammatory drugs. Studies have shown that FA exhibits anti-carcinogenic effects against azoxymethan-induced colon carcinogenesis in F344 rats (Kawabata et al., 2000). It has also been reported to depress 12-O-tetradecanoylphorbol-13-acetate (TPA)-promotion of skin tumorigenesis (Asanoma et al., 1993). Recently Sultana et al., (2005) showed that 10–50 □M of FA significantly protects against amyloid beta-peptide (A□) toxicity by modulating oxidative stress and by inducing the expression of protecting proteins in hippocampal cultures. In vivo, long term administration of FA effectively protects against A□ (1-42) toxicity by inhibiting microglial activation (Kim et al., 2004).
The consumption of green tea has recently attracted much attention in the occidental culture because of its beneficial health effects. The polyphenolic compounds found in green tea include epigallocatechin-3-gallate (EGCG), epicatechin-3-gallate (ECG), epigallocatechin (EGC) and epicatechin (EC), and their intake has been associated with reduced risk of coronary artery disease (Miura et al., 2001). EGCG is the most abundant and active catechin derivative, and has been shown to possess both anti-inflammatory and anti-atherogenic properties in experimental studies conducted in vitro and in vivo (Hayek et al., 1997; Tedeschi et al., 2002). A recent study reports that EGCG up-regulates hemeoxygenase-1 (HO-1) expression by activation of the Nrf2/ARE pathway in endothelial cell, conferring resistance against H2O2-induced cell death (Wu et al., 2006), suggesting a hormetic mechanism of action.
Luteolin (3′,4′,5′,7′-tetrahydroxyflavone) is a widespread flavonoid aglycon structurally related to quercetin. Topical application of luteolin causes significant reduction of skin tumor incidence and multiplicity in a mouse skin cancer model (Ueda et al., 2003). In cell culture studies, luteolin has been shown to be a potent inhibitor of cyclin-dependent kinases, to induce cell cycle arrest in human melanoma cells and apoptotic cell death in human myeloid leukemia cells (Huang et al., 1999; Ko et al., 2002). Luteolin also sensitizes cancer cell lines to TNFα-dependent apoptosis by inhibiting the NF-κB pathway (Shi et al., 2004). In addition to the anticancer properties luteolin has also shown to protect neuronal cell lines against H2O2 induced oxidative damage (Dajas et al., 2003) as well as N-methyl-4-phenyl-pyridinium (MPP+) induced toxicity (Wruck et al., 2007). In the MPP+ model the ERK-dependent Nrf2 activation is necessary for the beneficial effect of luteolin (Wruck et al., 2007).
Phenethyl isothiocyanate (PEITC) occurs naturally in cruciferous vegetables such as chinese cabbage, turnips, rutabagas, watercress and radishes (Tookey et al., 1980; Carlson et al., 1981; Sones et al., 1984). PEITC is liberated from its glucosinolate precursor gluconasturtiin by hydrolysis following disruption of the plant tissue and liberation of the plant enzyme myrosinase (Tookey et al., 1980). PEITC has been shown to inhibit the tumorigenic effects of various carcinogens. Induction of mammary tumors by 7,12-dimethylbenz[a]anthracene in Sprague-Dawley rats is inhibited by pretreatment with PEITC (Wattenberg, 1977). Dietary PEITC inhibits tomach and pulmonary adenomas induced by 7,12-dimethylbenz[a]anthracene in ICR/Ha mice (Wattenberg, 1977). Pretreatment with PEITC inhibits lung tumors induced by the tobacco-specific nitrosamine 4-(methylnitrosamino)-l-(3-pyridyl)-1-butanone (NNK) in F344 rats and in A/J mice (Morse et al., 1989a; Morse et al., 1989b). It is well documented that in rodents aryl isothiocyanates exert chemopreventive effects against lung (Morse et al., 1989a; Jiao et al., 1994; Nishikawa et al., 1996), esophagus (Morse et al., 1993; Stoner et al., 1998), mammary (Wattenberg, 1992) and stomach (Wattenberg, 1992) carcinogenesis. PEITC significantly inhibits pancreatic carcinogenesis in hamsters (Nishikawa et al., 1996). It has also been reported that synthetic analogues of PEITC with longer and modified alkyl chains [e.g. 3- phenylpropyl isothiocyanate (PPITC) and 4-phenylbutyl isothiocyanate (PBITC)] have increased ability to reduce DNA alkylation, and tumor inhibition efficacy than PEITC (Morse et al., 1989a; Jiao et al., 1994; Nishikawa et al., 1996; Son et al., 2000).
Piceatannol (trans-3,4,3′,5′-tetrahydroxystilbene) isolated from the seeds of Euphorbia lagascae, is a structural homolog of resveratrol (Ferrigni et al., 1984). Piceatannol (PIC) is an anti-inflammatory, immunomodulatory and anti-proliferative compound. PIC treatment attenuates the intracellular accumulation of ROS induced by treatment of PC12 cells with Aβ, and inhibits Aβ-induced apoptosis (Kim et al., 2007). The protective effect afforded by PIC is reportedly stronger than the effect of resveratrol (Kim et al., 2007). It has also been reported that PIC inhibits the release of NO, PGE2 and pro-inflammatory cytokines in a dose-dependent manner (Jin et al., 2006).
Neuroprotective Actions of Phytochemicals in Experimental Models
Extracts of various fruits and vegetables exhibit neuroprotective properties in cell culture and animal models that are relevant to the pathogenesis of many different neurodegenerative conditions including stroke, and Alzheimer’s and Parkinson’s diseases (Joseph et al., 2005; Mattson and Cheng, 2006). Supplementation of the diet of 19 month-old rats with strawberry, blueberry or spinach extracts for 8 weeks resulted in the reversal of age-related deficits in several neuronal and behavioral parameters including: oxotremorine enhancement of K+-evoked release of dopamine from striatal slices, carbachol-stimulated GTPase activity, calcium buffering in striatal synaptosomes, motor behavioral performance and Morris water maze performance (Joseph et al., 1999). Blueberry supplementation prevented learning and memory deficits in a mouse model of Alzheimer’s disease (Joseph et al., 2003). In addition, dietary supplementation with blueberry extract increased the survival of dopamine-producing neurons in a model relevant to Parkinson’s disease therapy (McGuire et al., 2006). Interestingly, blueberry extract increased thermal stress resistance and increased lifespan in the nematode C. Elegans (Wilson et al., 2006), suggesting evolutionary conservation of phytochemical hormetic response pathways.
Green tea contains chemicals capable of activating adaptive cellular stress responses and to protect neurons against a range of oxidative and metabolic insults. Examples of neuroprotective effects of chemicals in green tea include: protection of dopaminergic neurons from damage induced by 6-hydroxydopamine in a rat model of Parkinson’s disease (Guo et al., 2007); protection of retinal neurons against ischemia –reperfusion injury (Zhang et al., 2007); reduction of mutant huntingtin misfolding and neurotoxicity in Huntington’s disease models (Ehrnhoefer et al., 2006); direct protection of neurons against Ab toxicity (Bastianetto et al., 2006); protection against A□-induced cognitive impairment in a rat model relevant to Alzheimer’s disease (Haque et al., 2008). The neuroprotective phytochemicals in green tea include catechins and epicatechins that can induce the production of cytoprotective proteins. (Mandel et al., 2005).
Several beneficial effects of curcumin for the nervous system have been reported. In an animal model of stroke curcumin treatment protected neurons against ischemic cell death and ameliorated behavioral deficits (Wang et al., 2005). In a rat model of Parkinson’s disease curcumin protected dopaminergic neurons against 6-hydroxydopamine toxicity (Zbarsky et al., 2005). A hormetic mechanism of action of curcumin is suggested from studies showing that levels of expression of the stress response protein HO-1 were increased in cultured hippocampal neurons treated with curcumin (Scapagnini et al., 2006). Moreover, curcumin has been shown to reverse chronic stress-induced impairment of hippocampal neurogenesis and increase expression of brain-derived neurotrophic factor (BDNF) in an animal model of depression (Xu et al., 2007).
Sulforaphane is a phytochemical present in high amounts in broccoli sprouts and cruciferous vegetables. It is known to activate the Nrf2-ARE stress response pathway in a variety of cells including neurons (see next section). Sulforaphane has been reported to protect cultured neurons against oxidative stress (Kraft et al., 2004) and dopaminergic neurons against mitochondrial toxins (Han et al., 2007). Administration of sulforaphane to mice can protect photoreceptors against degeneration in a retinal degeneration model (Kong et al., 2007). Sulforaphane induced the expression of phase II enzymes and protected neurons against death in a Drosophila model of Parkinson’s disease (Trinh et al., 2008).
Many of the phytochemicals that can kill tumor cells (see previous section) have been shown to have neuroprotective actions at lower doses. For example, a chalcone (safflor yellow B) can protect neurons against ischemic brain injury (Wang et al., 2007) and piceatannol can protect cultured neurons against Aβ-induced death (Kim et al., 2007). Neuroprotective effects of resveratrol have been reported by several different laboratories. It protected cultured PC12 neural cells against Aβ toxicity (Jang et al., 2003) and dopaminergic neurons in midbrain slice cultures against several different insults (Okawara et al., 2007). Resveratrol also protected hippocampal neurons against nitric oxide-mediate death (Bastianetto et al., 2000), prevented axon degeneration (Araki et al., 2004) and protected nematode and mammalian neurons against mutant polyglutamine toxicity (Parker et al., 2005).
Nrf2/ARE as a Prototypical Hormetic Signaling Pathway
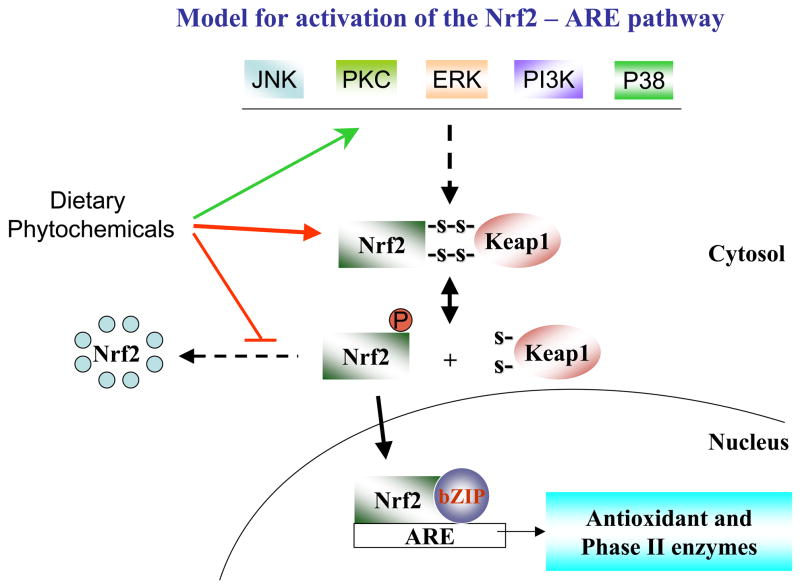
The mechanisms underlying the beneficial health effects of dietary phytochemicals have only recently begun to be understood. Although further research is clearly needed to expand our understanding, the involvement of specific cellular signaling pathways in the biological actions of phytochemicals is becoming increasingly obvious. Herbivorous and omnivorous animals, for which plants are a primary source of nutrients, have evolved complex mechanisms to neutralize the potentially harmful effects of phytochemicals (Fig. 2). One of the most important defensive signaling pathways in animals is the NF-E2-related factor 2 (Nrf2) /antioxidant response element (ARE) pathway. Nrf2 regulates the expression of phase II detoxifying enzymes and antioxidants in response to noxious stimuli. Based on a body of emerging evidence it seems that in many cases the beneficial effects of low doses of phytochemicals rely on their ability to activate the Nrf2/ARE pathway. Several upstream signaling cascades such as p38, protein kinase C (PKC), extracellular signal-regulated protein kinase (ERK), c-jun N-terminal kinase (JNK) and phosphatidylinositol-3-kinase (PI3K) may either individually, or in a combined manner, activate Nrf2. Suppression of the inhibitory action of Keap1 on Nrf2, and inhibition of the proteasomal degradation of Nrf2 are additional mechanisms by which naturally occurring dietary agents may enhance the levels of Nrf2 activity (Fig. 3).
Figure 2.
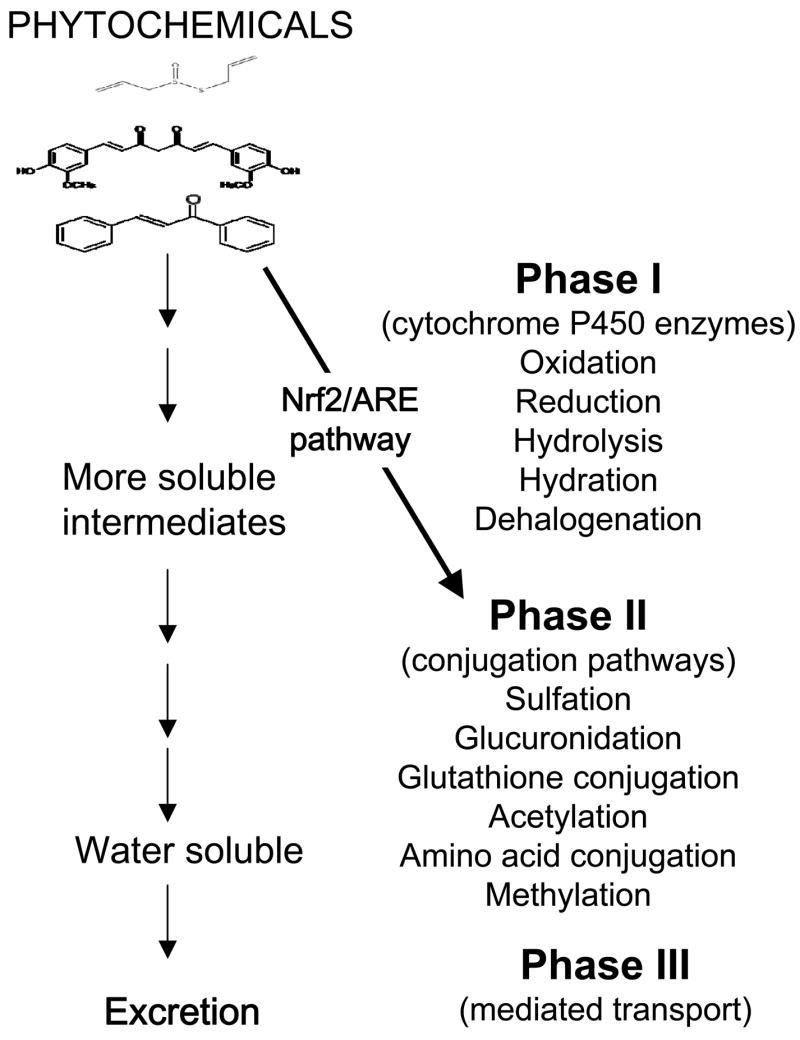
Detoxification pathways. Potentially noxious chemicals ingested with food undergo enzymatic metabolism in a coordinated process involving phase I, II and III enzymes; metabolites are then excreted from the body. Some such phytochemicals induce the expression of phase II enzymes via the Nrf2/ARE pathway.
Figure 3.
Model of activation of Nrf2-mediated ARE pathway by phytochemicals. Phytochemicals may act directly on the Nrf2-Keap1 complex, or alternatively on upstream kinases such as PI3K, p38, ERK, PKC, JNK, causing the release of Nrf2 from the inhibitory complex. Additionally, certain phytochemicals may act at the level of the proteasome to inhibit proteolytic degradation of Nrf2 and prolong its half-life. Activated Nrf2 translocates into the nucleus where it interacts with small MAF family proteins bound to the antioxidant response element (ARE), allowing transcription of target genes including those including antioxidant and phase II enzymes.
Sulporaphane (SFN) and PEITC, both major components of cruciferous vegetables, have been shown to be potent inducers of ARE-driven phase II gene expression (Hu et al., 2006a; Juge et al., 2007). PEITC induction of ARE-driven HO-1 expression in human prostate cancer requires JNK activation (Xu et al., 2006). At non-toxic concentrations, curcumin induces heme-oxygenase 1 (HO-1) expression by activating the Nrf2/ARE pathway both in vitro (Pae et al., 2007) and in vivo (Farombi et al., 2007). SFN and tert-butylhydroquinone (tBHQ) stimulate the MEK-ERK2 pathway in human hepatoma (HepG2) and murine hepatoma (Hepa1c1c7) cells (Yu et al., 1999a). Indeed, incubation with a pharmacological inhibitor of ERK or co-transfection with a dominant negative mutant ERK2 abrogates SFN and tBHQ induced quinine reductase activity (Yu et al., 1999a). The requirement of Nrf2 in SFN and PEITC phase II gene expression induction is clearly demonstrated by the use of Nrf2 knock-out mice. A host of phase II genes such as UDP-glucuronosyltransferase (UGT), glutathione S-transferase (GST) and gamma-glutamylcysteine synthetase (GCS), are found to be highly induced in wild type animals following phytochemical administration, but are unchanged in Nrf2 null mice (McWalter et al., 2004; Hu et al., 2006a; Hu et al., 2006b). SFN can act at different levels of the Nrf2 pathway. Indeed SFN suppresses Nrf2 proteosomal degradation leading to a prolonged half life and transcriptional activity (Jeong et al., 2005). In addition SFN has also been shown to directly covalently bind the thiol groups or the inhibitor Keap1 thus causing the release of Nrf2 and its subsequent nuclear relocalization (Dinkova-Kostova et al., 2002). In a similar way electrophilic phytochemicals may originate thiyl radicals resulting in Nrf2 activation and the induction of phase II detoxifying enzyme expression (Foresti et al., 2005; Wu et al., 2006). An example is chalcone, it possesses a highly electrophilic α,β-unsaturated carbonyl moiety, which is necessary for Nrf2 activation and the induction of phase II detoxifying enzyme expression (Foresti et al., 2005; Wu et al., 2006). Recently it has been shown that this moiety is also responsible for chalcone’s ability to inhibit NF-□B (Liu et al., 2007).
Green tea components such as epigallocatechin gallate (EGCG), flavonoids such as kaempferol and genistein, tBHQ and curcumin exemplify another major category of phytochemical agents that can activate the Nrf2 pathway – polyphenols. By comparing the global gene expression profile between wild type and Nrf2 mutant mice it has become clear that these compounds, and part of their protective mechanisms, depend upon their ability to activate phase II detoxifying enzymes via Nrf2 (Shen et al., 2005; Nair et al., 2006; Shen et al., 2006). Although the end point is similar, the mechanism leading to Nrf2 activation can be different (Nair et al., 2007). Flavonoids have been shown to induce the expression of NQO1 and GST via Nrf2, possibly involving upstream PKC modulation (Lee-Hilz et al., 2006). EGCG upregulates levels of HO-1 in endothelial cells acting on PI3K and ERK2 signaling cascades (Wu et al., 2006). Modulation of MAPKs by the flavonoid polyphenols kaempferol and genistein in PC3 cells has been shown (Gopalakrishnan et al., 2006; Gopalakrishnan and Tony Kong, 2008).
Curcumin (diferuloyl methane) is the principal active component of the spice turmeric and is obtained from the rhizome of Curcuma longa. Curcumin has been reported to activate the expression of several intracellular defense systems both in vitro and in vivo. Curcumin supplementation in mice results in increased expression of the detoxification enzymes glutathione-s-transferases, glutathione reductase, epoxide hydrolase, HO-1, catalase and NQO1 in liver, small intestine and kidney tissues (Shen et al., 2006). In vitro, curcumin has been shown to activate NQO1 and HO-1 in numerous cell types. In human monocytes, curcumin induced ARE-driven HO-1 and NQO1 expression requires PKC activation (Rushworth et al., 2006). Inactivation of the Nrf2-KEAP1 complex, resulting in increased nuclear localization of Nrf2, has also been implicated in curcumin and caffeic acid phenylethyl ester (CAPE) induced HO-1 expression via the MAPK pathway (Jeong et al., 2005). Diallyl sulfides (DAs) such as diallyl sulfide (DAS), diallyl disulfide (DADS), and diallyl trisulfide (DATS) are active principles in garlic and onions. They are lipophilic thioesters derived from oxidized allicin, which is produced when garlic cloves are crushed. DAs has been shown to be protective against numerous chemically induced cancers and are potent inducers of phase II detoxifying enzymes (Gong et al., 2004). Extensive research from several groups has demonstrated that these diallyl sulfides can induce NQO1 and HO-1 in an Nrf2/ARE dependent fashion. These agents stimulate Nrf2 expression and its nuclear translocation, and this effect can be abated by MAPK inhibitors (Chen et al., 2004). In addition to the examples reported above, numerous other phytochemicals such as indole-3-carbinol, coffee diterpenes such as cafestol, and sesquiterpenes such as parthenolide have been shown to induce Nrf2-mediated ARE-driven gene expression (Cavin et al., 2002; Aggarwal and Ichikawa, 2005; Jeong et al., 2006).
Two additional pathways that are known to play important roles in neuronal stress adaptation are those involving the NF-κB and FOXO transcription factors (Brunet et al., 2004; van der Horst and Burgering, 2007; Mattson and Meffert, 2006; Camandola and Mattson, 2007). Recent findings suggest that the latter two pathways mediate neuroprotective actions of at least some phytochemicals. Activation of NF-κB typically up-regulates the expression of pro-survival genes such as Bcl-2 and manganese superoxide dismutase. In cancer cells and some immune cells curcumin, sulforaphane and tea polyphenols inhibit NF-κB and may thereby trigger apoptosis (Park and Dong, 2003; Katula et al., 2005; Jagetia and Aggarwal, 2007). However, in neurons activation of NF-κB can prevent cell death induced by a range of insults including exposure to excitotoxins and oxidative stress (Mattson et al., 1997; Yu et al., 1999b). FOXO transcription factors can be activated by resveratrol resulting in the up-regulation of genes involved in energy metabolism and antioxidant pathways (Frescas et al., 2005; Robb et al., 2008). Much further work will be required to establish if and how neuroprotective actions of various phytochemicals involve NF-κB, FOXO and other transcription factors.
Conclusions
A better understanding of the cellular effects exerted by dietary phytochemicals is vital to properly utilize such compounds as promising agents that promote health and prevent or support conventional therapy for diseases. From the results collected to date, some clear trends are emerging. Although some phytochemicals possess direct free radical-scavenging properties at high concentrations, in lower amounts typical of those obtained in the diet, phytochemicals may activate one or more adaptive cellular stress responses pathways. Activation of such hormetic pathways in neurons results in the production of several types of cytoprotective proteins including neurotrophic factors, protein chaperones, antioxidant and phase II enzymes and anti-apoptotic proteins. One specific pathway that is receiving considerable attention in regards to hormesis in the nervous system involves the transcription factor Nrf2 which binds the ARE, thereby inducing the expression of genes encoding phase II detoxifying enzymes. Preclinical and clinical studies of the therapeutic potential of phytochemicals that activate the Nrf2/ARE pathway (curcumin, for example) in several different neurodegenerative disorders are in progress. Other hormetic pathways involved in neuronal stress resistance and plasticity include those that activate FOXO and NF-κB transcription factors. Using neurohormetic phytochemicals as base compounds for medicinal chemistry (Ohori et al., 2006; Milne et al., 2007) will likely result in the development of a range of drugs that enhance neuroplasticity and protect against synaptic dysfunction and neurodegeneration.
References
- Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;9:1201–1215. doi: 10.4161/cc.4.9.1993. [DOI] [PubMed] [Google Scholar]
- Akihisa T, Tokuda H, Ukiya M, et al. Chalcones, coumarins, and flavanones from the exudate of Angelica keiskei and their chemopreventive effects. Cancer Lett. 2003;201:133–137. doi: 10.1016/s0304-3835(03)00466-x. [DOI] [PubMed] [Google Scholar]
- Alcaraz MJ, Vicente AM, Araico A. Role of nuclear factor-kappaB and heme oxygenase-1 in the mechanism of action of an anti-inflammatory chalcone derivative in RAW 264.7 cells. Br J Pharmacol. 2004;142:1191–1199. doi: 10.1038/sj.bjp.0705821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araico A, Terencio MC, Alcaraz MJ. Phenylsulphonyl urenyl chalcone derivatives as dual inhibitors of cyclo-oxygenase-2 and 5-lipoxygenase. Life Sci. 2006;78:2911–2918. doi: 10.1016/j.lfs.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- Asanoma M, Takahashi K, Miyabe M, et al. Inhibitory effect of topical application of polymerized ferulic acid, a synthetic lignin, on tumor promotion in mouse skin two stage tumorigenesis. Carcinogenesis. 1993;14:1321–1325. doi: 10.1093/carcin/15.9.2069. [DOI] [PubMed] [Google Scholar]
- Ban HS, Suzuki K, Lim SS. Inhibition of lipopolysaccharide-induced expression of inducible nitric oxide synthase and tumor necrosis factor-alpha by 2′-hydroxychalcone derivatives in RAW 264.7 cells. Biochem Pharmacol. 2004;67:1549–1557. doi: 10.1016/j.bcp.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Bastianetto S, Zheng WH, Quirion R. Neuroprotective abilities of resveratrol and other red wine constituents against nitric oxide-related toxicity in cultured hippocampal neurons. Br J Pharmacol. 2000;131:711–720. doi: 10.1038/sj.bjp.0703626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastianetto S, Yao ZX, Papadopoulos V, Quirion R. Neuroprotective effects of green and black teas and their catechin gallate esters against beta-amyloid-induced toxicity. Eur J Neurosci. 2006;23:55–64. doi: 10.1111/j.1460-9568.2005.04532.x. [DOI] [PubMed] [Google Scholar]
- Berger KJ, Guss DA. Mycotoxins revisited: Part I. J Emerg Med. 2005;28:53–62. doi: 10.1016/j.jemermed.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Camandola S, Mattson MP. NF-kappa B as a therapeutic target in neurodegenerative diseases. Expert Opin Ther Targets. 2007;11:123–132. doi: 10.1517/14728222.11.2.123. [DOI] [PubMed] [Google Scholar]
- Carlson DG, Daxenbichler ME, VanEtten CH, Tookey HL, Williams PH. Glucosinolates in crucifer vegetables: turnips and rutabagas. J Agric Food Chem. 1981;29:1235–1239. doi: 10.1021/jf00108a034. [DOI] [PubMed] [Google Scholar]
- Cavin C, Holzhaeuser D, Scharf G, et al. Cafestol and kahweol, two coffee specific diterpenes with anticarcinogenic activity. Food Chem Toxicol. 2002;8:1155–1163. doi: 10.1016/s0278-6915(02)00029-7. [DOI] [PubMed] [Google Scholar]
- Chen C, Pung D, Leong V, Hebbar V, et al. Induction of detoxifying enzymes by garlic organosulfur compounds through transcription factor Nrf2: effect of chemical structure and stress signals. Free Radic Biol Med. 2004;37:1578–1590. doi: 10.1016/j.freeradbiomed.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Cook R, Calabrese EJ. The importance of hormesis to public health. Cien saude Colet. 2007;12:955–963. doi: 10.1590/s1413-81232007000400017. [DOI] [PubMed] [Google Scholar]
- Dajas F, Rivera F, Blasina F, Arredondo F, et al. Cell culture protection and in vivo neuroprotective capacity of flavonoids. Neurotox Res. 2003;5:425–432. doi: 10.1007/BF03033172. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnhoefer DE, Duennwald M, Markovic P, Wacker JL, Engemann S, et al. Green tea (−)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington’s disease models. Hum Mol Genet. 2006;15:2743–2751. doi: 10.1093/hmg/ddl210. [DOI] [PubMed] [Google Scholar]
- Farombi EO, Shrotriya S, Na HK, et al. Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through Nrf2-mediated induction of heme oxygenase-1. Food Chem Toxicol. 2008;46:1279–1287. doi: 10.1016/j.fct.2007.09.095. [DOI] [PubMed] [Google Scholar]
- Ferrigni NR, McLaughlin JL, Powell RG, Smith CR., Jr Use of potato disc and brine shrimp bioassays to detect activity and isolate piceatannol as the antileukemic principle from the seeds of Euphorbia lagascae. J Natl Prod. 1984;47:347–352. doi: 10.1021/np50032a019. [DOI] [PubMed] [Google Scholar]
- Fontana L, Klein S. Aging, Adiposity, and Calorie Restriction. JAMA. 2007;297:986–994. doi: 10.1001/jama.297.9.986. [DOI] [PubMed] [Google Scholar]
- Foresti R, Hoque M, Monti D, Green CJ, Motterlini R. Differential activation of heme oxygenase-1 by chalcones and rosolic acid in endothelial cells. J Pharmacol Exp Ther. 2005;312:686–693. doi: 10.1124/jpet.104.074153. [DOI] [PubMed] [Google Scholar]
- Frescas D, Valenti L, Accili D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem. 2005;280:20589–20595. doi: 10.1074/jbc.M412357200. [DOI] [PubMed] [Google Scholar]
- Gong P, Hu B, Cederbaum AI. Diallyl sulfide induces heme oxygenase-1 through MAPK pathway. Arch Biochem Biophys. 2004;432:252–260. doi: 10.1016/j.abb.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan A, Xu CJ, Nair SS, Chen C, et al. Modulation of activator protein-1 (AP-1) and MAPK pathway by flavonoids in human prostate cancer PC3 cells. Arch Pharm Res. 2006;8:633–644. doi: 10.1007/BF02968247. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan A, Tony Kong AN. Anticarcinogenesis by dietary phytochemicals: Cytoprotection by Nrf2 in normal cells and cytotoxicity by modulation of transcription factors NF-kappaB and AP-1 in abnormal cancer cells. Food Chem Toxicol. 2008;46:1257–1270. doi: 10.1016/j.fct.2007.09.082. [DOI] [PubMed] [Google Scholar]
- Guo S, Yan J, Yang T, Yang X, Bezard E, Zhao B. Protective effects of green tea polyphenols in the 6-OHDA rat model of Parkinson’s disease through inhibition of ROS-NO pathway. Biol Psychiatry. 2007;62:1353–1362. doi: 10.1016/j.biopsych.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Haque AM, Hashimoto M, Katakura M, Hara Y, Shido O. Green tea catechins prevent cognitive deficits caused by Abeta(1-40) in rats. J Nutr Biochem. 2008 Feb 14; doi: 10.1016/j.jnutbio.2007.08.008. Epub. Ahead of print. [DOI] [PubMed] [Google Scholar]
- Han JM, Lee YJ, Lee SY, Kim EM, et al. Protective effect of sulforaphane against dopaminergic cell death. J Pharmacol Exp Ther. 2007;321:249–56. doi: 10.1124/jpet.106.110866. [DOI] [PubMed] [Google Scholar]
- Hayek T, Fuhrman B, Vaya J, et al. Reduced progression of atherosclerosis in apolipoprotein E-deficient mice following consumption of red wine, or its polyphenols quercetin or catechin, is associated with reduced susceptibility of LDL to oxidation and aggregation. Arterioscler Thromb Vasc Biol. 1997;17:2744– 2752. doi: 10.1161/01.atv.17.11.2744. [DOI] [PubMed] [Google Scholar]
- Hayes DP. Nutritional hormesis. Eur J Clin Nutr. 2007;61:147–159. doi: 10.1038/sj.ejcn.1602507. [DOI] [PubMed] [Google Scholar]
- Heber D. Vegetables, fruits and phytoestrogens in the prevention of diseases. J Postgrad Med. 2004;50:145–149. [PubMed] [Google Scholar]
- Hu R, Xu C, Shen G, Jain MR, et al. Identification of Nrf2-regulated genes induced by chemopreventive isothiocyanate PEITC by oligonucleotide microarray. Life Sci. 2006a;79:1944–1955. doi: 10.1016/j.lfs.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Hu R, Xu C, Shen G, Jain MR, et al. Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Cancer Lett. 2006b;243:170–192. doi: 10.1016/j.canlet.2005.11.050. [DOI] [PubMed] [Google Scholar]
- Huang YT, Hwang JJ, Lee PP, Ke FC, et al. Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor receptor. Br J Pharmacol. 1999;128:999–101. doi: 10.1038/sj.bjp.0702879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman MA. Animal self-medication and ethno-medicine: exploration and exploitation of the medicinal properties of plants. Proc Nutr Soc. 2003;62:371–381. doi: 10.1079/pns2003257. [DOI] [PubMed] [Google Scholar]
- Isman MB. The role of botanical insecticides, deterrents and repellents in modern agriculture and an increasingly regulated world. Annu Rev Entomol. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- Jagetia GC, Aggarwal BB. Spicing up” of the immune system by curcumin. J Clin Immunol. 2007;27:19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- Jang JH, Surh YJ. Protective effect of resveratrol on beta-amyloid-induced oxidative PC12 cell death. Free Radic Biol Med. 2003;34:1100–1110. doi: 10.1016/s0891-5849(03)00062-5. [DOI] [PubMed] [Google Scholar]
- Jeong WS, Keum YS, Chen C, Jain MR, et al. Differential expression and stability of endogenous nuclear factor E2-related factor 2 (Nrf2) by natural chemopreventive compounds in HepG2 human hepatoma cells. J Biochem Mol Biol. 2005;38:167–176. doi: 10.5483/bmbrep.2005.38.2.167. [DOI] [PubMed] [Google Scholar]
- Jiao D, Eklind KI, Choi CI, Desai DH, Amin SG, Chung FL. Structure-activity relationships of isothiocyanates as mechanism-based inhibitors of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice. Cancer Res. 1994;54:4327–433. [PubMed] [Google Scholar]
- Jin CY, Moon DO, Lee KJ, Kim MO, et al. Piceatannol attenuates lipopolysaccharide-induced NF-kappaB activation and NF-kappaB-related proinflammatory mediators in BV2 microglia. Pharmacol Res. 2006;54:461–467. doi: 10.1016/j.phrs.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, et al. Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. J Neurosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JA, Denisova NA, Arendash G, Gordon M, et al. Blueberry supplementation enhances signaling and prevents behavioral deficits in an Alzheimer disease model. Nutr Neurosci. 2003;6:153–162. doi: 10.1080/1028415031000111282. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Casadesus G. Reversing the deleterious effects of aging on neuronal communication and behavior: beneficial properties of fruit polyphenolic compounds. Am J Clin Nutr. 2005;81(1 Suppl):313S–316S. doi: 10.1093/ajcn/81.1.313S. [DOI] [PubMed] [Google Scholar]
- Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci. 2007;9:1105–1127. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katula KS, McCain JA, Radewicz AT. Relative ability of dietary compounds to modulate nuclear factor-kappaB activity as assessed in a cell-based reporter system. J Med Food. 2005;8:269–274. doi: 10.1089/jmf.2005.8.269. [DOI] [PubMed] [Google Scholar]
- Kawabata K, Yamamoto T, Hara A, et al. Modifying effects of ferulic acid on azoxymethane-induced colon carcinogenesis in F344 rats. Cancer Lett. 2000;157:15–21. doi: 10.1016/s0304-3835(00)00461-4. [DOI] [PubMed] [Google Scholar]
- Kim HS, Cho JY, Kim DH, et al. Inhibitory effects of long term administration of ferulic acid on microglial activation induced by intercerebroventricular injection of beta-amyloid peptide (1-42) in mice. Biol Pharm Bull. 2004;27:120–121. doi: 10.1248/bpb.27.120. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Lee KW, Lee HJ. Protective effects of piceatannol against beta-amyloid-induced neuronal cell death. Ann N Y Acad Sci. 2007;1095:473–482. doi: 10.1196/annals.1397.051. [DOI] [PubMed] [Google Scholar]
- Ko WG, Kang TH, Lee SJ, Kim YC, Lee BH. Effects of luteolin on the inhibition of proliferation and induction of apoptosis in human myeloid leukaemia cells. Phytother Res. 2002;3:295–298. doi: 10.1002/ptr.871. [DOI] [PubMed] [Google Scholar]
- Kong L, Tanito M, Huang Z, Li F, et al. Delay of photoreceptor degeneration in tubby mouse by sulforaphane. J Neurochem. 2007;101:1041–1052. doi: 10.1111/j.1471-4159.2007.04481.x. [DOI] [PubMed] [Google Scholar]
- Kraft AD, Johnson DA, Johnson JA. Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci. 2004;24:1101–1112. doi: 10.1523/JNEUROSCI.3817-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Hilz YY, Boerboom AM, Westphal AH, et al. Pro-oxidant activity of flavonoids induces EpRE-mediated gene expression. Chem Res Toxicol. 2006;19:1499–1505. doi: 10.1021/tx060157q. [DOI] [PubMed] [Google Scholar]
- Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr. 2004;134:3479S–3485S. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- Liu YC, Hsieh CW, Wu CC, Wung BS. Chalcone inhibits the activation of NF-kappaB and STAT3 in endothelial cells via endogenous electrophile. Life Sci. 2007;80:1420–1430. doi: 10.1016/j.lfs.2006.12.040. [DOI] [PubMed] [Google Scholar]
- Madan B, Batra S, Ghosh B. 2′-hydroxychalcone inhibits nuclear factor-kappaB and blocks tumor necrosis factor-alpha- and lipopolysaccharide-induced adhesion of neutrophils to human umbilical vein endothelial cells. Mol Pharmacol. 2000;58:526–534. doi: 10.1124/mol.58.3.526. [DOI] [PubMed] [Google Scholar]
- Mandel SA, Avramovich-Tirosh Y, Reznichenko L, Zheng H, et al. Multifunctional activities of green tea catechins in neuroprotection. Modulation of cell survival genes, iron-dependent oxidative stress and PKC signaling pathway. Neurosignals. 2005;14:46–60. doi: 10.1159/000085385. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Goodman Y, Luo H, Fu W, Furukawa K. Activation of NF-kappaB protects hippocampal neurons against oxidative stress-induced apoptosis: evidence for induction of manganese superoxide dismutase and suppression of peroxynitrite production and protein tyrosine nitration. J Neurosci Res. 1997;49:681–697. doi: 10.1002/(SICI)1097-4547(19970915)49:6<681::AID-JNR3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Cheng A. Neurohormetic phytochemicals: Low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosc. 2006;29:632–639. doi: 10.1016/j.tins.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13:852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Hormesis defined. Ageing Res Rev. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SO, Sortwell CE, Shukitt-Hale B, Joseph JA, et al. Dietary supplementation with blueberry extract improves survival of transplanted dopamine neurons. Nutr Neurosci. 2006;9:251–258. doi: 10.1080/10284150601086134. [DOI] [PubMed] [Google Scholar]
- McWalter GK, Higgins LG, McLellan LI, Henderson CJ, et al. Transcription factor Nrf2 is essential for induction of NAD(P)H:quinine oxidoreductase 1, glutathione S-transferases, and glutamate cysteine ligase by broccoli seeds and isothiocyanates. J Nutr. 2004;134:3499S–3506S. doi: 10.1093/jn/134.12.3499S. [DOI] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, Carney DP, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura Y, Chiba T, Tomita I, et al. Tea catechins prevent the development of atherosclerosis in apoprotein E-deficient mice. J Nutr. 2001;131:27– 32. doi: 10.1093/jn/131.1.27. [DOI] [PubMed] [Google Scholar]
- Morse MA, Eklind KI, Amin SG, Hecht SS, Chung FL. Effects of alkyl chain length on the inhibition of NNK-induced lung neoplasia in A/J mice by arylalkyl isothiocyanates. Carcinogenesis. 1989a;10:1757–1759. doi: 10.1093/carcin/10.9.1757. [DOI] [PubMed] [Google Scholar]
- Morse MA, Wang CX, Stoner GD, Mandal S, et al. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced DNA adduct formation and tumorigenicity in the lung of F344 rats by dietary phenethyl isothiocyanate. Cancer Res. 1989b;49:549–553. [PubMed] [Google Scholar]
- Morse MA, Zu H, Galati AJ, Schmidt CJ, Stoner GD. Dose-related inhibition by dietary phenethyl isothiocyanate of esophageal tumorigenesis and DNA methylation induced by N-nitrosomethylbenzylamine in rats. Cancer Lett. 1993;72:103–110. doi: 10.1016/0304-3835(93)90018-5. [DOI] [PubMed] [Google Scholar]
- Murakami A, Nakamura Y, Koshimizu K, et al. FA15, a hydrophobic derivative of ferulic acid, suppresses inflammatory responses and skin tumore promotion: comparison with ferulic acid. Cancer Lett. 2002;180:121–129. doi: 10.1016/s0304-3835(01)00858-8. [DOI] [PubMed] [Google Scholar]
- Nair S, Xu C, Shen G, Hebbar V, et al. Pharmacogenomics of phenolic antioxidant butylated hydroxyanisole (BHA) in the small intestine and liver of Nrf2 knockout and C57BL/6J mice. Pharm Res. 2006;11:2621–2637. doi: 10.1007/s11095-006-9099-x. [DOI] [PubMed] [Google Scholar]
- Nair S, Li W, Kong AN. Natural dietary anti-cancer chemopreventive compounds: redox-mediated differential signaling mechanisms in cytoprotection of normal cells versus cytotoxicity in tumor cells. Acta Pharmacol Sin. 2007;28:59–72. doi: 10.1111/j.1745-7254.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- Nishikawa A, Furukawa F, Uneyama C, Ikezaki S, et al. Chemopreventive effects of phenethyl isothiocyanate on lung and pancreatic tumorigenesis in N-nitrosobis(2-oxopropyl)amine-treated hamsters. Carcinogenesis. 1996;17:1381–1384. doi: 10.1093/carcin/17.6.1381. [DOI] [PubMed] [Google Scholar]
- Nishimura R, Tabata K, Arakawa M, et al. Isobavachalcone, a chalcone constituent of Angelica keiskei, induces apoptosis in neuroblastoma. Biol Pharm Bull. 2007;30:1878–1883. doi: 10.1248/bpb.30.1878. [DOI] [PubMed] [Google Scholar]
- Ohori H, Yamakoshi H, Tomizawa M, Shibuya M, et al. Synthesis and biological analysis of new curcumin analogues bearing an enhanced potential for the medicinal treatment of cancer. Mol Cancer Ther. 2006;5:2563–2571. doi: 10.1158/1535-7163.MCT-06-0174. [DOI] [PubMed] [Google Scholar]
- Okawara M, Katsuki H, Kurimoto E, Shibata H, Kume T, Akaike A. Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochem Pharmacol. 2007;73:550–560. doi: 10.1016/j.bcp.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Ou L, Kong LY, Zhang XM, Niwa M. Oxidation of ferulic acid by momordic charantia peroxidase and related anti-inflammation activity changes. Biol Pharm Bull. 2003;26:1511–1516. doi: 10.1248/bpb.26.1511. [DOI] [PubMed] [Google Scholar]
- Pae HO, Jeong GS, Jeong SO, et al. Roles of heme oxygenase-1 in curcumin-induced growth inhibition in rat smooth muscle cells. Exp Mol Med. 2007;39:267–277. doi: 10.1038/emm.2007.30. [DOI] [PubMed] [Google Scholar]
- Park AM, Dong Z. Signal transduction pathways: targets for green and black tea polyphenols. J Biochem Mol Biol. 2003;36:66–77. [PubMed] [Google Scholar]
- Parker JA, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, Néri C. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet. 2005;37:349–350. doi: 10.1038/ng1534. [DOI] [PubMed] [Google Scholar]
- Penniston KL, Tanumihardjo SA. The acute and chronic toxic effects of vitamin A. Am J Clin Nutr. 2006;83:191–201. doi: 10.1093/ajcn/83.2.191. [DOI] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Koltai E, Taylor AW, Goto S. Exercise, oxidative stress and hormesis. Ageing Res Rev. 2008;7:34–42. doi: 10.1016/j.arr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Robb EL, Page MM, Wiens BE, Stuart JA. Molecular mechanisms of oxidative stress resistance induced by resveratrol: Specific and progressive induction of MnSOD. Biochem Biophys Res Commun. 2008;367:406–412. doi: 10.1016/j.bbrc.2007.12.138. [DOI] [PubMed] [Google Scholar]
- Rushworth SA, Ogborne RM, Charalambos CA, O’Connell MA. Role of protein kinase C delta in curcumin-induced antioxidant response element-mediated gene expression in human monocytes. Biochem Biophys Res Commun. 2006;341:1007–1016. doi: 10.1016/j.bbrc.2006.01.065. [DOI] [PubMed] [Google Scholar]
- Scapagnini G, Colombrita C, Amadio M, D’Agata V, et al. Curcumin activates defensive genes and protects neurons against oxidative stress. Antioxid Redox Signal. 2006;8:395–403. doi: 10.1089/ars.2006.8.395. [DOI] [PubMed] [Google Scholar]
- Schwarting AE. Poisonous seeds and fruits. Prog Chem Toxicol. 1963;18:385–401. doi: 10.1016/b978-0-12-536501-7.50015-1. [DOI] [PubMed] [Google Scholar]
- Shen G, Xu C, Hu R, Jain MR, et al. Comparison of (−)-epigallocatechin-3-gallate elicited liver and small intestine gene expression profiles between C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Pharm Res. 2005;11:1805–1820. doi: 10.1007/s11095-005-7546-8. [DOI] [PubMed] [Google Scholar]
- Shen G, Xu C, Hu R, Jain MR, et al. Modulation of nuclear factor E2-related factor 2-mediated gene expression in mice liver and small intestine by cancer chemopreventive agent curcumin. Mol Cancer Ther. 2006;1:39–51. doi: 10.1158/1535-7163.MCT-05-0293. [DOI] [PubMed] [Google Scholar]
- Shi RX, Ong CN, Shen HM. Luteolin sensitizes tumor necrosis factor-alpha-induced apoptosis in human tumor cells. Oncogene. 2004;23:7712–7721. doi: 10.1038/sj.onc.1208046. [DOI] [PubMed] [Google Scholar]
- Son HY, Nishikawa A, Furukawa F, Lee IS, et al. Modifying effects of 4-phenylbutyl isothiocyanate on N-nitrosobis(2-oxopropyl)amine-induced tumorigenesis in hamsters. Cancer Lett. 2000;160:141–147. doi: 10.1016/s0304-3835(00)00570-x. [DOI] [PubMed] [Google Scholar]
- Sones K, Heaney RK, Fenwick GR. An estimate of the mean daily intake of glucosinolates from cruciferous vegetables in the UK. J Scr Food Agric. 1984;35:712–720. [Google Scholar]
- Srinivasan M, Sudheer AR, Menon VP. Ferulic Acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr. 2007;40:92–100. doi: 10.3164/jcbn.40.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner GD, Adams C, Kresty LA, Amin SG, et al. Inhibition of N′-nitrosonornicotine-induced esophageal tumorigenesis by 3-phenylpropyl isothiocyanate. Carcinogenesis. 1998;12:2139–2143. doi: 10.1093/carcin/19.12.2139. [DOI] [PubMed] [Google Scholar]
- Sudakin DL. Biopesticides. Toxicol Rev. 2003;22:83–90. doi: 10.2165/00139709-200322020-00003. [DOI] [PubMed] [Google Scholar]
- Sultana R, Ravagna A, Mohmmad-Abdul H, et al. Ferulic acid ethyl ester protect neurons against amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: relationship to antioxidant activity. J Neurochem. 2005;92:749–758. doi: 10.1111/j.1471-4159.2004.02899.x. [DOI] [PubMed] [Google Scholar]
- Tedeschi E, Suzuki H, Menegazzi M. Antiinflammatory action of EGCG, the main component of green tea, through STAT-1 inhibition. Ann N Y Acad Sci. 2002;973:435– 437. doi: 10.1111/j.1749-6632.2002.tb04678.x. [DOI] [PubMed] [Google Scholar]
- Tetsuka T, Baier LD, Morrison AR. Antioxidants inhibit interleukin-1 induced cyclooxygenase and nitric oxide synthase expression in rat mesanglial cells. Evidence for post-transcriptional regulation. J Biol Chem. 1996;271:1168–1169. doi: 10.1074/jbc.271.20.11689. [DOI] [PubMed] [Google Scholar]
- Tookey HL, VanEtten CH, Daxenbichler ME. Glucosinolates. In: Liener IE, editor. Toxic Constituents of Plant Stuffs. Academic Press; New York, NY: 1980. pp. 103–142. [Google Scholar]
- Trinh K, Moore K, Wes PD, Muchowski PJ, Dey J, Andrews L, Pallanck LJ. Induction of the phase II detoxification pathway suppresses neuron loss in Drosophila models of Parkinson’s disease. J Neurosci. 2008;28:465–472. doi: 10.1523/JNEUROSCI.4778-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja N, Singh MB, Misra MK, Bhalla PL, Tuteja R. Molecular mechanisms of DNA damage and repair: progress in plants. Crit Rev Biochem Mol Biol. 2001;36:337–397. doi: 10.1080/20014091074219. [DOI] [PubMed] [Google Scholar]
- Ueda H, Yamazaki C, Yamazaki M. Inhibitory effect of Perilla leaf extract and luteolin on mouse skin tumor promotion. Biol Pharm Bull. 2003;4:560–563. doi: 10.1248/bpb.26.560. [DOI] [PubMed] [Google Scholar]
- van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8:440–450. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- Wang Q, Sun AY, Simonyi A, Jensen MD, et al. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J Neurosci Res. 2005;82:138–148. doi: 10.1002/jnr.20610. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhang D, Li G, Liu J, Tian J, Fu F, Liu K. Neuroprotective effects of on brain ischemic injury. Exp Brain Res. 2007;177:533–539. doi: 10.1007/s00221-006-0705-2. [DOI] [PubMed] [Google Scholar]
- Wattenberg LW. Inhibition of carcinogenic effects of polycyclic hydrocarbons by benzyl isothiocyanate and related compounds. J Natl Cancer Inst. 1977;58:395–398. doi: 10.1093/jnci/58.2.395. [DOI] [PubMed] [Google Scholar]
- Wattenberg LW. Inhibition of carcinogenesis by minor dietary constituents. Cancer Res. 1992;52:2085S–2091S. [PubMed] [Google Scholar]
- Wattenberg LW, Coccia JB, Galbraith AR. Inhibition of carcinogen-induced pulmonary and mammary carcinogenesis by chalcone administered subsequent to carcinogen exposure. Cancer Lett. 1994;83:165–169. doi: 10.1016/0304-3835(94)90314-x. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Shukitt-Hale B, Kalt W, Ingram DK, Joseph JA, Wolkow CA. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell. 2006;5:59–68. doi: 10.1111/j.1474-9726.2006.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wruck CJ, Claussen M, Fuhrmann G, Römer L, et al. Luteolin protects rat PC12 and C6 cells against MPP+ induced toxicity via an ERK dependent Keap1-Nrf2-ARE pathway. J Neural Transm Suppl. 2007;72:57–67. doi: 10.1007/978-3-211-73574-9_9. [DOI] [PubMed] [Google Scholar]
- Wu CC, Hsu MC, Hsieh CW, et al. Upregulation of heme oxygenase-1 by Epigallocatechin-3-gallate via the phosphatidylinositol 3-kinase/Akt and ERK pathways. Life Sci. 2006;78:2889–2897. doi: 10.1016/j.lfs.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Xu C, Yuan X, Pan Z, Shen G, et al. Mechanism of action of isothiocyanates: the induction of ARE-regulated genes is associated with activation of ERK and JNK and the phosphorylation and nuclear translocation of Nrf2. Mol Cancer Ther. 2006;8:1918–1926. doi: 10.1158/1535-7163.MCT-05-0497. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ku B, Cui L, Li X, Barish PA, Foster TC, Ogle WO. Curcumin reverses impaired hippocampal neurogenesis and increases serotonin receptor 1A mRNA and brain-derived neurotrophic factor expression in chronically stressed rats. Brain Res. 2007;1162:9–18. doi: 10.1016/j.brainres.2007.05.071. [DOI] [PubMed] [Google Scholar]
- Ye CL, Liu JW, Wei DZ. In vitro anti-tumor activity of 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone against six established human cancer cell lines. Pharmacol Res. 2004;50:505–510. doi: 10.1016/j.phrs.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Ye CL, Liu JW, Wei DZ. In vivo antitumor activity by 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone in a solid human carcinoma xenograft model. Cancer Chemother Pharmacol. 2005;55:447–452. doi: 10.1007/s00280-004-0917-8. [DOI] [PubMed] [Google Scholar]
- Yu R, Lei W, Mandlekar S, Weber MJ, Der CJ, Wu J, Kong AN. Role of mitogen-activated protein kinase pathway in the induction of phase II detoxifying enzymes by chemical. J Biol Chem. 1999a;274:27545–27552. doi: 10.1074/jbc.274.39.27545. [DOI] [PubMed] [Google Scholar]
- Yu Z, Zhou D, Bruce-Keller AJ, Kindy MS, Mattson MP. Lack of the p50 subunit of nuclear factor-kappaB increases the vulnerability of hippocampal neurons to excitotoxic injury. J Neurosci. 1999b;19:8856–8865. doi: 10.1523/JNEUROSCI.19-20-08856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbarsky V, Datla KP, Parkar S, Rai DK, Aruoma OI, Dexter DT. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic Res. 2005;39:1119–1125. doi: 10.1080/10715760500233113. [DOI] [PubMed] [Google Scholar]
- Zhang B, Safa R, Rusciano D, Osborne NN. Epigallocatechin gallate, an active ingredient from green tea, attenuates damaging influences to the retina caused by ischemia/reperfusion. Brain Res. 2007;1159:40–53. doi: 10.1016/j.brainres.2007.05.029. [DOI] [PubMed] [Google Scholar]