Abstract
Serum 25-hydroxyvitamin D (25OHD) was measured in 128 youth with type 1 diabetes (T1D). Less than 25% of the patients were vitamin D sufficient. Given that individuals with T1D possess multiple risk factors for skeletal fragility, ensuring vitamin D sufficiency throughout childhood and adolescence in this population seems especially warranted.
Keywords: diabetes, pediatrics, vitamin D, bone
Chronic severe vitamin D deficiency in infants and children causes bone deformation from poor mineralization (i.e., rickets). Less severe vitamin D insufficiency prevents youth from attaining their optimal peak bone mass and may contribute to increased fracture risk later in life (1). Vitamin D inadequacy constitutes a largely unrecognized epidemic in many populations (2, 3).
Type 1 diabetes (T1D) also negatively impacts bone health and is associated with a modest reduction in bone mineral density (BMD) and strength (4, 5) as well as an increase in fracture risk among middle age and older individuals (6). To date, several potential mechanisms for reduced BMD associated with diabetes have been proposed, including advanced glycation end products in bone collagen (7), hypercalciuria associated with glycosuria (8), inflammatory cytokines (9), and diabetic microangiopathy with reduced blood flow to bone (10). It is not known, however, if aggressively treating diabetes can help preserve skeletal health (11).
Given the negative impact of vitamin D inadequacy and T1D on bone health, youth with both conditions have multiple risk factors for increased skeletal fragility. Studies examining vitamin D inadequacy in youth with T1D have been somewhat limited. In one study from Italy (12) and a second study from Sweden (13), the mean level of vitamin D was found to be lower in patients with T1D at the time of diagnosis compared with controls. In a third more recent study from Australia, 43% of adolescents with T1D were found to be vitamin D deficient (14). Therefore, our aims were to assess vitamin D status in youth with T1D from the northeastern United States and to examine the influence of specific patient and disease characteristics on vitamin D status.
Methods
Participants were enrolled in the cross-sectional study at the time of a regularly scheduled medical visit to the Pediatric, Adolescent, and Young Adult Section at the Joslin. The study sample included youth with recently diagnosed T1D and youth with established T1D. A research assistant obtained written, informed consent from the parent and assent from the child. Participants and their families then completed a set of questionnaires and youth provided a blood sample for analysis. The institutional review board at the Joslin approved the study protocol.
Serum 25-hydroxyvitamin D (25OHD) is the standard indicator of vitamin D status, composed of cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2). Levels of 25OHD were measured using the radioimmunoassay method (Diasorin) that detects both forms of 25OHD. The criteria used to define vitamin D sufficiency, insufficiency, and deficiency were 25OHD levels of ≥30 ng/mL, 21–29 ng/mL, and ≤20 ng/mL, respectively (1, 15).
Because vitamin D status is associated with sunlight exposure which varies by season, we categorized each participant’s study visit according to the following division of the calendar year: winter (December 22-March 21), spring (March 22-June 21), summer (June 22-September 21), and fall (September 22-December 21).
A1c was measured using high-performance liquid chromatography standardized to the DCCT assay (reference range: 4–6%; Tosoh Medics, Inc, Foster City, CA). An age- and sex-adjusted z-BMI was calculated from weight and height.
Statistical analysis was performed with SAS version 8.2 (SAS Institute, Cary, NC). Means ± SD and percentages are presented unless otherwise indicated. Group comparisons were performed using unpaired t tests, ANOVA, χ2 analysis, and multivariate models.
Results
Of the 128 participants with T1D, the majority had inadequate levels of vitamin D: sufficiency, 24% (n=31); insufficiency, 61% (n=78); deficiency, 15% (n=19). The Table displays patient characteristics for the entire sample and each of the three vitamin D subgroups.
Table.
Participant Characteristics.
| Total Sample N=128 | Vitamin D Sufficient n=31 (24%) | Vitamin D Insufficient n=78 (61%) | Vitamin D Deficient n=19 (15%) | P | |
|---|---|---|---|---|---|
| Age, yrs | |||||
| Mean ± SD | 10.8 ± 4.3 | 8.4 ± 4.1 | 11.2 ± 3.8 | 13.2 ± 4.7 | <0.001 |
| Range | 1.6–17.5 | 1.6–15.0 | 3.0–17.4 | 1.8–17.5 | |
|
| |||||
| Sex | |||||
| Male | 69 (54) | 19 (61) | 41 (53) | 9 (47) | NS |
| Female | 59 (46) | 12 (39) | 37 (47) | 10 (53) | |
|
| |||||
| Ethnicity | |||||
| White | 113 (88) | 28 (90) | 69 (88) | 16 (84) | NS |
| Other | 15 (12) | 3 (10) | 9 (12) | 3 (16) | |
|
| |||||
| Visit Season | |||||
| Spring | 31 (24) | 6 (19) | 19 (24) | 6 (32) | |
| Summer | 19 (15) | 6 (19) | 12 (15) | 1 (5) | NS |
| Fall | 29 (23) | 11 (35) | 15 (19) | 3 (16) | |
| Winter | 49 (38) | 8 (26) | 32 (41) | 9 (47) | |
|
| |||||
| z-BMI (SDS) | |||||
| Mean ± SD | 0.4 ± 1.0 | 0.1 ± 1.1 | 0.5 ± 0.9 | 0.6 ± 1.1 | NS |
| Range | −2.8–2.3 | −2.3–2.0 | −2.8–2.3 | −1.9–2.2 | |
|
| |||||
| Duration of T1D (yrs) | |||||
| Mean ± SD | 4.1 ± 5.6 | 1.7 ± 4.3 | 4.5 ± 5.6 | 6.7 ± 5.9 | <0.01 |
| Range | 0–14.6 | 0–13.5 | 0–14.5 | 0–14.6 | |
|
| |||||
| A1c (%) | |||||
| Mean ±SD | 9.8 ± 2.2 | 10.7 ± 2.1 | 9.7 ± 2.2 | 9.3 ± 1.9 | 0.05 |
| Range | 6.5–15.8 | 7.2–15.8 | 6.5–15.3 | 6.8–13.6 | |
|
| |||||
| 25OHD (ng/mL) | |||||
| Mean ± SD | 26.8 ± 6.7 | 35.5 ± 5.4 | 25.7 ± 2.5 | 17.2 ± 2.9 | <0.001 |
| Range | 11.0–52.6 | 30.0–52.6 | 21.4–29.9 | 11.0–20.8 | |
Data are presented as n (%) unless otherwise indicated.
In bivariate analyses, sex, ethnicity, visit season, and z-BMI were similar among participants with vitamin D sufficiency, insufficiency, and deficiency. Participants with vitamin D deficiency, however, were significantly older (P<.001), had longer diabetes duration (P<.01), and had lower A1c (P=.05).
In a multivariate model controlling for age, sex, ethnicity, visit season, z-BMI, diabetes duration, and A1c, age was most significantly associated with 25OHD (P<.0001); older age was associated with lower 25OHD. Only ethnicity (P=.05) was also significantly associated with 25OHD, with lower 25OHD more common among non-white patients.
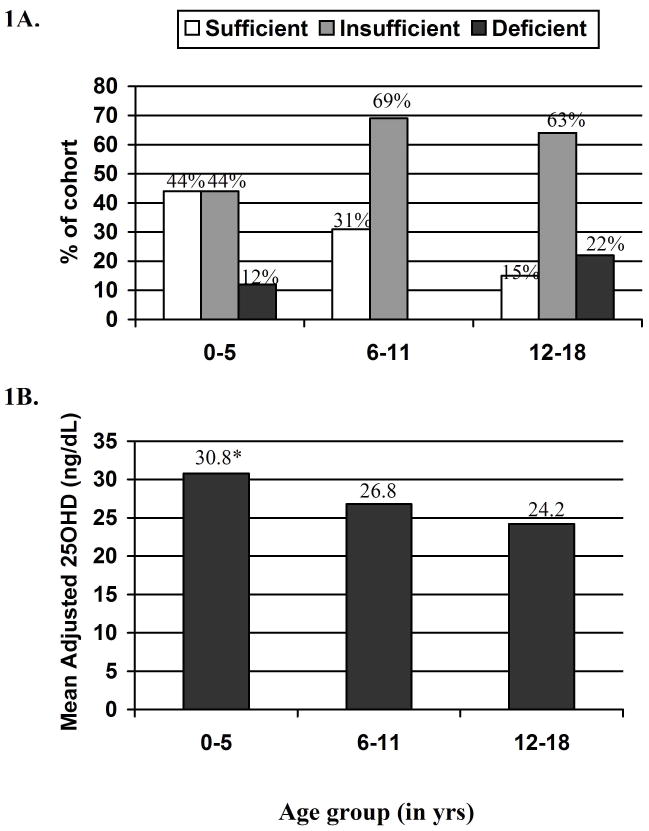
To further explore the relationship between age and 25OHD, participants were stratified into three groups by age: youngest (0–5 years), middle (6–11 years), and oldest (12–18 years). The percentage of participants in each age group meeting criteria for vitamin D sufficiency, insufficiency, or deficiency is shown in Figure 1A. Inadequate 25OHD concentrations were most prevalent in the oldest age group, with only 15% meeting criteria for vitamin D sufficiency. Mean adjusted 25OHD concentrations for the three age groups are shown in Figure 1B. Mean adjusted 25OHD concentration was significantly lower in the oldest group than in the youngest group (P<.01).
Figure 1.
Figure 1A Vitamin D status. Among 0–5, 6–11, and 12–18 year olds, the percentage of participants with either vitamin D insufficiency or deficiency was 56%, 69%, and 85%, respectively. B, Adjusted 25OHD levels. A mean adjusted 25OHD value was calculated for each age group controlling for patient ethnicity, visit season, sex, z-BMI, A1c, and diabetes duration. The 12–18 year olds had significantly lower 25OHD levels compared with 0–5 year olds (<0.01).
Discussion
It is important for those who care for children, particularly children with T1D, to be aware of the high prevalence of vitamin D inadequacy and its adverse effect on skeletal health. In our sample of youth, vitamin D inadequacy was most common among adolescents aged 12–18 years, with more than 4 out of 5 meeting criteria for vitamin D insufficiency or deficiency. In comparison, a recent study of adolescent youth without diabetes from Philadelphia found that 51% of white participants had vitamin D levels <30 ng/mL when tested between the months of November-March (16). For the white participants in our cohort tested during the winter, 84% had 25OHD levels <30 ng/mL. Thus, our data suggest excessive vitamin D inadequacy in youth with T1D compared with a geographically-, age-, and race-matched sample.
Although glycemic control, duration of diabetes, and age were associated with vitamin D inadequacy in bivariate analyses, only age remained a significant predictor in multivariate analyses. The youngest participants were over-represented by youth with short duration diabetes and higher A1c levels reflective of their recently diagnosed diabetes.
In addition to inadequate levels of vitamin D, adolescent patients with T1D potentially possess multiple risk factors for increased skeletal fragility. Previous studies have shown that the early adolescent years are often associated with a rapid decrease in vitamin D-fortified milk intake (17). Sugar-free colas, which are frequently consumed by adolescents with diabetes, convey additional risk for poor bone health as they contain phosphoric acid, which is known to reduce intestinal calcium absorption (18). Hyperglycemia, hypercalciuria resulting in a calcium deficit, inflammatory cytokines, and microangiopathy could also potentially impair bone strength. Given that many of these risk factors may not be modifiable due to the inherent presence of diabetes, ensuring vitamin D sufficiency throughout childhood and during the time of maximal bone mineral accrual seems particularly warranted in this population.
Future studies need to confirm our findings of vitamin D inadequacy in youth with T1D, identify mechanisms leading to insufficient or deficient states, and assess BMD in youth with T1D by DEXA.
Acknowledgments
We acknowledge contributions from the laboratory of Thomas O. Carpenter, MD, at Yale University School of Medicine where the vitamin D metabolite assays were performed. In addition, we recognize contributions from the Pediatric Team at the Joslin Diabetes Center.
This study was supported by grants from the NIH: RO1DK046887 (to LMBL) and K12DK63696 (to BMS). Support was also received from the Charles H. Hood Foundation and Eli Lilly and Company. The[H1] authors declare no affiliations, financial agreements, or other involvements that would constitute a conflict of interest.
Abbreviations
- A1c
hemoglobin A1c
- BMD
bone mineral density
- T1D
type 1 diabetes
- 25OHD
25-hydroxyvitamin D
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–73. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 2.El Hajj FG, Nabulsi M, Choucair M, Salamoun M, Hajj SC, Kizirian A, et al. Hypovitaminosis D in healthy schoolchildren. Pediatrics. 2001;107:E53. doi: 10.1542/peds.107.4.e53. [DOI] [PubMed] [Google Scholar]
- 3.Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med. 2002;112:659–62. doi: 10.1016/s0002-9343(02)01091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuominen JT, Impivaara O, Puukka P, Ronnemaa T. Bone mineral density in patients with type 1 and type 2 diabetes. Diabetes Care. 1999;22:1196–200. doi: 10.2337/diacare.22.7.1196. [DOI] [PubMed] [Google Scholar]
- 5.Miazgowski T, Czekalski S. A 2-year follow-up study on bone mineral density and markers of bone turnover in patients with long-standing insulin-dependent diabetes mellitus. Osteoporos Int. 1998;8:399–403. doi: 10.1007/s001980050082. [DOI] [PubMed] [Google Scholar]
- 6.Holmberg AH, Johnell O, Nilsson PM, Nilsson J, Berglund G, Akesson K. Risk factors for fragility fracture in middle age. A prospective population-based study of 33,000 men and women. Osteoporos Int. 2006;17:1065–77. doi: 10.1007/s00198-006-0137-7. [DOI] [PubMed] [Google Scholar]
- 7.Paul RG, Bailey AJ. Glycation of collagen: the basis of its central role in the late complications of ageing and diabetes. Int J Biochem Cell Biol. 1996;28:1297–310. doi: 10.1016/s1357-2725(96)00079-9. [DOI] [PubMed] [Google Scholar]
- 8.Ward DT, Yau SK, Mee AP, Mawer EB, Miller CA, Garland HO, et al. Functional, molecular, and biochemical characterization of streptozotocin-induced diabetes. J Am Soc Nephrol. 2001;12:779–90. doi: 10.1681/ASN.V124779. [DOI] [PubMed] [Google Scholar]
- 9.Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332:305–11. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- 10.McNair P, Christensen MS, Christiansen C, Madsbad S, Transbol I. Is diabetic osteoporosis due to microangiopathy? Lancet. 1981;1:1271. doi: 10.1016/s0140-6736(81)92447-8. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz AV. Diabetes Mellitus: Does it Affect Bone? Calcif Tissue Int. 2003;73:515–9. doi: 10.1007/s00223-003-0023-7. [DOI] [PubMed] [Google Scholar]
- 12.Pozzilli P, Manfrini S, Crino A, Picardi A, Leomanni C, Cherubini V, et al. Low levels of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 in patients with newly diagnosed type 1 diabetes. Horm Metab Res. 2005;37:680–3. doi: 10.1055/s-2005-870578. [DOI] [PubMed] [Google Scholar]
- 13.Littorin B, Blom P, Scholin A, Arnqvist HJ, Blohme G, Bolinder J, et al. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide Diabetes Incidence Study in Sweden (DISS) Diabetologia. 2006;49:2847–52. doi: 10.1007/s00125-006-0426-x. [DOI] [PubMed] [Google Scholar]
- 14.Greer RM, Rogers MA, Bowling FG, Buntain HM, Harris M, Leong GM, et al. Australian children and adolescents with type 1 diabetes have low vitamin D levels. Med J Aust. 2007;187:59–60. doi: 10.5694/j.1326-5377.2007.tb01130.x. [DOI] [PubMed] [Google Scholar]
- 15.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–6. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 16.Weng FL, Shults J, Leonard MB, Stallings VA, Zemel BS. Risk factors for low serum 25-hydroxyvitamin D concentrations in otherwise healthy children and adolescents. Am J Clin Nutr. 2007;86:150–8. doi: 10.1093/ajcn/86.1.150. [DOI] [PubMed] [Google Scholar]
- 17.Bowman SA. Beverage choices of young females: changes and impact on nutrient intakes. J Am Diet Assoc. 2002;102:1234–9. doi: 10.1016/s0002-8223(02)90273-7. [DOI] [PubMed] [Google Scholar]
- 18.Wyshak G. Teenaged girls, carbonated beverage consumption, and bone fractures. Arch Pediatr Adolesc Med. 2000;154:610–3. doi: 10.1001/archpedi.154.6.610. [DOI] [PubMed] [Google Scholar]