Abstract
During phagocytosis, neutrophils undergo a burst of respiration in which oxygen is reduced to superoxide (), which dismutates to form H2O2. Myeloperoxidase (MPO) is discharged from the cytoplasmic granules into the phagosome following particle ingestion. It is thought to utilize H2O2 to oxidize halides, which then react with and kill ingested microbes. Recent studies have provided new information as to the concentration of and proteins, and the pH, within the vacuole. This study was conducted to examine the antimicrobial effect of , H2O2 and hypochlorous acid under these conditions and it was found that the previously described bactericidal effect of these agents was reversed in the presence of granule proteins or MPO. To establish which cellular proteins were iodinated by MPO, cellular proteins and bacterial proteins, iodinated in neutrophils phagocytosing bacteria in the presence of 125I, were separated by 2D gel electrophoresis. Iodinated spots were detected by autoradiography and the oxidized proteins were identified by MS. The targets of these iodination reactions were largely those of the host cell rather than those of the engulfed microbe.
INTRODUCTION
Phagocytosis results in assembly and activation of the respiratory burst NADPH oxidase at the membrane of the phagocytic vacuole. The respiratory burst is required for optimal antimicrobial function by neutrophils, and its importance is demonstrated by the syndrome of chronic granulomatous disease (CGD), a rare condition in which its absence predisposes patients to severe infection (Thrasher et al., 1994). Activation of the oxidase is associated with the generation of various reduced oxygen species (ROS) (Root et al., 1975). These have widely been thought to be responsible for the killing of phagocytosed micro-organisms, either directly (Babior et al., 1973, 1974) or by acting as substrates for myeloperoxidase (MPO)-mediated halogenation (Klebanoff, 1975).
The first product of the oxidase is , the product of the univalent reduction of oxygen (Babior et al., 1973). has minimal antibacterial activity (Rosen & Klebanoff, 1979; Hurst & Barrette, 1989) and dismutates to produce H2O2. H2O2 is thought to be acted upon by MPO, and released into the vacuole from the cytoplasmic granules, to produce hypochlorous acid (HOCl), a potent antimicrobial oxidant (Klebanoff, 1967a, 1968).
Recently an attempt was made to define the conditions that pertain within the phagocytic vacuole at the time at which the respiratory burst takes place (Reeves et al., 2002). It was found that large amounts of , of approximately 4 mols l−1, are produced, that the concentration of granule proteins is as much as 500 mg ml−1, and the pH is between 7·4 and 8·0. This study was undertaken to examine the antibacterial action of and H2O2, and products of chloride oxidation (HOCl), under these conditions. 125I was used to identify the protein targets of MPO-induced iodination.
METHODS
In vitro killing of Staphylococcus aureus and Escherichia coli by human neutrophils
Neutrophils were purified from fresh human blood by dextran sedimentation and centrifugation through Ficoll/Hypaque as described previously (Segal & Jones, 1980). Cells (5 × 108) were incubated at 37 °C in 1 ml PBS (140 mM NaCl, 10 mM KCl, 10 mM NaH2PO4, 5 mM glucose, pH 7·3) in a rapidly stirred chamber. IgG opsonized S. aureus (NCTC 12981) or E. coli (ATCC 11775) (1 × 108 c.f.u. ml−1) was added and killing was measured as described by Segal et al. (1981) omitting lysostaphin. Results were calculated as the mean (±se) from at least three experiments with colony counts performed in triplicate for each sample and expressed as a percentage of the original numbers at time zero.
Preparation of granules and MPO purification
Diisopropyl fluorophosphate (DIFP; 1 mM) was added to 1 × 1010 neutrophils, which were mixed and left on ice for 10 min. The cells were then resuspended in Break Buffer (10 mM KCl, 3 mM NaCl, 4 mM MgCl2, 10 mM PIPES, pH 7·3) containing protease inhibitors [10 μgml−1 leupeptin, N-p-tosyl-l-lysine chloromethyl ketone (TLCK), pepstatin A and aprotinin] and disrupted by nitrogen cavitation after 400 p.s.i. (2760 kPa) for 20 min. A post-nuclear supernatant was prepared (400 g, °15 min, 4 8C) and layered on top of a discontinuous gradient of 30, 43 and 55% sucrose (w/w in Break Buffer) and centrifuged (1 h/100 000 g/4 °C) in a Beckman SW41 head. The azurophil and specific granules were collected from the interfaces of the 55 and 43% sucrose, respectively. The granules were washed in PBS, pelleted and stored at −80 °C at a final concentration of 25 mg ml−1.
For the preparation of MPO, azurophilic granules of 1 × 1010 neutrophils were homogenized in 120 ml Break Buffer containing 0·75% cetyltrimethylammonium bromide (CETAB) and centrifuged (30 000 g/10 min/4 °C). Ammonium sulphate was added to the supernatant to 50% final concentration. The precipitate was removed by centrifugation at 30 000 g/10 min/4 °C. Further ammonium sulphate (100 mg ml−1) was added to the supernatant, at which concentration the MPO came out of solution and was pelleted by centrifugation (30 000 g/10 min/4 °C). The pellet was resolubilized in 50 mM phosphate buffer (pH 7·2). The MPO was purified by ion-exchange chromatography on Fast Flow S-Sepharose (1·5 × 10 cm column, flow rate of 1 ml min−1, 1 ml fractions collected, eluted with a 100 ml linear gradient of 50-500 mM phosphate buffer, pH 7·2, at 4 °C). Peak fractions were pooled, the ionic concentration was diluted to 50 mM and the pH was adjusted to pH 6·5. The sample was then loaded onto a Mono S column (1 ml column, flow rate of 0·5 ml min−1, eluted with a linear gradient of 50–500 mM phosphate buffer, pH 6·5, 1 ml fractions collected). The peak fractions of MPO with a purity index (A430/A280)of 0·82 (Olsen & Little, 1982) were pooled and stored at −80 °C.
Killing by, and oxidative capacity of, purified MPO
Bacteria (2 × 107 c.f.u. ml−1) from an overnight culture were washed twice and resuspended in PBS at pH 7·5, 6·5 or 5·5 together with purified MPO (5 mg ml−1). H2O2 was then added at concentrations of 0·1, 1·0, 10 or 100 mM. After mixing at 37 °C for 0, 1, 2, 4, 8 and 16 min, aliquots were removed and diluted 1/10 in ice-cold LB broth (Difco). Serial tenfold dilutions were then made, and plated in triplicate on LB agar plates. Colony counts were performed in triplicate or quadruplicate for each sample and results were calculated as the mean (±se) from at least three experiments. The pH remained stable during assays to within 0·15 pH units of the starting pH.
The oxidative capacity (COX) of purified MPO was determined in the presence of 10 mM taurine by the addition of potassium iodide in molar excess, to detect the reaction product N-chlorotaurine. The product triiodide () was measured by its A350, which is the wavelength of maximum absorption (λmax)of (ε = 22 900 mol−1 cm−1) (Nagl et al., 2000). Values measured from 1, 10 and 100 mM H2O2 in PBS at pH 5·5, 6·5 and 7·5 without MPO were subtracted from these values to ensure determination of HOCl and N-chlorotaurine, respectively.
In vitro killing of S. aureus and E. coli by H2O2, HOCl and superoxide (KO2).
From an overnight culture, bacteria (2 × 107 c.f.u. ml−1) were washed twice in PBS and resuspended in PBS at pH 7·5, 6·5 or 5·5. To investigate the pH-dependency of killing, the suspended bacteria were incubated with gentle mixing at 37 °C for 32 min. Aliquots were removed periodically and surviving bacteria were counted by serial dilution and colony counting.
Increasing concentrations of H2O2 (1·0, 10 or 100 mM) or HOCl (1 or 5 μM) were added and incubated at 37 °C for 0, 1, 2, 4, 8, 16 and 32 min. Aliquots were removed and plated out as described above. The pH remained stable during assays to within 0·2 pH units of the starting pH.
This experiment was repeated with 100 mM H2O2 and up to 1 mM HOCl in the presence of a mixture of azurophil and specific demembraned granules (25 mg ml−1). Membranes were removed by Percoll granule disruption as described by Vita et al. (1997). The granules were purified in the presence of protease inhibitors to prevent killing of bacteria by these enzymes. Due to the viscosity of the granule protein at high concentration, for technical handling purposes the concentration used was 25 mg ml−1. Bacteria (2 × 107 c.f.u. ml−1) were added to the granule protein prior to the addition of H2O2 or HOCl.
Killing of bacteria by was performed similarly to that described for H2O2 and HOCl. As a source of , KO2 was employed and added as a powder to the reactions. Since concentrations of KO2 greater than 50 mM elevated the pH, bacteria were suspended in PBS at a pH of 6·5 prior to the addition of 100 mM KO2, which resulted in a rise in the pH to approximately 7·5.
Iodination studies
Iodination studies were performed as described by Klebanoff & Clark (1976). Neutrophils (1 × 107) were resuspended in 1 ml PBS supplemented with 40 nM KI and 100 μCi (3700 kBq) 125I. The cell mixture was placed in a magnetically stirring oxygenated chamber at 37 °C and IgG opsonized S. aureus was added at a ratio of 10 : 1. After 4 min, the mixture was taken into 1 ml cold PBS containing 10% trichloroacetic acid (TCA). This experiment was also carried out with IgG opsonized E. coli, added at a ratio of 100 : 1.
Two-dimensional electrophoresis of proteins using immobilized pH gradients
Samples were centrifuged (8000 g, 5 min, 4 °C). The pellet was washed three times with ice-cold 80% acetone and air-dried. The pellet was resuspended in 300 μl IEF sample buffer (8 M urea, 2 M CHAPS, 1% Triton X-100, 65 mM DTT, 10 mM Tris base, 0·8% Ampholyte), sonicated briefly and centrifuged (10 000 g/5 min/4 °C). Samples (0·5 mg/270 μl) were pipetted on top of IPG (3–10) strips, and IEF was performed for 40 000 V h at 16 °C.
Prior to electrophoresis in the second dimension, IPG strips were equilibrated twice in 10 ml equilibration buffer [30% (v/v) glycerol, 2% (w/v) SDS, 6 M urea, 50 mM Tris/HCl, pH 6·8] for 20 min. The first equilibration was in 10 ml equilibration buffer containing 2% (w/v) DTT and the second contained 2·5% (w/v) iodoacetamide. The IPG gel strips were electrophoresed into a 12% SDS-PAGE gel and stained with Coomassie R-250.
MALDI-TOF MS
The protein bands/spots of interest were excised from the SDS gel and digested according to the protocol described by Rosenfeld et al. (1992).
The following peptides were used as external standards for MALDI spectra calibration: human angiotensin I and II, ACTH (clip 18–39), [Glu]-fibrinopeptide B, renin substrate tetradecapeptide and insulin B chain. The amount of each peptide was 25 pmol per spot. MALDI-TOF mass spectra of the peptides were obtained using a Biflex III mass spectrometer (Bruker). All spectra were acquired in a positive-ion reflector. Typically 200 shots were recorded. Proteins were identified by comparing mass fingerprints to NCBI's database using Matrix Science, Msfit and PeptIdent searching machines (http://www.matrixscience.com/).
Statistical analysis
Statistical comparisons were made with Student's t test.
RESULTS AND DISCUSSION
Vacuolar conditions
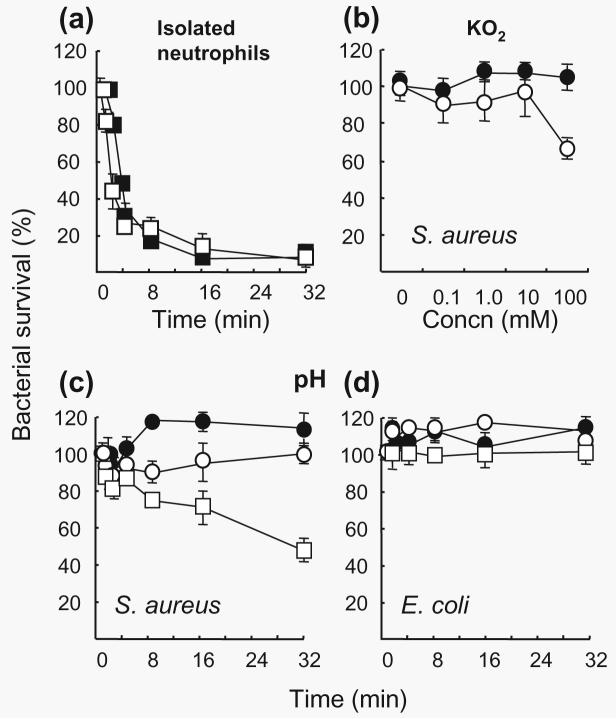
The kinetics of bacterial killing by neutrophils is illustrated in Fig. 1(a). Killing occurred quickly, with over 50% killed after just 2 min and 20% remaining after 4 min as described previously (Segal et al., 1981).
Fig. 1.
Effect of pH on bacterial viability, destruction by stimulated neutrophils and bactericidal effects of . (a) IgG opsonized S. aureus (■) or E. coli (□) (1 × 108 c.f.u. ml−1) was mixed at a ratio of one target organism to five neutrophils in 1 ml PBS (pH 7·3) for the indicated periods of time and bacterial viability was determined. The mean (±se) of three experiments is shown. No significant difference was observed between killing of S. aureus and E. coli. (b) Bactericidal effect of was determined by suspending S. aureus (1 × 107 c.f.u.) in 0· 01 M phosphate buffer (pH 7·5) (●) or buffer containing different concentrations of KO2 (○). Each point is the mean of triplicate experiments (±se). (c, d) To determine the effect of pH on bacterial viability, S. aureus or E. coli (1 × 107 c.f.u. ml−1) was incubated at 37 °C in 0·01 M phosphate buffer, pH 5·5 (□), 6·5 (○) or 7·5 (●). Reduction in survival of S. aureus at pH 5·5 compared to 6·5 was found to be significant, P < 0·033.
Fig. 1(c, d) shows that pH did not affect the viability of E. coli or S. aureus, except after prolonged exposure approximately 50% of S. aureus was killed at pH 5·5 (P < 0·05) after 32 min.
Bactericidal effects of ,H2O2 and HOCl
The bactericidal effect of increasing concentrations of was investigated at pH 7·5 after 6 min. Fig. 1(b) shows that itself is relatively non-toxic.
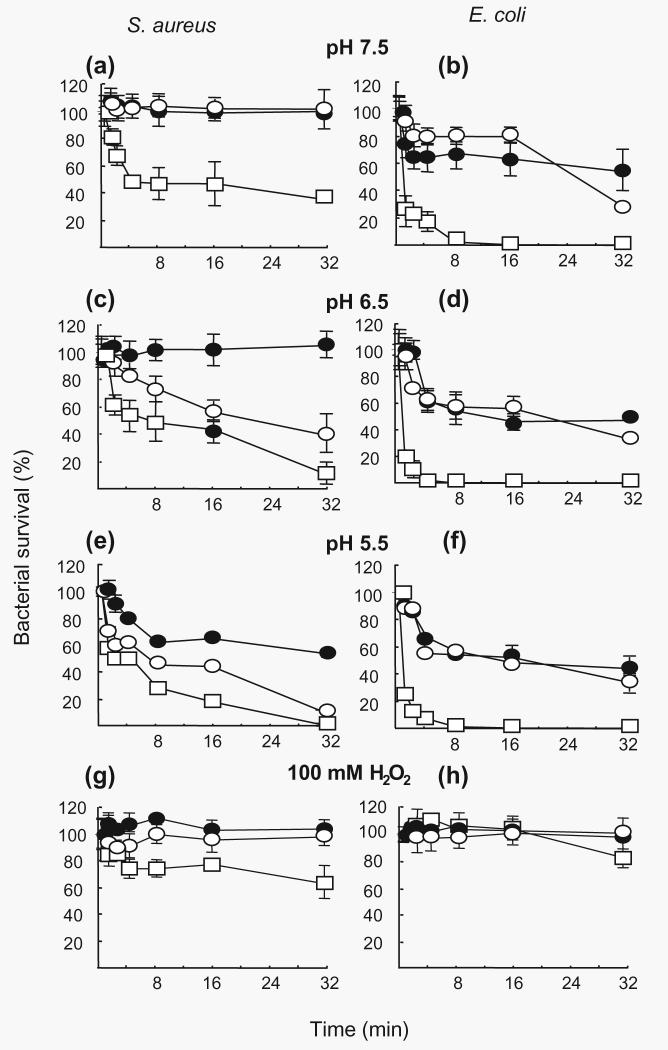
Fig. 2(a–f) shows the killing of S. aureus and E. coli exposed to increasing concentrations of H2O2 at pH values of 7·5, 6·5 or 5·5. The bactericidal effect of H2O2 was both dose- and pH-dependent. As the pH was elevated to 7·5, a concentration of 100 mM H2O2 was required to reduce the survival of S. aureus by 50%. The effect of 100 mM H2O2 on S. aureus and E. coli was totally eliminated in the presence of granule protein (Fig. 2g, h).
Fig. 2.
Bactericidal activity of H2O2 in the presence and absence of granule protein. The reaction mixture contained S. aureus (a, c, e) or E. coli (b, d, f) (2 × 107 c.f.u. ml−1) in 0·01 M phosphate buffer at pH 5·5, 6·5 or 7·5 with 1 (●), 10 (○) or 100 (□) mM H2O2 added at 37 °C. Aliquots were removed at the times indicated. The experiment was repeated with 100 mM H2O2 and granule protein (g, h) (25 mg ml−1) at pH 7·5 (●), 6·5 (○) or 5·5 (□). Each value is derived from triplicate plating. The mean values (±se) from four experiments are shown. Changes in viability greater than 50% were always significant (P ≤ 0·05).
The result of incubating S. aureus and E. coli in the presence of 1 and 5 μM HOCl at pH 7·5, 6·5 or 5·5 is illustrated in Fig. 3(a–d). This agent was rapidly lethal. However, when added to bacteria in the presence of granule proteins no killing was evident (result not shown). No killing was seen even when 1 mM HOCl was used at pH 7·5 and 5·5 in the presence of granule protein (Fig. 3e–h).
Fig. 3.
Bactericidal activity of HOCl in the presence and absence of granule protein. The reaction mixture 0·01 M phosphate buffer, pH 7·5 (●), 6·5 (○) or 5·5 (□), contained S. aureus (a, c) or E. coli (b, d) (2 × 107 c.f.u. ml−1) and 1 or 5 μM HOCl. Inhibition of killing of S. aureus (e, g) (2 × 107 c.f.u. ml−1) or E. coli (f, h) by HOCl was observed in 0·01 M phosphate buffer, pH 7·5 or 5·5, with added granule protein. Bacteria (2 × 107 c.f.u. ml−1) were exposed to 100 μM(●), 250 μM(○), 500 μM(▲) or 1 mM (△) HOCl in the presence of granule protein (25 mg ml−1). Each line is representative of the mean (±SE) of three experiments.
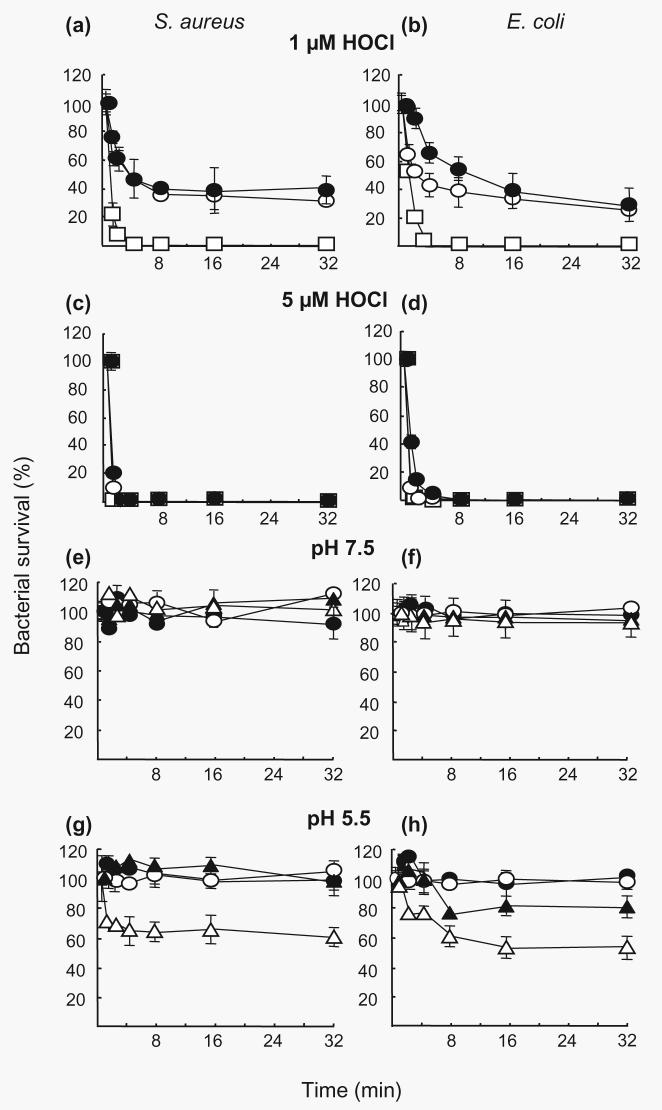
Bactericidal effects of the MPO system
Bacterial killing by the MPO/H2O2/Cl− system was assessed using purified neutrophil granule MPO. Bacteria were washed and suspended in PBS at pH 5·5, 6·5 and 7·5 containing MPO (5 mg ml−1) and reactions were started by the addition of 0·1, 1, 10 or 100 mM H2O2. The bactericidal effect proved to be dependent upon the concentration of H2O2 and the pH. In the presence of 0·1 (result not shown) and up to 1 mM H2O2 no bacterial killing was observed despite the low pH of 5·5 and the presence of Cl− (Fig. 4a–c). With the use of 10 mM H2O2 there was a marked bactericidal effect at pH 5·5 and 6·5. Finally, the addition of 100 mM H2O2 resulted in total killing of bacteria at low pH.
Fig. 4.
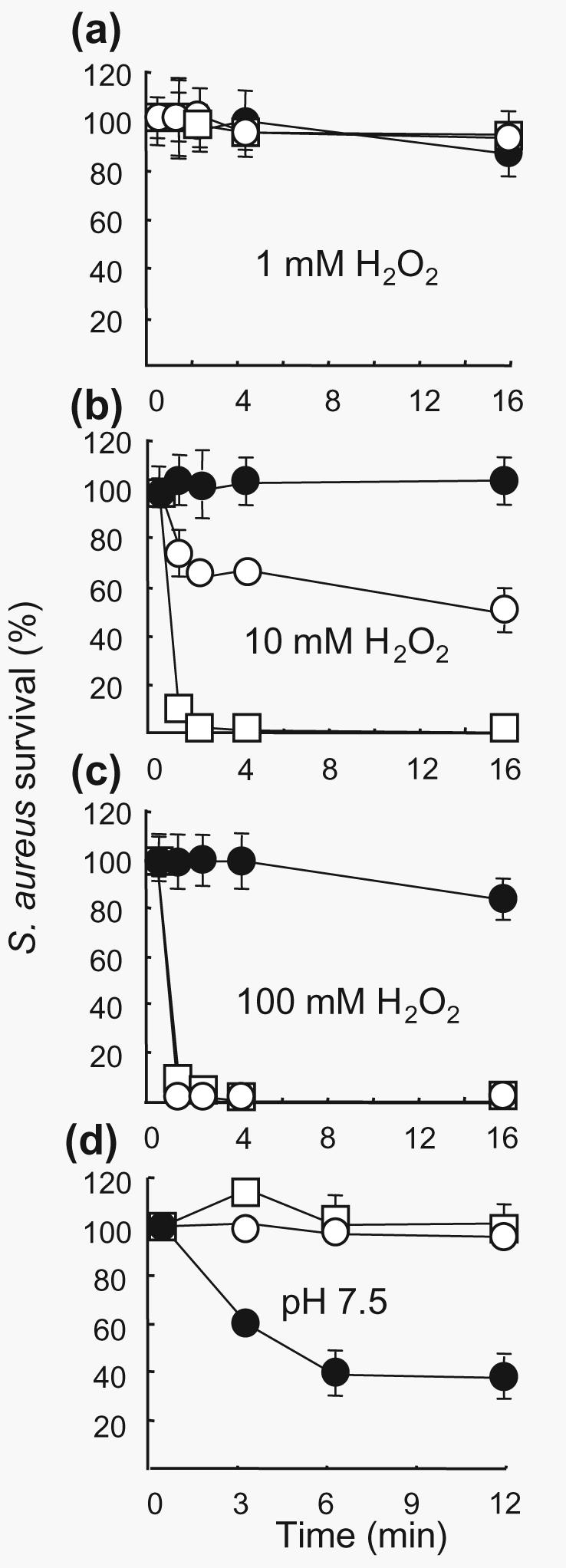
Bacterial killing by the MPO/H2O2/Cl− system. S. aureus (1 × 107 c.f.u. ml−1) in 0·01 M phosphate buffer at pH 7·5 (●), 6·5 (○) or 5·5 (□) was mixed with MPO (5 mg ml−1). H2O2 at a concentration of 1 (a), 10 (b) or 100 (c) mM was added. (d) MPO itself had no effect on bacterial viability (□), whilst the bactericidal effect of 100 mM H2O2 (●) was prevented in the presence of MPO (5 mg ml−1) (○) at pH 7·5. Each line is representative of the mean (±se) of three experiments.
Quantification of HOCl production by MPO under exactly the same experimental conditions showed the in vitro activity of MPO to be pH- and H2O2-dependent (Table 1), with HOCl production increasing with decreasing pH and increasing H2O2 concentration. Most of the HOCl was produced within the first few seconds after the addition of H2O2.
Table 1. Quantification of hypochlorite production.
Hypochlorite (mM) produced by purified MPO (5 mgml−1) in PBS at pH values of 5·5, 6·5 and 7·5 and in the presence of 1, 10 and 100 mM H2O2 at 37 °C. Measurements were made at 0·3, 2·0 and 16 min. Mean values (±se) of three independent experiments. nd, None detected.
| H2O2 (mM) | Minutes | pH 7·5 | pH 6·5 | pH 5·5 |
|---|---|---|---|---|
| 1 | 0·3 | 0·09 ± 0·16 | 0·35 ± 0·12 | 0·19 ± 0·11 |
| 2·0 | 0·02 ± 0·16 | 0·23 ± 0·12 | 0·24 ± 0·19 | |
| 16·0 | nd | 0·28 ± 0·12 | 0·22 ± 0·08 | |
| 10 | 0·3 | 1·14 ± 0·22 | 2·02 ± 0·79 | 4·84 ± 0·09 |
| 2·0 | 0·99 ± 0·10 | 1·86 ± 0·57 | 4·44 ± 0·09 | |
| 16·0 | 1·30 ± 0·05 | 1·57 ± 0·16 | 3·49 ± 0·09 | |
| 100 | 0·3 | 1·29 ± 0·40 | 14·80 ± 1·13 | 13·26 ± 1·58 |
| 2·0 | 0·18 ± 0·37 | 8·64 ± 0·59 | 7·61 ± 1·32 | |
| 16·0 | 0·82 ± 0·26 | 6·80 ± 0·55 | 4·16 ± 0·37 |
MPO inhibited killing of bacteria by high concentrations of H2O2 at physiological pH. A pH of 7·5 and 100 mM H2O2 resulted in a reduction of the bacterial colony count by 60% (Figs 2a and 4d). This was reduced to 10% or less in the presence of MPO (Fig. 4c, d). The lack of killing at pH 7·5 was coupled with low levels of HOCl production (Table 1). Maximally 1 mM HOCl was produced at pH 7·5, a concentration incapable of bacterial killing in the presence of granule protein (Fig. 3e–h).
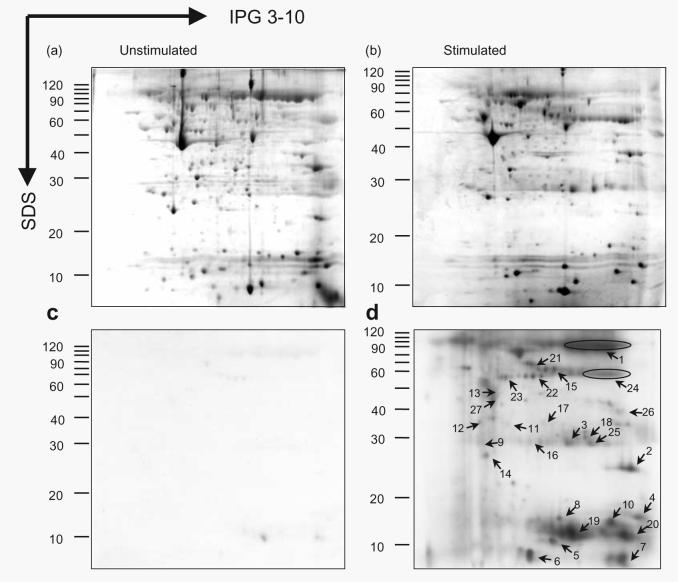
Identification of iodinated proteins
Proteins that were iodinated when neutrophils phagocytosed opsonized bacteria were cut from 2D PAGE gels, digested and identified by MALDI-TOF MS.
A variety of iodinated proteins were present in phagocytosing cells when compared with resting cells (Fig. 5). At least 40 spots, varying from very high molecular masses to about 8 kDa, became apparent (Fig. 5c, d). The identity of the iodinated spots is shown in Table 2. They mainly belonged to the contents of the azurophilic and specific granules (mainly lacoferrin, lysozyme, gelatinase-associated lipocalin and lysozyme). Other neutrophilic intracellular cytoskeletal proteins (profilin, annexin and actin) and plasma proteins including fibrinogen and fibrin were also identified. In contrast, the main bacterial-associated protein was the opsonizing IgG. Most of the iodinated proteins appear to be located within the phagocytic vacuole, or in the case of the cytoskeletal proteins, just surrounding it. Proteins like haemoglobin and fibrinogen would either be iodinated after their uptake with engulfed particles, or iodinated by MPO and H2O2 secreted into the extracellular space.
Fig. 5.
2D gel electrophoresis and autoradiographs of neutrophils before and after phagocytosis of S. aureus. Neutrophils (1 × 107) in 1 ml PBS (pH 7·3) containing 100 μCi (3700 kBq) 125I were mixed in a rapidly stirring oxygenated chamber at 37 °C without (a, c) or with (b, d) IgG opsonized S. aureus (1 × 108 c.f.u.). After 4 min the suspension was taken into 10% TCA. Coomassie blue 2D stained gels (a, b) and corresponding autoradiographs (c, d) (216 h exposure) are shown. Iodinated proteins (labelled 1–27) were excised from the SDS gel and identified.
Table 2. List of proteins that were iodinated following phagocytosis of S. aureus or E. coli by isolated neutrophils.
Iodinated proteins were trypsin-digested and mass-spectrometric peptide maps were acquired. Proteins were identified by comparing mass fingerprints to the NCBI database.
| Neutrophilic | Other human | Bacterial |
|---|---|---|
| Granule-associated | 14. GDP-dissociation inhibitor | S. aureus |
| 1. Lactoferrin | 15. Glucose-6P-dehydrogenase | 27. Ornithine transcarbamoylase |
| 2. Gelatinase-associated lipocalin | 16. Glutathione-S-transferase P | |
| 3. Cathepsin G | 17. Esterase D | E. coli * |
| 4. Lysozyme | 18. Phosphoglycerate mutase 1 | Outer-membrane protein A |
| 5, 6. Calgranulin A | 19. Haemoglobin beta | OMP-NMPC |
| 7. Elastase fragment | 20. Haemoglobin alpha 2 | OMP W precursor |
| 8. Myeloperoxidase | 21. Albumin | Superoxide dismutase |
| 22. Fibrin | Malate dehydrogenase | |
| Cytoskeletal | 23. Fibrinogen | Asparaginase |
| 9. Grancalcin | 24, 25. IgG | Hydroperoxide reductase |
| 10. Profilin | 26. Fructose-bisphosphate aldolase A | Fructose-bisphosphate aldolase |
| 11. Annexin III | Chaperonin CPN10 | |
| 12. Annexin V | ||
| 13. Actin |
These proteins are not shown on the gel.
Iodination of granular and cytosolic proteins, as well as extracellular human proteins, was much more obvious than that of bacterial proteins. Only if a great excess of bacteria (2·5 × 109 c.f.u. ml−1) was used was the iodination of outer-membrane proteins (OMP-A, OMP-NMPC) and other enzymes of E. coli observed (Table 2). At such high numbers of bacteria to neutrophils (100 : 1), frustrated phagocytosis takes place (Henson, 1971) with degranulation and H2O2 release to the outside of the cell, and under these conditions iodination of these organisms is probably occurring in the extracellular medium.
There is no doubt that the generation of ROS is essential for efficient killing of bacteria (Klebanoff, 1967a, 1968) and fungi (Lehrer, 1969) by neutrophils. The question is how these ROS accomplish this. The current view is that HOCl formed by oxidation of Cl− by H2O2 plays a primary role in this killing. However, initial experiments to demonstrate the toxicity of the MPO/H2O2/Cl− system were performed with very low concentrations of enzyme. MPO was used in the range of 50 μg (Thomas, 1979; Kettle & Winterbourn, 1988) rather than the 100-fold higher concentration present within the vacuole (Reeves et al., 2002). Most importantly, the pH was 5·0-5·5 (Klebanoff, 1967b, 1968, 1970) or less (Belding & Klebanoff, 1970), rather than the 7·6–8·0 that pertains in the vacuole (Segal et al., 1981; Cech & Lehrer, 1984; Jiang et al., 1997). In this study, 100 mM H2O2 and up to 5 μM HOCl demonstrated bactericidal activity, which decreased significantly with increasing pH, an effect related to the higher activity of HOCl than OCL− (Dychdala, 2001).
Markedly more pronounced than the influence of pH are physiological concentrations of granule proteins (which include about 20% MPO) or pure MPO. Oxidants like HOCl are known to react with thio groups, thioethers, and aliphatic or aromatic groups (Test et al., 1984). Most of these reactions lead to an immediate loss in oxidative capacity resulting in the loss of microbicidal properties. in vitro experiments employing a lower granule protein concentration (25 mg ml−1) than that present within the phagocytic vacuole strongly suggest that the enormous amount of protein will consume the available HOCl immediately in vivo. Thus estimates of approximately 28 μM HOCl production (Jiang et al., 1997) would be totally ineffective against bacteria within the confines of the vacuole.
Furthermore, the target proteins of iodination reactions are largely those of the engulfing neutrophil rather than the microbial prey. This was demonstrated by results of this, and a previous study (Segal et al., 1983). Regarding chlorination, Chapman et al. (2002) established that 94% of the total chlorinated tyrosine residues formed during phagocytosis were those of neutrophil proteins.
How do these new data fit in with the current dogma on the role of ROS in microbial killing? Confirmation of the involvement of MPO in the killing process was made through the use of MPO knockout mice (Aratani et al., 1999), in which killing of Candida albicans was defective. However, deficiency of MPO is a common condition in humans and does not lead to obvious susceptibility to bacterial infection (Forehand et al., 1995). Therefore an alternative system must dominate to compensate for this deficiency. A more recent study using elastase- and cathepsin-G-deficient mice showed that killing of C. albicans was grossly defective despite perfectly normal iodination (Reeves et al., 2002), implicating granule proteases and questioning the conventional theory of MPO action.
Doubt has also been cast on another aspect of oxidative killing. It was thought that patients with CGD were more susceptible to catalase-positive microbes because the catalase-negative organisms generated H2O2 as substrate for MPO-mediated halogenation (Mandell & Hook, 1969), thereby providing the substrate for their own destruction. However, catalase-deficient S. aureus (Messina et al., 2002) and Aspergillus nidulans (Chang et al., 1998) were shown to be at least as virulent as the catalase-positive variety in a mouse model of CGD.
An alternative role for MPO has been suggested in which it protects the microbicidal enzymes against oxidative damage (Reeves et al., 2002) by ROS. In addition to its peroxidase activity, MPO can also act as a catalase. This latter role may dominate under the alkaline conditions in the vacuole, in which the concentration of H2O2 is high and where the catalase activity of MPO can be constantly regenerated through the reduction by (Kettle & Winterbourn, 2001). This theory is supported by the observation that HOCl decreased markedly the activity of proteolytic enzymes (Schiller et al., 2000). Similarly, mechanisms of oxidant denaturation of degradative enzymes could explain the predisposition to atherosclerosis seen in MPO-deficient mice (Brennan et al., 2001). In conclusion, results obtained support the novel concept that the function of the neutrophil oxidative pathway is to provide optimal conditions for bacterial killing by proteases rather than their direct oxidative destruction.
ACKNOWLEDGEMENTS
We are grateful to The Wellcome Trust, the Chronic Granulomatous Disease Research Trust and the Austrian Science Fund (grant no. J-1845-MED) for providing financial support.
Abbreviations
- CGD
chronic granulomatous disease
superoxide
- MALDI-TOF
matrix-assisted laser desorption ionization time-of-flight
- MPO
myeloperoxidase
- ROS
reduced oxygen species.
REFERENCES
- Aratani Y, Koyama H, Nyui SI, Suzuki K, Kura F, Maeda N. Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect Immun. 1999;67:1828–1836. doi: 10.1128/iai.67.4.1828-1836.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior BM, Kipnes RS, Curnutte JT. Biological defence mechanisms: the production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973;52:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior BM, Curnutte JT, Kipnes RS. Biological defense mechanisms. Evidence for the participation of superoxide in bacterial killing by xanthine oxidase. J Lab Clin Med. 1974;85:235–244. [PubMed] [Google Scholar]
- Belding ME, Klebanoff SJ. Peroxidase-mediated virucidal systems. Science. 1970;167:195–196. doi: 10.1126/science.167.3915.195. [DOI] [PubMed] [Google Scholar]
- Brennan ML, Anderson MM, Shih DM. Increased atherosclerosis in myeloperoxidase-deficient mice. J Clin Invest. 2001;107:419–430. doi: 10.1172/JCI8797. 10 other authors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech P, Lehrer RI. Phagolysosomal pH of human neutrophils. Blood. 1984;63:88–95. [PubMed] [Google Scholar]
- Chang Y, Segal B, Holland S, Miller G, Kwon-Chung K. Virulence of catalase-deficient Aspergillus nidulans in p47phox−/−mice. Implications for fungal pathogenicity and host defence in chronic granulomatous disease. J Clin Invest. 1998;101:1843–1850. doi: 10.1172/JCI2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman AL, Hampton MB, Senthilmohan R, Winterbourn CC, Kettle AJ. Chlorination of bacterial and neutrophil proteins during phagocytosis and killing of Staphylococcus aureus. J Biol Chem. 2002;277:9757–9762. doi: 10.1074/jbc.M106134200. [DOI] [PubMed] [Google Scholar]
- Dychdala GR. Chlorine and chlorine compounds. In: Block SS, editor. Disinfection, Sterilization and Preservation. 5th edn Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 135–158. [Google Scholar]
- Forehand JR, Nauseef WM, Curnutte JT, Johnston RB. Inherited disorders of phagocyte killing. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 7th edn McGraw-Hill; New York: 1995. pp. 3995–4026. [Google Scholar]
- Henson PM. Interaction of cells with immune complexes: adherence, release of constituents and tissue injury. J Exp Med. 1971;134:114–118. [PMC free article] [PubMed] [Google Scholar]
- Hurst JK, Barrette WC., Jr Leukocytic oxygen activation and microbicidal oxidative toxins. Crit Rev Biochem Mol Biol. 1989;24:271–328. doi: 10.3109/10409238909082555. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Griffin D, Barofsky D, Hurst J. Intraphagosomal chlorination dynamics and yields determined using unique fluorescent bacterial mimics. Chem Res Toxicol. 1997;10:1080–1089. doi: 10.1021/tx9700984. [DOI] [PubMed] [Google Scholar]
- Kettle AJ, Winterbourn CC. Superoxide modulates the activity of myeloperoxidase and optimizes the production of hypochlorous acid. Biochem J. 1988;252:529–536. doi: 10.1042/bj2520529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettle AJ, Winterbourn CC. A kinetic analysis of the catalase activity of myeloperoxidase. Biochemistry. 2001;40:10204–10212. doi: 10.1021/bi010940b. [DOI] [PubMed] [Google Scholar]
- Klebanoff SJ. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967a;126:1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff SJ. A peroxidase-mediated antimicrobial system in leukocytes. J Clin Invest. 1967b;126:1063–1078. [Google Scholar]
- Klebanoff SJ. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968;95:2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff SJ. Myeloperoxidase: contribution to the microbicidal activity of intact leukocytes. Science. 1970;169:1095–1097. doi: 10.1126/science.169.3950.1095. [DOI] [PubMed] [Google Scholar]
- Klebanoff SJ. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975;12:117–142. [PubMed] [Google Scholar]
- Klebanoff SJ, Clark RA. Iodination by human polymorphonuclear leukocytes: a re-evaluation. J Lab Clin Med. 1976;89:675–686. [PubMed] [Google Scholar]
- Lehrer RI. Antifungal effects of peroxidase systems. J Bacteriol. 1969;99:361–365. doi: 10.1128/jb.99.2.361-365.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell GL, Hook EW. Leukocyte bactericidal activity in chronic granulomatous disease: correlation of bacterial hydrogen peroxide production and susceptibility to intracellular killing. J Bacteriol. 1969;100:531–532. doi: 10.1128/jb.100.1.531-532.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina CGM, Reeves EP, Roes J, Segal AW. Catalase negative Staphylococcus aureus retain virulence in mouse model of chronic granulomatous disease. FEBS Lett. 2002;518:107–110. doi: 10.1016/s0014-5793(02)02658-3. [DOI] [PubMed] [Google Scholar]
- Nagl M, Hess M, Pfaller K, Hengster P, Gottardi W. Bactericidal activity of micromolar N-chlorotaurine: evidence for its antimicrobial function in the human defence system. Antimicrob Agents Chemother. 2000;44:2507–2513. doi: 10.1128/aac.44.9.2507-2513.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R, Little C. Purification and some of the properties of myeloperoxidase and eosinophil peroxidase from human blood. Biochem J. 1982;209:781–787. doi: 10.1042/bj2090781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves EP, Lu H, Hugues Lortat J. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–296. doi: 10.1038/416291a. 7 other authors. [DOI] [PubMed] [Google Scholar]
- Root RK, Metcalf J, Oshino N, Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation and some regulating factors. J Clin Invest. 1975;55:945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H, Klebanoff SJ. Bactericidal activity of a superoxide anion-generating system. A model for the polymorphonuclear leukocyte. J Exp Med. 1979;149:27–32. doi: 10.1084/jem.149.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld J, Capdevielle J, Guillemot JC, Ferrara P. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal Biochem. 1992;203:173–179. doi: 10.1016/0003-2697(92)90061-b. [DOI] [PubMed] [Google Scholar]
- Schiller J, Benard S, Reichl S, Arnhold J, Arnold K. Cartilage degradation by stimulated human neutrophils: reactive oxygen species decrease markedly the activity of proteolytic enzymes. Chem Biol. 2000;7:557–568. doi: 10.1016/s1074-5521(00)00013-2. [DOI] [PubMed] [Google Scholar]
- Segal AW, Jones OT. Rapid incorporation of the human neutrophil plasma membrane cytochrome b into phagocytic vacuoles. Biochem Biophys Res Commun. 1980;92:710–715. doi: 10.1016/0006-291x(80)90391-5. [DOI] [PubMed] [Google Scholar]
- Segal AW, Geisow M, Garcia R, Harper A, Miller R. The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH. Nature. 1981;290:406–409. doi: 10.1038/290406a0. [DOI] [PubMed] [Google Scholar]
- Segal AW, Garcia RC, Harper AM, Banga JP. Iodination by stimulated human neutrophils. Studies on its stoichiometry, subcellular localization and relevance to microbial killing. Biochem J. 1983;210:215–225. doi: 10.1042/bj2100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Test ST, Lampert MB, Ossanna PJ, Thoene JG, Weiss SJ. Generation of nitrogen-chlorine oxidants by human phagocytes. J Clin Invest. 1984;74:1341–1349. doi: 10.1172/JCI111544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EL. Myeloperoxidase, hydrogen peroxide, chloride antimicrobial system: nitrogen-chlorine derivatives of bacterial components in bacterial action against Escherichia coli. Infect Immun. 1979;23:522–531. doi: 10.1128/iai.23.2.522-531.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrasher AJ, Keep NH, Wientjes FB, Segal AW. Chronic granulomatous disease. Biochim Biophys Acta. 1994;1227:1–24. doi: 10.1016/0925-4439(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Vita F, Borelli V, Soranzo MR, Magnarin M, Bertoncin P, Zabucchi G. Preparation of membrane fractions from human neutrophil granules: a simple method. Methods Cell Sci. 1997;19:197–205. [Google Scholar]