Abstract
Background
Live attenuated influenza H5N1 vaccines have been produced and evaluated in mice and ferrets that were never exposed to influenza A virus infection (Suguitan et al., Plos Medicine, e360:1541, 2006). However, the preexisting influenza heterosubtypic immunity on live attenuated H5N1 vaccine induced immune response has not been evaluated.
Methodology and Principal Findings
Primary and recall B cell responses to live attenuated H5N1 vaccine viruses were examined using a sensitive antigen-specific B cell ELISpot assay to investigate the effect of preexisting heterosubtypic influenza immunity on the development of H5N1-specific B cell immune responses in ferrets. Live attenuated H5N1 A/Hong Kong/213/03 and A/Vietnam/1203/04 vaccine viruses induced measurable H5-specific IgM and IgG secreting B cells after intranasal vaccination. However, H5-specific IgG secreting cells were detected significantly earlier and at a greater frequency after H5N1 inoculation in ferrets previously primed with trivalent live attenuated influenza (H1N1, H3N2 and B) vaccine. Priming studies further revealed that the more rapid B cell responses to H5 resulted from cross-reactive B cell immunity to the hemagglutinin H1 protein. Moreover, vaccination with the H1N1 vaccine virus was able to induce protective responses capable of limiting replication of the H5N1 vaccine virus to a level comparable with prior vaccination with the H5N1 vaccine virus without affecting H5N1 vaccine virus induced antibody response.
Conclusion
The findings indicate that previous vaccination with seasonal influenza vaccine may accelerate onset of immunity by an H5N1 ca vaccine and the heterosubtypic immunity may be beneficial for pandemic preparedness.
Introduction
Influenza pandemics can occur when new influenza subtypes capable of both infecting and spreading easily among humans emerge with a new hemagglutinin (HA) subtype (antigenic shift) to which there is little or no population immunity. During the last century, three novel influenza A hemagglutinin subtypes (H1, H2 and H3) have appeared; an H1N1 strain caused the catastrophic “Spanish flu” pandemic in 1918 [1] followed by milder pandemics in 1957 and 1968 caused by H2N2 and H3N2 strains, respectively. Importantly, the origin of the pandemic H2N2 and H3N2 viruses has since been attributed to genetic reassortment events where circulating human influenza viruses acquired novel HA subtypes from avian influenza viruses [2], [3]. Alarmingly, in the past decade, a number of avian influenza viruses containing HA subtypes not typically found in humans have crossed species barrier and infected humans, raising concerns about a future pandemic. Highly pathogenic avian H5N1 influenza viruses have infected only a small number of individuals but are associated with a high mortality rate and are perceived as a potential major global health threat.
Several strategies have been used to develop vaccines against H5N1 viruses including inactivated whole virus vaccines, split or subunit vaccines, live attenuated influenza vaccine (LAIV), vectored vaccines, and DNA vaccines; many of these candidates have shown promise in preclinical studies [4]. Seasonal LAIV has demonstrated several attributes that would be important for an effective pandemic vaccine including efficacy, an ability to protect against antigenically drifted strains, an ability to elicit a rapid immune response in an immunologically naïve population, and a highly efficient production system for the vaccine [5], [6], [7], [8]. Several prototypic pandemic LAIV (pLAIV) 6∶2 reassortant viruses containing the H5N1 HA and NA gene segments have been produced on the backbone of six internal gene segments from the cold-adapted (ca) A/Ann Arbor/6/60 vaccine strain [9], the master donor virus (MDV-A) used to produce influenza A vaccine strains for the seasonal FluMist® influenza vaccines (MedImmune). These candidate H5N1 vaccine strains, A/HK/491/97 (HK97 ca), A/HK/213/03 (HK03 ca), and A/VN/1203/04 (VN04 ca), were found to provide complete protection against lethal challenge with homologous and heterologous wild-type (wt) H5N1 viruses in mice and offered complete protection against pulmonary replication of wt H5N1 virus in ferrets [7].
It has been observed that individuals who have recovered from influenza infections develop broad subtype-specific immunity that can protect them from subsequent infection by closely related drift variants of the same subtype [10], [11], [12]. Although not nearly as common, Schulman and Kilbourne [13] reported heterosubtypic immunity in mice, where protection was induced by an influenza virus belonging to a different subtype. Recently, there have been several reports describing heterosubtypic immunity against H5N1 infection induced by influenza virus infection or vaccines in mice [8], [14], [15], [16]. The mechanistic basis of this type of immunity remains undetermined, however, one study demonstrated a role of the N1 component of the vaccine [17] and other studies suggest that structural similarity of the H5 and H1 HA may mediate this type of protection [18], [19]. Kreijtz et al. [20] reported cross-recognition of avian H5N1 influenza virus by human cytotoxic T-lymphocyte populations directed against human influenza viruses and suggested that the preexisting cross-reactive T-cell immunity in humans may dampen the impact of a next pandemic if it is caused by an H5N1 virus. The ferret is considered to be a suitable mammalian host for seasonal influenza vaccine research [21], [22] and for efficacy studies of HPAI H5N1 vaccines [7], [23], [24]. Although ferrets immunized with a H1N1 ca strain were not protected from replication of a wild-type H5N1 virus [7], however, because LAIV has been shown to provide protection from strains that are antigenically different from the vaccine antigen, we investigated whether priming with a heterologous seasonal LAIV vaccine containing different subtypes could influence the immune response to H5N1 viruses in the ferret model. Such studies will also help us to understand whether live attenuated H5N1 vaccine could induce effective immune response in individuals that have immunity to seasonal influenza viruses.
HAI and microneutralization assays are frequently used to measure humoral antibody responses, however, these assays may not be sensitive enough to detect early and local antibody responses. To assess the presence and magnitude of heterosubtypic immunity following immunization with LAIV, a sensitive B cell ELISpot assay was developed that could detect early induction of immunity at a time when the HAI assay was less sensitive. Using this assay, we show that local B cell responses induced by the H5N1 VN04 ca and HK03 ca vaccine viruses can be detected at a virus-specific and HA-specific level. Previous infection with an H1N1 virus induced a faster and higher level B cell response to H5N1 vaccination and could prevent shedding of the H5N1 vaccine virus. The data implies that priming with a non-H5 vaccine may enable a more rapid memory response to an H5 vaccine, however, whether this would be beneficial to the effectiveness of an H5 vaccine remains to be determined.
Materials and Methods
Viruses
Influenza virus vaccine strains H1N1 A/New Caledonia/20/99 ca (NC99 ca), H3N2 A/Wyoming/03/03 ca (WY03 ca), H3N2 A/California/7/04 ca (CA04 ca), H5N1 A/Hong Kong/213/03 ca (HK03 ca), H5N1 A/Vietnam/1203/04 ca (VN04 ca), H2N2 A/AA/6/60 ca, (AA60 ca or MDV-A) [22] and H1N2 reassortant ca virus containing the H1 HA from A/New Caledonia/20/99 and the N2 NA from A/Wyoming/03/03 were generated by reverse genetics. All viruses were expanded at 33°C for 3 days in the allantoic cavity of 10-day-old embryonated SPAFAS hen's eggs (Norwich, CT). Allantoic fluids collected from infected eggs were examined by hemagglutination assay using 0.5% turkey (tRBC) or horse (hRBC) erythrocytes to determine HA titer. Infectious virus titer was determined by plaque assay (plaque forming unit, PFU) or 50% tissue culture infectious dose (TCID50) using Madin-Darby canine kidney (MDCK) cells. Viruses were inactivated by treatment with β-propriolactone (BPL) for use as antigen in ELISpot and ELISA assays. Trivalent LAIV (FluMist®) was manufactured by MedImmune and contained 107 TCID50 each of 6∶2 reassortant vaccine strains, A/New Caledonia/20/99 ca, A/California/7/04 ca, and B/Jilin/20/03 ca.
Animal studies
Ferrets between 7 and 10 weeks of age from Triple F Farms (Sayre, PA) were screened prior to use in experiments for preexisting antibodies to H1N1, H3N2 and H5N1 influenza viruses by hemagglutination inhibition (HAI) assay. Sero-negative ferrets were inoculated intranasally on day 0 with a predetermined does of a monovalent vaccine virus (105 to 107 PFU or TCID50 virus), trivalent LAIV, or medium (mock control). To examine the B cell response and virus replication after primary infection, animals were sacrificed 5 or 10 days post-inoculation to collect paratracheal lymph nodes (TLN) and whole blood for B cell ELIspot assays; serum was collected to measure serum antibody level and nasal turbinates were harvested to examine virus replication in the upper respiratory tract. To examine the effect of previous influenza virus infection on the induction of H5N1-specific immune responses, ferrets were given a second intranasal inoculation of 107 or 108 PFU of homologous or heterologous vaccine virus 4–6 weeks after the initial vaccination. Animals in these latter groups were sacrificed 3–10 days later to collect TLN, blood, serum, and nasal turbinate samples or were used to collect serum samples for up to three weeks after the second vaccination. All animal study protocols were approved by MedImmune's Institutional Animal Care and Use Committee and performed in an AAALAC certified facility.
Measurement of virus titers in animal tissues
Nasal turbinate tissues were homogenized in MEM medium and centrifuged at 400 g for 10 min. Serial 10-fold dilutions of supernatants collected from each preparation were inoculated into three 10- to 11-day old embryonated SPAFAS hen's eggs. After incubation at 33°C for 72 hr, allantoic fluid from each egg was collected for HA assay using 0.5% tRBC. Virus titers in the tissues are reported as a 50% egg infectious dose (EID50) per gram of tissue processed.
B cell ELISpot assay
AcroWell™ 96-well PVDF filter plates (Pall Life Sciences, Ann Arbor, MI) were coated with 50 µL/well PBS containing either 2,000 HA unit/mL of BPL-treated vaccine virus or 10 µg/mL recombinant HA protein derived from H5N1 A/VN/1203/2004 (rH5), H1N1 A/New Caledonia/20/99 (rH1), or H3N2 A/Wyoming/03/03 (rH3) that were purified from recombinant baculovirus infected insect cells (Protein Sciences, Meriden, CT). After overnight incubation at 4°C, plates were washed 3 times with PBS and blocked with RPMI-1640 medium containing 10% FBS for 2 hr at 37°C prior to the addition of cell samples.
Whole blood samples from ferrets were collected in EDTA tubes and processed using Lympholyte®-Mammal (Cedarlane, Ontario, Canada) to isolate peripheral blood mononuclear cells (PBMC). PBMC were washed once with RPMI-1640/10% FBS by centrifugation (300 g for 10 min), counted, and resuspended in complete medium (RPMI-1640, 10% FBS, 2 mM L-glutamine, 0.5 nM ß-mercaptoethanol and penicillin/streptomycin). TLN were harvested from each ferret and placed in cold PBS/5% FBS and the cells were released from TLN into the media by gently rubbing partially minced tissue against a sterile mesh screen with a glass pestle. The resultant cell suspension was collected, passed through a cell strainer to remove large debris, and pelleted by centrifugation (300 g for 10 min). Cell pellets were washed once, counted, and resuspended in the RPMI-1640 complete medium. PBMC and TLN cell suspensions were added to triplicate wells (100 µL/well) at a concentration of 3×106/mL for PBMC or 105 to 106/mL for TLN samples and incubated at 37°C, 5% CO2 for 5 hr. The plates were washed 5 times with PBS containing 0.05% Tween-20 (PBS-T) to remove the cells from the plate. To measure isotype-specific B cell responses, goat anti-ferret IgM (Rockland, Gilbertville, PA) or goat anti-ferret IgG (Bethyl Laboratories, Montgomery, TX) diluted 1∶1000 in PBS-T/1% BSA and incubated overnight at 4°C. After 5 washes with PBS-T, HRP-conjugated rabbit anti-goat Ig (Dako, Carpinteria, CA) diluted 1∶2000 in PBS-T/BSA was added to all wells and incubated at 37°C for 1 hr. Plates were washed 3 times with PBS-T and 3 times with PBS before development with AEC substrate (Vector Labs, Burlingame, CA) for 10 min at room temperature (RT). Wells were rinsed extensively with water and allowed to dry completely before spots in each well were counted using an ImmunoSpot plate reader (Cellular Technologies, Ltd., Cleveland, OH).
HAI and microneutralization assays
Prior to serologic analysis, ferret sera were treated with receptor-destroying enzyme (RDE) (Denka Seiken, Tokyo, Japan) that was reconstituted with 10 mL of 0.9% NaCl per vial. 0.1 mL serum was mixed with 0.15 mL RDE and incubated at 37°C for 18 hr and adjusted to a final 1∶4 dilution by adding 0.15 mL of 0.9% sodium citrate followed by incubation at 56°C for 45 min. Strain-specific serum HAI titers were determined using 0.5% tRBC or hRBC and the HAI titers are presented as the reciprocal value of the highest serum dilution that did not inhibit hemagglutination. Serum neutralizing antibody titers were determined by microneutralization assay using MDCK cells. RDE-treated ferret serum was 2-fold serially diluted, incubated with 100 TCID50 virus at 33°C for 1 hr and transferred onto MDCK cell monolayers in 96-well culture plates (Costar, Corning, NY). After 6 days' incubation at 33°C, the cell monolayers were fixed with 10% formaldehyde, incubated with chicken MDV-A polyclonal antibody followed by incubation with an HRP-conjugated rabbit anti-chicken IgG (Thermo, Rockford, IL), and developed with TMB substrate (Sigma, St. Louis, MO). The reaction was stopped with an equal volume of 0.1 N HCl and the absorbance at 450 nm was determined using a SpectraMax plate reader (Molecular Devices, Sunnyvale, CA). Neutralizing antibody titers were calculated as the highest serum dilution with a value less than that calculated by the formula of (average OD of virus-infected wells - average OD of cell control wells)/2+average OD of cell control wells.
ELISA analysis of HA-specific antibody responses
96-well EIA plates (Costar, Corning, NY) were coated with 0.025 µg/well of rH1, rH3 or rH5 in PBS overnight at 4°C. Plates were washed 3 times with PBS-T and blocked with SuperBlock Blocking Buffer (Pierce, Rockford, IL) for 1 hr at 37°C. RDE-treated ferret sera were 2-fold serially diluted with PBS-T, transferred to 96-well plates (50 µL/well), and incubated for 1 hr at 37°C. Plates were washed with PBS-T and incubated for 30 min at 37°C with 100 µL/well HRP-conjugated goat anti-ferret IgG (Bethyl Laboratories, Montgomery, TX) diluted 1∶10,000 in PBS-T/1% BSA. After washing with PBS-T, plates were developed with TMB substrate and read as described above in the microneutralization assay. Antibody titers are expressed as the highest dilution with an optical density (OD) reading greater than 2 times the mean OD+standard deviation of similarly diluted negative control samples.
Results
Detection of H5N1-specific B cell responses after immunization with pLAIV using a sensitive B cell ELIspot assay
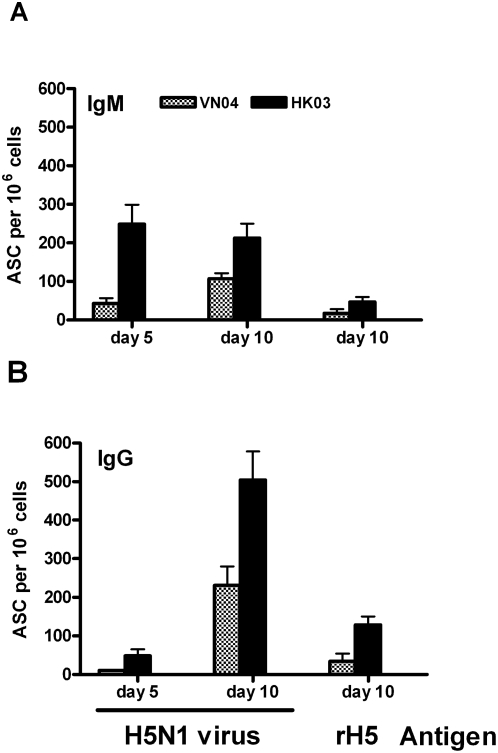
Pandemic live attenuated influenza vaccines (pLAIV) developed for H5N1 viruses confer protection against wild-type virus challenge in mice and ferrets [7], however, the level of the serum HAI antibody responses induced by the VN04 vaccine was low. We sought to implement a more sensitive ELISpot assay to measure whole virus- and HA-specific antibody secreting cells (ASC) in draining paratracheal lymph node (TLN) and PBMC from vaccinated ferrets. The ELISpot assay has been shown to be a sensitive tool for detecting cellular immunity following influenza vaccination in humans [25], [26], [27]. To test the sensitivity of this ELISpot assay, ferrets were inoculated intranasally with either the H5N1 VN04 ca or H5N1 HK03 ca vaccine virus and were sacrificed 5 or 10 days later to isolate TLN cells and PBMC. The ELISpot data obtained from TLN samples (Fig. 1) showed that H5N1 virus-specific ASC could be detected after vaccination with either of the H5N1 ca vaccine viruses and similar data were obtained from PBMC (data not shown). However, the magnitude and kinetics of IgM and IgG ASC induced by the VN04 ca vaccine appeared to be lower and slower than the HK03 vaccine virus on both days 5 and 10 post-vaccination (Fig 1). The virus-specific IgM ASC response was higher on day 5 than day 10 for HK03 ca virus immunized animals, but higher on day 10 than day 5 for VN04 ca virus immunized animals, indicating that the initial IgM response induced by the VN04 ca virus was slower and weaker than that induced by the HK03 ca virus. The IgG secreting ASC response was much higher on day 10 than day 5 for both HK03 ca and VN04 ca vaccine immunized animals. Again, HK03 ca vaccine induced more IgG ASC than VN04 ca vaccine (p<0.05). H5 HA-specific IgM and IgG ASC were also detected on day 10 for both viruses. However, the level of HA-specific ASC was only about 15% of the total virus-specific ASC, indicating a majority of the ASC induced by H5N1 ca virus vaccine were against antigens other than HA. The level of the H5 HA-specific ASC on day 5 was lower than on day 10 (data not shown). Thus, consistent with the HAI data, the ELISpot data also showed that the HK03 ca vaccine induced a better immune response than the VN04 ca vaccine.
Figure 1. H5N1-specific B cells were detected in ferrets infected with live attenuated H5N1 ca vaccines.
Ferrets were intranasally administered the H5N1 VN04 ca or HK03 ca viruses on day 0. Five and ten days post-inoculation, ferrets were sacrificed to collect paratracheal lymph nodes (TLN) and a B cell ELISpot assay was performed using lymphocytes isolated from TLN and BPL-inactivated H5N1 HK03 ca virus or rH5 HA antigens. The number of IgM ASC (A) and IgG ASC (B) are presented as per 106 lymphocytes.
LAIV-vaccinated animals show a more rapid immune responses to the H5N1 ca vaccine than unvaccinated animals
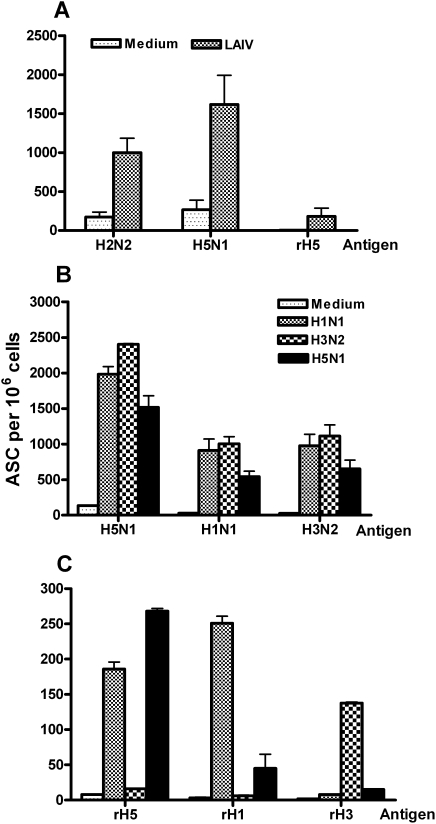
Most people have some immunity to influenza H1N1, H3N2, and B viruses due to previous natural infections or immunization with influenza vaccines. To mimic this sero-positive status and to determine whether preexisting heterosubtypic immunity affects responses to subsequent vaccination with H5N1ca vaccine viruses, ferrets were primed intranasally with live attenuated trivalent seasonal influenza vaccines (LAIV) or medium control prior to vaccination with the H5N1 HK03 or VN04 ca viruses. Five days after vaccination with H5N1 ca vaccine, TLN and PBMC samples were collected for analysis by B cell ELISpot assay to measure virus- and HA-specific IgG ASC. Responses detected using whole virus reagents (Fig. 2A) revealed that the number of H2N2 (AA60, MDV-A) and H5N1 (HK03) virus-specific IgG ASC were significantly higher in ferrets primed with LAIV (Fig. 2A). This was not completely unexpected because both the H1N1 and H3N2 viruses in LAIV share the same internal viral proteins such as the M1 and NP that likely stimulate rapid B cell responses. However, LAIV priming also significantly enhanced H5 HA-specific IgG ASC responses, which were not detected in the unprimed ferrets (Fig. 2A). This finding suggested that the enhanced H5 HA-specific responses could occur as a result of expansion of memory B cells that were elicited against the H1N1 and/or H3N2 influenza A virus components in the LAIV vaccine.
Figure 2. Previous exposure with seasonal LAIV or H1N1 ca virus induced a faster H5N1-specific immune response.
(A) Ferrets were intranasally inoculated with either medium or trivalent LAIV on day 0. Six weeks later, ferrets were intranasally inoculated with the H5N1 HK03 ca vaccine and five days later, lymphocytes isolated from TLN were examined for ASC against H2N2 MDV-A, H5N1 HK03 ca viruses and rH5 HA antigens by B cell ELISpot analysis. Ferrets were intranasally inoculated with medium or the monovalent H1N1 NC99, H3N2 CA04, H5N1 HK03 ca vaccine viruses on day 0 and intranasally inoculated with the H5N1 HK03 ca vaccine six weeks later. The B cell ELISpot analysis was performed with lymphocytes isolated from TLN using the indicated ca vaccine virus (B) or rHA (C) as antigens. The IgG antibody secreting B cells are presented as the number of ASC per 106 lymphocytes.
H1N1 vaccine primes faster B cell responses to the H5N1 vaccine than the H3N2 vaccinated animals
To determine whether the higher numbers of H5-specific B cell responses observed after trivalent LAIV priming were due to only one or both of the influenza A virus components, groups of ferrets were primed with monovalent H1N1 NC99 ca, H3N2 CA04 ca, H5N1 HK03 ca vaccine or medium six weeks prior to a second inoculation with the H5N1 HK03 ca vaccine. Five days after vaccination with the H5N1 HK03 ca vaccine (Fig. 2B), the level of virus-specific IgG ASC was very low in the ferrets that initially received medium, similar to that observed in the first study. In contrast, ferrets that were primed with the H1N1 NC99, H3N2 CA04 or H5N1 HK03 ca vaccine viruses, had significantly higher numbers of H5N1 HK03 ca virus-specific IgG ASC after vaccination with the H5N1 HK03 ca vaccine. The ferrets that were previously vaccinated with the H1N1, H3N2 and H5N1 ca vaccine viruses also had B cell response to the H1N1 and H3N2 vaccine virus (Fig. 2B). The number of the H5 HA-specific IgG ASC was the highest (approximately 10% of total virus-specific ASC) in the group that received 2 doses of HK03 ca virus (Fig. 2C). Interestingly, a significant number of H5 HA-specific IgG ASC (approximately 180 ASC per 106 cells) was also observed in the group that previously received the H1N1 NC99 ca vaccine virus. A much lower number of ASC was found in the group that received the H3N2 CA04 ca vaccine virus. As expected, a significant number of H1 and H3 HA-specific IgG ASC (approximately 250 and 140 per 106 cells, respectively) were detected in the groups that were primed with the H1N1 NC99 and H3N2 CA04 ca vaccine viruses, respectively.
H1N1 ca vaccine-induced faster B cell responses to the H5N1 vaccine virus is due to the H1 HA
The neuraminidases of the H5N1 and H1N1 viruses are of the same (N1) subtype. Several studies have reported a role of the N1 NA-mediated immunity against H5N1 infection in the murine model and in serology analysis of human serum samples [14], [17], [28]. Because it was shown earlier that previous exposure to H3N2 virus did not affect H5-specific ASC responses and the H1 HA and H5 HA share some structural similarity [18] and common epitopes [19], the effect of the H1N1-mediated priming on the H5N1 ca vaccines was investigated. To confirm that the enhanced B cell response to the H5N1 virus was due to a primary immune response to the H1 HA not the N1 NA, an H1N2 virus containing the HA from the H1N1 NC99 ca virus, the NA from the H3N2 WY03 ca virus and the internal protein gene segments from MDV-A was generated. Priming with this H1N2 virus in ferrets elicited a similar level of B cell response (122 ASC per million cells) as those (120 ASC per million cells) primed with the H5N1 HK03 vaccine virus (Fig. 3), confirming that the enhanced H5N1 B cell response was due to the H1 HA and not the N1 NA. The number of the H5 HA-specific IgG ASC in the H1N2 virus primed ferrets was higher than those primed with the H1N1 ca vaccine virus, which was possibly due to the better replication of the H1N2 ca virus than the H1N1 ca virus in the upper respiratory tract of ferrets (data not shown).
Figure 3. The priming effect of the H1N1 ca vaccine is induced by the H1 HA.
Ferrets were intranasally inoculated with the H1N1 NC99 ca virus, a reassortant H1N2 ca, H3N2 CA04 ca or H5N1 HK03 ca virus on day 0 and intranasally inoculated with the H5N1 HK03 ca virus five weeks later. The animals were sacrificed 5 days later and B cell ELISpot analysis was performed with lymphocytes isolated from TLN using rH5 HA as antigen. IgG antibody secreting B cells are presented as the number of ASC per 106 lymphocytes.
H5-, H1- and H3-specific serum antibody responses following prime-boost vaccination in ferrets
As described earlier, previous exposure to a virus containing the H1 HA resulted in an increased H5-specific B cell response. To examine if the increased ASC response reflected a greater antibody response, serum antibody titers were examined by HAI, microneutralization and ELISA assays. Groups of ferrets were immunized with medium, the H1N1 NC99 ca, H3N2 CA04 ca, H5N1 HK03 ca or VN04 ca virus, and 6 weeks later, a second dose of the H5N1 HK03 ca or VN04 ca vaccine was administered intranasally. Serum samples were collected 6 weeks after the 1st vaccination (post dose 1) and 1 and 3 weeks after the 2nd vaccination (Table 1). At 6 weeks after the first dose neutralizing antibodies against homologous vaccine virus were detected in the ferrets that received vaccine virus but not in the control group that received medium only. The H3N2 CA04 ca vaccine induced the highest mean neutralizing antibody (titer of 4064) and the H1N1 NC99 ca vaccine induced a mean neutralizing antibody titer of 50. Neither H1N1 nor H3N2 ca viruses induced antibodies that cross-neutralized the H5N1 HK03 ca virus. The H5N1 HK03 ca vaccine induced the H5-specific neutralizing antibody at a mean titer of 320 (Table 1). One week after administration of the H5N1 HK03 ca vaccine, the ferrets that received two doses of the HK03 ca vaccine had levels of H5-specific neutralizing antibody that were significantly higher than in animals that received a single dose of the H5N1 HK03 ca virus. In contrast to the B cell ELISpots results, animals primed with a dose of H1N1 ca and boosted with a second dose of H5N1 ca for one week had low titers of antibodies against the H5 antigen (Table 1). Previous vaccination with the H3N2 CA04 ca vaccine had little effect on the antibody response to the H5N1 ca vaccine. When measured at three weeks after vaccination with the H5N1 HK03 ca virus, the level of the H5-specific antibody in the animals that received a dose of medium, the H1N1 NC99 or H3N2 CA04 ca vaccines as the first dose was much lower (3- to 5-fold) than the animals that received two doses of the H5N1 HK03 ca vaccine virus.
Table 1. Serum antibody response to influenza viruses after one and two doses of intranasal vaccine.
| 1st dose vaccine (Day 0) | 2nd dose vaccine (Day 42) | Neutralizing antibody GMT against the indicated vaccine virus antigens | |||
| 6 wk post dose-1 | 1 wk post dose-2 | 3 wk post dose-2 | |||
| 1st Vac | H5N1 | H5N1 | H5N1 | ||
| Medium | H5N1 HK03 ca | <10 | <10 | 40 | 761 |
| H1N1 NC99 ca | 50 | <10 | 50 | 403 | |
| H3N2 CA04 ca | 4064 | <10 | 18 | 419 | |
| H5N1 HK03 ca | 320 | 320 | 2560 | 2281 | |
| Medium | H5N1 VN04 ca | <10 | <10 | 14 | 71 |
| H1N1 NC99 ca | 63 | <10 | 50 | 71 | |
| H5N1 VN04 ca | 45 | 45 | 806 | 403 | |
Groups of three ferrets were vaccinated intranasally with the indicated 1st dose of vaccine and 42 days later were inoculated with a 2nd dose of vaccine (H5N1 HK03 ca or VN04 ca). Serum samples were collected 6 weeks after the 1st dose (pre-dose 2), 1 week and 3 weeks after the 2nd dose, respectively, and antibody titers (geometric mean titers from 3 animals) against the first or second vaccine viruses were determined by microneutralization assay.
The effect of H1N1 ca vaccination on the H5N1 VN04 ca vaccine induced antibody response was also evaluated (Table 1). As expected, H1N1-specific antibody did not cross-react with the H5N1 VN04 ca virus. One week after the second vaccination with the H5N1 VN04 ca virus, H5N1-specific neutralizing (titer of 50) antibodies were detected in the ferrets that previously received the H1N1 NC99 ca vaccine, and this titer was significantly higher than the ferrets that received medium only (titer of 14, p<0.005) but much lower than the animals that received two doses of the H5N1 VN04 ca vaccine (titer of 806). However, three weeks after vaccination with the H5N1 VN04 ca vaccine there was no difference in antibody levels between the animals that received the first dose of medium or the H1N1 NC99 ca vaccine. Again, animals that received two doses of the H5N1 HK03 ca vaccine had neutralizing antibodies more than 5-fold higher than those that received the H1N1 or H3N2 ca vaccine as the first dose. Thus, the H1 HA induced enhanced production of H5N1-specific neutralizing antibody was temporary. Similar results were also obtained by the HAI assay (data not shown).
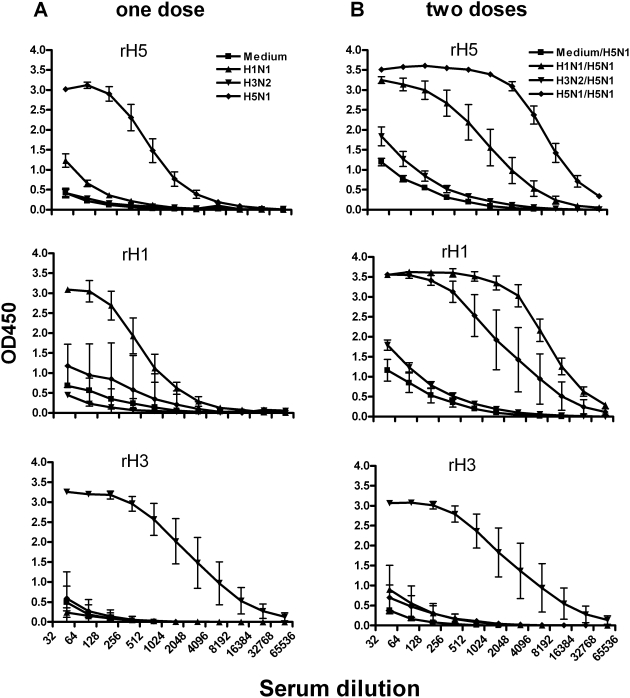
To further evaluate the H1 induced priming effect on the H5N1 antibody response, an ELISA assay was performed to measure levels of HA-specific serum IgG antibodies using rHA as the antigen (Fig. 4). Six weeks after the 1st vaccination, a substantial level of homologous HA-specific IgG was present (Fig. 4A). It was noted that the H1 and H5 specific antibodies had a low level of cross reactivity with the H5 and H1 antigens, respectively. The level of the H5-specific ELISA antibodies was much higher in the group that received the H1N1 NC99 ca virus as the first dose and the H5N1 HK03 ca virus as the second dose than the ferrets that previously received the H3N2 CA04 ca virus and those that did not receive any virus (medium), although the titer was lower than the ferrets that received 2 doses of the H5N1 HK03 ca vaccine (Fig. 4B). The antibodies from the ferrets that received 2 doses of the H5N1 HK03 ca vaccine also reacted with the rH1 HA. The H5-specific IgG antibodies continued to increase until 3 weeks following the 2nd dose and reached a level that was similar among all the groups (data not shown). Similar data were also obtained for ferrets that received the H5N1 VN04 ca vaccine as a second dose (data not shown). Thus, these data indicated that the H1- and H5-specific binding antibodies cross-reacted with each other and previous exposure to H1N1 vaccine appeared to result in a more rapid H5-specific humoral immune response to the H5 HA protein.
Figure 4. HA-specific antibodies measured by an ELISA assay.
Ferrets were intranasally inoculated with medium or the H1N1 NC99, H3N2 CA04, H5N1 HK03 ca vaccine viruses on day 0 and intranasally inoculated with the HK03 ca vaccine virus six weeks later. Serum samples were collected 6 weeks after the 1st dose (A) and one week after the 2nd dose (B). ELISA was performed with RDE-treated serum using rH5, rH1 or rH3 HA as antigens.
H1N1 vaccine-induced immunity prevented replication of the H5N1 ca vaccine virus
To determine whether previous vaccination with the H1N1 NC99 ca vaccine would affect subsequent replication of the H5N1 ca vaccine virus in the respiratory tract, groups of six naïve ferrets were primed with the H1N1 NC99 ca, H3N2 WY03 ca, H5N1 HK03 ca vaccine or medium only. Four weeks later, animals were inoculated intranasally with the H5N1 HK03 ca vaccine and sacrificed on day 3 post-inoculation to collect nasal turbinates to quantify replication of the H5N1 HK03 ca vaccine virus in the upper respiratory tract of ferrets. As shown in Table 2, the mean titer of the H5N1 HK03 ca vaccine virus detected in the upper respiratory tract of ferrets that had previously received medium was 104.5 EID50/g of tissue. In contrast, none of the ferrets that were primed with the H1N1 NC99 ca or the H5N1 HK03 ca vaccine had detectable virus in the upper respiratory tract. The H3N2 WY03 ca vaccine priming protected 2 of 6 ferrets and the H5N1 HK03 virus replicated to a mean titer of 102.2 EID50/g of tissue in this group of ferrets. These results indicated that heterosubtypic immunity from H1N1 ca virus infection or vaccination reduced H5N1 ca vaccine virus replication.
Table 2. Effect of the H1N1 and H3N2 ca vaccines on replication of the H5N1 HK03 ca virus in the upper respiratory tract of ferrets.
| Vaccine | # of animals per group | GMT of homologous HAI antibody | # of Animals with H5N1 detected in NT | Mean Virus Titer in NT (log10EID50/g±SE) |
| Medium | 6 | <4 | 6 | 4.5±0.5 |
| H1N1 NC99 ca | 6 | 47 | 0 | ≤1.5 |
| H3N2 WY03 ca | 6 | 203 | 4 | 2.2±0.7 |
| H5N1 HK03 ca | 6 | 40 | 0 | ≤1.5 |
Groups of ferrets were vaccinated with the indicated virus and four weeks later inoculated with the H5N1 HK03 ca vaccine. Antibody titers against homologous vaccine virus were determined by HAI assay and expressed as geometric mean titer. The H5N1 HK03 ca virus titer in nasal turbinates (NT) on day 3 post-inoculation is expressed as log10EID50 per gram of tissues calculated from the mean of 6 animals.
Discussion
In this study, we demonstrate that live attenuated H5N1 vaccine induced immunity can be detected by the B cell ELISpot assay. The protective level of ASC is not well established yet, however, the B cell response to the H5N1 HK03 ca vaccine is greater than to the VN04 ca vaccine and the magnitude of the ASC response correlates with serum antibody level as determined by microneutralization assay. The H5N1 HK03 and VN04 viruses differ by 9 amino acids in the HA molecule and the amino acid at position 223 is known to contribute to receptor binding specificity [29]. The HA of the HK03 virus that preferentially binds to sialic acid receptors with α2,6-linked oligosaccharide linkages contains serine at residue 223 and the HA of the VN04 virus that prefers an avian-like receptor with α2,3-linked oligosaccharide linkages contains asparagine at this residue [30], [31]. In addition, the length of the NA of the H5N1 HK03 virus differs from the VN04 virus; the VN04 virus, like most of the H5N1 isolates, has a deletion of 20 amino acids in the NA stalk whereas the HK03 virus does not have this deletion. The differences in the HA and NA sequences between the H5N1 HK03 and VN04 viruses presumably contribute to the observed difference in vaccine immunogenicity. Despite the lower immune response induced by VN04 ca virus, two doses of the H5N1 VN04 ca vaccine offered complete protection against homologous and heterologous H5N1 wt virus lethal challenge in mice and provided protection against replication of the H5N1 wt virus in the respiratory tracts of mice and ferrets [7].
In addition to the local draining lymph nodes (TLN), ASC were also detected in the PBMC of the vaccinated ferrets at a level slightly lower than those detected in TLN, therefore, only the data obtained with TLN are presented. Virus-specific memory B cells in the lungs can persist for a long time along with germinal center B cells and plasma cells and appear to be a unique feature of the mucosal memory response [16]. In response to re-encountered antigens, memory B cells robustly secrete antibodies against the pathogen and this memory response is much faster than that of primary B cells due to quantitative and qualitative changes in antigen-specific B cells and helper T cells. In this study, we found that previous exposure to the H1N1 ca virus could accelerate the memory B cell response to the H5N1 virus. The heterosubtypic antibody response as detected by ELISA in the serum could prevent replication of the H5N1 HK03 ca vaccine virus in the respiratory tract, suggesting that protective immunity was enhanced by a priming dose of H1N1 ca vaccine. A recent study also showed that the ferrets immunized with the H1N1 virus-like particles (VLP) had a low level of neutralizing antibody against the H5N1 virus and cleared the H5N1 challenge virus rapidly and had reduced morbidity [32]. There was a concern that heterosubtypic immunity might reduce vaccine efficacy by reducing vaccine virus replication in the upper respiratory tract. However, despite restricted replication of the H5N1 ca virus in the upper respiratory tract of the H1N1 exposed ferrets, the level of H5N1-specific neutralizing antibodies in the animals that previously received the H1N1 ca vaccine was similar to the seronegative animals. Our previous study indicated that two doses of the H1N1 A/New Caledonia/20/99 ca virus were unable to protect ferrets from replication of a high dose (107 TCID50) of H5N1 HK97 wt virus in the respiratory tracts of ferrets [7]. However, it remains to be determined whether the H1N1 ca virus could offer a protective benefit from a lower challenge dose of H5N1 wt virus. Despite the faster onset of immunity to the H5N1 ca vaccine, the antibodies produced in animals that received the H1N1 ca vaccine followed by the H5N1 ca vaccine are at least 5-fold lower that the animals that received two doses of the H5N1 ca vaccines. Thus, it is likely that the immunity provided by previous immunization with an H1N1 ca virus is limited and priming with seasonal LAIV cannot replace the use of 2 doses of an H5-specific vaccine.
It has been observed frequently that individuals recovered from influenza virus infection are protected against subsequent infection by antigenic drift variant viruses within the same subtype [10], [11], [12] and to a lesser extent from infection by a different subtype due to heterosubtypic immunity [13]. Recently, several reports have described heterosubtypic immunity from seasonal influenza vaccines to H5N1 infection in mice and humans [8], [14], [15], [16]. Ichinohe et al [14] showed that intranasal inoculation of an inactivated trivalent seasonal influenza vaccine provided cross-protection against H5N1 infection in mice. Such studies are difficult to conduct in humans. Ferrets develop symptoms upon influenza infection that resemble those of humans including sneezing, body temperature variation and weight loss and have been shown to be an appropriate model for influenza virus research. In this study, we demonstrated that the faster H5N1 B cell response induced by the H1N1 ca vaccine in ferrets was mediated by the H1 HA protein as demonstrated by a similar effect caused by an H1N2 virus. Although the accelerated H5N1 response following previous exposure to the H1N1 ca virus was barely detected by HAI and microneutralization assays, we found a temporal rise of mincroneutralizing antibody in H5N1 VN04 ca vaccinated ferrets that were previously exposed to the H1N1 NC04 ca virus (Table 1). We could demonstrate cross-reactivity of H1N1 and H5N1 ca vaccine induced HA antibodies by ELISA assay. These data confirmed that the H1 and H5 HA contain some conserved epitopes that could elicit cross-reactive antibodies [19], [33] because of their structure similarity [18].
N1 NA-induced protection against experimental H5N1 virus infection has been reported in mice and by the finding that human sera are capable of inhibiting the NA enzymatic activity of the H5N1 VN04 virus [17]. Our study was not designed to examine the contribution of the N1 protein of the H1N1 virus to H5N1 immunity. We cannot exclude the possibility that the N1-induced immunity might also contribute to the restricted replication of the H5N1 ca virus in the upper respiratory tract of ferrets.
Our current study indicates that previous exposure to the H3N2 ca virus was less protective than the H1N1 ca virus in restricting replication of the H5N1 ca vaccine virus in the upper respiratory tract of ferrets. However, replication of the H5N1 vaccine virus in ferrets previously primed with an H3N2 ca virus was also greatly reduced compared to the control animals. This could be because the H1N1, H3N2 and H5N1 ca vaccine viruses share 6 internal protein gene segments. As shown by the ELISpot assay, the number of ASC against the vaccine virus was much higher than HA-specific ASC (compare Fig. 2B with Fig. 2C). In addition to the protective immune response against the HA and NA surface proteins, influenza viruses also induce immune responses against conserved viral proteins such as NP and M1 that could result in heterosubtypic protection [34] to restrict H5N1 virus replication. An earlier report [35] showed that previous mucosal delivery of trivalent influenza vaccine offered protection against H5N1 wt virus lethal infection in the mouse model. We also showed previously that H2N2 AA ca vaccinated mice were partially protected from the lethal challenge of the H5N1 wt viruses [7]. This type of heterosubtypic response could be mediated by secondary CTL responses involving CD8+ or CD4+ T cells as reported previously [15], [36].
In summary, our study supports the notion that previous vaccination with seasonal influenza vaccine may accelerate onset of immunity by an H5N1 ca vaccine. Since the influenza pandemic vaccine may not be available until some time well into the first wave or early in the second wave of a pandemic, an earlier response may be of value in pandemic preparedness.
Acknowledgments
We are very grateful to Scott Jacobson, Kim Ngo, Brett Pickell, Ernesto Madariaga, Stephanie Gee and Nick Nguyen for performing the ferret studies, Anu Cherukuri for review of the manuscript, Jennifer Woo and members of HJ's group for technical support and discussions.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The funding was provided by MedImmune. This research was supported in part by the Intramural Research Program of NIAID, NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Taubenberger JK. The origin and virulence of the 1918 “Spanish” influenza virus. Proc Am Philos Soc. 2006;150:86–112. [PMC free article] [PubMed] [Google Scholar]
- 2.Scholtissek C, Rohde W, Von Hoyningen V, Rott R. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology. 1978;87:13–20. doi: 10.1016/0042-6822(78)90153-8. [DOI] [PubMed] [Google Scholar]
- 3.Schafer JR, Kawaoka Y, Bean WJ, Suss J, Senne D, et al. Origin of the pandemic 1957 H2 influenza A virus and the persistence of its possible progenitors in the avian reservoir. Virology. 1993;194:781–788. doi: 10.1006/viro.1993.1319. [DOI] [PubMed] [Google Scholar]
- 4.Subbarao K, Joseph T. Scientific barriers to developing vaccines against avian influenza viruses. Nat Rev Immunol. 2007;7:267–278. doi: 10.1038/nri2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belshe RB, Gruber WC, Mendelman PM, Mehta HB, Mahmood K, et al. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J Infect Dis. 2000;181:1133–1137. doi: 10.1086/315323. [DOI] [PubMed] [Google Scholar]
- 6.Brokstad KA, Cox RJ, Eriksson JC, Olofsson J, Jonsson R, et al. High prevalence of influenza specific antibody secreting cells in nasal mucosa. Scand J Immunol. 2001;54:243–247. doi: 10.1046/j.1365-3083.2001.00947.x. [DOI] [PubMed] [Google Scholar]
- 7.Suguitan AL, Jr, McAuliffe J, Mills KL, Jin H, Duke G, et al. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 2006;3:e360. doi: 10.1371/journal.pmed.0030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu X, Edwards LE, Desheva JA, Nguyen DC, Rekstin A, et al. Cross-protective immunity in mice induced by live-attenuated or inactivated vaccines against highly pathogenic influenza A (H5N1) viruses. Vaccine. 2006;24:6588–6593. doi: 10.1016/j.vaccine.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 9.Maassab HF. Adaptation and growth characteristics of influenza virus at 25 degrees C. Nature. 1967;213:612–614. doi: 10.1038/213612a0. [DOI] [PubMed] [Google Scholar]
- 10.Liew FY, Russell SM, Appleyard G, Brand CM, Beale J. Cross-protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA antibody rather than serum antibody or cytotoxic T cell reactivity. Eur J Immunol. 1984;14:350–356. doi: 10.1002/eji.1830140414. [DOI] [PubMed] [Google Scholar]
- 11.McMichael AJ, Gotch F, Cullen P, Askonas B, Webster RG. The human cytotoxic T cell response to influenza A vaccination. Clin Exp Immunol. 1981;43:276–284. [PMC free article] [PubMed] [Google Scholar]
- 12.Couch RB, Kasel JA. Immunity to influenza in man. Annu Rev Microbiol. 1983;37:529–549. doi: 10.1146/annurev.mi.37.100183.002525. [DOI] [PubMed] [Google Scholar]
- 13.Schulman JL, Kilbourne ED. Induction of Partial Specific Heterotypic Immunity in Mice by a Single Infection with Influenza a Virus. J Bacteriol. 1965;89:170–174. doi: 10.1128/jb.89.1.170-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichinohe T, Tamura S, Kawaguchi A, Ninomiya A, Imai M, et al. Cross-protection against H5N1 influenza virus infection is afforded by intranasal inoculation with seasonal trivalent inactivated influenza vaccine. J Infect Dis. 2007;196:1313–1320. doi: 10.1086/521304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreijtz JH, Bodewes R, van Amerongen G, Kuiken T, Fouchier RA, et al. Primary influenza A virus infection induces cross-protective immunity against a lethal infection with a heterosubtypic virus strain in mice. Vaccine. 2007;25:612–620. doi: 10.1016/j.vaccine.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi Y. Memory B cells in systemic and mucosal immune response: implications for successful vaccination. Biosci Biotechnol Biochem. 2007;71:2358–2366. doi: 10.1271/bbb.70142. [DOI] [PubMed] [Google Scholar]
- 17.Sandbulte MR, Jimenez GS, Boon AC, Smith LR, Treanor JJ, et al. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med. 2007;4:e59. doi: 10.1371/journal.pmed.0040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ha Y, Stevens DJ, Skehel JJ, Wiley DC. X-ray structures of H5 avian and H9 swine influenza virus hemagglutinins bound to avian and human receptor analogs. Proc Natl Acad Sci U S A. 2001;98:11181–11186. doi: 10.1073/pnas.201401198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smirnov YA, Lipatov AS, Gitelman AK, Okuno Y, Van Beek R, et al. An epitope shared by the hemagglutinins of H1, H2, H5, and H6 subtypes of influenza A virus. Acta Virol. 1999;43:237–244. [PubMed] [Google Scholar]
- 20.Kreijtz JH, de Mutsert G, van Baalen CA, Fouchier RA, Osterhaus AD, et al. Cross-recognition of avian H5N1 influenza virus by human cytotoxic T-lymphocyte populations directed to human influenza A virus. J Virol. 2008;82:5161–5166. doi: 10.1128/JVI.02694-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann E, Mahmood K, Chen Z, Yang C, Spaete J, et al. Multiple gene segments control the temperature sensitivity and attenuation phenotypes of ca B/Ann Arbor/1/66. J Virol. 2005;79:11014–11021. doi: 10.1128/JVI.79.17.11014-11021.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin H, Lu B, Zhou H, Ma C, Zhao J, et al. Multiple amino acid residues confer temperature sensitivity to human influenza virus vaccine strains (FluMist) derived from cold-adapted A/Ann Arbor/6/60. Virology. 2003;306:18–24. doi: 10.1016/s0042-6822(02)00035-1. [DOI] [PubMed] [Google Scholar]
- 23.Govorkova EA, Webby RJ, Humberd J, Seiler JP, Webster RG. Immunization with reverse-genetics-produced H5N1 influenza vaccine protects ferrets against homologous and heterologous challenge. J Infect Dis. 2006;194:159–167. doi: 10.1086/505225. [DOI] [PubMed] [Google Scholar]
- 24.Hampson AW. Ferrets and the challenges of H5N1 vaccine formulation. J Infect Dis. 2006;194:143–145. doi: 10.1086/505229. [DOI] [PubMed] [Google Scholar]
- 25.Lindemann M, Witzke O, Lutkes P, Fiedler M, Kreuzfelder E, et al. ELISpot assay as a sensitive tool to detect cellular immunity following influenza vaccination in kidney transplant recipients. Clin Immunol. 2006;120:342–348. doi: 10.1016/j.clim.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Cox RJ, Haaheim LR, Ericsson JC, Madhun AS, Brokstad KA. The humoral and cellular responses induced locally and systemically after parenteral influenza vaccination in man. Vaccine. 2006;24:6577–6580. doi: 10.1016/j.vaccine.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki S, Jaimes MC, Holmes TH, Dekker CL, Mahmood K, et al. Comparison of the influenza virus-specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza virus vaccines. J Virol. 2007;81:215–228. doi: 10.1128/JVI.01957-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gioia C, Castilletti C, Tempestilli M, Piacentini P, Bordi L, et al. Cross-subtype immunity against avian influenza in persons recently vaccinated for influenza. Emerg Infect Dis. 2008;14:121–128. doi: 10.3201/eid1401.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann E, Lipatov AS, Webby RJ, Govorkova EA, Webster RG. Role of specific hemagglutinin amino acids in the immunogenicity and protection of H5N1 influenza virus vaccines. Proc Natl Acad Sci U S A. 2005;102:12915–12920. doi: 10.1073/pnas.0506416102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, et al. H5N1 Virus Attachment to Lower Respiratory Tract. Science. 2006;312:399. doi: 10.1126/science.1125548. [DOI] [PubMed] [Google Scholar]
- 31.Yamada S, Suzuki Y, Suzuki T, Le MQ, Nidom CA, et al. Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature. 2006;444:378–382. doi: 10.1038/nature05264. [DOI] [PubMed] [Google Scholar]
- 32.Haynes JR, Dokken L, Wiley JA, Cawthon AG, Bigger J, et al. Influenza-pseudotyped Gag virus-like particle vaccines provide broad protection against highly pathogenic avian influenza challenge. Vaccine. 2008 doi: 10.1016/j.vaccine.2008.11.011. In Press. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed R, Oldstone MB, Palese P. Protective immunity and susceptibility to infectious diseases: lessons from the 1918 influenza pandemic. Nat Immunol. 2007;8:1188–1193. doi: 10.1038/ni1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scherle PA, Gerhard W. Functional analysis of influenza-specific helper T cell clones in vivo. T cells specific for internal viral proteins provide cognate help for B cell responses to hemagglutinin. J Exp Med. 1986;164:1114–1128. doi: 10.1084/jem.164.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tumpey TM, Renshaw M, Clements JD, Katz JM. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J Virol. 2001;75:5141–5150. doi: 10.1128/JVI.75.11.5141-5150.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamura S, Tanimoto T, Kurata T. Mechanisms of broad cross-protection provided by influenza virus infection and their application to vaccines. Jpn J Infect Dis. 2005;58:195–207. [PubMed] [Google Scholar]